Abstract
Background/Aims
Optimal dosing regimens for 25-OH vitamin D (VitD) deficiency are unknown in hemodialysis (HD) patients. Our aim was to evaluate the efficacy of prescribing ergocalciferol supplementation based on KDOQI guidelines for chronic kidney disease (CKD) stages III–IV in HD patients.
Methods
We conducted a retrospective study of 96 urban, predominately African-American HD patients at a single-center dialysis unit with VitD insufficiency or deficiency treated with ergocalciferol. Patients were classified as either compliant or non-compliant with supplementation as determined by review of pharmacy records. The primary outcome was VitD levels 6 months after initiation of treatment and secondary outcomes were VitD levels at 11 months, bone/mineral and anemia parameters.
Results
The population was predominately African-American (69%) and Hispanic (28%). There were 61 individuals in the compliant group and 35 individuals in the non-compliant group. The compliant group was older but otherwise similar in demographics and co-morbid conditions to the non-compliant group. After 6 months of treatment, the compliant group had a significant increase in VitD level (14.7 ± 6.0 to 28.7 ± 10.0 ng/ml, p < 0.0001) compared to the non-compliant group (14.7 ± 5.5 to 14.8 ± 7.1 ng/ml, p = 0.95). There were no differences in the incidence of hypercalcemia between the two groups. Except for a decrease in phosphorus in the compliant group (5.6 ± 1.6 to 4.9 ± 1.7 mg/dl, p = 0.004), there were no significant difference in bone/mineral or anemia parameters including dosing of darbepoetin.
Conclusion
An ergocalciferol-prescribing strategy using the KDOQI guidelines for stage III–IV kidney disease in HD patients with VitD deficiency or insufficiency is inadequate to achieve repletion or maintenance of normal VitD levels.
Keywords: Vitamin D deficiency, End-stage renal disease, Hemodialysis
Introduction
Worldwide, an estimated 1 billion people have low serum 25-hydroxyvitamin D (25(OH)D) levels, with serum levels <30 ng/ml considered to be insufficient [1–3]. In the United States, inadequate vitamin D levels have been increasingly prevalent and affect approximately 40% of the population, with African-Americans and Hispanics having an even higher prevalence than Caucasians [4–6]. In addition to its known role in bone and mineral disease, insufficient levels of vitamin D have now been linked to cardiovascular disease, cancer, myopathy, diabetes mellitus, infection, multiple sclerosis, autoimmune disease, lung function, and mental health [1, 3, 7, 8].
In patients with end-stage renal disease (ESRD) on hemodialysis (HD), vitamin D insufficiency is widely prevalent. Reports have shown that in this population, at least 75% of the patients are vitamin D insufficient, likely due to inadequate sunlight exposure, inefficient 25(OH)D formation by the skin, and dietary deficiency [2, 9–11]. African-American dialysis patients have particularly high rates of insufficiency, but previous studies of vitamin D supplementation have focused primarily on Caucasian populations [12]. Additionally, evolving research has found that many cells and tissues, such as those of the prostate, colon, breast, lung, pancreas, parathyroid, and lymphocytes, contain vitamin D receptors (VDRs) and can convert 25(OH)D into 1,25(OH)2 D [13]. Interestingly, it has been postulated that these cells also possess the ability utilize 25(OH)D directly, meaning, 25(OH)D does not need to be converted to 1,25(OH)2 D in order activate VDRs [9, 14]. In addition, 25(OH)D metabolism outside of the renal-hydroxylase system may produce active metabolites, other than 1,25(OH)2 D, that activate VDRs [15].
Studies regarding vitamin D supplementation, using inactivated vitamin D (cholecalciferol or ergocalciferol) in insufficient ESRD patients have shown significant and favorable effects on laboratory parameters of bone and mineral metabolism, reduced doses of activated vitamin D analogs, phosphorus binders, and erythropoiesis-stimulating agents (ESAs), improvements in glycemic control, improvements in serum albumin levels, and reduced circulating levels of inflammatory markers such as C-reactive protein [2, 10, 12]. Vitamin D deficiency has also been suggested to be a risk factor for mortality [16]. However, specific guidelines for repletion of 25(OH)D levels with inactive vitamin D in patients with ESRD do not exist [3, 17, 18].
