Abstract
Cutis laxa (CL), a disease characterized by redundant and inelastic skin, displays extensive locus heterogeneity. Together with geroderma osteodysplasticum and arterial tortuosity syndrome, which show phenotypic overlap with CL, eleven CL-related genes have been identified to date, which encode proteins within 3 groups. Elastin, fibulin-4, fibulin-5 and and latent transforming growth factor-beta-binding protein 4 are secreted proteins which form elastic fibers and are involved in the sequestration and subsequent activation of transforming growth factor-beta (TGFβ). Proteins within the second group, localized to the secretory pathway, perform transport and membrane trafficking functions necessary for the modification and secretion of elastic fiber components. Key proteins include a subunit of the vacuolar-type proton pump, which ensures the efficient secretion of tropoelastin, the precursor or elastin. A copper transporter is required for the activity of lysyl oxidases, which crosslink collagen and elastin. A Rab6-interacting goglin recruits kinesin motors to Golgi-vesicles facilitating the transport from the Golgi to the plasma membrane. The Rab and Ras interactor 2 regulates the activity of Rab5, a small guanosine triphosphatase essential for the endocytosis of various cell surface receptors, including integrins. Proteins of the third group related to CL perform metabolic functions within the mitochondria, inhibiting the accumulation of reactive oxygen species. Two of these proteins catalyze subsequent steps in the conversion of glutamate to proline. The third transports dehydroascorbate into mitochondria. Recent studies on CL-related proteins highlight the intricate connections among membrane trafficking, metabolism, extracellular matrix assembly, and TGFβ signaling.
1. Introduction
Cutis laxa (CL) is a group of disorders characterized by redundant, pendulous, prematurely wrinkled and inelastic skin. CL can either be inherited or acquired secondary to a range of inflammatory conditions. Considerable progress has been made in identifying the genes responsible for inherited forms of CL (Table 1). A detailed review of the clinical manifestations of the different types of inherited CL and related syndromes has recently been published (Berk et al., 2012). Recent reviews also discussed the genetic heterogeneity of CL and its implications for the complexity of elastic fiber biogenesis (Uitto et al., 2013; Urban, 2012). Complementary human genetic, biochemical, cell biological and animal model studies now suggest that many forms of inherited CL display abnormalities both of elastic fibers biogenesis and of transforming growth factor-β (TGFβ) signaling, membrane trafficking and mitochondrial metabolism, highlighting the integration of these processes at multiple levels. The goal of this review is to discuss these latest insights.
Table 1.
Cutis laxa and related genes
| Disease* | Distinguishing Clinical Features | Mutated Genes | Gene function | References |
|---|---|---|---|---|
| ADCL | Pulmonary and cardiovascular manifestations absent, milder or later onset | ELN | Structural protein of the elastic fibers | (Callewaert et al., 2011; Hu et al., 2010) |
| ARCL1A | Supravalvular aortic stenosis, lethal developmental emphysema | FBLN5 | Accessory protein of the elastic fibers, binds ELN, FBN1, LOXL1, LTBP2 and LTBP4 | (Loeys et al., 2002) |
| ARCL1B | Arterial tortuosity, lethal pulmonary hypertension, bone fragility | FBLN4 EFEMP2 | Accessory protein of the elastic fibers, binds LOX and FBN1 | (Dasouki et al., 2007; Hucthagowder et al., 2006; Renard et al., 2010) |
| ARCL1C/URDS | Severe gastrointestinal and urinary malformations, lethal developmental emphysema mild cardiovascular involvement | LTBP4 | Accessory protein of the elastic fibers, binds TGFB1, FN, FBN1, and FBLN5 | (Urban et al., 2009) |
| ARCL2A | Growth and developmental delay, abnormal glycosylation of serum proteins | ATP6V0A2 | A subunit of the vacuolar-type proton pump, acidifies vesicles in the secretory pathway | (Hucthagowder et al., 2009; Kornak et al., 2008) |
| ARCL2B | Growth and developmental delay, triangular face, normal glycosylation | PYCR1 | Mitochondrial enzyme in the proline-biosynthetic pathway | (Guernsey et al., 2009; Reversade et al., 2009) |
| XLCL | Occipital exostoses, pili torti | ATP7A | Copper transporter required for lysyl oxidase activity | (Byers et al., 1980; Kennerson et al., 2010; Moller et al., 2005) |
| DBS/ARCL3 | Corneal clouding, athetoid movements | ALDH18A1 | Mitochondrial enzyme in the proline-biosynthetic pathway | (Bicknell et al., 2008; Skidmore et al., 2011) |
| GO | Bone fragility, short stature | GORAB | Localized to the Golgi, binds RAB6, a G-protein involved in vesicle trafficking | (Hennies et al., 2008) |
| MACS | Macrocephaly, alopecia, scoliosis | RIN2 | Localized to the Golgi, is a guanine nucleotide exchange factor of RAB5 | (Basel-Vanagaite et al., 2009) |
| ATS | Triangular face, arterial tortuosity, normal lungs | SLC2A10 | Transports dehydroascorbate into mitochondria | (Coucke et al., 2006; Lee et al., 2010) |
ADCL, autosomal dominant cutis laxa; ARCL, autosomal recessive cutis laxa; URDS, Urban-Rifkin-Davis syndrome; XLCL, X-linked cutis laxa; DBS, DeBarsy syndrome; GO, geroderma osteodysplasticum; MACS, macrocephaly alopecia cutis laxa scoliosis syndrome;ATS
2. CL genes in elastic fiber biogenesis
The skin manifestations in inherited CL are usually congenital and generalized, and are commonly associated with involvement of elastic tissues within the respiratory and cardiovascular systems (Berk et al., 2012). The phenotype is consistent with a generalized elastinopathy. However, some forms of CL are also associated with growth and developmental delay, craniofacial anomalies, reduced bone density, joint and ligament laxity, hernias and gastrointestinal and urinary tract lesions suggesting broader connective tissue involvement (Table 1). Histological and electron microscopic studies have shown a range of elastic fiber abnormalities in CL, consistent with defective assembly of these extracellular matrix (ECM) structures (Figure 1). Consistently, several genes that encode proteins associated with elastic fibers, including elastin, fibulin-4, fibulin-5 and latent TGFβ-binding protein 4 (LTBP4), have been identified to harbor mutations causing CL (Table 1). The mechanisms by which mutations in these genes lead to abnormal elastic fiber assembly and altered TGFβ signaling is beginning to emerge.
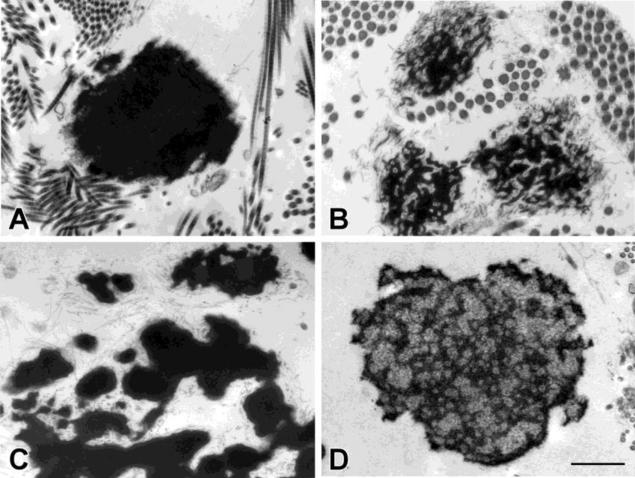
Figure 1.
