Abstract
Over a century ago, Gowers described two young patients in whom distal muscles weakness involved the hand, foot, sternocleidomastoid, and facial muscles in the other case the shoulder and distal leg musculature. Soon after, , similar distal myopathy cases were reported whereby the absence of sensory symptoms and of pathologic changes in the peripheral nerves and spinal cord at postmortem examination allowed differentiation from Charcot-Marie-Tooth disease. In 1951, Welander described autosomal dominant (AD) distal arm myopathy in a large Scandanavian cohort. Since then the number of well-characterized distal myopathies has continued to grow such that the distal myopathies have formed a clinically and genetically heterogeneous group of disorders. Affected kindred commonly manifest weakness that is limited to foot and toe muscles even in advanced stages of the disease, with variable mild proximal leg, distal arm, neck and laryngeal muscle involvement in selected individuals. An interesting consequence of the molecular characterization of the distal myopathies has been the recognition that mutation in a single gene can lead to more than one clinical disorder. For example, Myoshi myopathy (MM) and limb girdle muscular dystrophy (LGMD) type 2B are allelic disorders due to defects in the gene that encodes dysferlin. The six well described distal myopathy syndromes are shown in Table 1. Table 2 lists advances in our understanding of the myofibrillar myopathy group and Table 3 includes more recently delineated and less common distal myopathies. In the same manner, the first section of this review pertains to the more traditional six distal myopathies followed by discussion of the myofibrillar myopathies. In the third section, we review other clinically and genetically distinctive distal myopathy syndromes usually based upon single or smaller family cohorts. The fourth section considers other neuromuscular disorders that are important to recognize as they display prominent distal limb weakness.
Keywords: Distal myopathy, Welander myoapthy, Myoshi myoapthy, LGMD type 2B, Nonaka myoapthy, Laing myoapthy, Markesberry-Griggs myoapthy, Udd distal myopathy, myofibrillar myopathy, αβ-Crystallin, desmin, filamin C, selenoprotein, ZASP, BAG3, FHL1
APPROACH
In approaching patients with distal weakness, we have to consider disorders affecting motor neurons, peripheral nerves, neuromuscular junction, or muscle (6) and the reader is referred for a full discussion to the chapter titled “Approach to Muscle Disease” in this issue. Some myopathies with pattern 2 have predominantly distal presentations including distal muscular dystrophies, myofibrillar myopathies, myotonic dystrophy type 1, and some forms of hereditary inclusion body myopathies (HIBM). Pattern 3 or scapuloperoneal pattern has proximal arm and distal leg involvement. In the presence of facial weakness, we consider facioscapulohumeral muscular dystrophy likely. Emery-Dreifuss muscular dystrophy is usually associated with contractures and cardiac involvement. Late onset acid maltase deficiency can rarely have a scapuloperoneal presentation as well. Pattern 4 consists of distal arm involvement and proximal leg weakness as is typical for the sporadic inclusion body myositis (IBM) in which there is prominent finger flexor, wrist flexor, and knee extensor weakness. Pattern 5 is associated with ptosis and ophthalmoplegia, and includes patients with oculopharyngeal dystrophy and mitochondrial myopathy.
The presence of rimmed vacuoles (Table 4) significantly helps to further narrow down these diagnostic possibilities. Welander myopathy is nearly always in cases from Scandinavia and presents with distal hand involvement. The Markesbery-Griggs and Udd types are autosomal dominant late-onset distal leg myopathies caused by mutations in the genes encoding Z disk associated protein (ZASP) and titin, respectively (7,8,9). Limb girdle muscular dystrophy 1A due to autosomal dominant mutations in the myotilin gene is associated with adult onset of proximal or distal weakness and rimmed vacuoles and occasional nemaline rod-like inclusions (10). Histopathologically, myotilinopathy and ZASPopathy can be placed into the category of myofibrillar myopathy (Table 2) (7–11). Another group of disorders with rimmed vacuoles on biopsy are the hereditary inclusion body myopathies (h-IBM) (7). Nonaka myopathy or hIBM2 is autosomal recessive with anterior leg involvement (Table 1 and Table 4). Hereditary IBM3 caused by heavy chain 2 myosin mutations is associated with congenital arthrogryposis and later onset ophthalmoplegia. One autosomal dominant late-onset multisystem form of HIBM is variably associated with Paget disease and frontotemporal dementia (IBMPFD) and is due to valosin-containing protein (VCP) mutations (Table 3). Immunostaining using VCP antibodies demonstrates the presence of VCP-positive cytoplasmic aggregates in scattered muscle fibers including those with no clear vacuoles or other morphological changes (12). Patients with IBMPFD can present with proximal, distal, scapuloperoneal, or axial weakness (13) and can have dilated cardiomyopathy with inclusion bodies (14).
Table 4.
Muscle disorders with rimmed vacuoles on biopsy
| Inclusion body myositis |
| Hereditary inclusion body myopathy (h-IBM) |
| h-IBM2 or Nonaka type distal myopathy (GNE) |
| hIBM with Paget disease and frontotemporal dementia[*] (VCP) |
| h-IBM3 (myosin heavy chain IIa)[*] |
| Distal muscular dystrophies |
| Welander type[*] |
| Markesbery-Griggs type (ZASPopathy)[*] |
| Udd type (titinopathy)[*] |
| Myofibrillar myopathy |
| Myotilinopathy (LGMD1A)[*] |
| Zaspopathy[*] |
| Desminopathy[*] |
| Filaminopathy[*] |
| Bag3-opathy[*] |
| αB-crystallin[*] |
| SEPN1 |
| Other muscular dystrophies/myopathies |
| Reducing body myopathy (FHL1-opathy) |
| Emery-Dreifuss (emerinopathy, laminopathy[*]) |
| LGMD2G (telethoninopathy) |
| Oculopharyngeal muscular dystrophy (PABP2-GCG triplet)[*] |
| Oculopharyngodistal muscular dystrophy |
| Pompe disease (acid maltase deficiency) |
| Danon disease (LAMP-2) |
| X-linked myopathy with excessive autophagy (VMA21) |
Autosomal dominant.
TABLE 2.
Classification of myofibrillar myopathies
| Type | Inheritance Gene localization |
Initial weakness |
CK | Biopsy | |
|---|---|---|---|---|---|
| Desmin — adult onset (h IBM1& LGMD 1D/E)α MFM1 |
AD or AR (6%) |
2q35 | Hands or legs | Moderately, increased <5× normal |
Myopathy, occasional rimmed vacuoles; sub- sarcolemmal granules, desmin bodies |
| αB-crystallin — early - mid adult MFM2 |
AD or AR |
11q22 | Proximal & leg distal |
Mild elevation | Myopathy, desmin increase |
| Myotilin adult (LGMD 1A) β MFM3 |
AD or sporadic |
5q31.2 | proximal or distal nasal, dysarthria |
Normal to 15 × elevated |
Myofibrillar myopathy, rimmed vacuoles, hyaline / rod inclusions, desmin |
| ZASP— late adult MFM4 |
AD | 10q23.2 | proximal or in 9% distal |
Normal or mild elevation |
Myofibrillar myopathy, small vacuoles, desmin aggregates |
| Myofibrillar with Cardiomyopathy - adult |
AD | 10q22.3 Similar to ZASP |
Distal | Normal or mild elevation |
Myofibrillar myopathy |
| Filamin C Mid to late adult MFM5 (see Table 3) |
AD | 7q32.1 | proximal & respiratory |
2–8 × elevation |
Myopathy, hyaline mass, vacuoles, rods & desmin aggregates |
| BAG3 Childhood MFM6 |
AD | 10q25.2-q26.2 | Proximal > distal, cardiac |
3–15 × elevation |
Myopathy, congophilia, desmin accumulation, small vacuoles |
| Scapuloperoneal aka hyaline body myopathy — adult |
AD | Xq26 FHL1 |
Distal legs Scapular winging |
1.5–10 × elevation |
Myopathy, hyaline bodies with focal desmin inclusions |
| SEPN1 – child - aka Congenital muscular dystrophy with desmin inclusions |
AR | 1p36-p35 | proximal, rigid spine & cardiac |
Normal or mild elevation |
Myopathy, vacuoles, desmin inclusions |
α Autosomal dominant hereditary IBM1, with early quadriceps muscle involvement and later ankle dorsiflexion weakness, has been linked to a mutation in the desmin gene (93). Autosomal dominant LGMD 1D/E, with cardiac conduction defect and dilated cardiomyopathy, is also linked to the desmin gene (155). A dominant neurogenic Kaeser type scapuloperoneal phenotype also been described to harbor a desmin gene mutation (99).
β Autosomal dominant LGMD 1A has been linked to a mutation in myotilin, the causative gene of myofibrillar myopathy 3.
BAG3, BCL2-associated athanogene 3;CK; creatine kinase; FHL1; Four-and-a-half-LIM protein 1, hIBM, hereditary inclusion body myopathy; LGMD, limb girdle muscular dystrophy; SEPN1, Selenoprotein N, 1; ZASP, Z-band alternatively spliced PDZ-motif-containing protein aka LDB3, Lim domain-binding 3.
TABLE 1.
Classification of classic distal myopathies
| Type | Inheritance | Gene localization |
Initial weakness |
CK | Biopsy |
|---|---|---|---|---|---|
| Welander— late adult type 1 |
AD1 | 2p13 | Hands, fingers, wrist extensors |
Normal or mild elevation |
Myopathic; rimmed vacuoles in some |
| Udd— late adult type 2a |
AD | 2q31 titin |
Legs, anterior compartment |
Normal or mild elevation |
Myopathic; rimmed vacuoles in some cases |
| Markesbery— Griggs late adult type 2b |
AD | 10q22.3-q23.2 ZASP |
Legs, anterior compartment |
Normal or mild elevation |
Vacuolar myopathy; myofibrillar features |
| Nonaka— early adult onset or sporadic type 1 (h IBM2) α |
AR | 9p13.3 GNE |
Legs, anterior compartment |
Mild to moderate Vacuolar myopathy increase, <5 × NL |
|
| Miyoshi— early adult onset type 2 (LGMD 2B) β |
AR or sporadic |
2p13 Dysferlin |
Legs, posterior compartment |
10–150 × NL | Myopathic, usually no vacuoles; “endstage” gastrocnemius |
| Laing— early adult onset type 3 (MPD1) |
AD | 14q11.2 MYH7 |
Legs, anterior compartment, neck flexors |
Mild increase, <3× NL |
Moderate myopathic changes; no vacuoles in most |
α Autosomal recessive familial hereditary IBM2, also known as quadriceps sparing myopathy, has been genetically linked with the Nonaka distal myopathy (69, 72, 73).
β LGMD type 2B has been genetically linked with Miyoshi distal myopathy (86).
CK; creatine kinase; hIBM, hereditary inclusion body myopathy; LGMD, limb girdle muscular dystrophy; NL, normal.
TABLE 3.
Classification of less common distal myopathies
| Type | Inheritance | Gene localization |
Initial weakness |
CK | Biopsy |
|---|---|---|---|---|---|
| Myopathy with Anterior leg sparing -child to young adult (see Table 2) |
AD | 7q32 Filamin C |
Calf & hands |
Normal or mild elevation |
Fiber size Variability |
| Myopathy with Paget’s & dementia young adult |
AD | 9p13 VCP |
Proximal & distal leg |
Normal to 8 × elevation |
Myopathy with vacuoles |
| Distal Myopathy with Vocal Cord & Pharyngeal Weakness, MPD2 – late adult onset |
ADl | 5q31 Matrin 3 |
Legs, hands or vocal cords |
Normal to 8 × elevation |
Myopathy with vacuoles |
| Miyoshi-like myopathy 3 early adult onset |
AR | 11p14.3 Anoctamin 5 (ANO 5) |
Posterior legs | 3–100 × elevated |
Myopathy with sarcolemmal lesion |
| Distal nebulin myopathy -child or adult* |
AR | 2q21.2-q22 Nebulin |
Toe & finger extensor |
Normal | Myopathy with small rods |
| LGMD 2G puberty onset |
AR | 17q12 Telethonin |
Leg: proximal & anterior distal |
3–17 × elevation |
Myopathy, rimmed vacuoles |
| Distal myopathy type 3 (MPD3) early adult onset |
AD | 8p22-q11 & 12q13-q22 |
Asymmetric Distal leg & hand |
Normal or mild elevation |
Myopathy with vacuoles |
allelic with rod body myopathy
CK; creatine kinase; LGMD, limb girdle muscular dystrophy.
CLASSIC DISTAL MYOPATHIES
WELANDER DISTAL MYOPATHY: LATE-ADULT ONSET, TYPE 1
Welander (5) described a large number of patients with AD distal myopathy in 72 Swedish families with symptoms onset in the mid-fifth decade (range < 30 to 77 years). Although WDM is mainly seen in Sweden, 12 Finnish families with onset in hands and fingers long extensor muscles were reported to co-segregate to chromosome 2p13 haplotype (15, 16). Proximal limb involvement rarely occurs in WDM even with advanced disease except in severe homozygous cases. Ankle dorsiflexion weakness occurs in 25% of cases and may be the initial presenting symptoms in 10%. Flexor muscles of wrists and fingers are later on affected in 40% of cases but to a lesser extent than extensors. Tendon reflexes remain present except for loss of ankle and brachioradialis reflexes late in the disease. Though sensation is normal, deficits on quantitative temperature and vibration testing are detectable (17).
