Abstract
In this issue, Stachniak et al. determine that the chemogenetic silencer, hM4Di-DREADD, suppresses presynaptic glutamate release and by generating an axon targeted hM4Di variant they demonstrate it can be used to locally silence synaptic transmission in neural circuits.
“Silence is all we dread.
There’s Ransom in a Voice —
But Silence is Infinity.”
Emily Dickinson
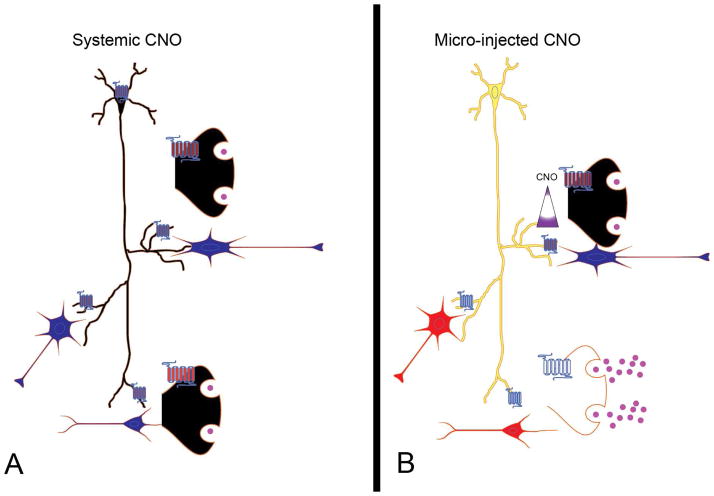
Some decades ago, Francis Crick presciently predicted that in order for scientists to elucidate the ‘neuronal codes’ that specify behavior, perception and consciousness: “a method (is needed) by which all neurons of just one type could be inactivated, leaving the others more or less unaltered” (Crick, 1979). Now, of course, both optogenetic (Zhang et al., 2007) and chemogenetic (Armbruster et al., 2007) technologies are widely available for silencing neurons “of just one type”. Simply inhibiting cell body firing and observing the resulting behavioral phenotype, unfortunately, does little to elucidate which specific projections or target areas might be responsible for the observed effects (see Fig 1A). Ideally, what is needed is a technology that can specifically and reversibly silence presynaptic nerve terminals, thereby ‘silencing synapses’ projecting to distinct neuronal populations (Fig 1B). A technological leap forward is now reported by Stachniak et al. who made via clever modifications of the DREADD chemogenetic platform to achieve synaptic silencing.
Fig 1. Spatially precise synaptic silencing.
As shown in A, hM4Di is expressed mainly in the cell bodies and axon. Systemic CNO administration leads to hyperpolarization and suppression of electrical activity (depicted in the diagram by the Black colored neuron) in a prototypic glutamatergic neuron. As described by Stachniak et al. a global suppression of presynaptic glutamate release also occurs (black synaptic terminals) via synaptic silencing. The net effect is less excitatory drive of anatomically distinct neurons (which are colored blue to indicate lower excitatory drive). In B, a glutamatergic neuron expresses hM4DNRXN mainly in axonal projections. Here a microinfusion of CNO does not induce somatic hyperpolarization and as a result the overall activity of the neuron is unchanged (colored yellow indicating robust spontaneous activity). Microinfusion of CNO suppresses presynaptic release of glutamate leading to less excitatory drive of only neurons in the region perfused (Blue) without altering activity of other neurons (Red).