Electron microscopic analyses of elastic fibers in skin punch biopsies from unaffected and CL patients. (A) An unaffected individual; (B) A patient with a heterozygous mutation in the elastin gene; (C) A patient with a homozygous mutation in the LTBP4 gene; (D) Dermal elastic fibers from a patient with CL of an unknown cause. Bar = 500 nm.
Autosomal dominant CL (ADCL) is most commonly caused by frameshift mutations within the last 5 exons of the elastin gene (ELN) (Callewaert et al., 2011; Hadj-Rabia et al., 2013). These mutations lead to the replacement of the C-terminus of the elastin precursor, tropoelastin, with a missense peptide sequence as a consequence of a stable mutant mRNA and protein (Callewaert et al., 2011; Szabo et al., 2006). ADCL-causing mutations have multiple cellular and biochemical effects. The most upstream (exon 30) mutations result in the longest missense peptide sequence, which activates the unfolded protein response (UPR) and leads to increased rates of apoptosis both in patient-derived skin fibroblasts (Callewaert et al., 2011) and in a transgenic mouse model (Hu et al., 2010). Despite activation of the UPR, the mutant tropoelastin is partially secreted by ADCL cells and alters the assembly of the elastic fibers by interfering with the binding of tropoelastin to fibulin-5 and fibrillin-1 (Sato et al., 2006). In addition, ADCL mutations increase the coacervation of tropoelastin in a dominant manner (Callewaert et al., 2011). The effects of these biochemical changes include ultrastructural disorganization and altered mechanical properties of the elastic fibers (Callewaert et al., 2011; Hu et al., 2010). In transgenic mouse models, the mutant tropoelastin was observed to contribute to lung elastic fibers, but was largely excluded from fibers in the skin and blood vessels (Hu et al., 2010; Sugitani et al., 2012). Although these findings point to tissue-specific mechanisms of elastic fiber assembly, the precise molecular determinants of such tissue specificity remain to be determined. Nevertheless, it is clear that ADCL-related ELN mutations are characterized by a complex molecular disease mechanism with a gain of function in polymerization and a loss of function in elastin-microfibril interaction coupled with some non-specific toxic effects associated with protein misfolding.
The profound functional consequences of alterations at the C-terminus of tropoelastin in ADCL strengthen an existing body of evidence for the essential nature of this region for a variety of activities. For example, the last 17 amino acids of tropoelastin are key for cell attachment both via heparan sulfate proteoglycans (HSPGs) (Broekelmann et al., 2005) and through non-RGD-mediated αvβ3 integrin binding (Bax et al., 2009). Consistent with the dynamic, cell-mediated nature of elastic fiber assembly (Czirok et al., 2006; Kozel et al., 2006), antibodies against this C-terminal cell interaction domain interfere with elastin deposition (Brown-Augsburger et al., 1996). Additionally, the hydrophobic domain encoded by exon 30 is essential for tropoelastin polymerization, and hence, fiber formation (Kozel et al., 2003).
Whereas the molecular mechanisms of ADCL are quite complex, autosomal recessive CL (ARCL) involves a simpler, loss of function mechanism. Type 1 ARCL (ARCL1), characterized by severe cardiovascular or pulmonary manifestations (Table 1), can be caused by mutations in fibulin-5 (FBLN5, ARCL1A) (Loeys et al., 2002), fibulin-4 (FBLN4/EFEMP2, ARCL1B) (Hucthagowder et al., 2006), and LTBP4 (ARCL1C) (Urban et al., 2009).
Loss of function mutations in FBLN5 result in ARCL1A associated with severe, often fatal, infantile respiratory distress related to developmental emphysema. Other common manifestations include supravalvular aortic stenosis, pulmonary artery stenosis and inguinal hernias (Callewaert et al., 2013; Claus et al., 2008; Elahi et al., 2006; Loeys et al., 2002). Fibulin-5 knockout mice show a similar phenotype with skin laxity, developmental emphysema, elongation, tortuosity and altered branching of the large arteries (Nakamura et al., 2002; Yanagisawa et al., 2002), an increased angiogenic phenotype (Sullivan et al., 2007), and pelvic prolapse (Drewes et al., 2007). ARCL1A-causing mutations affect the folding and secretion of fibulin-5 resulting in a failure of elastin to be properly integrated with microfibrils (Choi et al., 2009; Hu et al., 2006; Lotery et al., 2006). Biochemical studies have highlighted a number of activities of fibulin-5, including its ability to bind lysyl oxidase-like-1 (LOXL1) (Hirai et al., 2007b; Liu et al., 2004), LOXL2, and LOXL4 (Hirai et al., 2007b), fibrillin-1 (El-Hallous et al., 2007; Freeman et al., 2005; Ono et al., 2009), tropoelastin (Hirai et al., 2007b; Yanagisawa et al., 2002), LTBP2 (Hirai et al., 2007a) and LTBP4 (Noda et al., 2013). The binding of fibulin-5 increases the coacervation and crosslinking of tropoelastin, facilitating elastic fiber formation (Hirai et al., 2007b; Wachi et al., 2008). Furthermore, an RGD motif in fibulin-5 binds αvβ3, αvβ5, α4β1, α5β1 and α9β1 integrins (Lomas et al., 2007; Nakamura et al., 2002). In vascular smooth muscle cells (VSMC), the main integrins responsible for attachment to fibulin-5 are α4β1 and α5β1, however, binding to fibulin-5 does not result in integrin activation (Lomas et al., 2007). Additionally, in vivo evidence suggests that fibulin-5-integrin interactions are not necessary for elastic fiber formation, as mice carrying homozygous RGD>RGE mutations in fibulin-5 are normal (Budatha et al., 2011).
Fibulin-5-deficient tissues show increased angiogenesis and vascular invasion, with elevated VEGF and angiopoietin expression playing a role (Sullivan et al., 2007). Conversely, transplanted tumors show decreased growth in fibulin-5 knockout mice associated with increased reactive oxygen species (ROS) production dependent on β1-integrin and fibronectin (Schluterman et al., 2010). Fibulin-5 also binds the extracellular superoxide dismutase, Sod3. In the absence of fibulin-5, the localization of Sod3 to the ECM is lost, leading to an elevated extracellular superoxide levels in vessels (Nguyen et al., 2004). However, it remains unclear if any of these physiological changes contribute to disease in human patients with fibulin-5 mutations.
