Abstract
Androgen administration to castrated individuals was purported to decrease activity in the serotonin system. However, we found that androgen administration to castrated male macaques increased fenfluramine-induced serotonin release as reflected by increased prolactin secretion. In this study, we sought to define the effects of androgens and aromatase inhibition on serotonin-related gene expression in the dorsal raphe, as well as serotonergic innervation of the LC. Male Japanese macaques (Macaca fuscata) were castrated for 5–7 months and then treated for 3 months with [1] placebo, [2] testosterone (T), [3] dihydrotestosterone (DHT; non- aromatizable androgen) and ATD (steroidal aromatase inhibitor), or [4] Flutamide (FLUT; androgen antagonist) and ATD (n=5/group). This study reports the expression of serotonin-related genes: tryptophan hydroxylase 2 (TPH2), serotonin reuptake transporter (SERT) and the serotonin 1A autoreceptor (5HT1A) using digoxigenin-ISH and image analysis. To examine the production of serotonin and the serotonergic innervation of a target area underlying arousal and vigilance, we measured the serotonin axon density entering the LC with ICC and image analysis. TPH2 and SERT expression were significantly elevated in T- and DHT+ATD- treated groups over placebo- and FLUT+ATD- treated groups in the dorsal raphe (p<0.007). There was no difference in 5HT1A expression between the groups. There was a significant decrease in the pixel area of serotonin axons and in the number of varicosities in the LC across the treatment groups with T > placebo >DHT+ATD = FLUT+ATD treatments. Comparatively, T- and DHT+ATD -treated groups had elevated TPH2 and SERT gene expression, but the DHT+ATD group had markedly suppressed serotonin axon density relative to the T-treated group. Further comparison with previously published data indicated that TPH2 and SERT expression reflected yawning and basal prolactin secretion. The serotonin axon density in the LC agreed with the area under the fenfluramine-stimulated prolactin curve, providing a morphological basis for the pharmacological results. This suggested that androgen activity increased TPH2 and SERT gene expression but, aromatase activity, and neural production of estradiol (E), may subserve axonal serotonin and determination of the compartment acted upon by fenfluramine. In summary, androgens stimulated serotonin-related gene expression, but aromatase inhibition dissociated gene expression from the serotonin innervation of the LC terminal field and fenfluramine-stimulated prolactin secretion.
Keywords: TPH2, SERT, 5HT1A, androgen, serotonin, aromatase, locus ceruleus, fenfluramine, prolactin
Introduction
Previous studies with humans, macaques and rodents have measured various endpoints and inferred that androgens decrease CNS serotonin function (Brown and LInnoila, 1990, Virkkunen et al., 1995, Coccaro et al., 1997, Clark and Henderson, 2003). Furthermore, low CNS serotonin was thought to play a causal role in androgen-induced aggressive behavior in humans, non-human primates, foxes and rodents (Martinez-Conde et al., 1985, Brown and LInnoila, 1990, Popova et al., 1991, Higley et al., 1996, Clark and Henderson, 2003, Popova, 2006). However, a few studies contradicted this dogma. For example, depletion of serotonin with PCPA in rats did not increase aggression unless androgens were present (Kubala et al.). Also in rodents, low doses of anabolic steroids increased serotonin activity (Thiblin et al., 1999) and T increased serotonin content in the frontal cortex and hypothalamus (Kubala et al., 2008). One study found elevated T during the mating season of rhesus macaques that was associated with elevation of a serotonin metabolite, 5HIAA, and elevated aggression (Mehlman et al., 1997). We hypothesized that the effect of testosterone (T) on serotonin is more complex than often suggested from studies of one serotonergic endpoint.
Other data suggest that serotonin is reduced in castrated or androgen-deprived men. Fenfluramine-induced prolactin secretion involves serotonin release with reuptake block; and castration reduced fenfluramine-induced prolactin secretion compared to normal men (Foresta et al., 1987). An increased incidence of depression and anxiety were observed in men with hypogonadism, and serotonin supports these functions (Ponholzer et al., 2009, Giltay et al., 2010). Also, castration increased 5HT1A autoreceptor expression in the rat raphe, an indirect indication of lower serotonin release from dendrites (Zhang et al., 1999). Therefore, although many studies link elevated androgens and reduced serotonin, other studies point to reduced serotonin in the absence of androgens.