As first described, the hM4Di-DREADD, when stimulated by clozapine-N-oxide (CNO), activates G-protein inwardly rectifying potassium (GIRK) channels thereby hyperpolarizing and attenuating neuronal activity (Armbruster et al., 2007). hM4Di is now routinely used as a tool to diminish the activity of genetically-defined neurons in vitro and in vivo (Atasoy et al., 2012; Ferguson et al., 2011; Krashes et al., 2011; Ray et al., 2011)(Carter et al., 2013). The robust effects of hM4Di activation on physiology (see Ray et al., 2011 for example) and behavior (see Carter et al., 2013 for instance) have been difficult to reconcile with the relatively modest ability of hM4Di to hyperpolarize and attenuate neuronal firing in vitro (see for instance Ferguson et al., 2011; Krashes et al., 2011; Ray et al., 2011). Here, Stachniak et al. confirm that hM4Di activation leads to hyperpolarization and attenuation of neuronal firing, but also discovered a much more potent action of hM4Dias an effective synaptic silencer in slice preparations and in vivo. It is this silencing of synaptic transmission that will ultimately expand the utility of DREADD-based technology for deconstructing the neuronal code.
Using postsynaptic current as the readout, they first tested whether the CNO-induced activation of hM4Di in a presynaptic neuron suppresses synaptic transmission to postsynaptic neurons located in the same or in different layers of the cortex. They discovered that CNO-induced activation of hM4Di L2/3 presynaptic cortical neurons robustly inhibited the postsynaptic current in both L2/3 neurons and L5 neurons. They also demonstrated that this inhibition of the postsynaptic current was not due to blockade of either the initiation or the propagation of the axonal action potential. Instead, hM4Di appeared to act by suppressing L2/3 synaptic glutamate release. Importantly, neither CNO administered in control slices nor basal hM4Di activity had any significant effect on synaptic glutamate release from L2/3 glutamatergic neurons.
To determine if this ‘synaptic silencing’ by hM4Di could be useful for studies in vivo, they chose a well characterized and popular neural circuit for food intake and examined synaptic transmission from Agouti Related Peptide (AgRP)-expressing neurons in the arcuate nucleus of the hypothalamus (ARC) to paraventricular hypothalamic (PVH) neurons (Stachniak et al, 2014). Prior studies showed that PVH neurons receive axonal projections from AgRP neurons and mediate food intake evoked by activation of AgRP neurons(Atasoy et al., 2012). Stachniak et al then co-expressed channelrhodopsin2 (ChR2) and hM4Di in AgRP neurons and examined the effect of CNO on light-evoked food intake. Astoundingly, they found that microinfusion of CNO above the PVH, but not in an area only 300–500 μm distant, reduced feeding by ~50% during AgRP neuron photostimulation. Taken together, the results obtained from studies in the cortex and hypothalamus demonstrate that hM4Di can effectively suppress presynaptic transmission both ex vivo and in vivo. In support of this notion, a recent report(Mahler et al., 2014) showed that microinfusion of CNO suppresses terminal dopamine release in hM4Di-expressing dopaminergic axons.
As hM4Diis normally localized to both neuronal cell bodies and axons(Mahler et al., 2014; Zhu et al., 2014) it would be more useful to target it specifically to the axonal compartment to achieve selective suppression of synaptic transmission. To achieve this Stachniak et al. developed anaxon-preferring variant of hM4Di they refer to as hM4DNRXN, as it contained the axonal C-terminal targeting sequence of Neurexin1a. This hM4DiNRXN variant displayed reduced somatic expression and enhanced selective axonal expression. Activation of the hM4DNRXN variant by CNO (1μM) robustly inhibited synaptic transmission, but did not induce somatic hyperpolarization and thereby selectively silenced synaptic transmission.
Using this synaptic silencing tool, Stachniak et al then deconstructed the neural circuit downstream of the PVH to demonstrate the further utility of this improved DREADD. Thus, the hM4DNRXN variant was expressed in PVHSIM1(Single-minded homolog 1, SIM1) neurons, and CNO was microinfused into the multiple brain regions targeted by PVHSIM1 axon projections. The authors reported that inhibition of the PVHSIM1 → NTS/DVC (Nucleus of the solitary tract and dorsal vagal complex, NTS/DVC) axon projection was not sufficient to evoke food intake behavior. However, they identified another “hotspot”, the caudal ventrolateral periaqueductal gray and dorsal raphe complex, as a key node downstream of ARCAGRP → PVH neural circuit that controls food intake.