Mutations in FBLN4cause ARCL1B, a disease associated with widespread systemic involvement, including arterial tortuosity, aortic aneurysm, pulmonary hypertension, developmental emphysema, bone fragility, aranchnodactyly, joint laxity and diaphragmatic and inguinal hernias (Dasouki et al., 2007; Hoyer et al., 2009; Hucthagowder et al., 2006; Renard et al., 2010). Knockout and hypomorphic mice replicate many of these phenotypes (Hanada et al., 2007; Horiguchi et al., 2009; Huang et al., 2010; McLaughlin et al., 2006). Fibulin-4 binds tropoelastin (McLaughlin et al., 2006), fibrillin-1 (Choudhury et al., 2009; El-Hallous et al., 2007; Ono et al., 2009) and LTBP1 (Massam-Wu et al., 2010). A somewhat different set of ligands, distinct binding affinities for shared ligands and cell type- and developmental stage-specific expression of fibulin-5 and fibulin-4 ensure that these proteins perform non-overlapping functions in elastic fiber assembly. For example, fibulin-5 has higher affinity for tropoelastin than fibrillin-1, whereas fibulin-4 preferentially binds fibrillin-1 over tropoelastin. Fibulin-4 can form a ternary complex with tropoelastin and LOX, facilitating the crosslinking of elastin (Choudhury et al., 2009; Horiguchi et al., 2009). In addition, fibulin-5 enhances tropoelastin coacervation (Hirai et al., 2007b; Wachi et al., 2008). Both fibulin-4 and fibulin-5 limit tropoelastin droplet size during the maturation of coacervates (Cirulis et al., 2008). In both human mutations and animal models, the absence of fibulin-5 leads to elastin deposits that are large and not integrated with the microfibril scaffold (Hu et al., 2006; Nakamura et al., 2002; Yanagisawa et al., 2002). In contrast, fibulin-4 mutant patients and mice show greatly reduced amounts of elastic fibers, a decrease in elastin-specific desmosine crosslinks and disorganization of the elastic fiber ultrastructure (Horiguchi et al., 2009; Hucthagowder et al., 2006; McLaughlin et al., 2006).
In a VSMC conditional knockout of fibulin-4, the VSMC were shown to be undifferentiated, together with enhanced angiotensin production, increased ERK signaling and cell proliferation (Huang et al., 2010; Huang et al., 2013). Aortic aneurysms caused by fibulin-4 deficiency can be prevented by treatment with an angiotensin converting enzyme inhibitor (captopril) or an angiotensin II type 1 receptor inhibitor (losartan) (Huang et al., 2013; Moller et al., 2005), indicating a role for fibulin-4 in the regulation of angiotensin signaling and VSMC homeostasis and identifying a potential approach for the treatment of patients with fibulin-4 mutations.
Mutations in LTBP4 cause ARCL1C, also known as Urban-Rifkin-Davis syndrome, characterized by severe developmental emphysema, severe diverticulosis, tortuosity, enlargement and stenosis of the gastrointestinal tract, diverticulosis of the bladder and diaphragmatic and inguinal hernias (Callewaert et al., 2013; Urban et al., 2009). Respiratory failure and, less frequently, intestinal perforation are causes of premature death. LTBP4 utilizes 3 alternative promoters producing one small (LTBP4S) and 2 large (LTBP4L) isoforms. Human mutations identified to date affect all isoforms. A knockout mouse has only been reported for Ltbp4S (Sterner-Kock et al., 2002), which nevertheless replicates the elastic fiber phenotypes seen in humans, such as developmental emphysema and the abnormal morphology of elastic fibers with large elastin deposits with a smooth surface devoid of microfibrils (Dabovic et al., 2009; Urban et al., 2009). However, the structural anomalies of the gastrointestinal and urinary systems observed in humans with LTBP4 mutations are not present in Ltbp4S−/− mice, suggesting that normal development of the digestive system and bladder primarily requires LTBP4L.
An increasing body of evidence shows that LTBP4 has at least two functions, one related to TGFβ sequestration, and the other for facilitating elastic fiber assembly. Indeed, the developmental emphysema observed in Ltbp4S−/− mice could be partially reversed by reducing the TGFβ2 dose, but the elastic fiber abnormality was not corrected (Dabovic et al., 2009). LTBP4 isoforms are functionally specialized, with LTBP4L preferentially binding TGFβ, and LTBP4S associated with the ECM (Kantola et al., 2010). Consistent with phenotypic similarities between ARCL1A and ARCL1C and between Fbln5−/− and Ltbp4S−/− mice, LTBP4 binds fibulin-5 (Noda et al., 2013) and fibrillin-1 (Isogai et al., 2003), facilitating the incorporation of fibulin-5/elastin complexes onto microfibrils. LTBP4 is also known to interact with fibronectin and HSPGs and is capable of supporting cell adhesion (Kantola et al., 2008). Although the importance of these functions for elastic fiber formation or TGFβ signaling remains unclear, a human mutation eliminating the C-terminal cell-attachment region results in microfibrillar bundles of abnormally thick and wavy morphology (Callewaert et al., 2013) suggesting that a balanced binding of cells and ECM molecules is necessary for LTBP4 to contribute to normal deposition of elastic fibers.
4. CL and TGFβ signaling
TGFβs are a family of cytokines involved in reciprocal interactions with the ECM. They are secreted in a latent form strongly but non-covalently bound to their propeptides, also known as latency-associated peptides, which in turn interact with latent TGFβ-binding proteins (LTBPs). LTBPs target latent TGFβs to the ECM by binding to fibrillin-1, fibronectin and HSPGs. Activation of latent TGFβs can occur by integrin-mediated force generation, proteolytic degradation, exposure to ROS or interaction with matricellular proteins, such as thrombospondin-1 (Horiguchi et al., 2012).
TGFβs, in turn, up-regulate the expression of many genes necessary for the production of elastic fibers including fibronectin (Ignotz et al., 1987), LTBPs (Ahmed et al., 1998; Weikkolainen et al., 2003), ELN (Kahari et al., 1992; Kucich et al., 1997), LOXs (Boak et al., 1994; Kim et al., 2008) and FBLN5 (Kuang et al., 2006). This regulation occurs at the transcriptional (Ahmed et al., 1998; Ignotz et al., 1987; Kim et al., 2008; Kuang et al., 2006) or posttranscriptional level depending on the gene (Kahari et al., 1992; Kucich et al., 1997). Posttranscriptional regulation of ELN and fibrillin-1 (FBN1) is likely achieved in part by TGFβ-mediated suppression of the miR29 family of micro-RNAs (van Rooij et al., 2008), which have binding sites in several mRNAs encoding elastic fiber proteins (Table 2).
Table 2.