Serum creatine kinase (CK) level has been shown to be normal or slightly elevated (18, 19). Motor and sensory nerve conduction studies (NCS) are typically normal, and needle electromyography (EMG) shows occasional spontaneous activity comprised of fibrillation potentials, and myopathic motor units potentials (MUP), and although some authors have reported a mixed myopathic and neuropathic recruitment pattern (18–21). T1 and T2 magnetic resonance imaging (MRI) of muscle in 11 patients showed signal abnormalities in the distal anterior and posterior compartments of the legs including the gastrocnemius, soleus, tibialis anterior (TA), and extensor digitorum longus (EDL), as well hamstrings and posterior compartment muscles of the legs (22).
Muscle biopsy shows slight to severe myopathic features, including variability in fiber size, increased connective tissue and fat deposition, central nuclei, and split fibers (5). Vacuoles, a common feature in several of the distal dystrophies, have been noted by some (5, 19, 23) but not all investigators (21, 24, 25) and they are not generally a conspicuous histological feature. A disorganization and loss of myofibrils with accumulation of Z-disk material is noted at the ultrastuctural level (23). Since then, rimmed vacuoles and 15- to 18-nm cytoplasmic and nuclear filaments have been noted by electron microscopy (18, 19) indicating that these are not specific to inclusion body myositis (IBM). The main pathologic feature that distinguishes IBM from WDM is inflammatory cell infiltration in the former (19) besides the clinical phenotype. Groups of small angular fibers can occur suggestive of a neurogenic component (23). Sural nerve biopsy can demonstrate a moderate reduction in myelinated nerve fibers without any axonal degeneration or demyelination and remyelination (23).
The clinical progression of WDM is so slow in most cases that most affected patients continue to work without a reduction in life expectancy; those with atypical relative rapid progression may be homozygous for the genetic defect (5, 16).
TIBIAL MUSCULAR DYSTROPHY: LATE ONSET DISTAL MYOPTHY TYPE 2
In the 1970’s, Non-Scandinavian AD late-onset distal myopathy was described in English families by Sumner (26) and in French-English and Finnish families by Markesbery (27). Other large pedigrees and several sporadic cases were reported and renamed tibial distal muscular dystrophy (TMD) to emphasize the dystrophic features (28, 29). Despite phenotypic overlap, recent idenfication of 2 distinct gene mutations led to the definition of 2 subtypes: type 2a related to titin gene defect and type 2b due to ZASP point mutation.
UDD-LATE ONSET DISTAL MYOPTHY TYPE 2a
The prevalence of AD TMD is 5–15 per 100,000 in Finland. Weakness begins in ankle dorsiflexor muscles typically after age 40. In non-scandinavian cases, weakness may over time involve finger and wrist extensor muscles; later, proximal involvement can supervene. Whereas most Finnish patients progressed more slowly and rarely involved the upper extremity or proximal muscles (4), some western Finnish cases exhibited severe limb girdle syndrome (28–30).
Serum CK is normal or slightly elevated and EMG reveals an irritative myopathy. Muscle biopsy shows dystrophic tissue with myofibers having single and multiple vacuoles. Magnetic resonance imaging in 22 affected patients showed fatty replacement of the TA muscle and EDL in 8 and in the hamstring and posterior compartment of the legs in 14 others (22).
Tibial muscular dystrophy 2a is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal muscle protein titin (31), with a locus at 2q31, composed of 363 exons. Mutation in Mex6 titin leads to abnormal titin-calpain3 interaction (32). The Finnish mutation is due to a deletion/insertion of 11 consecutive base pairs changing four amino acid residues without interrupting the reading frame.
One French cohort had a point mutation in Mex6 that introduced a potentially harmful praline in the beta sheet structure. Mutations of the Mex6 exon correspond to M-line titin in some cases, affecting the calpain3 binding site at the N2-A line in I-band titin. There is secondary calpain3 protein reduction in the homozygous state and apoptotic myonuclei.
MARKESBERY-GRIGGS- LATE ONSET DISTAL MYOPTHY TYPE 2b
TMD2b has been reported in English, French and Finnish families. Men are marginally more severely affected in comparison to women. Like TMD2a, weakness in TMD 2b begins in the anterior leg compartment after age 40. Hands weakness affects distal finger and wrist extensors and late in the course the proximal arms and legs. Progression is faster than in TMD2a leading to disability.
CK is normal or mildly elevated and muscle biopsy demonstrates vacuolar myopathy with myofibrillar features. One patient described by Markesbery (27) had a cardiomyopathy with heart block and heart failure requiring pacemaker insertion. At postmortem examination, vacuoles were present in cardiac and skeletal muscle. Muscle imaging shows considerable involvement of posterior and anterior compartments of the lower leg at a younger age (8). Later in the course of the disease proximal muscles are affected with mild to moderate fatty degeneration and atrophy of gluteus maximus, hamstring, vastus medialis and lateralis muscles, besides severe end-stage replacement in lateral gastrocnemius, soleus, lateral peroneal and anterior compartment muscles. Deep long toe flexor and tibialis posterior muscles are relatively preserved.
No conclusive mutation in titin or other genes was noted in the original English-Finnish cases (27). Because the pathology was compatible with MFM, both myotilin and ZASP (Z-band alternatively spliced PDZ-motif-containing protein) were sequenced. A previously identified mutation in ZASP (A165V mutation) was detected in originally affected family members (8) with full penetrance by the age of 60 years. Immunohistochemical studies revealed strong accumulation of myotilin, αB-crystallin (αBC), and desmin in affected muscle fibers but as with myotilinopathy, abnormal myotilin aggregation was more prominent than abnormal expression of desmin, αBC or ZASP. Although occasional punctate aberrant cytoplasmic labelling is observed, dystrophin C-terminus does not consistently localize to the accumulated aggregates. Cardiomyopathy is not a regular feature since different isoforms are predominantly expressed in cardiac and skeletal muscle. Whereas mutations in exons 4, 6, 10 and 15, which are expressed in cardiac muscle isoforms, were associated with dilated cardiomyopathy, the A165V substitution in skeletal muscle specific exon 6 cause a myopathy dominated by skeletal muscle involvement.
NONAKA DISTAL MYOPATHY: EARLY ADULT ONSET, TYPE 1, DISTAL MYOPATHY WITH RIMMED VACUOLES
Early adult-onset AR distal muscular dystrophy was reported in Japanese families from 1963 to 1975 (33, 34), however they were not widely appreciated until later reports by Nonaka and colleagues (35–37). Similar patients were later described in the United States (38–40), South America (41) and Europe (42, 43). Until identification of the responsible gene defect, weakness onset was thought to be in the second to third decade. Distal myopathy with rimmed vacuoles (DMRV) or Nonaka myopathy (NM) is characterized by its unique distribution of muscular weakness and wasting manifesting as foot drop and steppage gait. The hamstring and TA muscles are most severely affected initially. Though it was initially thought even at late stages to spare knee extensors, discovery of the gene defect led to the realization that the quadriceps muscle can be involved in rare cases. Finger and hand muscles can also be involved but less than the legs. The degree of progression tends to be more aggressive in non-Japanese cases. With rare exceptions (44) weakness remained confined to distal in the Japanese cases, whereas non-Japanese cases eventually develop significant proximal weakness in the legs, arms, and neck muscles, with loss of ambulation (38). Complete heart block producing syncope and requiring a pacemaker has been reported (45) and disability may supervene within 10 to 20 years of onset.
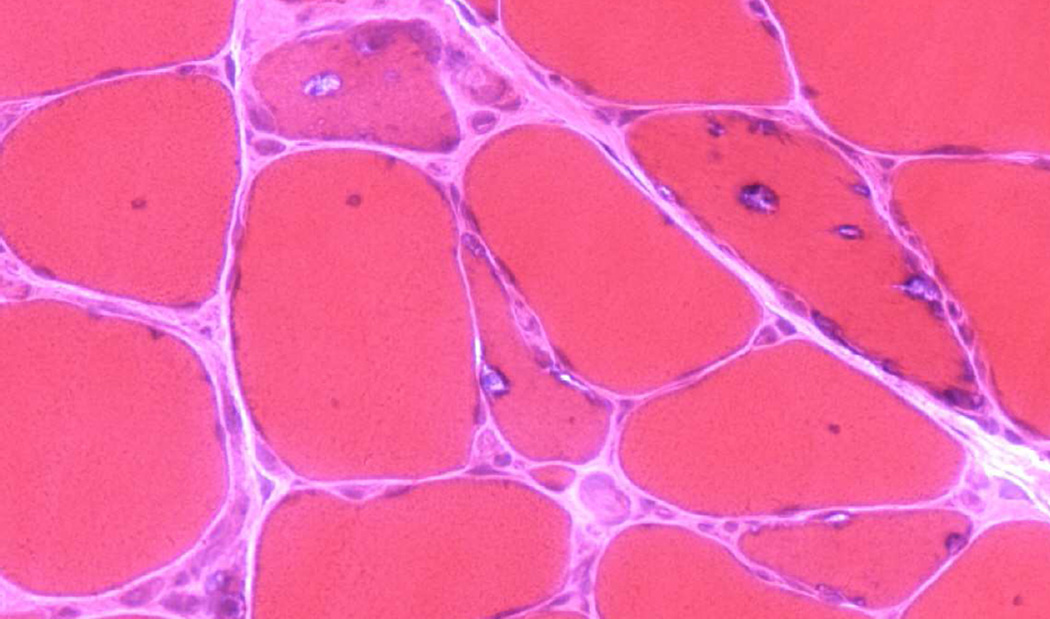
Serum CK is slightly or moderately elevated, but not more than five times the upper normal limit. Needle electromyographic examination reveals fibrillation potentials and myopathic MUP. Muscle biopsy in Japanese and non-Japanese cases demonstrates dystrophic myopathy and rimmed vacuoles due to the deposition therein of granular material with the characteristic of basophilia after hematoxylin and eosin (H&E), purple-red coloration with the modified Gomori trichrome, and acid phosphatase reactivity (Fig. 1). The autophagic vacuoles have nuclear and cytoplasmic 15- to 18-nm filamentous inclusions on electron microscopy (EM) (44, 46, 47) which are not unique to IBM as they are also seen in WDM and DMRV or NM (48). In rare cases, there may be additionally inflammatory muscle pathology with rimmed vacuoles as in sporadic IBM (49, 50).
FIG. 1.
Nonaka distal myopathy (early adult onset type 1). Muscle fiber size variability and rimmed vacuoles on hematoxylin & eosin.
Almost all cases of DMRV are caused by mutations in the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene (GNE) located within the 1.5-Mb region between markers D9S2178 and D9S1791 on chromosome 9 (51). It is allelic to autosomal recessive (AR) hIBM2 wherein several mutations have been detected including one at M712T, which is the most common mutation in early-onset Jewish hereditary IBM type 2 (52). HIBM2, the most common form of hereditary inclusion-body myopathy, was originally described in Persian-Jewish families with distal leg onset in the second–third decade. Weakness and atrophy progressed proximal with relative sparing of the quadriceps (53, 54). A homozygous T to C substitution at nucleotide position 2186 in the GNE gene, converting methionine to threonine at codon 712, has been found in all Middle Eastern families of both Jewish and non-Jewish descent, whereas affected individuals of other ethnicities are usually compound heterozygous or homozygous for different mutations (55). A year later, GNE mutations were also identified in patients with distal myopathy with rimmed vacuoles also known as Nonaka myopathy (56). In the Japanese patients, Nonaka myopathy is most commonly associated with V572L homozygous or compound heterozygous mutation. However, the identification of the causative gene defect has allowed recognition of phenotypic variants of this disorder in the age of onset, degree of progression of symptoms, and distribution of muscle weakness. For example, a minority of patients lack distal weakness or have distinctive quadriceps involvement, as well as patients with unusual facial weakness. 57). The age at onset of symptoms is sometimes delayed even to late adulthood and patients may remain asymptomatic in their 6th–7th decade of life (56, 57).
Two individuals with DMRV patients and undetectable GNE mutation were postulated to be caused by mutations in the non-coding and intron sites, or abnormal transcription or translation of the GNE gene, or in other genes with a role similar to GNE (52). Conversely, a GNE mutation detected in a non-DMRV patient showed predominant involvement of proximal leg muscles sparing the TA and gastrocnemius muscles. Autosomal dominant inheritance, late onset, severe cardiac involvement, and proximal leg muscle involvement distinguish DMRV from other myopathies with prominent rimmed vacuoles. Genetic analysis was instrumental in confirming the diagnosis of DMRV in two GNE compound heterozygote Japanese patients with the unusual feature of inflammation in muscle biopsy and otherwise typical DMRV (49). Endomysial and perivascular inflammation is distinctly uncommon in NM, but occasionally noted in hIBM.