The purpose of this study is to assess the efficacy of a prescription-based ergocalciferol repletion strategy in a population of urban, primarily African-American ESRD patients using the KDOQI Clinical Practice Guidelines’ (for Bone Metabolism and Disease in Chronic Kidney Disease) recommendations for vitamin D supplementation in patients with CKD stages III–IV. The effect of ergocalciferol replacement on laboratory parameters and ESA, activated vitamin D, and phosphorus binder doses were also examined.
Methods
Study Design
This study is a retrospective cohort study of participants who were on HD at a single dialysis unit associated with an academic institution in Chicago, Ill., USA (latitude 41). Each HD patient had a baseline 25(OH)D level drawn before starting ergocalciferol supplementation. Oral ergocalciferol replacement was given according to KDOQI guidelines for chronic kidney disease (CKD) stages III–IV: patients with 25(OH)D levels <5 ng/ml were given 50,000 IU weekly for 12 weeks then once monthly for 3 months; patients with 25(OH)D levels of 5–15 ng/ml were given 50,000 IU weekly for 4 weeks followed by 5 monthly doses of 50,000 IU, and patients with 25(OH)D levels 16–30 ng/ml were given 6 monthly doses of 50,000 IU. Patients with normal 25(OH)D levels at baseline were not prescribed ergocalciferol supplementation and were therefore not included in this analysis. The 25(OH)D levels were reassessed at 6 months and patients were again given ergocalciferol according to the KDOQI dosing protocol for CKD stages III–IV. Relevant laboratory and clinical variables were assessed at baseline and 6, 9, and 11 months when 25(OH)D levels were measured. The institutional review board at the University of Illinois at Chicago approved the study.
Variables and Data Sources
Serum 25(OH)D levels were measured in September 2009 (study baseline). At this time, doses of medications including darbepoetin, doxercalciferol, cinacalcet, and sevelamer were also recorded. Baseline laboratory parameters including calcium, parathyroid hormone (PTH), phosphorus, albumin, iron, total iron-binding capacity, ferritin, and hemoglobin were determined based on the monthly blood draw performed by the HD unit. Demographic data including age, gender, race, dialysis vintage, and co-morbidities including diabetes, hypertension, coronary artery disease, congestive heart failure, cerebrovascular accident, hyperlipidemia, failed kidney transplant, and cancer were abstracted from review of the electronic medical record. Medication doses and laboratory variables were reassessed at 6 and 11 months after treatment initiation in all participants.
Patient compliance with the prescribed ergocalciferol regimen was determined from University of Illinois Pharmacy dispensing records for approximately 65% of the patients. For the patients who, due to insurance constraints, obtained the ergocalciferol from outside pharmacies, compliance was assessed via patient interview and requests for additional refills per the electronic medical record. Participants were considered to be compliant if the ergocalciferol course prescribed was completed and non-compliant if the course was either not started or partially completed.
Statistical Methods
We examined differences between the groups using one-way analysis of variance (ANOVA) for normally distributed continuous variables and Wilcoxon signed rank test for non-normally distributed variables. χ2 or Fisher’s exact test was used for categorical variables. Analyses were carried out with SAS version 9.2 (SAS Institute, Inc., Cary, N.C., USA).
Results
Participants and Baseline Demographic and Clinical Data
Of the 96 participants included in the study, 42 (44%) had 25(OH)D insufficiency (16–30 ng/ml), 53 (55%) had 25(OH)D deficiency (5–15 ng/ml), and 1 (1%) had severe 25(OH)D deficiency with a level <5 ng/ml (table 1). 66 (69%) participants were African-American and 27 (28%) were Hispanic. When participants were divided into groups based on medication compliance, the non-compliant group was younger (52.5 ± 14.6 vs. 59.7 ± 15.9 years, p = 0.03) but otherwise similar to the compliant group in terms of gender, race, dialysis vintage, and co-morbidities. Baseline bone metabolism parameters were similar between the two groups (table 2). The baseline hemoglobin was similar among the two groups (non-compliant 11.3 ± 1.4 g/dl, compliant 11.3 ± 1.4 g/dl, p = 0.94). Median baseline darbepoetin usage was 30 μg/week with an IQR 12.5–50 for the non-compliant group and 25 μg/week with an IQR 18–40 (p = 0.98).