Elastic fiber genes as targets of miR29 regulation*
| Number of binding sites (target score) | ||||
|---|---|---|---|---|
| Gene | miR29a-3p | miR29b-3p | miR29c-3p | miR29b-5p |
| ELN | 3 (78) | 3 (77) | 3 (78) | 0 |
| FBN1 | 2 (86) | 2 (85) | 2 (85) | 1 (78) |
| LOX | 3 (81) | 3 (81) | 3 (80) | 0 |
| LTBP1 | 0 | 0 | 0 | 1 (53) |
Data based on miRDB searches (http://mirdb.org/miRDB/)
During the course of normal development or injury, sequestration of TGFβ by elastic fibers serves as a negative feedback signal indicating that sufficient amounts and quality of ECM has been produced. Consistently, loss of function mutations in genes responsible for TGFβ sequestration, including FBN1 and LTBP4, result in elevated TGFβ signaling (Dabovic et al., 2009; Neptune et al., 2003; Urban et al., 2009). However, recent studies have shown elevated TGFβ in several types of CL caused by mutations in genes not directly involved in TGFβ sequestration, but rather required for the biogenesis of elastic fibers including ELN (Callewaert et al., 2011; Hu et al., 2010), fibulin-4 (Hanada et al., 2007; Renard et al., 2010) and ATP6V0A2 (Fischer et al., 2012). Thus, impaired elastic fiber function sensed by cells, in turn up-regulates TGFβ activity. Sensing of ECM dysfunction may involve integrin-mediated activation of TGFβ itself, which is dependent on the mechanical properties of the ECM, as well as on force generation by the cells (Hinz, 2009). Consistently, mutations in actin (ACTA2) and myosin (MYH11) genes, required for the contractility of VSMC, are mutated in familial thoracic aortic aneurysms and are associated with increased TGFβ signaling (Renard et al., 2013).
5. The CL proteins and TGFβ in evolution
In humans, there are three fibrillins and four LTBPs. These proteins contain multiple TGFβ-binding (TB) domains interspersed with numerous calcium-binding epidermal growth factor domains. The TB domain, which is found in no other proteins, emerged over 600 million years ago in the Eumetazoa (Peterson and Butterfield, 2005) within a single fibrillin gene (Piha-Gossack et al., 2012). Diversification of the TB domain led to the emergence of the first LTBP-like protein in sea urchins (Robertson et al., 2011). TGFβ also appeared at this time suggesting co-evolution of their functions (Robertson et al., 2011). Elastin appeared later still, with the divergence of jawless fish (agnatha) and jawed vertebrates, known as gnathostomes within the phylum Chordata (Keeley, 2013). This time period coincided with a duplication event leading to two fibrillins (Piha-Gossack et al., 2012), and the appearance of a closed circulatory system (Faury, 2001). The single fibrillin had also acquired a unique hybrid domain and RGD-intergin binding sites in two of the TB domains by this time (Piha-Gossack et al., 2012). Like fibrillin, fibulin-like proteins also existed in the Eumetazoa, much earlier than the emergence of elastin supporting a functional role for fibulins independent of elastic fiber assembly (Segade, 2010). Interestingly, diversification of the fibulins also occurred at the same time as the evolution of elastin. The appearance of specialized domains and the occurrence of duplication events around the time of the evolution of elastin underscores the potential functional significance of these specialized regions and duplicated proteins in elastic fiber assembly.
6. CL and the secretory pathway
Several CL-related genes are required for intracellular protein trafficking, highlighting the importance of the secretory pathway in elastic fiber biogenesis. Loss of function mutations in ATP7A, a copper transporter localized to the Golgi apparatus, cause Menkes disease or a milder disease, occipital horn syndrome (OHS) (Das et al., 1995). OHS, also known as X-linked CL displays LOX deficiency associated with impaired crosslinking of elastin and collagen (Byers et al., 1980). Recessive mutations in the gene for the A2 subunit of the vacuolar proton pump, ATP6V0A2, cause ARCL2A (Kornak et al., 2008). ATP6V0A2-deficient cells show accumulation of tropoelastin in the Golgi, Golgi fragmentation and an accumulation of lamellar bodies, leading to impaired secretion of tropoelastin, but relatively preserved production of fibrillin-1 and LOXs (Hucthagowder et al., 2009). Macrocephaly alopecia CL scoliosis (MACS) syndrome is caused by a recessive mutation in RIN2 (Ras and Rab interactor 2) (Basel-Vanagaite et al., 2009). Subsequently, a recessive RIN2 mutation was also described in an Ehlers-Danlos syndrome-like condition (Syx et al., 2010). Tissues and cells from individuals with RIN2 mutations show abnormal endoplasmic reticulum, Golgi apparatus, elastic and collagen fibers (Basel-Vanagaite et al., 2009; Syx et al., 2010). Geroderma osteodysplasticum, a disease related to CL, is caused by recessive mutations in the GORAB (goglin, Rab6 interacting) (Hennies et al., 2008). GORAB is localized to the Golgi apparatus where it interacts with Rab6 G-proteins, which recruit kinesin motor proteins required for the trafficking of secretory vesicles to the plasma membrane (Grigoriev et al., 2007).
7. CL and metabolism
Three conditions related to CL are caused by mutations in molecules required for cellular metabolism. ARCL2B, is caused by recessive mutations in gene for pyrroline-5-carboxylate reductase 1 (PYCR1) (Guernsey et al., 2009; Reversade et al., 2009), a mitochondrial enzyme that catalyzes the final step of proline biosynthesis (De Ingeniis et al., 2012). PYCR1 mutant cells do not show overt proline deficiency, but are sensitive to oxidative stress, suggesting that PYCR1 is required for the maintenance of mitochondrial antioxidant balance (Reversade et al., 2009). De Barsy syndrome, also known as ARCL3, can be caused by mutations in ALDH18A1 (Bicknell et al., 2008; Skidmore et al., 2011), the gene for another mitochondrial enzyme in the proline biosynthetic pathway, Δ1-pyrroline-5-carboxylate synthase (P5CS). In ALDH18A1 mutant cells, the assembly of collagen type I and elastin into ECM fibers is diminished (Bicknell et al., 2008; Skidmore et al., 2011). Arterial tortuosity syndrome (ATS), related to CL, is caused by mutations in the SLC2A10 gene (Coucke et al., 2006), which encodes the facilitative glucose transporter family member 10 (GLUT10). Patients show disorganized elastic fibers in the arterial wall and elevated TGFβ signaling. Surprisingly, mice with inactivating missense mutations in Slc2a10 do not show phenotypes characteristic of ATS (Callewaert et al., 2008; Cheng et al., 2009). In contrast, slc2a10 knockdown in zebrafish produces disorganization of the vasculature, wavy notochord and cardiac edema, as well as mitochondrial dysfunction and reduced TGFβ signaling (Willaert et al., 2012). SLC2A10 was shown to be transport dehydroascorbate (oxidized vitamin C) into mitochondria to limit the production of ROS (Lee et al., 2010). Thus, together with PYCR1, SLC2A10 is also required for the maintenance of mitochondrial redox balance.