The study by Stachniak et al. addresses two important issues related to DREADD technology. First, they provide convincing evidence that activation of hM4Di in presynaptic terminals can suppress synaptic transmission without disturbing somatic or axonal membrane potentials. Therefore, hM4Di likely silences neuronal activity in vivo via both hyperpolarization and suppression of presynaptic neurotransmitter release in a manner analogous to that achieved by presynaptic G-protein coupled receptors. This makes hM4Di unique among the currently available optogenetic and chemogenetic tools which silence neuronal activity via hyperpolarizing neurons to suppress action potentials. Secondly, they demonstrate that microinfusion of CNO into discrete brain regions is a reliable way to achieve very precise spatio-temporal control of neuronal activity—in agreement with a recent study (Mahler et al., 2014).
This study also raises an intriguing question regarding the mechanism by which hM4Di inhibits presynaptic neurotransmitter release. Although GPCRs are known to be ubiquitously expressed on presynaptic terminals and to modulate synaptic neurotransmitter release, how hM4Diregulates neurotransmitter release is unknown. It is not likely via GIRKS as GIRK1 is primary localized in postsynaptic rather than presynaptic terminals (Drake et al., 1997) and GPCR agonist-induced presynaptic inhibition is unchanged in GIRK2 knockout mice (Luscher et al., 1997). Conceivably, hM4Di could induce presynaptic silencing via inhibition of cAMP-mediated signaling, which has been shown to modulate the activity of voltage-gated calcium channels in a model system (Hilfiker et al., 2001). Alternatively, hM4Di could inhibit the SNARE exocytotic fusion machinery downstream of calcium entry through the action G protein βγ subunits(Gerachshenko et al., 2005)
Whatever the mechanism, it is clear that hM4Di can effectively suppress presynaptic transmission both ex vivo and in vivo, and this makes it useful for many applications. Further, the axon-selective hM4DNRXN variant developed in this study is an exceedingly useful tool to functionally dissect neuronal circuitry by the targeted inhibition of presynaptic transmission without compromising the activities of other synapses originating from the same neurons.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Bartleson C, Kowalchyk JA, Yoon EJ, Preininger AM, Alford S, Hamm HE, Martin TF. G protein betagamma directly regulates SNARE protein fusion machinery for secretory granule exocytosis. Nat Neurosci. 2005;8:421–425. doi: 10.1038/nn1423. [DOI] [PubMed] [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FH. Thinking about the brain. Sci Am. 1979;241:219–232. doi: 10.1038/scientificamerican0979-219. [DOI] [PubMed] [Google Scholar]
- Drake CT, Bausch SB, Milner TA, Chavkin C. GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1997;94:1007–1012. doi: 10.1073/pnas.94.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerachshenko T, Blackmer T, Yoon EJ, Bartleson C, Hamm HE, Alford S. G betagamma acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci. 2005;8:597–605. doi: 10.1038/nn1439. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Czernik AJ, Greengard P, Augustine GJ. Tonically active protein kinase A regulates neurotransmitter release at the squid giant synapse. J Physiol. 2001;531:141–146. doi: 10.1111/j.1469-7793.2001.0141j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes M, Koda S, ChiangPing Y, Rogan SC, Adams A, Maratos-Flier E, Roth BL, Lowell B. Rapid, Reversible Activation of AgRP Neurons Drives Feeding Behavior. J Clin Invest. 2011 doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachniak TJ, Ghosh A, Sternson SM. Chemogenetic synaptic silencing of neural circuits localizes a hypothalamus→midbrain pathway for feeding behavior. Neuron. 82 doi: 10.1016/j.neuron.2014.04.008. (20140) in this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhu H, Pleil KE, Urban DJ, Moy SS, Kash TL, Roth BL. Chemogenetic Inactivation of Ventral Hippocampal Glutamatergic Neurons Disrupts Consolidation of Contextual Fear Memory. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]