Table 1.
Baseline demographic characteristics and baseline vitamin D levels for the cohort
| Variable | Non- or partially compliant participants (n = 35) | Compliant participants (n = 61) | p value |
|---|---|---|---|
| Age, years | 52.5±14.6 | 59.7±15.9 | 0.0313 |
| Male | 16 (45.7) | 35 (57.4) | 0.27 |
| Race | 0.86 | ||
| African-American | 23 (65.7) | 43 (70.5) | |
| Hispanic | 11 (31.4) | 16 (26.2) | |
| Caucasian | 1 (2.9) | 2 (3.3) | |
| Dialysis vintage, years | 5.7±4.6 | 4.6±4.5 | 0.28 |
| Comorbidities | |||
| Diabetes mellitus | 19 (54.3) | 38 (62.3) | 0.44 |
| Hypertension | 29 (82.9) | 55 (90.2) | 0.30 |
| Coronary artery disease | 7 (20.0) | 15 (24.6) | 0.61 |
| Heart failure | 7 (20.00) | 11 (18.0) | 0.81 |
| Cerebrovascular accident | 4 (11.4) | 7 (11.5) | 0.99 |
| Hyperlipidemia | 9 (25.7) | 16 (26.2) | 0.96 |
| Failed renal transplant | 4 (11.4) | 7 (11.5) | 0.99 |
| Cancer | 2 (5.7) | 5 (8.2) | 0.65 |
| 25(OH)D at baseline, ng/ml | 0.18 | ||
| <5 | 1 (2.9) | 0 (0) | |
| 5–15 | 16 (45.7) | 37 (60.7) | |
| 16–30 | 18 (51.4) | 24 (39.3) | |
Values are means ± SD or n (%).
Table 2.
Changes in bone parameters and medication doses from baseline to month 6 by compliance group
| Variable | Non- or partially compliant participants (n = 35)
|
Compliant participants (n = 61)
|
||||
|---|---|---|---|---|---|---|
| baseline | 6 months | p | baseline | 6 months | p | |
| 25(OH)D, ng/ml | 14.7±5.5 | 14.8±7.1 | 0.95 | 14.7±6.0 | 28.7±10.0 | <0.0001 |
| PTH, pg/ml | 354 (214–830) | 505 (326–805) | 0.70 | 362 (223–832) | 374 (226–696) | 0.76 |
| Calcium, mg/dl | 8.3±1.0 | 8.2±0.9 | 0.38 | 8.5±0.6 | 8.6±0.6 | 0.35 |
| Albumin, g/dl | 3.4±0.3 | 3.4±0.3 | 0.69 | 3.3±0.4 | 3.5±0.5 | 0.01 |
| Corrected calcium, mg/dl | 8.8±1.0 | 8.7±0.9 | 0.49 | 9.1±0.8 | 9.0±0.7 | 0.28 |
| Phosphorus, mg/dl | 6.0±1.8 | 5.9±1.8 | 0.60 | 5.6±1.6 | 4.9±1.7 | 0.004 |
| Cinacalcet, mg | 0 (0–90) | 0 (0–90) | 0.25 | 0 (0–30) | 0 (0–30) | 0.46 |
| Doxercalciferol, μg | 0.56 (0–4.0) | 1.5 (0–4.0) | 0.63 | 1.62 (0–4.0) | 2.2 (0–4.0) | 0.49 |
| Sevelamer, n tablets | 6 (3–9) | 9 (6–9) | 0.06 | 6 (3–9) | 6 (6–9) | 0.0005 |
Values are means ± SD or median (interquartile range). There were no statistical differences between baseline values for the non-compliant group and compliant group.