8. Concluding remarks
Human genetic studies on CL patients have revealed a network of genes required for elastic fiber assembly, TGFβ sequestration and activation, vesicular trafficking in the Golgi apparatus and metabolic function of the mitochondria. The connections between secreted proteins (elastin, fibulin-4, fibulin-5, LTBP4) and their interactions with other elastic fiber-related proteins (fibronectin, lysyl oxidases, fibrillins, LTBPs) are well known and often involve direct binding. However, the temporal and spatial hierarchy of these interactions has not been defined yet. A combination of developmental and live imaging investigations will be necessary to have a comprehensive mechanistic view of elastic fiber assembly. The involvement of the posttranslational and sorting mechanisms that occur in the secretory pathway will also need to be investigated, as they may define the timing, order and precise biochemical milieu in which the components can interact. Finally, the role of small molecule metabolites (ROS, proline) as well as signaling molecules (angiotensin, TGFβ) in elastic fiber biogenesis and dysfunction will need to be elucidated, as these molecules are more amenable to therapeutic modification than structural elastic fiber proteins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed W, Kucich U, Abrams W, Bashir M, Rosenbloom J, Segade F, Mecham R, Rosenbloom J. Signaling pathway by which TGF-beta1 increases expression of latent TGF-beta binding protein-2 at the transcriptional level. Connective tissue research. 1998;37:263–276. doi: 10.3109/03008209809002444. [DOI] [PubMed] [Google Scholar]
- Basel-Vanagaite L, Sarig O, Hershkovitz D, Fuchs-Telem D, Rapaport D, Gat A, Isman G, Shirazi I, Shohat M, Enk CD, Birk E, Kohlhase J, Matysiak-Scholze U, Maya I, Knopf C, Peffekoven A, Hennies HC, Bergman R, Horowitz M, Ishida-Yamamoto A, Sprecher E. RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome. Am J Hum Genet. 2009;85:254–263. doi: 10.1016/j.ajhg.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax DV, Rodgers UR, Bilek MM, Weiss AS. Cell adhesion to tropoelastin is mediated via the C-terminal GRKRK motif and integrin alphaVbeta3. The Journal of biological chemistry. 2009;284:28616–28623. doi: 10.1074/jbc.M109.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z. Cutis laxa: a review. J Am Acad Dermatol. 2012;66:842, e841–817. doi: 10.1016/j.jaad.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bicknell LS, Pitt J, Aftimos S, Ramadas R, Maw MA, Robertson SP. A missense mutation in ALDH18A1, encoding Delta1-pyrroline-5-carboxylate synthase (P5CS), causes an autosomal recessive neurocutaneous syndrome. Eur J Hum Genet. 2008;16:1176–1186. doi: 10.1038/ejhg.2008.91. [DOI] [PubMed] [Google Scholar]
- Boak AM, Roy R, Berk J, Taylor L, Polgar P, Goldstein RH, Kagan HM. Regulation of lysyl oxidase expression in lung fibroblasts by transforming growth factor-beta 1 and prostaglandin E2. American journal of respiratory cell and molecular biology. 1994;11:751–755. doi: 10.1165/ajrcmb.11.6.7946403. [DOI] [PubMed] [Google Scholar]
- Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, Mecham RP. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. The Journal of biological chemistry. 2005;280:40939–40947. doi: 10.1074/jbc.M507309200. [DOI] [PubMed] [Google Scholar]
- Brown-Augsburger P, Broekelmann T, Rosenbloom J, Mecham RP. Functional domains on elastin and microfibril-associated glycoprotein involved in elastic fibre assembly. The Biochemical journal. 1996;318(Pt 1):149–155. doi: 10.1042/bj3180149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budatha M, Roshanravan S, Zheng Q, Weislander C, Chapman SL, Davis EC, Starcher B, Word RA, Yanagisawa H. Extracellular matrix proteases contribute to progression of pelvic organ prolapse in mice and humans. The Journal of clinical investigation. 2011;121:2048–2059. doi: 10.1172/JCI45636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers PH, Siegel RC, Holbrook KA, Narayanan AS, Bornstein P, Hall JG. X-linked cutis laxa: defective cross-link formation in collagen due to decreased lysyl oxidase activity. N Engl J Med. 1980;303:61–65. doi: 10.1056/NEJM198007103030201. [DOI] [PubMed] [Google Scholar]
- Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F, Mecham RP, Loeys B, Coucke PJ, De Paepe A, Urban Z. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum Mutat. 2011;32:445–455. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert B, Su CT, Van Damme T, Vlummens P, Malfait F, Vanakker O, Schulz B, Mac Neal M, Davis EC, Lee JG, Salhi A, Unger S, Heimdal K, De Almeida S, Kornak U, Gaspar H, Bresson JL, Prescott K, Gosendi ME, Mansour S, Pierard GE, Madan-Khetarpal S, Sciurba FC, Symoens S, Coucke PJ, Van Maldergem L, Urban Z, De Paepe A. Comprehensive clinical and molecular analysis of 12 families with type 1 recessive cutis laxa. Hum Mutat. 2013;34:111–121. doi: 10.1002/humu.22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert BL, Loeys BL, Casteleyn C, Willaert A, Dewint P, De Backer J, Sedlmeier R, Simoens P, De Paepe AM, Coucke PJ. Absence of arterial phenotype in mice with homozygous slc2A10 missense substitutions. Genesis. 2008;46:385–389. doi: 10.1002/dvg.20409. [DOI] [PubMed] [Google Scholar]
- Cheng CH, Kikuchi T, Chen YH, Sabbagha NG, Lee YC, Pan HJ, Chang C, Chen YT. Mutations in the SLC2A10 gene cause arterial abnormalities in mice. Cardiovascular research. 2009;81:381–388. doi: 10.1093/cvr/cvn319. [DOI] [PubMed] [Google Scholar]
- Choi J, Bergdahl A, Zheng Q, Starcher B, Yanagisawa H, Davis EC. Analysis of dermal elastic fibers in the absence of fibulin-5 reveals potential roles for fibulin-5 in elastic fiber assembly. Matrix Biol. 2009;28:211–220. doi: 10.1016/j.matbio.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury R, McGovern A, Ridley C, Cain SA, Baldwin A, Wang MC, Guo C, Mironov A, Jr., Drymoussi Z, Trump D, Shuttleworth A, Baldock C, Kielty CM. Differential regulation of elastic fiber formation by fibulin-4 and -5. The Journal of biological chemistry. 2009;284:24553–24567. doi: 10.1074/jbc.M109.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulis JT, Bellingham CM, Davis EC, Hubmacher D, Reinhardt DP, Mecham RP, Keeley FW. Fibrillins, fibulins, and matrix-associated glycoprotein modulate the kinetics and morphology of in vitro self-assembly of a recombinant elastin-like polypeptide. Biochemistry. 2008;47:12601–12613. doi: 10.1021/bi8005384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus S, Fischer J, Megarbane H, Megarbane A, Jobard F, Debret R, Peyrol S, Saker S, Devillers M, Sommer P, Damour O. A p.C217R mutation in fibulin-5 from cutis laxa patients is associated with incomplete extracellular matrix formation in a skin equivalent model. J Invest Dermatol. 2008;128:1442–1450. doi: 10.1038/sj.jid.5701211. [DOI] [PubMed] [Google Scholar]
- Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, Fox JE, Mancini GM, Kambouris M, Gardella R, Facchetti F, Willems PJ, Forsyth R, Dietz HC, Barlati S, Colombi M, Loeys B, De Paepe A. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- Czirok A, Zach J, Kozel BA, Mecham RP, Davis EC, Rongish BJ. Elastic fiber macro-assembly is a hierarchical, cell motion-mediated process. Journal of cellular physiology. 2006;207:97–106. doi: 10.1002/jcp.20573. [DOI] [PubMed] [Google Scholar]
- Dabovic B, Chen Y, Choi J, Vassallo M, Dietz HC, Ramirez F, von Melchner H, Davis EC, Rifkin DB. Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity. Journal of cellular physiology. 2009;219:14–22. doi: 10.1002/jcp.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Levinson B, Vulpe C, Whitney S, Gitschier J, Packman S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am J Hum Genet. 1995;56:570–576. [PMC free article] [PubMed] [Google Scholar]
- Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A. 2007;143A:2635–2641. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- De Ingeniis J, Ratnikov B, Richardson AD, Scott DA, Aza-Blanc P, De SK, Kazanov M, Pellecchia M, Ronai Z, Osterman AL, Smith JW. Functional specialization in proline biosynthesis of melanoma. PloS one. 2012;7:e45190. doi: 10.1371/journal.pone.0045190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewes PG, Yanagisawa H, Starcher B, Hornstra I, Csiszar K, Marinis SI, Keller P, Word RA. Pelvic organ prolapse in fibulin-5 knockout mice: pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. The American journal of pathology. 2007;170:578–589. doi: 10.2353/ajpath.2007.060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hallous E, Sasaki T, Hubmacher D, Getie M, Tiedemann K, Brinckmann J, Batge B, Davis EC, Reinhardt DP. Fibrillin-1 interactions with fibulins depend on the first hybrid domain and provide an adaptor function to tropoelastin. The Journal of biological chemistry. 2007;282:8935–8946. doi: 10.1074/jbc.M608204200. [DOI] [PubMed] [Google Scholar]
- Elahi E, Kalhor R, Banihosseini SS, Torabi N, Pour-Jafari H, Houshmand M, Amini SS, Ramezani A, Loeys B. Homozygous missense mutation in fibulin-5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. J Invest Dermatol. 2006;126:1506–1509. doi: 10.1038/sj.jid.5700247. [DOI] [PubMed] [Google Scholar]
- Faury G. Function-structure relationship of elastic arteries in evolution: from microfibrils to elastin and elastic fibres. Pathologie-biologie. 2001;49:310–325. doi: 10.1016/s0369-8114(01)00147-x. [DOI] [PubMed] [Google Scholar]
- Fischer B, Dimopoulou A, Egerer J, Gardeitchik T, Kidd A, Jost D, Kayserili H, Alanay Y, Tantcheva-Poor I, Mangold E, Daumer-Haas C, Phadke S, Peirano RI, Heusel J, Desphande C, Gupta N, Nanda A, Felix E, Berry-Kravis E, Kabra M, Wevers RA, van Maldergem L, Mundlos S, Morava E, Kornak U. Further characterization of ATP6V0A2-related autosomal recessive cutis laxa. Human genetics. 2012;131:1761–1773. doi: 10.1007/s00439-012-1197-8. [DOI] [PubMed] [Google Scholar]
- Freeman LJ, Lomas A, Hodson N, Sherratt MJ, Mellody KT, Weiss AS, Shuttleworth A, Kielty CM. Fibulin-5 interacts with fibrillin-1 molecules and microfibrils. The Biochemical journal. 2005;388:1–5. doi: 10.1042/BJ20050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, Akhmanova A. Rab6 regulates transport and targeting of exocytotic carriers. Developmental cell. 2007;13:305–314. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Guernsey DL, Jiang H, Evans SC, Ferguson M, Matsuoka M, Nightingale M, Rideout AL, Provost S, Bedard K, Orr A, Dube MP, Ludman M, Samuels ME. Mutation in pyrroline-5-carboxylate reductase 1 gene in families with cutis laxa type 2. Am J Hum Genet. 2009;85:120–129. doi: 10.1016/j.ajhg.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadj-Rabia S, Callewaert BL, Bourrat E, Kempers M, Plomp AS, Layet V, Bartholdi D, Renard M, De Backer J, Malfait F, Vanakker OM, Coucke PJ, De Paepe AM, Bodemer C. Twenty patients including 7 probands with autosomal dominant cutis laxa confirm clinical and molecular homogeneity. Orphanet journal of rare diseases. 2013;8:36. doi: 10.1186/1750-1172-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circulation research. 2007;100:738–746. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- Hennies HC, Kornak U, Zhang H, Egerer J, Zhang X, Seifert W, Kuhnisch J, Budde B, Natebus M, Brancati F, Wilcox WR, Muller D, Kaplan PB, Rajab A, Zampino G, Fodale V, Dallapiccola B, Newman W, Metcalfe K, Clayton-Smith J, Tassabehji M, Steinmann B, Barr FA, Nurnberg P, Wieacker P, Mundlos S. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008;40:1410–1412. doi: 10.1038/ng.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Current rheumatology reports. 2009;11:120–126. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- Hirai M, Horiguchi M, Ohbayashi T, Kita T, Chien KR, Nakamura T. Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly. The EMBO journal. 2007a;26:3283–3295. doi: 10.1038/sj.emboj.7601768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Ohbayashi T, Horiguchi M, Okawa K, Hagiwara A, Chien KR, Kita T, Nakamura T. Fibulin-5/DANCE has an elastogenic organizer activity that is abrogated by proteolytic cleavage in vivo. The Journal of cell biology. 2007b;176:1061–1071. doi: 10.1083/jcb.200611026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19029–19034. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M, Ota M, Rifkin DB. Matrix control of transforming growth factor-beta function. Journal of biochemistry. 2012;152:321–329. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer J, Kraus C, Hammersen G, Geppert JP, Rauch A. Lethal cutis laxa with contractural arachnodactyly, overgrowth and soft tissue bleeding due to a novel homozygous fibulin-4 gene mutation. Clinical genetics. 2009;76:276–281. doi: 10.1111/j.1399-0004.2009.01204.x. [DOI] [PubMed] [Google Scholar]
- Hu Q, Loeys BL, Coucke PJ, De Paepe A, Mecham RP, Choi J, Davis EC, Urban Z. Fibulin-5 mutations: mechanisms of impaired elastic fiber formation in recessive cutis laxa. Hum Mol Genet. 2006;15:3379–3386. doi: 10.1093/hmg/ddl414. [DOI] [PubMed] [Google Scholar]