To safely correct GNE gene function, a patient with severe HIBM2 was treated on compassionate basis with intravenous infusion of seven doses of liposomal wild-type GNE gene (58). Quadriceps muscle expression of the delivered GNE, plasmid, and RNA was observed and sialic acid-related proteins were increased with stabilization in the decline of muscle strength. Further assessment of GNE gene lipoplex through a phase I trial in less advanced HIBM cases is in the planning stages. Since GNE gene encodes a protein with two enzymatic activities in sialic acid biosynthetic pathway, reduced sialylation of muscle glycoproteins may play a pivotal role in the h-IBM2/DMRV muscle phenotype. Oral supplementation with sialic acid metabolites in GNE knockout mice results in an increase of sialic acid in muscle to a nearly normal level and prevents development of the muscle phenotype (59). Sialic acid metabolites-treated mice have increased strength, muscle mass, mean muscle fiber cross sectional area, body weight and overall survival compared to untreated control litter mates. Following a phase I sequential dose-escalation study of oral sialic acid in patients with hereditary IBM that showed the drug to be safe and well-tolerated, there is an ongoing phase 2 study to evaluate the sialic acid-extended release tablets in patients with GNE myopathy http://clinicaltrials.gov/ct2/show/NCT01517880.
MIYOSHI DISTAL MYOPATHY: EARLY ADULT ONSET, TYPE 2
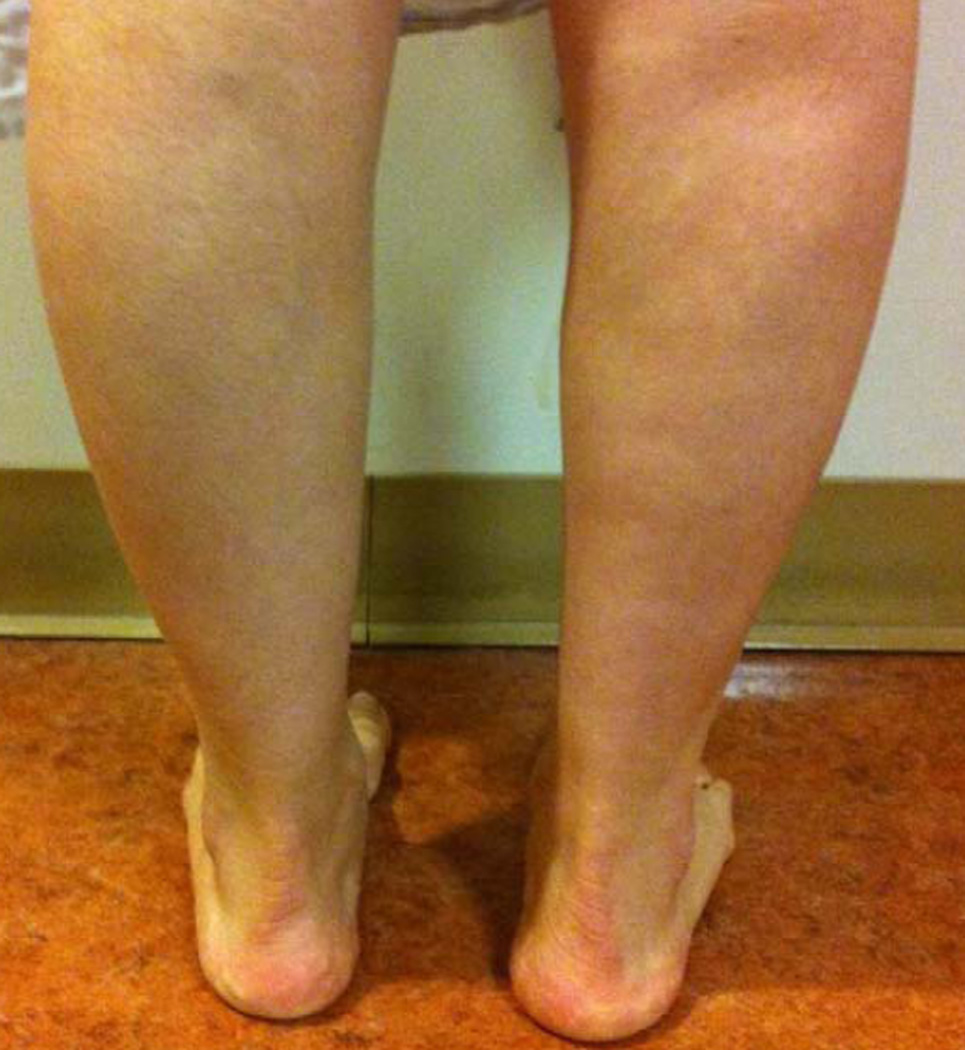
The early reports by Miyoshi and colleagues (60, 61) of this disorder went largely unnoticed for 2 decades until their cases were published in Western literature (62). Similar patients were later reported (63–67) from the U.S. and Europe. Weakness in the gastrocnemii muscles begins between ages 15 to 25 years with an AR inheritance pattern. Affected patients notice difficulty in walking on toes or climbing stairs, and calf myalgia (68). Gastrocnemius muscle hypertrophy is followed by wasting and loss of the ankle muscle stretch reflexes at a later point (Fig. 2). The muscles of the leg anterior compartment and those of the arms and hands remain relatively spared early in the disease. With disease progression, there is some proximal arm and leg weakness with the hamstring muscle group being weaker than the quadriceps (67). Progression is variable with some patients remaining fairly stable with distal weakness, and others experiencing a more aggressive relentless course involving proximal and distal muscles. A consistent finding is preservation of the deltoid muscle despite biceps atrophy (69).
FIG. 2.
Miyoshi distal myopathy. Asymmetric atrophy of posterior compartment gastrocnemii.
The serum CK, which is markedly elevated 10 to 150 fold the upper normal limit, may be a prelude to the disease in asymptomatic patients (64). Needle EMG reveals myopathic MUP and recruitment pattern. Examination of the gastrocnemius muscle typically demonstrates high amplitude long-duration polyphasic MUP with a reduced recruitment pattern reflecting chronicity and severity. Muscle MRI confirms selective involvement of the posterior compartment muscles of the leg compared to those of the anterior compartment (66). ‘Diamond on quadriceps’ sign was present in 21 out of 31 (68%) patients with dysferlinopathy that included 62% of LGMD2B and 71% of MM. The quadriceps femoris muscle had uniform texture and smooth surface at rest but when contracted, a portion of the muscle bulged out both clinically and radiographically toward the anterolateral aspect at midthigh (70).
Biopsy of a severely weak and wasted gastrocnemius muscle typically shows end stage findings including extensive fibrosis, fatty replacement, with few if any myofibers. Biopsy of an uninvolved quadriceps muscle shows minimal myopathic changes, including variability of myofibers size and internal nuclei but absent indirect immunofluorescence for dysferlin staining of the muscle membrane (71). If possible, the biceps femoris muscle should be biopsied with an expectation of showing diagnostic histopathologic findings. While perimysial and perivascular inflammation is not uncommon, vacuoles are an unexpected finding in MM (72).
The gene for MM and LGMD2B both mapped to chromosome 2p12–14, and the protein product of this gene was found to be dysferlin (73). It is a very large gene with 55 exons and > 150 kb yielding a protein with 2,080 amino acids. Although both MM and LGMD2B patients begin in late childhood or early adulthood, with marked elevation in serum CK, and in general both progress slowly, the latter differs in onset in proximal not distal muscles (74). LGMD2B accounts for 5–25% of all LGMD and for further discussion of MM the reader is referred to the section titled “Limb Girdle Muscular Dystrophies” in this issue. Muscle biopsy is an excellent way to confirm MM or LGMD2B in clinically suspected cases as they have absence of dysferlin staining indicating a primary dysferlinopathy, while reduced levels of dysferlin may be secondary to a secondary disorder such as limb-girdle muscular dystrophy (75). Blood monocyte testing for dysferlin western blot is helpful in distinguishing truly abnormal dysferlin immunostaining in muscle from false positives (76). Although the function of dysferlin is not known, it is highly expressed in skeletal muscle, where it is important for sarcolemmal maintenance. The predicted cytoplasmic component contains calcium-binding motifs homologous to C2 domains that are believed to trigger calcium-signaled membrane fusion and trafficking (77) suggesting a role for dysferlin in muscle membrane fusion events and repair. Consistent with this observation is that dysferlin is membrane associated (78) and it has been shown to form a protein complex with integrins at the monocyte cell membrane, and its depletion impairs cell adhesion (79).
LAING DISTAL MYOPATHY: EARLY ONSET, TYPE 3
In 1995 Laing and colleagues (80) reported affected members of a three generation English/Welsh family with AD distal myopathy. Age at onset was 4 to 25 years with selective weakness of the toe and ankle extensor and neck flexor. This was followed after several years by progressive weakness of the finger extensor muscles. Finger flexor and intrinsic hand muscles were relatively spared, although hip abductors and external rotator and shoulder abductor muscles were mildly affected. Tendon reflexes were preserved, and plantar responses were flexor. Disease progression was gradual with a moderate degree of incapacity; the oldest affected member re-examined after 25 years was still ambulatory, but had difficulty maintaining an erect posture when standing. Dilated or hypertrophic cardiomyopathy has been reported.
Serum CK levels were normal or minimally elevated. Electrodiagnostic studies showed normal NCS, with occasional fibrillation potentials and positive sharp wave discharges at rest, markedly myopathic MUP in affected distal limb muscles and early recruitment. Muscle biopsy of the quadriceps shows non-specific myopathy with occasional necrotic and regenerating fibers, excessive variation in fiber size, myofibers with central nuclei, without vacuoles in most cases or fiber type grouping. On modified Gomori trichrome, hyaline inclusions stain light green. Ultrastructural examination shows 15 to 20 nm intranuclear tubulofilamentous inclusions. TA muscle pathology reveals end stage myopathy with normal dystrophin and desmin immunohistochemistry. Muscle MRI studies (81) showed markedly increased signal intensity and severe atrophy of both TA and EHL muscles, as well as the extensor digitorum longus. There was selective moderately increased signal intensity and atrophy of the medial head of gastrocnemius muscles, with similar involvement of the sartorius muscles. The only abnormality in the arms was moderate symmetric atrophy without signal change in the extensor digitorum communis muscles.
Linkage to MYH7 and D14S64 was found on chromosome 14, and later refined to the 14q11.2-q13 locus (80–81). Two muscle genes known to lie within the linked region were the alpha and beta cardiac myosin genes MYH6 and MYH7. Subsequent studies identified five novel heterozygous mutations in the light meromyosin (LMM) regions of the MYH7 tail in six of seven families (82–86) from Europe and Western Australia. MYH7 codes for the isoform of myosin present in slow type 1 skeletal muscle fibers in skeletal and cardiac ventricle muscle, mutations of which lead to hypertrophic cardiomyopathy (HCM). Although HCM was not present in a study cohort (82), atrophy, grouping, and occasionally depletion of type 1 myofibers, was seen in muscle biopsies of four families. The pathophysiology of the mutations in MYH7 was not well understood, however some introduced proline, which is incompatible with coiled coils. All five Laing mutations, including others that resulted in single amino acid deletions, significantly decreased the probability of coiled coil formation over segments of the myosin tail.
Mutation in the MYH7 rod domain at chromosome 14q has been linked to hyaline body myopathy (HBM), a rare congenital AD disorder characterized either by early nonprogressive proximal and distal weakness with significant wasting and loss of subcutaneous fat, or early progressive scapuloperoneal weakness with and loss of ambulation by late teens (86), and subsarcolemmal inclusions known as hyaline inclusion bodies exclusively in type 1 fibers. Mutation in the rod and light meromysin (LMM) domain of myosin heavy chain IIa also lead to HBM (87).
MYOFIBRILLAR MYOPATHY (MFM)
Myofibrillary myopathy consists of a pathological pattern of myofibrillary dissolution and degradation on EM leading in most cases to the accumulation of myotilin, desmin and αBC (11, 88). Myotilin is a Z-disk-associated protein that cross-links actin filaments and binds to α-actinin and γ-filamin (89, 90). Desmin is an intermediate filament protein of skeletal, cardiac, and some smooth muscles cells which links Z-bands with the plasmalemma and the nucleus. αBC is a member of the small heat-shock protein family and is a molecular chaperone. Similar pathologic alterations are seen in spheroid body myopathy, cytoplasmic body myopathy, Mallory body myopathy, and myopathy with granulofilamentous inclusions (91). Following the first description of the inclusions and material around them reactive for desmin (92), the term myofibrillary myopathy was coined (91). The first to missense mutations in desmin was described in 1998 (93). In that same year, missense mutation in αBC was first to report in a French family (94). In 2003, Selcen and Engel described the second and third mutation in αBC in two patients with progressive myopathy (95).