Changes in Serum 25(OH)D Levels with Ergocalciferol Repletion
When 25(OH)D levels were reassessed at 6 months, participants who had been compliant (n = 61) with the ergocalciferol regimen had a significant increase in serum 25(OH)D levels (14.7 ± 6.0 to 28.7 ± 10.0 ng/ml, p < 0.0001) while those that were not compliant had no significant change (14.7 ± 5.5 to 14.8 ± 7.1 ng/ml, p = 0.95) (table 1). At 6 months, 41% of the compliant group achieved a normal 25(OH)D compared to 6% in the non-compliant group (p < 0.0001) (fig. 1a).
Fig. 1.
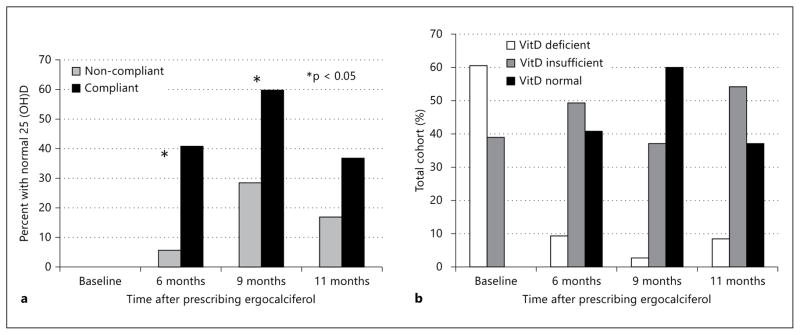
Changes in the proportion of individuals (%) over time by (a) compliance group and by (b) 25(OH)D category. * p < 0.05.
After 6 months, those individuals with persistently low 25(OH)D levels were prescribed a second course of ergocalciferol as described earlier. All patients had 25(OH)D levels reassessed at 9 months and 60% of the compliant group had normal 25(OH)D levels while 37% were insufficient and 3% were deficient (fig. 1b). At 11 months of follow-up, cohort 25(OH)D levels decreased, with only 37% of the compliant group maintaining a normal 25(OH)D level while 25(OH)D insufficiency and deficiency increased to 54% and 9%, respectively (fig. 1b).
Changes in Calcium, Phosphorus, PTH, and Medications with Ergocalciferol Repletion
Table 2 lists the changes in bone laboratory parameters and medication doses during the period of follow-up. At 6 months of follow-up, there was no significant change in the PTH, calcium, or corrected calcium levels in either the non-compliant or compliant groups. The compliant group did show a significant increase in albumin. The compliant group also had a significant decrease in serum phosphorus (5.6 ± 1.6 vs. 4.9 ± 1.7 mg/dl, p = 0.004) while sevelamer use significantly increased (p = 0.0005). There was no significant change in dose of doxercalciferol or cinacalcet.
For safety, calcium levels were monitored closely for all patients during the study period. At our center, the normal range for serum calcium is between 8.6 and 10.6 mg/dl. Anyone with a calcium level >10.6 was labeled having a high calcium level that could potentially be from ergocalciferol supplementation. In our cohort, there were 5 of 30 (17%) in the non-compliant group and 5 of 56 (9%) in the compliant group that had a high calcium level anytime during the study period but this difference was not significant (p = 0.35). Only 2 individuals had a serum calcium level >11 including one at 11.3 (compliant group) and another at 11.2 (non-compliant group).
Changes in Hemoglobin and ESA Use with Ergocalciferol Repletion
Hemoglobin levels were similar between the two groups during the entire study cohort. ESA use was evaluated between the two groups. We examined weekly usage, weekly usage by weight, and weekly usage by weight by hemoglobin. Overall, there were no differences in darbepoetin dosage between the two groups. A subgroup analysis of compliant versus non-compliant patients with baseline 25(OH)D levels <15 ng/ml also showed no difference in hemoglobin levels (11.2 ± 1.7 vs. 11.4 ± 1.1 g/dl, p = 0.64) or darbepoetin dosing (0.38 ± 0.32 vs. 0.53 ± 0.65 μg/kg/month, p = 0.39) in the first 6 months.