- Hu Q, Shifren A, Sens C, Choi J, Szabo Z, Starcher BC, Knutsen RH, Shipley JM, Davis EC, Mecham RP, Urban Z. Mechanisms of emphysema in autosomal dominant cutis laxa. Matrix Biol. 2010;29:621–628. doi: 10.1016/j.matbio.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circulation research. 2010;106:583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Yamashiro Y, Papke CL, Ikeda Y, Lin Y, Patel M, Inagami T, Le VP, Wagenseil JE, Yanagisawa H. Angiotensin-converting enzyme-induced activation of local Angiotensin signaling is required for ascending aortic aneurysms in fibulin-4-deficient mice. Science translational medicine. 2013;5:183ra158. doi: 10.1126/scitranslmed.3005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucthagowder V, Morava E, Kornak U, Lefeber DJ, Fischer B, Dimopoulou A, Aldinger A, Choi J, Davis EC, Abuelo DN, Adamowicz M, Al-Aama J, Basel-Vanagaite L, Fernandez B, Greally MT, Gillessen-Kaesbach G, Kayserili H, Lemyre E, Tekin M, Turkmen S, Tuysuz B, Yuksel-Konuk B, Mundlos S, Van Maldergem L, Wevers RA, Urban Z. Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Hum Mol Genet. 2009;18:2149–2165. doi: 10.1093/hmg/ddp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucthagowder V, Sausgruber N, Kim KH, Angle B, Marmorstein LY, Urban Z. Fibulin-4: a novel gene for an autosomal recessive cutis laxa syndrome. Am J Hum Genet. 2006;78:1075–1080. doi: 10.1086/504304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. The Journal of biological chemistry. 1987;262:6443–6446. [PubMed] [Google Scholar]
- Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. The Journal of biological chemistry. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- Kahari VM, Olsen DR, Rhudy RW, Carrillo P, Chen YQ, Uitto J. Transforming growth factor-beta up-regulates elastin gene expression in human skin fibroblasts. Evidence for post-transcriptional modulation. Laboratory investigation; a journal of technical methods and pathology. 1992;66:580–588. [PubMed] [Google Scholar]
- Kantola AK, Keski-Oja J, Koli K. Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Experimental cell research. 2008;314:2488–2500. doi: 10.1016/j.yexcr.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Kantola AK, Ryynanen MJ, Lhota F, Keski-Oja J, Koli K. Independent regulation of short and long forms of latent TGF-beta binding protein (LTBP)-4 in cultured fibroblasts and human tissues. Journal of cellular physiology. 2010;223:727–736. doi: 10.1002/jcp.22082. [DOI] [PubMed] [Google Scholar]
- Keeley FW. The evolution of elastin. In: Keeley FW, Mecham RP, editors. Evolution of extracellular matrix. 1 ed. Springer Verlag; Berlin Heidelberg: 2013. pp. 73–119. [Google Scholar]
- Kennerson ML, Nicholson GA, Kaler SG, Kowalski B, Mercer JF, Tang J, Llanos RM, Chu S, Takata RI, Speck-Martins CE, Baets J, Almeida-Souza L, Fischer D, Timmerman V, Taylor PE, Scherer SS, Ferguson TA, Bird TD, De Jonghe P, Feely SM, Shy ME, Garbern JY. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet. 2010;86:343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Lee DC, Yang SJ, Lee JJ, Bae EM, Kim DM, Min SH, Kim SJ, Kang DC, Sang BC, Myung PK, Park KC, Yeom YI. Lysyl oxidase like 4, a novel target gene of TGF-beta1 signaling, can negatively regulate TGF-beta1-induced cell motility in PLC/PRF/5 hepatoma cells. Biochemical and biophysical research communications. 2008;373:521–527. doi: 10.1016/j.bbrc.2008.06.071. [DOI] [PubMed] [Google Scholar]
- Kornak U, Reynders E, Dimopoulou A, van Reeuwijk J, Fischer B, Rajab A, Budde B, Nurnberg P, Foulquier F, Lefeber D, Urban Z, Gruenewald S, Annaert W, Brunner HG, van Bokhoven H, Wevers R, Morava E, Matthijs G, Van Maldergem L, Mundlos S. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet. 2008;40:32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
- Kozel BA, Rongish BJ, Czirok A, Zach J, Little CD, Davis EC, Knutsen RH, Wagenseil JE, Levy MA, Mecham RP. Elastic fiber formation: a dynamic view of extracellular matrix assembly using timer reporters. Journal of cellular physiology. 2006;207:87–96. doi: 10.1002/jcp.20546. [DOI] [PubMed] [Google Scholar]
- Kozel BA, Wachi H, Davis EC, Mecham RP. Domains in tropoelastin that mediate elastin deposition in vitro and in vivo. The Journal of biological chemistry. 2003;278:18491–18498. doi: 10.1074/jbc.M212715200. [DOI] [PubMed] [Google Scholar]
- Kuang PP, Joyce-Brady M, Zhang XH, Jean JC, Goldstein RH. Fibulin-5 gene expression in human lung fibroblasts is regulated by TGF-beta and phosphatidylinositol 3-kinase activity. American journal of physiology. Cell physiology. 2006;291:C1412–1421. doi: 10.1152/ajpcell.00087.2006. [DOI] [PubMed] [Google Scholar]
- Kucich U, Rosenbloom JC, Abrams WR, Bashir MM, Rosenbloom J. Stabilization of elastin mRNA by TGF-beta: initial characterization of signaling pathway. American journal of respiratory cell and molecular biology. 1997;17:10–16. doi: 10.1165/ajrcmb.17.1.2816. [DOI] [PubMed] [Google Scholar]
- Lee YC, Huang HY, Chang CJ, Cheng CH, Chen YT. Mitochondrial GLUT10 facilitates dehydroascorbic acid import and protects cells against oxidative stress: mechanistic insight into arterial tortuosity syndrome. Hum Mol Genet. 2010;19:3721–3733. doi: 10.1093/hmg/ddq286. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet. 2002;11:2113–2118. doi: 10.1093/hmg/11.18.2113. [DOI] [PubMed] [Google Scholar]
- Lomas AC, Mellody KT, Freeman LJ, Bax DV, Shuttleworth CA, Kielty CM. Fibulin-5 binds human smooth-muscle cells through alpha5beta1 and alpha4beta1 integrins, but does not support receptor activation. The Biochemical journal. 2007;405:417–428. doi: 10.1042/BJ20070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery AJ, Baas D, Ridley C, Jones RP, Klaver CC, Stone E, Nakamura T, Luff A, Griffiths H, Wang T, Bergen AA, Trump D. Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum Mutat. 2006;27:568–574. doi: 10.1002/humu.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massam-Wu T, Chiu M, Choudhury R, Chaudhry SS, Baldwin AK, McGovern A, Baldock C, Shuttleworth CA, Kielty CM. Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta. Journal of cell science. 2010;123:3006–3018. doi: 10.1242/jcs.073437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Molecular and cellular biology. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller LB, Bukrinsky JT, Molgaard A, Paulsen M, Lund C, Tumer Z, Larsen S, Horn N. Identification and analysis of 21 novel disease-causing amino acid substitutions in the conserved part of ATP7A. Hum Mutat. 2005;26:84–93. doi: 10.1002/humu.20190. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J, Jr, Honjo T, Chien KR. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Itoh S, Jeney V, Yanagisawa H, Fujimoto M, Ushio-Fukai M, Fukai T. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circulation research. 2004;95:1067–1074. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- Noda K, Dabovic B, Takagi K, Inoue T, Horiguchi M, Hirai M, Fujikawa Y, Akama TO, Kusumoto K, Zilberberg L, Sakai LY, Koli K, Naitoh M, von Melchner H, Suzuki S, Rifkin DB, Nakamura T. Latent TGF-beta binding protein 4 promotes elastic fiber assembly by interacting with fibulin-5. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2852–2857. doi: 10.1073/pnas.1215779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono RN, Sengle G, Charbonneau NL, Carlberg V, Bachinger HP, Sasaki T, Lee-Arteaga S, Zilberberg L, Rifkin DB, Ramirez F, Chu ML, Sakai LY. Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites. The Journal of biological chemistry. 2009;284:16872–16881. doi: 10.1074/jbc.M809348200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson KJ, Butterfield NJ. Origin of the Eumetazoa: testing ecological predictions of molecular clocks against the Proterozoic fossil record. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9547–9552. doi: 10.1073/pnas.0503660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piha-Gossack A, Sossin W, Reinhardt DP. The evolution of extracellular fibrillins and their functional domains. PloS one. 2012;7:e33560. doi: 10.1371/journal.pone.0033560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Callewaert B, Baetens M, Campens L, Macdermot K, Fryns JP, Bonduelle M, Dietz HC, Gaspar IM, Cavaco D, Stattin EL, Schrander-Stumpel C, Coucke P, Loeys B, De Paepe A, De Backer J. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFbeta signaling in FTAAD. International journal of cardiology. 2013;165:314–321. doi: 10.1016/j.ijcard.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Holm T, Veith R, Callewaert BL, Ades LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A, Trapane P, Earing MG, Coucke PJ, Sakai LY, Dietz HC, De Paepe AM, Loeys BL. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet. 2010;18:895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, Escande-Beillard N, Dimopoulou A, Fischer B, Chng SC, Li Y, Shboul M, Tham PY, Kayserili H, Al-Gazali L, Shahwan M, Brancati F, Lee H, O'Connor BD, Schmidt-von Kegler M, Merriman B, Nelson SF, Masri A, Alkazaleh F, Guerra D, Ferrari P, Nanda A, Rajab A, Markie D, Gray M, Nelson J, Grix A, Sommer A, Savarirayan R, Janecke AR, Steichen E, Sillence D, Hausser I, Budde B, Nurnberg G, Nurnberg P, Seemann P, Kunkel D, Zambruno G, Dallapiccola B, Schuelke M, Robertson S, Hamamy H, Wollnik B, Van Maldergem L, Mundlos S, Kornak U. Mutations in PYCR1 cause cutis laxa with progeroid features. Nat Genet. 2009;41:1016–1021. doi: 10.1038/ng.413. [DOI] [PubMed] [Google Scholar]
- Robertson I, Jensen S, Handford P. TB domain proteins: evolutionary insights into the multifaceted roles of fibrillins and LTBPs. The Biochemical journal. 2011;433:263–276. doi: 10.1042/BJ20101320. [DOI] [PubMed] [Google Scholar]
- Sato F, Wachi H, Starcher BC, Seyama Y. Biochemical analysis of elastic fiber formation with a frameshift-mutated tropoelastin (fmTE) at the C-terminus of tropoelastin. Journal of Health Science. 2006;52:259–267. [Google Scholar]
- Schluterman MK, Chapman SL, Korpanty G, Ozumi K, Fukai T, Yanagisawa H, Brekken RA. Loss of fibulin-5 binding to beta1 integrins inhibits tumor growth by increasing the level of ROS. Disease models & mechanisms. 2010;3:333–342. doi: 10.1242/dmm.003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segade F. Molecular evolution of the fibulins: implications on the functionality of the elastic fibulins. Gene. 2010;464:17–31. doi: 10.1016/j.gene.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Skidmore DL, Chitayat D, Morgan T, Hinek A, Fischer B, Dimopoulou A, Somers G, Halliday W, Blaser S, Diambomba Y, Lemire EG, Kornak U, Robertson SP. Further expansion of the phenotypic spectrum associated with mutations in ALDH18A1, encoding Delta(1)-pyrroline-5-carboxylate synthase (P5CS). Am J Med Genet A. 2011;155A:1848–1856. doi: 10.1002/ajmg.a.34057. [DOI] [PubMed] [Google Scholar]
- Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes & development. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugitani H, Hirano E, Knutsen RH, Shifren A, Wagenseil JE, Ciliberto C, Kozel BA, Urban Z, Davis EC, Broekelmann TJ, Mecham RP. Alternative splicing and tissue-specific elastin misassembly act as biological modifiers of human elastin gene frameshift mutations associated with dominant cutis laxa. The Journal of biological chemistry. 2012;287:22055–22067. doi: 10.1074/jbc.M111.327940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KM, Bissonnette R, Yanagisawa H, Hussain SN, Davis EC. Fibulin-5 functions as an endogenous angiogenesis inhibitor. Laboratory investigation; a journal of technical methods and pathology. 2007;87:818–827. doi: 10.1038/labinvest.3700594. [DOI] [PubMed] [Google Scholar]
- Syx D, Malfait F, Van Laer L, Hellemans J, Hermanns-Le T, Willaert A, Benmansour A, De Paepe A, Verloes A. The RIN2 syndrome: a new autosomal recessive connective tissue disorder caused by deficiency of Ras and Rab interactor 2 (RIN2). Human genetics. 2010;128:79–88. doi: 10.1007/s00439-010-0829-0. [DOI] [PubMed] [Google Scholar]
- Szabo Z, Crepeau MW, Mitchell AL, Stephan MJ, Puntel RA, Yin Loke K, Kirk RC, Urban Z. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. Journal of medical genetics. 2006;43:255–258. doi: 10.1136/jmg.2005.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Li Q, Urban Z. The complexity of elastic fibre biogenesis in the skin--a perspective to the clinical heterogeneity of cutis laxa. Exp Dermatol. 2013;22:88–92. doi: 10.1111/exd.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban Z. The complexity of elastic fiber biogenesis: the paradigm of cutis laxa. J Invest Dermatol. 2012;132:E12–14. doi: 10.1038/skinbio.2012.4. [DOI] [PubMed] [Google Scholar]
- Urban Z, Hucthagowder V, Schurmann N, Todorovic V, Zilberberg L, Choi J, Sens C, Brown CW, Clark RD, Holland KE, Marble M, Sakai LY, Dabovic B, Rifkin DB, Davis EC. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85:593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, Hill JA, Olson EN. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachi H, Nonaka R, Sato F, Shibata-Sato K, Ishida M, Iketani S, Maeda I, Okamoto K, Urban Z, Onoue S, Seyama Y. Characterization of the molecular interaction between tropoelastin and DANCE/fibulin-5. Journal of biochemistry. 2008;143:633–639. doi: 10.1093/jb/mvn014. [DOI] [PubMed] [Google Scholar]
- Weikkolainen K, Keski-Oja J, Koli K. Expression of latent TGF-beta binding protein LTBP-1 is hormonally regulated in normal and transformed human lung fibroblasts. Growth factors. 2003;21:51–60. doi: 10.1080/08977198310001598778. [DOI] [PubMed] [Google Scholar]
- Willaert A, Khatri S, Callewaert BL, Coucke PJ, Crosby SD, Lee JG, Davis EC, Shiva S, Tsang M, De Paepe A, Urban Z. GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFbeta signaling. Hum Mol Genet. 2012;21:1248–1259. doi: 10.1093/hmg/ddr555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]