The myopathic manifestations of the disorder caused by either desmin or αBC mutations are identical, with albeit some heterogeneity. Some manifest as a relentlessly progressive adult onset myopathy with or without signs of cardiac involvement (96, 97), but in others the cardiac signs may the leading (93) or exclusive manifestation (98) with cardiomyopathy, congestive heart failure, heart block and arrhythmias, often requiring pacemaker insertion. The majority of desmin myopathy cases show autosomal-dominant inheritance, but rare autosomal-recessive cases and sporadic cases have been reported (88). In an adult-onset multi-generation kindred of scapuloperoneal syndrome type Kaeser, desmin gene mutation was later on described (99) and the same R350P desmin mutation was identified in four unrelated German families. Out of thirteen examined cases, proximal and distal leg weakness occurred in 11, being associated with proximal and distal arm weakness in 4, proximal arm weakness in another 4 and normal arm strength in 5. Two out of eleven cases had only proximal leg weakness and the other two cases that were not examined were also thought to have proximal leg weakness. Genotype-phenotype correlations in a total of 15 patients carrying the same mutation showed large clinical variability, even within the same family, ranging from scapuloperoneal (n=2), limb girdle (n=10) and distal phenotypes (n=3). Cardiac (41%) or respiratory involvement (41%) was common as was facial weakness, dysphagia and gynecomastia (99). Overall, affected men carried a higher risk of sudden cardiac death as compared to affected women. Moreover, muscle biopsy histology and immunohistochemistry in 8 cases revealed a wide spectrum of findings ranging from neurogenic-like atrophy with rimmed vacuoles (n=1) to degenerative myopathy with (n=5), or without (n=2) rimmed vacuoles. Accumulation of desmin was noted in 3 out of 4 cases including one case of degenerative myopathy without rimmed vacuoles and two with rimmed vacuoles. All three distal myopathy cases showed a degenerative rimmed vacuolar myopathy with (n=2) or without (n=1) neurogenic changes. One out of two tested cases had desmin positive inclusions on immunostaining.
More recently, mutations in ZASP (allelic with Markesberry-Griggs TMD2b), myotilin (allelic with LGMD 1A), filamin C, BCL2-associated athanogene 3 (BAG3), Selenoprotein N (SEPN1) and four-and-a-half-LIM protein 1 (FHL1) have been described to cause myofibrillar myopathy (Table 2). In the initial Mayo clinic series, two of the 63 patients carried truncation mutations the αBC gene, and four had a missense mutations in the head or tail region of desmin (11). In a subsequent report from the Mayo, mutations in αBC, desmin, myotilin, Zasp, or filamin-C were overall detected in 32 of 85 patients in the MFM cohort with the addition of 3 BAG3 cases (100).
Most patients develop weakness in the third to fifth decade, although there are reports of onset in infancy and later in life. The Scandinavian patients described by Edström and colleagues (92) had onset of weakness beginning at about age 40 in the distal upper arms. The cohort of 63 cases studied at the Mayo Clinic from 1977 to 2003 (11) had a mean age at onset of 54 years but only one patient presented before the age of 10 years, and three before the age of 20 years. One quarter of cases exhibited an autosomal dominant mode of transmission. Of 56 patients in whom the distribution of weakness was determined, an equal number (16 patients) had similar degrees of proximal and distal myopathy, or distal greater than proximal involvement. A minority (2 patients) had distal myopathy alone. Ten patients had cardiomyopathy at the time of diagnosis with congestive heart failure, arrhythmia or dilated cardiomyopathy. Serum CK was normal in 23 patients, and had raised values up to 7 folds in 30 patients. Electrodiagnostic studies showed myogenic and high amplitude and long duration MUP, with occasional fibrillation potentials, positive sharp wave, complex repetitive, and rare myotonic discharges. In two patients, abnormal electrical irritability was the only EMG abnormality. Thirteen patients had abnormal nerve conduction studies (11) consistent with polyneuropathy, including 4 with long-standing diabetes mellitus. Muscle biopsy tissue demonstrates variability in fiber size, increased internalized nuclei, and on occasion predominance of type 1 fiber or few rimmed vacuoles.
In addition, MFM is suspected when one of the characteristic pathologic alterations are detected best observed on H&E and modified Gomori trichrome stains. Hyaline lesions are blue to purple colored spherical, lobulated or serpentine on trichrome and eosinophilic on H&E with subsarcolemmal cytoplasmic granular inclusions and amorphous granules resembling on EM cytoplasmic, spheroid or Mallory bodies. Besides hyaline structures, trichrome also shows myofibers filled with non-hyaline lesions as dark green smudges of amorphous material and myofibers with small rimmed vacuoles and vacuoles filled with membranous material. There are also myofibers containing desmin; and congophilic amyloidogenic deposits. Excessive desmin accumulation was noted in cardiac muscle of patients with associated cardiomyopathy (101). Desmin accumulation noted in MFM is not specific as it may also be seen in other disorders such as X-linked myotubular myopathy, spinal muscular atrophy, nemaline body myopathy, IBM, and in regenerating muscle fibers (102). Besides desmin, there is overexpression of dystrophin, αBC, gelsolin, ubiquitin and N terminus of β-amyloid precursor protein in both lesion types in addition to cell division cycle 2 kinase and cyclin-dependent kinases (CDK) 2, 4 & 7 (11, 103). Neural cell adhesion molecule (NCAM) is overexpressed in nonhyaline lesions with depletion of actin, α-actinin, myosin and at times titin and nebulin. On the other hand, hyaline structures react to actin, α-actinin, filamin C, myosin and variably to desmin since they are composed of remnants of thick and thin filaments. Immunostains positivity to αBC (MFM1, MFM2, MFM4, MFM6 & SEPN1), myotilin (MFM2, MFM4, MFM5 & MFM6), filamin C (MFM5 & SEPN1), BAG3 with geloslin (MFM6) are noted in specific subtypes of MFM.
Electron microscopy demonstrates foci of myofibrillar destruction and hyaline structures that appear as spheroidal bodies (91). The foci of myofibrillary destruction consist of disrupted myofilaments, Z-disk– derived bodies, dappled dense structures of Z-disk origin, and streaming of the Z-disk. The spheroidal bodies are composed of compacted and degraded remnants of thick and thin filaments (91). Although some have demonstrated the accumulation of 8- to 10-nm filaments (104), others have not confirmed these intermediate-sized filaments despite extensive searching (91). In 19 patients with different genetically proven MFMs (9 desmin, 5 αBC, 3 ZASP, 2 myotilin), an ultrastructural study demonstrated a variety of findings that might guide efforts towards identifying the causal mutated gene (105). On EM, 15–18 nm diameter tubulofilamentous inclusions (with filamentous bundles) accumulated in the sarcoplasm and nuclei of myotilinopathies. The ultrastructural findings in desminopathies and αB-crystallinopathies were very similar and consisted of electrondense granulofilamentous accumulations of predominantly reticular material and sandwich formations. This refers to the granulofilamentous material, which is deposited parallel to and facing the Z-lines, with mitochondria at both sides alongside these deposits, forming sandwich-like structures. Desminopathies and αB-crystallinopathies differed in that early apoptotic nuclear changes were noted in the latter. ZASPopathies were characterized by myotilin antibody-labeled filamentous bundles and floccular accumulations of thin filamentous material.
The variability in age of weakness onset in MFM is exemplified by recessively-inherited SEPN1 which begins in infancy or childhood (Table 2). Mutations in SEPN1 gene have a pleomorphic presentation as Congenital Muscular Dystrophy with Spinal Rigidity (106, 107) multiminicore disease (108), congenital fiber-type disproportion myopathy (109) and desmin-related myopathy with Mallory body-like inclusions (110). In the latter, muscle inclusions are immunoreactive to desmin, dystrophin, and ubiquitin (111) and rimmed vacuoles are present (112). Onset is in childhood for a recently identified rare severe autosomal dominant MFM6, also known as BAG3 myopathy (100). The severe childhood onset phenotype is associated with rigid spine in 2/3, severe respiratory insufficiency in the teens and hypertrophic or restrictive cardiomyopathy in all 3 cases. An adult onset isolated dilated cardiomyopathy phenotype has been described in other BAG3 gene mutations. BAG3 is a member of antiapoptotic BAG protein family, is a Z-disk–associated protein and binds heat shock protein 70 serving as a co-chaperone factor controlling the chaperone activity of Hsp70 (113). Otherwise, currently described MFM are dominantly transmitted with an adult age of onset. The pattern of weakness and different levels of CK alteration are described in Table 2. In addition to myofibrillar changes, vacuoles are present in MFM1 though MFM6 and selenoproteinopathy (Table 4).
DISTAL MYOPATHIES NOT YET CLASSIFIED
In a multigenerational Finnish cohort with AD inheritance, late onset distal myopathy phenotype termed MPD3 has an onset earlier in men than women, ranging from 32 to 50 years (114, 115). Early symptoms were clumsiness of the hands and steppage gait. Weakness affects the thenar and hypothenar muscles progressing to claw hand contractures, glutei, and distally both of the anterior and posterior leg compartments. Forearm muscles, triceps, and infraspinatus, and proximal leg muscles are involved later on the course with frequent asymmetry. Cardiac and respiratory functions were not affected. Serum CK was normal or slightly elevated, while muscle MRI showed fatty degeneration of the affected muscles and muscle biopsy revealed frequent rimmed vacuoles and dystrophic changes. The exact gene responsible for this phenotype remains unknown. There was no evidence of linkage to Welander distal myopathy or tibial muscular dystrophy. Linkage was established at two separate chromosomal regions, 8p22-q11 and 12q13-q22 with 2 reasonable candidate genes including myosin light chain 1 slow-twitch muscle A (MLC1SA) on 12q13 and the muscle specific isoform of ankyrin 1 (ANK1) on 8p11. However, sequencing excluded pathogenic mutations in the coding regions of these 2 genes.
Feit and colleagues (116) reported a southeastern Tennessee family in whom 12 of 37 members in four generations displayed AD weakness of the feet and ankles, or the hands, with an age of onset from 35 to 57 years. There was symmetrical or asymmetrical peroneal weakness, with inversion of the ankles and unsteady gait, and sparing the gastrocnemius muscles. There was characteristic extensor hand weakness frequently involving the abductor pollicus brevis. Two individuals had voice change as the initial manifestation, and 10 others had later vocal cord dysfunction and pharyngeal involvement. Shoulder weakness was the only involved proximal muscle and often asymmetric. Ptosis was noted in one individual. Serum CK was normal in a third of case, and elevated from 2 to 8 fold in the others. Electrodiagnostic studies of the vocal and pharyngeal muscles showed myopathic potentials. Muscle biopsy in one half of the cases disclosed chronic non-inflammatory myopathy with characteristic subsarcolemmal rimmed vacuoles. The syndrome of vocal cord and pharyngeal weakness with AD distal myopathy (VCPDM), so termed, was mapped to chromosome 5q, and in keeping with earlier precedent, designated MPD2. Autosomal dominant distal atrophy with vocal cord paralysis was previously recognized in association with spinal muscular atrophy and in neuronal forms of Charcot Marie Tooth disease. Two disorders that map in a similar location include LGMD2F, at 5q33–34, and LGMD1A within the linkage interval of VCPDM. In the originally reported North American family and in an unrelated in a Bulgarian family, Senderek et al. identified a heterozygous C-to-G transversion at nucleotide 254 in exon 2 of the matrin 3 gene that resulted in a change from serine to cysteine at codon 85 (117). Matrin 3, an internal nuclear matrix protein, belongs to the family of nuclear matrins, a group of proteins present in the nuclear matrix of a variety of mammalian tissues and cells (Table 3).
Two large Italian families with AD adult-onset vacuolar distal myopathy were described (118, 119) with linkage to the 19p13.3 locus. The age at diagnosis was 27 to 73 years with a variety of severity. Asymptomatic individuals had mild scapular weakness with normal CK levels; those mildly affected had in addition mild distal leg weakness. Severely affected individuals presented with marked ankle dorsiflexor, neck flexor, shoulder and finger muscle weakness and wasting, with 2 fold elevations in the serum CK. Needle EMG in the latter revealed myopathic MUP of proximal and distal leg more than arm muscles. Muscle biopsy showed myopathic changes and rimmed vacuoles clustered along myofibers surfaces, with basophilic granular material that stained positive for acid phosphatase, sarcolemmal protein, laminin alpha 2-chain, and negative for thioflavin-S, Congo red, beta amyloid, tau protein. Ubiquitin was abnormally present at the surface of myofibers and in the lumen of vacuoles. Positivity was also noted for the 19 and 20S subunits of proteasome complex on most vacuole surfaces. These findings suggested an endolysosomal origin of the vacuoles caused by abnormality in the lysosomal degradation pathway. Linkage analysis in the family reported by Di Blasi (119) yielded positive lod scores at several markers on 19p13.3.
Autosomal dominant distal myopathy was reported in association with mutation in the caveolin-3 gene (120). Mutations in this gene have also been identified as a cause of LGMD1C, sporadic hyperCKemia, and rippling muscle disease (121, 122).
Felice et al. (123) reported a family with AD adult onset slowly progressive distal myopathy without linkage to known loci. Weakness commenced in the distal anterior leg compartment resulting in foot drop mild proximal leg involvement. Serum CK was 2 to 6 fold normal. Muscle biopsy showed nonspecific myopathic findings including increased variation in fiber size and increased internalized nuclei.
A very late onset (around age 60) AD distal myopathy was described in a French kindred (124). Serum CK was normal or slightly elevated and muscle biopsy revealed numerous rimmed and nonrimmed vacuoles accompanied by aggregates of desmin and dystrophin labeling in the cytosplasm of defective fibers.