Discussion
This study demonstrates that 25(OH)D deficiency is highly prevalent in our population of HD patients. It also demonstrates that a prescribing strategy for ergocalciferol replacement using dosing guidelines for CKD stages III–IV is inadequate for HD patients with 25(OH)D deficiency, as many deficient participants failed to reach normal serum 25(OH)D levels with replacement regimens designed for CKD patients. In addition, the decrease in the number of participants with normal 25(OH) D levels between 9 and 11 months of follow-up suggests that even after achievement of normal levels by repletion, HD patients may need ongoing oral supplementation to maintain normal levels. Finally, while favorable improvements in relevant laboratory parameters such as hemoglobin or PTH were not observed with ergocalciferol replacement in this study, neither were negative changes in parameters such as calcium.
The high prevalence of 25(OH)D deficiency and insufficiency in our HD population is consistent with that seen in several other studies [2, 10–12]. While the population in this study is at disproportionate risk for 25(OH) D deficiency and insufficiency due to the high percentage of African-American participants and the geographic location of the study, the results also highlight disease-specific risk factors for 25(OH)D deficiency including decreased consumption of 25(OH)D rich foods such as dairy due to dietary restrictions for phosphorus control and decreased sunlight exposure due to health factors. An alternative explanation for the high prevalence of vitamin D deficiency is suggested by a study by Bhan et al. [19] that found that African-American dialysis patients have lower levels of vitamin D-binding protein than Caucasian patients, and therefore have higher levels of free, bioavailable 25(OH)D than suggested by routinely measured total serum levels of 25(OH)D.
Our results also demonstrate the need for dosing guidelines for 25(OH)D replacement and maintenance specifically for HD patients. While compliant patients in this study did experience a significant increase in serum 25(OH)D levels with the KDOQI dosing regimen for CKD stages III–IV, most of the participants remained 25(OH)D-deficient at 6 months, requiring a second round of ergocalciferol replacement. Additionally, the number of participants with normal serum 25(OH)D levels fell between 9 and 11 months of follow-up, suggesting that HD patients need ongoing oral maintenance doses to maintain normal levels. Further studies are needed to delineate the optimal ergocalciferol repletion and maintenance dosing schedule in the ESRD population.
The prescribing strategy used in this study also highlights the need for the likelihood of patient compliance with a 25(OH)D regimen to be taken into account by healthcare providers. Other strategies, such as directly-observed therapy accomplished by provision of vitamin D supplementation during dialysis treatments, have been shown to result in sustained improvements in serum 25(OH)D levels in dialysis patients [20]. However, the additional burden on dialysis unit staff to dispense this medication during dialysis treatments must be balanced against the benefit of improved patient compliance when considering such a prescribing strategy.
Our patients who were compliant with prescribed ergocalciferol did not have hypercalcemia at a disproportionate rate compared to non-compliant patients, suggesting concordance with the findings of a randomized controlled trial of cholecalciferol supplementation that 25(OH)D supplementation is safe in this population [21]. The reason for the lack of an observed calcemic effect are suggested by a study by Armas et al. [22] that found that raising serum 25(OH)D to normal levels in HD patients using weekly cholecalciferol supplementation had no effect on intestinal absorption of calcium measured using a standardized labeled calcium meal.
Contrary to other studies, we did not find improvements in hemoglobin with ergocalciferol replacement [10, 12]. We did observe a small decrease in serum phosphorus levels in the compliant population. However, that group also used more phosphate binders which may explain the decrease. We also observed a small but statistically significant increase in serum albumin at 6 months of follow-up in compliant participants, consistent with other studies [9, 12]. Other parameters such as PTH and calcium did not change appreciably with an increase in 25(OH)D. However, Bhan et al. [19] found that bioavailable levels of 25(OH)D correlate better with PTH and calcium then total 25(OH)D levels. Since the bioavailability of 25(OH)D varies by race, especially in African-Americans [23], the correction of total 25(OH)D may not be an appropriate target for mineral metabolism.
Several studies have suggested that ESA use may decrease with ergocalciferol therapy [12, 24]. These studies compared ESA usage before and after supplementation but did not have a control group for comparison. Our results did not reveal any significant difference in darbepoetin use between the compliant and non-compliant group. One possibility for not seeing a significant drop in darbepoetin usage as seen in other studies is that less than half of the compliant group was able to reach 25(OH)D levels >30 ng/ml. Kumar et al. [24] reported a significant decrease in ESA usage for those with 25(OH)D levels >30 ng/ml compared to those treated and were still <30 ng/ml.