The serotonin system has different compartments that can show independent or coordinated regulation. The most accessible and commonly described compartments in vivo are (1) serotonin metabolites, which are dependent upon enzymatic degradation, (2) serotonin per se in different brain regions, which is dependent upon serotonin transport to terminal fields, and (3) fenfluramine-induced prolactin secretion, which is dependent upon a poorly defined releasable pool of serotonin, that in turn stimulates prolactin secretion. Moreover, testosterone (T) is metabolized to active ligands of the androgen receptor (AR) and the estrogen receptor (ER). In females, we showed that estradiol (E) increases TPH2 gene and protein expression, and increases SERT binding, both thought to be markers of increased serotonin neurotransmission (Lu et al., 2003, Sanchez et al., 2005).
The direct regulation of serotonin at the level of gene expression by androgens needed clarification in primates. We previously showed that androgen administration did not correlate with fenfluramine-stimulated serotonin release and prolactin secretion if aromatase was inhibited (Bethea et al., 2013b). This study continues our previous observations with direct measurements of serotonin-related gene expression in the dorsal raphe, and analysis of serotonin transport to a pivotal target field, the locus ceruleus (LC).
The midbrain LC contains norepinephrine (NE) producing neurons. In macaques, it increases arousal, vigilance and attention to external stimuli (Grant et al., 1988, Aston-Jones et al., 1991b, Rajkowski et al., 1994, Aston-Jones et al., 1996). The majority of input to the LC comes from the autonomic nervous system (Aston-Jones et al., 1991a). The LC also has serotonergic innervation, and the source of the serotonin neurons has been ascribed to the local pericoerulear area (Aston-Jones et al., 1991c, Miller et al., 2011). Others have suggested that as much as 50% of the serotonergic innervation of the LC originates in the dorsal raphe of rats (Kaehler et al., 1999, Kim et al., 2004). In general, serotonin appears to be inhibitory of LC activity (Aston-Jones et al., 1991a), but there is a complex interplay with other excitatory amino acids (Charlety et al., 1993) and CRF (Valentino et al., 1993, Valentino et al., 2001, Jedema and Grace, 2004, Reyes et al., 2005, Curtis et al., 2012), as well as different modes of NE discharge (Aston-Jones and Cohen, 2005).
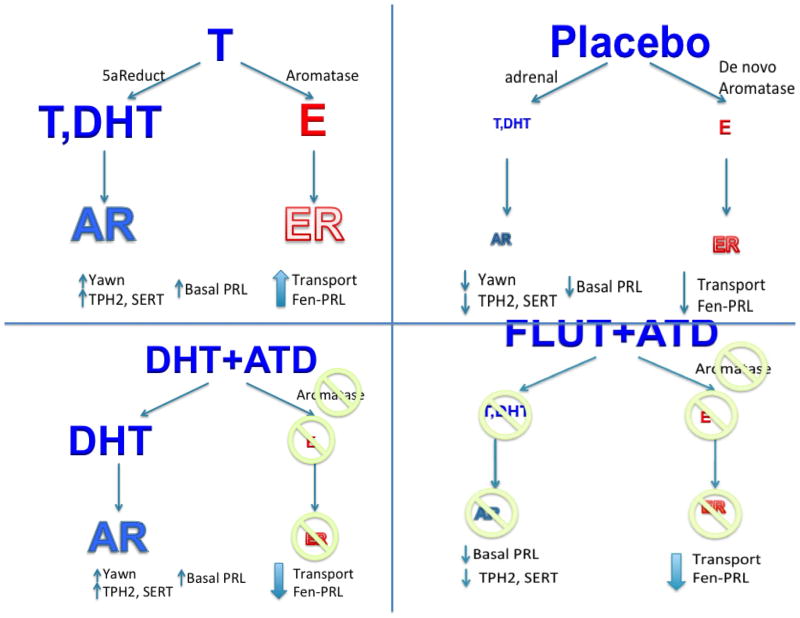
In order to examine serotonin gene expression and innervation under different conditions we used 2 different androgens (testosterone, T, and non-aromatizable dihydrotestosterone, DHT), an androgen antagonist (flutamide, FLUT), and an aromatase inhibitor (ATD). The objectives were to 1) remove endogenous androgens, but allow de novo neurosteroid production to proceed in castrated animals; 2) to restore androgen activity with T, which is converted to metabolites, DHT and 17β-estradiol (E); 3) to restore only androgen activity and eliminate all E activity by using the non-metabolizable androgen, DHT, together with aromatase block by ATD; and 4) to block all androgen activity and de novo E synthesis in the brain with FLUT + ATD. In this manner, we hoped to differentiate effects mediated by androgen versus estrogen activity (summary in Figure 8).