Chinnery et al. (125) studied a British family with AD distal anterior compartment weakness of the legs, and early respiratory muscle involvement. Age at onset varied from 32 to 75 years. Nighttime hypoventilation resulted from diaphragmatic muscle involvement. Progression of disease was variable with loss of ambulation in some within 7 to 20 years after onset. Serum CK values were normal or slightly elevated but muscle biopsy showed myopathic dystrophic features and occasional rimmed vacuoles with some Congophilic eosinophilic, desmin, beta-amyloid, and phosphorylated tau immunoreactivity. Linkage was excluded for all known distal myopathy loci.
An Australian kindred had slowly progressive symmetric, distal weakness and wasting of the anterior upper and posterior lower limbs, with sparing of tibialis anterior (126). All patients remained ambulatory and without any cardiac or respiratory muscle involvement. Serum CK levels were either normal or mildly elevated and EMG showed myopathic changes. Imaging studies showed widespread involvement of the posterior and lateral leg compartments but proximal muscles were abnormal only in advanced disease. Muscle histopathology showed either end stage muscle or nonspecific myopathic findings without inflammation or vacuoles. All known distal myopathy phenotype genes and linkage regions were formally excluded
Mitsuhashi et al. described distal myopathy in a 52-year-old man with distal predominant slowly progressive muscle weakness since age 36 (127). On muscle CT, the soleus, TA and paraspinal muscles, where type 1 fibers predominate, were almost totally replaced by fat tissue while quadriceps femoris, gastrocnemius and upper extremity muscles were relatively spared. Quadriceps muscle biopsy revealed multi-minicores in addition to occasional larger cores, in about 70% of the type 1 fibers. A novel heterozygous nucleotide change c.5869T > A (p.S1957T) was identified in RYR1. Although pathogenicity was not confirmed, this nucleotide change was absent in 100 control DNA.
OTHER MYOPATHIES WITH DISTAL WEAKNESS
These myopathies that cause distal weakness as summarized in Table 5.
Table 5.
Other myopathies that can have distal weakness
| Myotonic dystrophy (DM) |
| Facioscapulohumeral dystrophy (FSH)a |
| Scapuloperoneal syndromesa |
| Oculopharyngeal dystrophy |
| Oculopharyngodistal myopathy (recessive) |
| Emery-Dreifuss humeroperonal dystrophya |
| Inflammatory myopathy |
| Inclusion body myositis (IBM) |
| Polymyositis |
| Metabolic myopathy |
| Debrancher deficiency |
| Acid-maltase deficiency |
| Phosphorylase b Kinase |
| Mitochondrial myopathy |
| Congenital myopathy |
| Nemaline myopathya |
| Central core myopathya |
| Centronuclear myopathy type 2 (Dynamin 2; 19p13) |
| Nephropathic cystinosis |
| Myasthenia gravis |
| Cytoplasmic body myopathy (Myofibrillary inclusions in Type I muscle fibers; Dominant) |
| Hyperthyroid myopathy |
| hIBM3 (Myosin heavy chain IIa; Chromosome 17p13; Dominant) |
| hIBM and respiratory failure (6q27; Dominant) |
| Distal weakness (distal myopathy or motor neuropathy; KLHL9; Chromosome 9p22; Dominant) |
| Distal weakness, hoarseness & hearing loss (MYH14; Chromosome 19q13.33; Dominant) |
Scapuloperoneal distribution of weakness can occu.
Childhood-onset Distal Myopathy
There have been reports of infants with foot drop, finger, and hand weakness before age 2, with predominant ankle dorsiflexor, wrist and finger extensor muscle weakness, AD transmission, and very slow progression (128–130). Muscle biopsy and needle EMG showed a myopathic process without vacuolization. All patients remained ambulatory.
In a large Dutch family with AD juvenile-onset distal myopathy, weakness was slowly progressive, affecting flexor and extensor distal muscle groups, and without any functional limitations in adult life (131). Myopathic and neuropathic features were found on muscle biopsy and postmortem examination. Although the reports of childhood-onset distal myopathy preceded desmin immunostaining of skeletal muscle tissue, there were no clues on light microscopy to suggest excessive desmin.
Other Muscular Dystrophies
Weakness of distal muscle groups may be prominent in some forms of muscular dystrophy. In myotonic dystrophy, wrist and finger extensors and ankle dorsiflexors are typically weaker than proximal limb muscles, especially early in the disease (132). Because the prevalence of myotonic dystrophy is 5 per 100,000, it is probably the most commonly seen myopathic condition with prominent distal weakness, especially in the young and middle-aged groups. Rare patients with the phenotypic appearance of myotonic dystrophy and distal weakness but without clinical or electrical myotonia have been described (133).
Patients with fascioscapulohumeral (FSH) dystrophy can develop weakness of ankle dorsiflexion and wrist and finger extension along with typical facial and scapular muscle involvement. Rarely, they can present with ankle weakness which is part of the diagnostic criteria for FSH dystrophy (134). FSH dystrophy type 1 has been mapped to chromosome 4q35 (135) and is due to D4Z4 contraction. For a discussion of FSHD1 and FSHD2, the reader is referred to the section in this issue titled “Fascioscapulomuneral Muscular Dystrophy.” Patients with the so-called myopathic form of the scapuloperoneal syndrome have significant ankle weakness (136).
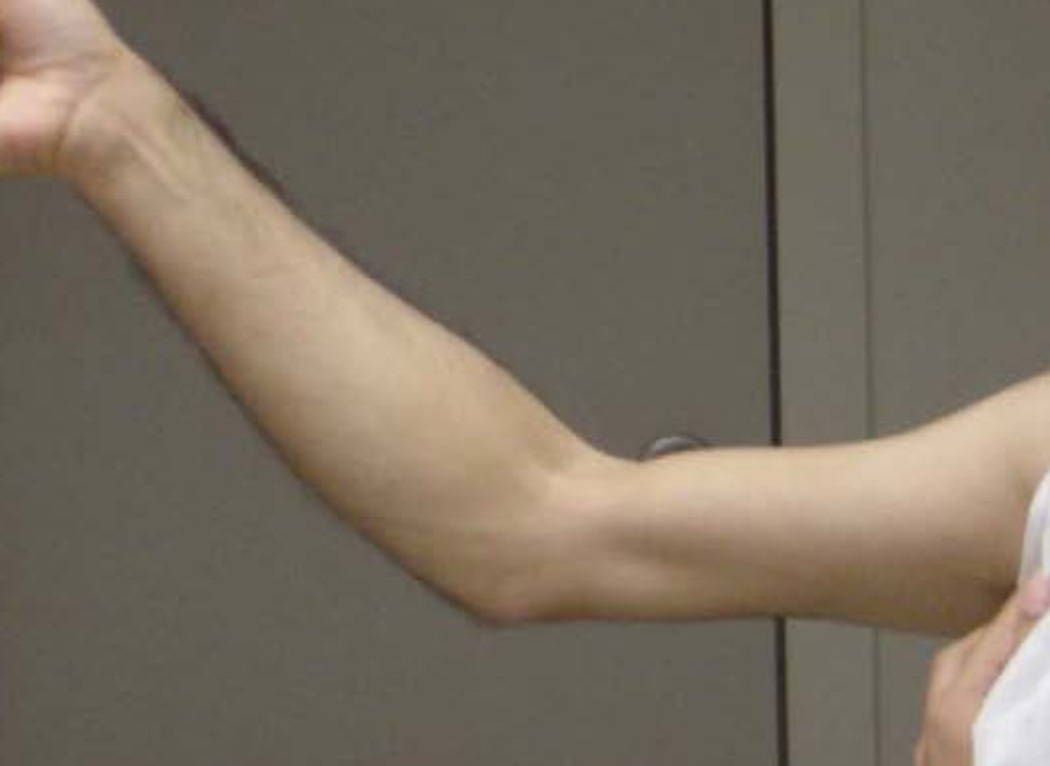
Patients with the X-linked Emery-Dreifuss disease, also known as humeroperoneal muscular dystrophy, present with ankle dorsiflexion, triceps and biceps weakness, along with contractures at the elbow (FIG. 3) and ankle (137). Marked contractures are also seen in an autosomal dominant variant of Emery-Dreifuss muscular dystrophy due to lamin A/C gene mutation. Some pedigrees of oculopharyngeal muscular dystrophy also have significant distal extremity weakness (138–140).
FIG 3.
X-linked Emery-Dreifuss muscular dystrophy. Atrophy of humeral compartment and elbow contracture.
Inflammatory Myopathies
Patients with polymyositis can manifest initial weakness in the hands and ankles (141, 142) with concurrent evidence of inflammatory myopathy on proximal muscle biopsy, and response to corticosteroid therapy. However, sporadic IBM is the more frequent cause of adult onset distal limb weakness (143) typically after age 50, with early weakness and atrophy of wrist and finger flexor muscles, as well as in the quadriceps, TA, and extensor digitorum hallucis muscles, the so called pseudo-Babinski sign. Both knee extensor and forearm and finger flexor weakness are part of the original diagnostic criteria for IBM (144). Muscle biopsy shows endomysial inflammation with invasion of non-necrotic muscle fibers, eosinophilic cytoplasmic inclusions, and rimmed vacuoles within the muscle fibers that contain amyloid deposits. Electron microscopy shows accumulation of cytoplasmic and intranuclear 15- to 21-nm filaments. Table 4 lists other disorders characterized by rimmed vacuoles on biopsy. Amyloidogenic green-birefringent deposits with Congo red stain may be detected in IBM biopsies (145). “Alzheimer-characteristic” proteins in vacuolated muscle fibers including β-amyloid and paired helical filament-tau are noted in IBM muscle tissue (144). The lack of response to immunosuppressive treatment distinguishes IBM from polymyositis and dermatomyositis (146). For a full discussion of IBM, the reader is referred to the section in this issue titled “Sporadic Inclusion Body Myositis.”
Larue et al reported four patients aged over 50 with chronic myopathy suggestive of sporadic IBM (147). Patients had progressive and selective weakness of the quadriceps femoris muscles and asymmetric atrophy of the forearm muscles especially the flexor compartment. Biopsy revealed granulomatous myositis, with in one case evidence for systemic sarcoidosis. Corticosteroid treatment was associated with a partial but significant improvement in two patients.
Metabolic and Congenital Myopathies
Debrancher enzyme deficiency (148) and adult-onset acid-maltase deficiency can present with a scapuloperoneal pattern of weakness (149). Patients with the AR lysosomal storage disorder, nephropathic cystinosis, develop a distal myopathy as a late complication of the disease. Non-progressive congenital muscle diseases such as nemaline rod (150, 151) central core (152); and centronuclear myopathy (153) can have significant involvement of distal limb muscles.
Myasthenia Gravis
Although most patients with myasthenia gravis (MG) present with ocular, bulbar, and proximal limb muscle weakness, the weakness can at times be prominent in distal limbs (154). Of 234 myasthenic patients, 7 (3%) had primarily distal muscle weakness mainly in finger extensor and interossei muscles; and another had involvement of ankle dorsiflexor muscles; six of whom improved with immunosuppressive therapy.
Except for hand extension weakness in Welander myopathy, the classic distal myopathies manifest as distal leg weakness beginning in early or late adult life.
Myoshi myopathy, manifesting as calf muscle weakness and atrophy after a hypertrophic phase, is allelic to LGMD2B as both diseases are caused by mutation in the gene encoding for dysferlin.
Myofibrillary myopathy patients present in the third to fifth decade with distal myopathy, frequent cardiomyopathy, and pathological evidence of myofibrillary degradation.
Mutation in genes encoding for αBC, desmin, myotilin, ZASP, filamin-C, BAG3 and SEPN1are responsible for myofibrillary myopathies.
Myotonic dystrophy is the most common adult muscular dystrophy and early in the disease, wrist and finger extensors and ankle dorsiflexors are weaker than proximal muscles.
Acknowledgments
This publication [or project] was supported by an Institutional Clinical and Translational Science Award, NIH/NCATS Grant Number UL1TR000001. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mazen M. Dimachkie, Professor and Director of Neuromuscular Section, Department of Neurology, The University of Kansas Medical Center, Kansas City, KS, 66160.
Richard J. Barohn, Gertrude and Dewey Ziegler Professor, Chairman, Department of Neurology, The University of Kansas Medical Center, Kansas City, KS, 66160.