One of the strengths of this study is the use of a control group to compare the effectiveness of this prescribing strategy and its effects of bone metabolism and anemia management. Except for age where the non-compliant group was younger, the two groups were similar in terms of gender, race, and co-morbidities. The control group also showed no significant change in 25(OH)D levels at baseline and after 6 months of treatment, reinforcing their non-compliance and supporting the use of them as a control group. While non-compliance may influence bone metabolism parameters such as serum phosphorus levels, binder usage, and PTH, anemia management is typically under the control of the medical team by administering intravenous iron and darbepoetin during dialysis.
There are several limitations to this study. First, the majority of participants in this study were African-American or Hispanic, which may affect generalizability of the results to other racial or ethnic populations, especially since it is recognized that African-Americans are at increased risk for 25(OH)D deficiency [6]. Second, because the prevalence of 25(OH)D deficiency varies with latitude [25, 26], patients in other geographic locations may have different rates of 25(OH)D insufficiency or deficiency. However, 25(OH)D deficiency has been noted to be highly prevalent regardless of geographic location in CKD patients [27]. Similarly, seasonal variation in vitamin D levels is well known with a higher prevalence of vitamin D deficiency during the winter compared to the summer in HD patients [28]. In our cohort, our 6-month follow-up period included the fall and winter months where vitamin D levels would be expected to decrease. However, over 40% of our compliant patients achieved normal 25(OH)D levels while our non-compliant group showed no significant change. Furthermore, at 9 and 11 months (June and August 2010, respectively), more compliant and non-compliant patients had normal 25(OH)D levels. However, the compliant group had a significantly higher proportion at 9 months, suggesting an incremental effect of treatment on 25(OH)D levels.
In conclusion, 25(OH)D deficiency and insufficiency is a common problem in HD patients. Oral ergocalciferol replacement appears to be safe but dosing strategies need to be adjusted for HD patients as protocols developed for CKD stages III–IV patients appear to be inadequate in the ESRD population. Future studies should be designed to address questions of dosing, clinical outcomes, and cost-effectiveness in 25(OH)D-deficient HD patients.
Acknowledgments
A.P. is supported by a grant from the National Cancer Institute (KM1CA156717) and S.A. by a grant from the National Institute of Diabetes and Digestive Kidney Diseases (DK084121).
Footnotes
Portions of these results were presented in abstract and poster form at the American Society of Nephrology Meeting, Denver, Colo., November 2010.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI, the NIDDK, or the NIH.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Blair D, Byham-Gray L, Lewis E, McCaffrey S. Prevalence of vitamin D [25(OH)D] deficiency and effects of supplementation with ergocalciferol (vitamin D2) in stage 5 chronic kidney disease patients. J Ren Nutr. 2008;18:375–382. doi: 10.1053/j.jrn.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 3.National Kidney F. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 4.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 5.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab. 2010;95:471–478. doi: 10.1210/jc.2009-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D for health and in chronic kidney disease. Semin Dial. 2005;18:266–275. doi: 10.1111/j.1525-139X.2005.18402.x. [DOI] [PubMed] [Google Scholar]
- 8.Baeke F, Gysemans C, Korf H, Mathieu C. Vitamin D insufficiency: implications for the immune system. Pediatr Nephrol. 2010;25:1597–1606. doi: 10.1007/s00467-010-1452-y. [DOI] [PubMed] [Google Scholar]
- 9.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. Daily oral 25-hydroxycholecalciferol supplementation for vitamin D deficiency in haemodialysis patients: effects on mineral metabolism and bone markers. Nephrol Dial Transplant. 2008;23:3670–3676. doi: 10.1093/ndt/gfn339. [DOI] [PubMed] [Google Scholar]