REFERENCES
- 1.Gowers WR. A lecture on myopathy and a distal form. Br Med J. 1902;2:89–92. doi: 10.1136/bmj.2.2167.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejerine J, Thomas A. Un cas de myopathie à topographie type Aran-Duchenne suivi d’autopsie. Rev Neurol. 1904;12:1187–1190. [Google Scholar]
- 3.Cambell CM. A case of muscular dystrophy affecting hand and feet. Rev Neurol Psychiatry. 1906;4:192–202. [Google Scholar]
- 4.Spiller WG. Myopathy of a distal type and its relation to the neural form of muscular atrophy (Charcot-Marie-Tooth type) J Nerv Mend Dis. 1907;34:14–30. [Google Scholar]
- 5.Welander L. Myopathia distalis tarda hereditaria. Acta Med Scan. 1951;141(Suppl 265):1–124. [PubMed] [Google Scholar]
- 6.Barohn RJ, Watts GDJ, Amato AA. A case of late-onset proximal and distal muscle weakness. Neurology. 2009 Nov 10;73(19):1592–1597. doi: 10.1212/WNL.0b013e3181c0d4cb. [DOI] [PubMed] [Google Scholar]
- 7.Udd B. 165th ENMC International Workshop: Distal myopathies 6–8 February 2009 in Naarden, the Netherlands. Neuromuscul Disord. 2009;19:429–438. doi: 10.1016/j.nmd.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Griggs R, Vihola A, Hackman P, Talvinen K, Haravuori H, Faulkner G, Eymard B, Richard I, Selcen D, Engel A, Carpen O, Udd B. Zaspopathy in a large classic late-onset distal myopathy family. Brain. 2007 Jun;130(Pt 6):1477–1484. doi: 10.1093/brain/awm006. [DOI] [PubMed] [Google Scholar]
- 9.Selcen D, Engel AG. Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann Neurol. 2005;5:269–276. doi: 10.1002/ana.20376. [DOI] [PubMed] [Google Scholar]
- 10.Selcen D, Engel AG. Mutations in myotilin cause myofibrillar myopathy. Neurology. 2004;62:1363–1371. doi: 10.1212/01.wnl.0000123576.74801.75. [DOI] [PubMed] [Google Scholar]
- 11.Selcen D, Ohno, Engel AG. Myofibrillar myopathy: clinical, morphological and genetic studies in 63 patients. Brain. 2004;127:439–451. doi: 10.1093/brain/awh052. [DOI] [PubMed] [Google Scholar]
- 12.Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- 13.Stojkovic T, Hammoud EH, Pascale Richard P, et al. Clinical outcome in 19 French and Spanish patients with valosin-containing protein myopathy associated with Paget’s disease of bone and frontotemporal dementia. Neuromuscul Disord. 2009;19:316–323. doi: 10.1016/j.nmd.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Hübbers CU, Clemen CS, Kesper K, Böddrich A, Hofmann A, Kämäräinen O, Tolksdorf K, Stumpf M, Reichelt J, Roth U, Krause S, Watts G, Kimonis V, Wattjes MP, Reimann J, Thal DR, Biermann K, Evert BO, Lochmüller H, Wanker EE, Schoser BG, Noegel AA, Schröder R. Pathological consequences of VCP mutations on human striated muscle. Brain. 2007 Feb;130(Pt 2):381–393. doi: 10.1093/brain/awl238. [DOI] [PubMed] [Google Scholar]
- 15.Ahlberg G, Von Tell D, Borg K, et al. Genetic linkage of Welander distal myopathy to chromosome 2p13. Ann Neurol. 1999;46:399–404. doi: 10.1002/1531-8249(199909)46:3<399::aid-ana16>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Von Tell D, Somer H, Udd B, et al. Welander distal myopathy outside the Swedish population: phenotype and genotype. Neuromuscul Disord. 2002;12:544–547. doi: 10.1016/s0960-8966(01)00338-8. [DOI] [PubMed] [Google Scholar]
- 17.Borg K, Borg J, Lindblom U. Sensory involvement in distal myopathy (Welander) J Neurol Sci. 1987;80:323–332. doi: 10.1016/0022-510x(87)90166-3. [DOI] [PubMed] [Google Scholar]
- 18.Borg K, Tome F, Edström L. Intranuclear and cytoplasmic filamentous inclusions in distal myopathy (Welander) Acta Neuropathol. 1991;82:102–106. doi: 10.1007/BF00293951. [DOI] [PubMed] [Google Scholar]
- 19.Lindberg C, Borg K, Edström L, et al. Inclusion body myositis and Welander distal myopathy: a clinical, neurophysiological and morphological comparison. J Neurol Sci. 1991;103:76–81. doi: 10.1016/0022-510x(91)90287-h. [DOI] [PubMed] [Google Scholar]
- 20.Borg K, Åhlberg G, Borg J, Edström L. Welander’s distal myopathy: clinical, neurophysiological and muscle biopsy observations in young and middle aged adults with early symptoms. J Neurol Neurosurg Psychiatry. 1991;54:494–498. doi: 10.1136/jnnp.54.6.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edström L. Histochemical and histopathological changes in skeletal muscle in late-onset hereditary distal myopathy (Welander) J Neurol Sci. 1975;26:147–157. doi: 10.1016/0022-510x(75)90027-1. [DOI] [PubMed] [Google Scholar]
- 22.Mahjneh I, Lamminen AE, Udd B, et al. Muscle magnetic imaging shows distinct diagnostic patterns in Welander and tibial muscular dystrophy. Acta Neurol Scand. 2004;110:87–93. doi: 10.1111/j.1600-0404.2004.00283.x. [DOI] [PubMed] [Google Scholar]
- 23.Borg K, Solders G, Borg J, et al. Neurogenic involvement in distal myopathy (Welander) J Neurol Sci. 1989;91:53–70. doi: 10.1016/0022-510x(89)90075-0. [DOI] [PubMed] [Google Scholar]
- 24.Dahlgaard E. Myopathia distalis tarda hereditaria. Acta Psychiatr Neurol Scand. 1960;35:440–447. doi: 10.1111/j.1600-0447.1960.tb07613.x. [DOI] [PubMed] [Google Scholar]
- 25.Barrows HS, Duemler LP. Late distal myopathy. Report of a case. Neurology. 1962;12:547–550. doi: 10.1212/wnl.12.8.547. [DOI] [PubMed] [Google Scholar]
- 26.Sumner D, Crawfurd M, Harriman DGF. Distal muscular dystrophy in an English family. Brain. 1971;94:51–60. doi: 10.1093/brain/94.1.51. [DOI] [PubMed] [Google Scholar]
- 27.Markesbery WR, Griggs RC, Leach RP, et al. Late onset hereditary distal myopathy. Neurology. 1974;23:127–134. doi: 10.1212/wnl.24.2.127. [DOI] [PubMed] [Google Scholar]
- 28.Udd B, Partanen J, Halonen P, et al. Tibial muscular dystrophy: late adult-onset distal myopathy in 66 Finnish patients. Arch Neurol. 1993;50:604–608. doi: 10.1001/archneur.1993.00540060044015. [DOI] [PubMed] [Google Scholar]
- 29.Partanen J, Laulumaa V, Paljärve, et al. Late onset foot-drop muscular dystrophy with rimmed vacuoles. J Neurol Sci. 1994;125:158–167. doi: 10.1016/0022-510x(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 30.Udd B, Kääriänen H, Somer H. Muscular dystrophy with separate clinical phenotypes in a large family. Muscle Nerve. 1991;14:1050–1058. doi: 10.1002/mus.880141103. [DOI] [PubMed] [Google Scholar]
- 31.Hackman P, Vihola A, Haravuori H, et al. Tibial muscular dystrophy is a titinopathy caused by mutations in TTN, the gene encoding the giant skeletal-muscle protein titin. Am J Hum Genet. 2002;71:492–500. doi: 10.1086/342380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Udd B, Bushby K, Nonaka I, Griggs R. 104th European Neuromuscular Center (ENMC) International Workshop: distal myopathies, 8–10th March 2002 in Naarden, The Netherlands. Neuromusc Disord. 2002;12:897–904. doi: 10.1016/s0960-8966(02)00116-5. [DOI] [PubMed] [Google Scholar]
- 33.Murone I, Sato T, Shirakawa K, et al. Distal myopathy—a case of non-hereditary distal myopathy. Clin Neurol (Tokyo) 1963:378–386. [Google Scholar]
- 34.Sasaki K, Mori H, Takahashi K, et al. Distal myopathy—report of four cases. Clin Neurol (Tokyo) 1969;9:627–637. [Google Scholar]
- 35.Nonaka I, Sunohara N, Ishiura S, et al. Familial distal myopathy with rimmed vacuole and lamellar (myeloid) body formation. J Neurol Sci. 1981;51:141–155. doi: 10.1016/0022-510x(81)90067-8. [DOI] [PubMed] [Google Scholar]
- 36.Nonaka I, Sunohara N, Satoyoshi E, et al. Autosomal recessive distal muscular dystrophy: a comparative study with distal myopathy with rimmed vacuole formation. Ann Neurol. 1985;17:51–59. doi: 10.1002/ana.410170113. [DOI] [PubMed] [Google Scholar]
- 37.Sunohara N, Nonaka I, Kamei N, et al. Distal myopathy with rimmed vacuole formation: a follow-up study. Brain. 1989;112:65–83. doi: 10.1093/brain/112.1.65. [DOI] [PubMed] [Google Scholar]
- 38.Markesbery WR, Griggs RC, Herr B. Distal myopathy: electron microscopic and histochemical studies. Neurology. 1977;27:727–735. doi: 10.1212/wnl.27.8.727. [DOI] [PubMed] [Google Scholar]
- 39.Miller RG, Blank NK, Layzer RB. Sporadic distal myopathy with early adult onset. Ann Neurol. 1979;5:220–227. doi: 10.1002/ana.410050303. [DOI] [PubMed] [Google Scholar]
- 40.Krendel D, Gilchrist J, Bossen E. Distal vacuolar myopathy with complete heart block. Arch Neurol. 1988;45:698–699. doi: 10.1001/archneur.1988.00520300118032. [DOI] [PubMed] [Google Scholar]
- 41.Isaacs H, Badenhorst M, Whistler T. Autosomal recessive distal myopathy. J Clin Pathol. 1988;41:188–194. doi: 10.1136/jcp.41.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scoppetta C, Vaccario ML, Casali C, et al. Distal muscular dystrophy with autosomal recessive inheritance. Muscle Nerve. 1984;7:478–481. doi: 10.1002/mus.880070610. [DOI] [PubMed] [Google Scholar]
- 43.Somer H. Distal myopathies: 25th ENMC international workshop. Neuromusc Disord. 1995;5:249–252. doi: 10.1016/0960-8966(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 44.Mizusawa H, Kurisaki H, Takatsu M, et al. Rimmed vacuolar distal myopathy: a clinical, electrophysiological, histopathological and computed tomographic study of seven cases. J Neurol. 1987;234:129–136. doi: 10.1007/BF00314131. [DOI] [PubMed] [Google Scholar]
- 45.Sunohara N, Nonaka I, Kamei N, et al. Distal myopathy with rimmed vacuole formation: a follow-up study. Brain. 1989;112:65–83. doi: 10.1093/brain/112.1.65. [DOI] [PubMed] [Google Scholar]
- 46.Kumamota T, Fukuhara N, Naguishima M, et al. Distal myopathy: histochemical and ultrastructural studies. Arch Neurol. 1982;51:141–155. doi: 10.1001/archneur.1982.00510180045011. [DOI] [PubMed] [Google Scholar]
- 47.Matsubara S, Tannabe H. Hereditary distal myopathy with filamentous inclusions. Acta Neurol Scand. 1982;65:363–368. doi: 10.1111/j.1600-0404.1982.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 48.Jongen PJH, Laak HJT, Stadhouders AM. Rimed basophilic vacuoles and filamentous inclusions in neuromuscular disorders. Neuromusc Disord. 1995;5:31–38. doi: 10.1016/0960-8966(94)e0019-5. [DOI] [PubMed] [Google Scholar]
- 49.Yabe I, Higashi T, Kikuchi S, et al. GNE mutations causing distal myopathy with rimmed vacuoles with inflammation. Neurology. 2003;61:384–386. doi: 10.1212/01.wnl.0000061520.63546.8f. [DOI] [PubMed] [Google Scholar]
- 50.Krause S, Schlotter-Weigel B, Walter MC, et al. A novel homozygous missense mutation in the GNE gene of a patient with quadriceps-sparing hereditary inclusion body myopathy associated with muscle inflammation. Neuromuscul Disord. 2003;13:830–834. doi: 10.1016/s0960-8966(03)00140-8. [DOI] [PubMed] [Google Scholar]
- 51.Asaka T, Ikeuchi K, Okino S, et al. Homozygosity and linkage disequilibrium mapping of autosomal recessive distal myopathy (Nonaka distal myopathy) J Hum Genet. 2001;46:649–655. doi: 10.1007/s100380170016. [DOI] [PubMed] [Google Scholar]
- 52.Tomimitsu H, Shimizu J, Ishikawa K, et al. Distal myopathy with rimmed vacuoles (DMRV): new GNE mutations and splice variant. Neurology. 2004;62:1607–1610. doi: 10.1212/01.wnl.0000123115.23652.6c. [DOI] [PubMed] [Google Scholar]
- 53.Argov Z, Yarom R. “Rimmed vacuole myopathy” A unique disorder in Iranian Jews. J Neurol Sci. 1984;64:33–43. doi: 10.1016/0022-510x(84)90053-4. [DOI] [PubMed] [Google Scholar]
- 54.Askanas V, Engel WK. Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis. Curr Opin Rheumatol. 1998;10:530–542. doi: 10.1097/00002281-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Eisenberg I, Avidan N, Potikha T, Hochner H, Chen M, Olender T, et al. The UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase gene is mutated in recessive hereditary inclusion body myopathy. Nat Genet. 2001;29:83–87. doi: 10.1038/ng718. [DOI] [PubMed] [Google Scholar]
- 56.Nishino I, Noguchi S, Murayama K, Driss A, Sugie K, Oya Y, et al. Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology. 2002;59:1689–1693. doi: 10.1212/01.wnl.0000041631.28557.c6. [DOI] [PubMed] [Google Scholar]
- 57.Argov Z, Eisenberg I, Grabov-Nardini G, Sadeh M, Wirguin I, Soffer D, et al. Hereditary inclusion body myopathy: the Middle Eastern genetic cluster. Neurology. 2003;60:1519–1523. doi: 10.1212/01.wnl.0000061617.71839.42. [DOI] [PubMed] [Google Scholar]
- 58.Nemunaitis G, Jay CM, Maples PB, Gahl WA, Huizing M, Yardeni T, Tong AW, Phadke AP, Pappen BO, Bedell C, Allen H, Hernandez C, Templeton NS, Kuhn J, Senzer N, Nemunaitis J. Hereditary Inclusion Body Myopathy: Single Patient Response to Intravenous Dosing of GNE Gene Lipoplex. Hum Gene Ther. 2011 Nov;22(11):1331–1341. doi: 10.1089/hum.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malicdan MC, Noguchi S, Hayashi YK, Nonaka I, Nishino I. Prophylactic treatment with sialic acid metabolites precludes the development of the myopathic phenotype in the DMRV-hIBM mouse model. Nat Med. 2009 Jun;15(6):690–695. doi: 10.1038/nm.1956. [DOI] [PubMed] [Google Scholar]
- 60.Miyoshi K, Saijo K, Kuryu Y, et al. Four cases of distal myopathy in two families. Jpn J Human Genet. 1967;12:113. [Google Scholar]
- 61.Miyoshi K, Tada Y, Iwasa M, et al. Autosomal recessive distal myopathy observed characteristically in Japan. Jpn J Human Genet. 1975;20:62–63. [Google Scholar]
- 62.Miyoshi K, Kawai H, Iwasa M, et al. Autosomal recessive distal muscular dystrophy. Brain. 1986;109:31–54. doi: 10.1093/brain/109.1.31. [DOI] [PubMed] [Google Scholar]
- 63.Kuhn E, Schroder M. A new type of distal myopathy in two brothers. J Neurol. 1981;226:181–185. doi: 10.1007/BF00313379. [DOI] [PubMed] [Google Scholar]
- 64.Galassi G, Rowland LP, Hays A, et al. High serum levels of creatine kinase: asymptomatic prelude to distal myopathy. Muscle Nerve. 1987;10:346–350. doi: 10.1002/mus.880100411. [DOI] [PubMed] [Google Scholar]
- 65.Barohn RJ, Miller RG, Griggs RC. Autosomal recessive distal dystrophy. Neurology. 1991;41:1365–1370. doi: 10.1212/wnl.41.9.1365. [DOI] [PubMed] [Google Scholar]
- 66.Meola G, Sansone V, Rotondo G, et al. Computerized tomography and magnetic resonance muscle imaging in Miyoshi’s myopathy. Muscle Nerve. 1996;19:1476–1480. doi: 10.1002/(SICI)1097-4598(199611)19:11<1476::AID-MUS12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 67.Linssen WHJP, Notermans NC, Van der Graaf Y, et al. Miyoshi-type distal muscular dystrophy. Clinical spectrum in 24 Dutch patients. Brain. 1997;120:1989–1996. doi: 10.1093/brain/120.11.1989. [DOI] [PubMed] [Google Scholar]
- 68.Fallon KE, Collins Purdam C. Miyoshi myopathy-an unusual cause of calf pain and tightness. Clin J Sport Med. 2004;14:45–47. doi: 10.1097/00042752-200401000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Rosales XQ, Gastier-Foster JM, Lewis S, Vinod M, Thrush DL, Astbury C, Pyatt R, Reshmi S, Sahenk Z, Mendell JR. Novel diagnostic features of dysferlinopathies. Muscle Nerve. 2010 Jul;42(1):14–21. doi: 10.1002/mus.21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pradhan S. Clinical and magnetic resonance imaging features of ‘diamond on quadriceps’ sign in dysferlinopathy. Neurol India. 2009 Mar-Apr;57(2):172–175. doi: 10.4103/0028-3886.51287. [DOI] [PubMed] [Google Scholar]
- 71.Soares CN, De Freitas MR, Nascimento OJ, et al. Myopathy of distal lower limbs:the clinical variant of Miyoshi Arq Neuropsiquiatr. 2003;61:946–949. doi: 10.1590/s0004-282x2003000600011. [DOI] [PubMed] [Google Scholar]
- 72.Shaibani A, Harati Y, Amato A, Ferrante M. Miyoshi myopathy with vacuoles. Neurology. 1997;47(Suppl):A195. [Google Scholar]
- 73.Liu J, Aoki M, Illa I, et al. Dysferlin, a novel skeletal muscle gene, is mutated in Miyoshi myopathy and limb girdle muscular dystrophy. Nature Genetics. 1998;20:31–36. doi: 10.1038/1682. [DOI] [PubMed] [Google Scholar]
- 74.Aoki M, Liu J, Richard I, et al. Genomic organization of the dysferlin gene and novel mutations in Miyoshi myopathy. Neurology. 2001;57:271–278. doi: 10.1212/wnl.57.2.271. [DOI] [PubMed] [Google Scholar]
- 75.Piccolo F, Moore SA, Ford GC, et al. Intracellular accumulation and reduced sarcolemmal expression of dysferlin in limb-girdle muscular dystrophies. Ann Neurol. 2000;48:902–912. [PubMed] [Google Scholar]
- 76.Gallardo E, de Luna N, Diaz-Manera J, et al. Comparison of dysferlin expression in human skeletal muscle with that in monocytes for the diagnosis of dysferlin myopathy. PLoS One. 2011;6(12):e29061. doi: 10.1371/journal.pone.0029061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+ binding domain. J Biol Chem. 1998;273:15879–15882. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 78.Matsuda C, Aoki M, Hayashi YK, et al. Dysferlin is a surface membrane-associated protein that is absent in Miyoshi myopathy. Neurology. 1999;53:1119–1122. doi: 10.1212/wnl.53.5.1119. [DOI] [PubMed] [Google Scholar]
- 79.de Morrée A, Flix B, Bagaric I, et al. Dysferlin regulates cell adhesion in human monocytes. J Biol Chem. 2013 May 17;288(20):14147–14157. doi: 10.1074/jbc.M112.448589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laing NG, Laing BA, Meredith C, et al. Autosomal dominant distal myopathy: Linkage to chromosome 14. Am J Hum Genet. 1995;56:422–427. [PMC free article] [PubMed] [Google Scholar]
- 81.Mastaglia FL, Phillips BA, Cala LA, et al. Early onset chromosome 14-linked distal myopathy (Laing) Neuromusc Disord. 2002;12:350–357. doi: 10.1016/s0960-8966(01)00287-5. [DOI] [PubMed] [Google Scholar]
- 82.Meredith C, Herrmann R, Parry C, et al. Mutations in the slow skeletal muscle fiber myosin heavy chain gene (MYH7) cause Laing early-onset distal myopathy (MPD1) Am J Hum Genet. 2004;75:703–708. doi: 10.1086/424760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Voit T, Kutz P, Leube B, et al. Autosomal dominant distal myopathy: further evidence of a chromosome 14 locus. Neuromuscul Disord. 2001;11:11–19. doi: 10.1016/s0960-8966(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 84.Zimprich F, Djamshidian A, Hainfellner JA, et al. An autosomal dominant early adult-onset distal muscular dystrophy. Muscle Nerve. 2000;23:1876–1879. doi: 10.1002/1097-4598(200012)23:12<1876::aid-mus13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 85.Hedera P, Petty EM, Bui MR, et al. The second kindred with autosomal dominant distal myopathy linked to chromosome 14q. Genetic and clinical analysis. Arch Neurol. 2003;60:1321–1325. doi: 10.1001/archneur.60.9.1321. [DOI] [PubMed] [Google Scholar]
- 86.Scoppetta C, Casali C, La Cesa I, et al. Infantile autosomal dominant distal myopathy. Acta Neurol Scand. 1995;92:122–126. doi: 10.1111/j.1600-0404.1995.tb01024.x. [DOI] [PubMed] [Google Scholar]
- 87.Bohlega S, Abu-Amero SN, Wakil SM, et al. Mutation of the slow myosin heavy chain domain underlies hyaline body myopathy. Neurology. 2004;62:1518–1521. doi: 10.1212/01.wnl.0000123255.92062.37. [DOI] [PubMed] [Google Scholar]
- 88.Goldfard LG, Vicart P, Goebel HH, et al. Desmin myopathy. Brain. 2004;127:723–734. doi: 10.1093/brain/awh033. [DOI] [PubMed] [Google Scholar]
- 89.Salmikangas P, van der Ven PFM, Lalowski M, et al. Myotilin, the limb girdle dystrophy 1A (LGMD1A) protein, cross-links actin filaments and control sarcomere assembly. Hum Mol Genet. 2003;12:189–203. doi: 10.1093/hmg/ddg020. [DOI] [PubMed] [Google Scholar]
- 90.van der Ven PFM, Wiesner S, Salmikangas P, et al. Indications for a novel muscular dystrophy pathway: gamma-filamin, the muscle-specific filamin isoform, interacts with myotilin. J Cell Biol. 2000;151:235–248. doi: 10.1083/jcb.151.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakano S, Engel AG, Waclwik AJ, Emslie-Smith AM, Busis NA. Myofibrillary myopathy with abnormal foci of desmin positivity. I. Light and electron microscopy analysis of 10 cases. J Neuropath Exp Pathol. 1996;55:549–562. doi: 10.1097/00005072-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Edström L, Thornell LE, Eriksson A. A new type of hereditary distal myopathy with characteristic sarcoplasmic bodies and intermediate (Skeletin) filaments. J Neurol Sci. 1980;47:171–190. doi: 10.1016/0022-510x(80)90002-7. [DOI] [PubMed] [Google Scholar]
- 93.Goldfarb LG, Park KY, CervenaÂkova L, Gorokhova S, Lee H-S, Vasconcelos O, et al. Missense mutations in desmin associated with familial cardiac and skeletal myopathy. Nature Genet. 1998;19:402–403. doi: 10.1038/1300. [DOI] [PubMed] [Google Scholar]
- 94.Vicart P, Caron A, Guicheney P, et al. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 95.Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003 Dec;54(6):804–10. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- 96.Dalakas MC, Park K-Y, Semino-Mora, Les HS, et al. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. New Engl J Med. 2000;342:770–780. doi: 10.1056/NEJM200003163421104. [DOI] [PubMed] [Google Scholar]
- 97.Dalakas MC, Dagvadorj A, Goudeau B, et al. Progressive skeletal myopathy, a phenotypic variant of desmin myopathy associated with desmin mutations. Neuromusc Disord. 2003;13:252–258. doi: 10.1016/s0960-8966(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 98.Li D, TApscoft T, Gonzalez O, et al. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100:461–464. doi: 10.1161/01.cir.100.5.461. [DOI] [PubMed] [Google Scholar]
- 99.Walter MC, Reilich P, Huebner A, Fischer D, Schröder R, Vorgerd M, Kress W, Born C, Schoser BG, Krause KH, Klutzny U, Bulst S, Frey JR, Lochmüller H. Scapuloperoneal syndrome type Kaeser and a wide phenotypic spectrum of adult-onset, dominant myopathies are associated with the desmin mutation R350P. Brain. 2007 Jun;130(Pt 6):1485–1496. doi: 10.1093/brain/awm039. [DOI] [PubMed] [Google Scholar]
- 100.Selcen D, Muntoni F, Burton BK, Pegoraro E, Sewry C, Bite AV, Engel AG. Mutation in BAG3 causes severe dominant childhood muscular dystrophy. Ann Neurol. 2009 Jan;65(1):83–89. doi: 10.1002/ana.21553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muntoni F, Catani G, Mateddu A, et al. Familial cardiomyopathy, mental retardation and myopathy associated with desmin-type intermediate filaments. Neuromusc Disord. 1994;4:233–241. doi: 10.1016/0960-8966(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 102.Goebel HH. Desmin-related neuromuscular disorders. Muscle Nerve. 1995 Nov;18(11):1306–1320. doi: 10.1002/mus.880181114. [DOI] [PubMed] [Google Scholar]
- 103.DeBleecker JL, Engel AG, Ertl BB. Myofibrillary myopathy with abnormal foci of desmin positivity. Immunocytochemical analysis reveals accumulation of multiple other proteins. J Neuropath Exp Pathol. 1996;55:563–577. doi: 10.1097/00005072-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Porte A, Stoeckel ME, Sacrez A, Batzenschager A. Unusual cardiomyopathy characterized by aberrant accumulation of desmin-type intermediate filaments. Virchows Arch. 1980;386:43–58. [Google Scholar]