- 10.Saab G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW. Prevalence of vitamin D deficiency and the safety and effectiveness of monthly ergocalciferol in hemodialysis patients. Nephron Clin Pract. 2007;105:c132–c138. doi: 10.1159/000098645. [DOI] [PubMed] [Google Scholar]
- 11.Del Valle E, Negri AL, Aguirre C, Fradinger E, Zanchetta JR. Prevalence of 25(OH) vitamin D insufficiency and deficiency in chronic kidney disease stage 5 patients on hemodialysis. Hemodial Int. 2007;11:315–321. doi: 10.1111/j.1542-4758.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 12.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A. Cholecalciferol supplementation in hemodialysis patients: effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol. 2010;5:905–911. doi: 10.2215/CJN.06510909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C. Evidence for persistent vitamin D 1α-hydroxylation in hemodialysis patients: evolution of serum 1,25-dihydroxycholecalciferol after 6 months of 25-hydroxycholecalciferol treatment. Nephron Clin Pract. 2008;110:c58–c65. doi: 10.1159/000151534. [DOI] [PubMed] [Google Scholar]
- 14.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D3 suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–659. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- 15.Kronfol NO, Hersh WR, Barakat MM. Effects of 25(OH)-vitamin D3 in hypocalcemic patients on chronic hemodialysis. ASAIO Trans. 1987;33:289–292. [PubMed] [Google Scholar]
- 16.Pecovnik-Balon B, Jakopin E, Bevc S, Knehtl M, Gorenjak M. Vitamin D as a novel nontraditional risk factor for mortality in hemodialysis patients. Ther Apher Dial. 2009;13:268–272. doi: 10.1111/j.1744-9987.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 17.KDIGO clinical practice guideline for the diagnosis, evaluation prevention, and treatment of chronic kidney disease-mineral and bone disorder. Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 18.Tokmak F, Quack I, Schieren G, Sellin L, Rattensperger D, Holland-Letz T, Weiner SM, Rump LC. High-dose cholecalciferol to correct vitamin D deficiency in haemodialysis patients. Nephrol Dial Transplant. 2008;23:4016–4020. doi: 10.1093/ndt/gfn367. [DOI] [PubMed] [Google Scholar]
- 19.Bhan I, Powe CE, Berg AH, Ankers E, Wenger JB, Karumanchi SA, Thadhani RI. Bioavailable vitamin D is more tightly linked to mineral metabolism than total vitamin D in incident hemodialysis patients. Kidney Int. 2012;82:84–89. doi: 10.1038/ki.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armas LA, Andukuri R, Barger-Lux J, Heaney RP, Lund R. 25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol. 2012;7:1428–1434. doi: 10.2215/CJN.12761211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delanaye P, Weekers L, Warling X, Moonen M, Smelten N, Medart L, Krzesinski JM, Cavalier E. Cholecalciferol in haemodialysis patients: a randomized, double-blind, proof-of-concept and safety study. Nephrol Dial Transplant. 2013 doi: 10.1093/ndt/gft001. (E-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.Armas LA, Zena M, Lund R, Heaney RP. Calcium absorption response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol. 2013 doi: 10.2215/CJN.08610812. (E-pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown AJ, Coyne DW. Bioavailable vitamin D in chronic kidney disease. Kidney Int. 2012;82:5–7. doi: 10.1038/ki.2012.135. [DOI] [PubMed] [Google Scholar]
- 24.Kumar VA, Kujubu DA, Sim JJ, Rasgon SA, Yang PS. Vitamin D supplementation and recombinant human erythropoietin utilization in vitamin D-deficient hemodialysis patients. J Nephrol. 2011;24:98–105. doi: 10.5301/jn.2010.1830. [DOI] [PubMed] [Google Scholar]
- 25.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55:1091–1097. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 26.Scharla SH. Prevalence of subclinical vitamin D deficiency in different European countries. Osteoporos Int. 1998;8(suppl 2):S7–S12. doi: 10.1007/pl00022726. [DOI] [PubMed] [Google Scholar]
- 27.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM. Prevalence of calcidiol deficiency in CKD: a cross-sectional study across latitudes in the United States. Am J Kidney Dis. 2005;45:1026–1033. doi: 10.1053/j.ajkd.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Tolouian R, Rao DS, Goggins M, Bhat S, Gupta A. Seasonal variation of vitamin D in patients on hemodialysis. Clin Nephrol. 2010;74:19–24. doi: 10.5414/cnp74019. [DOI] [PubMed] [Google Scholar]