- 105.Claeys KG, Fardeau M, Schröder R, Suominen T, Tolksdorf K, Behin A, Dubourg O, Eymard B, Maisonobe T, Stojkovic T, Faulkner G, Richard P, Vicart P, Udd B, Voit T, Stoltenburg G. Electron microscopy in myofibrillar myopathies reveals clues to the mutated gene. Neuromuscul Disord. 2008 Aug;18(8):656–666. doi: 10.1016/j.nmd.2008.06.367. [DOI] [PubMed] [Google Scholar]
- 106.Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, et al. Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet. 2001;29:17–18. doi: 10.1038/ng713. [DOI] [PubMed] [Google Scholar]
- 107.Lescure A, Gautheret D, Carbon P, Krol A. Novel selenoproteins identified in silico and in vivo by using a conserved RNA structural motif. J Biol Chem. 274(1999):38147–38154. doi: 10.1074/jbc.274.53.38147. [DOI] [PubMed] [Google Scholar]
- 108.Ferreiro A, Quijano-Roy Q, Pichereau C, Moghadaszadeh B, Goemans N, Bönnemann C, et al. Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet. 2002;71:739–749. doi: 10.1086/342719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clarke NF, Kidson W, Quijano-Roy S, Estournet B, Ferreiro A, Guicheney P, et al. SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann Neurol. 2006;59:546–552. doi: 10.1002/ana.20761. [DOI] [PubMed] [Google Scholar]
- 110.Ferreiro A, Ceuterick-de Groote C, Marks JJ, Goemans N, Schreiber G, Hanefeld F, et al. Desmin-related myopathy with Mallory body-like inclusions is caused by mutations of the selenoprotein N gene. 497 Ann Neurol. 2004;55:676–686. doi: 10.1002/ana.20077. [DOI] [PubMed] [Google Scholar]
- 111.Fidzianska A, Ryniewicz B, Barcikowska M, et al. A new familial congenital myopathy in children with desmin and dystrophin reacting plaques. J Neurol Sci. 1995;131:88–95. doi: 10.1016/0022-510x(95)00090-o. [DOI] [PubMed] [Google Scholar]
- 112.Fidzianska A, Goebel HH, Osborn M, et al. Mallory body-like inclusions in a hereditary congenital neuromuscular disease. Muscle Nerve. 1983;6:195–200. doi: 10.1002/mus.880060305. [DOI] [PubMed] [Google Scholar]
- 113.Takayama S, Xie Z, Reed JC. An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators. J Biol Chem. 1999;274:781–786. doi: 10.1074/jbc.274.2.781. [DOI] [PubMed] [Google Scholar]
- 114.Mahjneh I, Haravuori H, Paetau A, et al. A distinct phenotype of distal myopathy in a large Finnish family. Neurology. 2003;61:87–92. doi: 10.1212/01.wnl.0000073618.91577.e8. [DOI] [PubMed] [Google Scholar]
- 115.Haravuori H, Siitonen HA, Mahjneh I, et al. Linkage to two separate loci in a family with a novel distal myopathy phenotype (MPD3) Neuromusc Disord. 2004;14:183–187. doi: 10.1016/j.nmd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 116.Feit H, Silbergleit A, Schneider LB, et al. Vocal cord and pharyngeal weakness with autosomal dominant distal myopathy: clinical description and gene localization to 5q31. Am J Hum Genet. 1998;63:1732–1742. doi: 10.1086/302166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Senderek J, Garvey SM, Krieger M, Guergueltcheva V, Urtizberea A, Roos A, Elbracht M, Stendel C, Tournev I, Mihailova V, Feit H, Tramonte J and 11 others. Autosomal-dominant distal myopathy associated with a recurrent missense mutation in the gene encoding the nuclear matrix protein, matrin 3. Am. J. Hum. Genet. 2009;84:511–518. doi: 10.1016/j.ajhg.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Servidei S, Capon F, Spinazzola A, et al. A distinctive autosomal dominant vacuolar neuromyopathy linked to 19p13. Neurology. 1999;53:830–837. doi: 10.1212/wnl.53.4.830. [DOI] [PubMed] [Google Scholar]
- 119.Di Blasi C, Moghadaszadeh B, Ciano C, et al. Abnormal lysosomal and ubiquitin-protease pathways in 19p13.3 distal myopathy. Ann Neurol. 2004;56:133–138. doi: 10.1002/ana.20158. [DOI] [PubMed] [Google Scholar]
- 120.Tateyama M, Aoki M, Nishino I, et al. Mutation in the caveolin-3 gene causes a peculiar form of distal myopathy. Neurology. 2002;58:323–325. doi: 10.1212/wnl.58.2.323. [DOI] [PubMed] [Google Scholar]
- 121.Fee DB, So YT, Baraza C, et al. Phenotypic variability associated with Arg26Gln mutation in caveolin3. Muscle Nerve. 2004;30:375–378. doi: 10.1002/mus.20092. [DOI] [PubMed] [Google Scholar]
- 122.Sotgia F, Woodman SE, Bonuccelli G, et al. Phenotypic behavior of caveolin-3 R26Q, a mutant associated with hyperCKemia, distal myopathy, and rippling muscle disease. Am J Physiol Cell Physiol. 2003;285:C1150–C1160. doi: 10.1152/ajpcell.00166.2003. [DOI] [PubMed] [Google Scholar]
- 123.Felice K, Meredith C, Binz N, et al. Autosomal dominant distal myopathy not linked to the known distal myopathy loci. Neuromusc Disord. 1999;9:59–65. doi: 10.1016/s0960-8966(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 124.Penisson-BesnierI, Dumez C, Chateau D, et al. Autosomal dominant late adult onset distal leg myopathy. Neuromusc Disord. 1998;8:459–466. doi: 10.1016/s0960-8966(98)00063-7. [DOI] [PubMed] [Google Scholar]
- 125.Chinnery P, Johnson M, Walls T, et al. A novel autosomal dominant distal myopathy with early respiratory failure: clinico-pathologic characteristics and exclusion of linkage to candidate genetic loci. Neurology. 2001;49:443–452. [PubMed] [Google Scholar]
- 126.Williams DR, Reardon K, Roberts L, Dennet X, Duff R, Laing NG, Byrne E. A new dominant distal myopathy affecting posterior leg and anterior upper limb muscles. Neurology. 2005 Apr 12;64(7):1245–1254. doi: 10.1212/01.WNL.0000156524.95261.B9. [DOI] [PubMed] [Google Scholar]
- 127.Mitsuhashi S, Nonaka I, Wu S, Moreno CA, Shalaby S, Hayashi YK, Noguchi S, Nishino I. Distal myopathy in multi-minicore disease. Intern Med. 2009;48(19):1759–1762. doi: 10.2169/internalmedicine.48.2425. [DOI] [PubMed] [Google Scholar]
- 128.Magee KE, DeJong RN. Hereditary distal myopathy with onset in infancy. Arch Neurol. 1965;13:387–390. doi: 10.1001/archneur.1965.00470040053008. [DOI] [PubMed] [Google Scholar]
- 129.Van der Does de Willebois AEM, Bethlem J, Meyer AEFH, et al. Distal myopathy with onset in early infancy. Neurology. 1968;18:383–390. doi: 10.1212/wnl.18.4.383. [DOI] [PubMed] [Google Scholar]
- 130.Bautista J, Rafel E, Castilla J, et al. Hereditary distal myopathy with onset in early infancy. J Neurol Sci. 1978;37:149–158. doi: 10.1016/0022-510x(78)90199-5. [DOI] [PubMed] [Google Scholar]
- 131.Biemond A. Myopathia distalis juvenilis hereditaria. Acta Psychiatr Neurol Scand. 1955;30:25–38. doi: 10.1111/j.1600-0447.1955.tb06044.x. [DOI] [PubMed] [Google Scholar]
- 132.Morgenlander JC, Massey JM. Myotonic dystrophy. Semin Neurol. 1991;11:236–243. doi: 10.1055/s-2008-1041227. [DOI] [PubMed] [Google Scholar]
- 133.Schotland D, Rowland L. Muscular dystrophy: features of ocular myopathy, distal myopathy, and myotonic dystrophy. Arch Neurol. 1964;10:433–445. doi: 10.1001/archneur.1964.00460170003001. [DOI] [PubMed] [Google Scholar]
- 134.Tawil R, McDermott MP, Mendell JR, et al. Fascioscapulohumeral muscular dystrophy (FSHMD): Design of natural history study and results of baseline testing. Neurology. 1994;44:442–446. doi: 10.1212/wnl.44.3_part_1.442. [DOI] [PubMed] [Google Scholar]
- 135.Wijmenga C, Padberg GW, Moerer P, et al. Mapping of fascioscapulohumeral muscular dystrophy gene to chromosome 4q35-qter by multipoint linkage analysis and in situ hybridization. Genomics. 1991;9:570–575. doi: 10.1016/0888-7543(91)90348-i. [DOI] [PubMed] [Google Scholar]
- 136.Thomas PK, Schott GD, Morgan-Hughes JA. Adult onset scapuloperoneal myopathy. J Neurol Neurosurg Psychiatry. 1975;38:1008–1015. doi: 10.1136/jnnp.38.10.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rowland LP, Fetell M, Olarte M, et al. Emery-Dreifuss muscular dystrophy. Ann Neurol. 1979;5:111–117. doi: 10.1002/ana.410050203. [DOI] [PubMed] [Google Scholar]
- 138.Satoyoshi E, Kinoshita M. Oculopharyngodistal myopathy. Arch Neurol. 1977;34:89–92. doi: 10.1001/archneur.1977.00500140043007. [DOI] [PubMed] [Google Scholar]
- 139.Fukuhara N, Kumamoto T, Tadao T, et al. Oculopharyngeal muscular dystrophy and distal myopathy: intrafamilial difference in the onset and distribution of muscular involvement. Acta Neurol Scand. 1982;65:458–467. doi: 10.1111/j.1600-0404.1982.tb03103.x. [DOI] [PubMed] [Google Scholar]
- 140.Vita G, Dattola R, Santoro M, et al. Familial oculopharyngeal muscular dystrophy with distal spread. J Neurol. 1983;230:57–64. doi: 10.1007/BF00313597. [DOI] [PubMed] [Google Scholar]
- 141.Hollinrake K. Polymyositis presenting as a distal muscle weakness—a case report. J Neurol Sci. 1969;8:479–484. doi: 10.1016/0022-510x(69)90007-0. [DOI] [PubMed] [Google Scholar]
- 142.Van Kasteren BJ. Polymyositis presenting with chronic progressive distal muscular weakness. J Neurol Sci. 1979;41:307–310. doi: 10.1016/0022-510x(79)90091-1. [DOI] [PubMed] [Google Scholar]
- 143.Dimachkie MM, Barohn RJ. Idiopathic inflammatory myopathies. Front Neurol Neurosci. 2009;26:126–146. doi: 10.1159/000212374. [DOI] [PubMed] [Google Scholar]
- 144.Griggs RC, Askanas V, DiMauro S, et al. Inclusion body myositis and myopathies. Ann Neurol. 1995;38:705–713. doi: 10.1002/ana.410380504. [DOI] [PubMed] [Google Scholar]
- 145.Mendell JR, Sahenk Z, Gales T. Amyloid filaments inclusion body myositis: novel findings provide insight into nature of filaments. Arch Neurol. 1991;48:1229–1234. doi: 10.1001/archneur.1991.00530240033013. [DOI] [PubMed] [Google Scholar]
- 146.Dimachkie MM. Idiopathic inflammatory myopathies. J Neuroimmunol. 2011;231(1–2):32–42. doi: 10.1016/j.jneuroim.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 147.Larue S, Maisonobe T, Benveniste O, Chapelon-Abric C, Lidove O, Papo T, Eymard B, Dubourg O. Distal muscle involvement in granulomatous myositis can mimic inclusion body myositis. J Neurol Neurosurg Psychiatry. 2011 Jun;82(6):674–677. doi: 10.1136/jnnp.2009.190751. [DOI] [PubMed] [Google Scholar]
- 148.DiMauro S, Hartwig G, Hays A, et al. Debrancher deficiency: neuromuscular disorder in 5 adults. Ann Neurol. 1979;5:422–436. doi: 10.1002/ana.410050504. [DOI] [PubMed] [Google Scholar]
- 149.Barohn RJ, McVey AL, DiMauro S. Adult acid maltase deficiency. Muscle Nerve. 1993;16:672–676. doi: 10.1002/mus.880160614. [DOI] [PubMed] [Google Scholar]
- 150.Hausmanowa-Petrusewicz I, Fidzianska A, Badurska B. Unusual course of nemaline myopathy. Neuromusc Disord. 1992;2:413–418. doi: 10.1016/s0960-8966(06)80013-1. [DOI] [PubMed] [Google Scholar]
- 151.Laing NG, Majda BT, Akkari PA, et al. Assignment of a Gene (NEM1) for autosomal dominant nemaline myopathy to chromosome 1. Am J Hum Genet. 1992;50:576–583. [PMC free article] [PubMed] [Google Scholar]
- 152.Kratz R, Brooke MH. Distal myopathy. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical Neurology. Vol. 40. North-Holland: Amsterdam; 1980. pp. 471–483. [Google Scholar]
- 153.Moxley RT, Griggs RC, Markesbery WR, et al. Metabolic implications of distal atrophy. Carbohydrate metabolism in centronuclear myopathy. J Neurol Sci. 1978;39:247–259. doi: 10.1016/0022-510x(78)90127-2. [DOI] [PubMed] [Google Scholar]
- 154.Nations SP, Wolfe GI, Amato AA, Jackson CE, Bryan WB, Barohn RJ. Clinical features of patients with distal myasthenia gravis. Neurology. 1997;48(Suppl):A64. doi: 10.1212/wnl.52.3.632. [DOI] [PubMed] [Google Scholar]