Figure 8.
This figure contains a graphic representation of the different treatments, the expected effects of the treatments and the outcomes. The androgens had a similar effect on yawning, basal prolactin secretion, TPH2 gene expression and SERT gene expression. However, divergence was observed in fenfluramine-induced prolactin secretion and serotonin innervation of the LC with aromatase inhibition.
We determined the expression of 3 genes related to serotonin neurotransmission: TPH2, SERT and 5HT1A. In addition, we measured the density of serotonin axons innervating the LC. Correlations between serotonin-related gene expression, serotonin axon density (this study), yawning, fenfluramine-induced prolactin, and basal prolactin secretion (previous study) were sought. We show that androgens increased serotonin-related gene expression. However, serotonin transport was altered by aromatase inhibition.
Methods and Materials
This experiment was approved by the IACUC of the Oregon National Primate Research Center and conducted in accordance with the 2011 Eight Edition of the National Institute of Health Guide for the Care and Use of Laboratory Animals. Male Japanese macaques (Macaca fuscata) were utilized for study.
Troop
The Japanese macaques were born and raised in a 2-acre outdoor corral at ONPRC with approximately 300 individuals. The troop has been the subject of extensive behavioral studies since it arrived at ONPRC in 1965 (Eaton, 1974, Eaton et al., 1990). The troop composition is relatively stable and the age structure is comparable to that of a natural troop (Maruhashi, 1982). Like other macaque species, the hierarchical organization of the troop is along matriarchal lineages. The matriarchal lines and dominance hierarchies within the troop are well documented, and have remained stable for the past 40 years. In the wild, males normally leave the natal troop and so their dominance is less a function of their mother’s status and more a function of their age, size and social skills. Males cannot leave our troop on their own so in that respect, there are more males than a natural troop although they are removed for research and sales with attention to troop stability.
Study Animals
Twenty adult male Japanese macaques were assigned to this project. Half were used in year 1 and half were used in year 2. At initial assignment, the animals were aged from 4.9–9.8 years (average 7.1 years) and weighed between 7.7 and 16.2 kg (average of 12.81 kg). This age range is considered adult. Microsatellite analysis of the entire Japanese macaque troop indicated that the animals were highly related.
All the animals were born in the outdoor 2-acre corral. Several years prior to this study, 6 of the males were removed and put together into an indoor/outdoor pen and they stayed together as one group for the entire study. The remaining 14 animals were brought in from the corral at the start of the study. The two groups could not be mixed due to fighting.
All 20 monkeys were acclimated to new indoor group housing and castrated. The animals were in a castrated state (with no treatment) for 5–7 mo before initiating this protocol [for details see (Bethea et al., 2013b)].
Animal Treatments
The experimental period was conducted during the mating season when aggression is highest amongst males in the troop. Although the animals were housed indoors, their annual rhythms continue to be manifested (Rostal et al., 1986). The groups were treated for 3 months. In year 1, half of the animals were treated with placebo or testosterone (T) (n=5/group) and then euthanized. In year 2, the remaining animals were treated with dihydrotestosterone (DHT) + aromatase inhibitor (ATD) or an androgen antagonist (Flutamide; FLUT) + ATD (n=5/group) and then euthanized. Focal observations using Observer Software were routinely conducted on all animals prior to a fenfluramine challenge and euthanasia. The rationale for the different treatment groups is explained below and illustrated in Figure 8. The doses administered were based upon previous studies reporting serum T concentrations in intact Japanese macaques (Eaton and Resko, 1974, Rostal et al., 1986) and dosing and treatment outcomes with ATD administration in macaques (Ellinwood et al., 1984). The dose of DHT was chosen to significantly elevate DHT over the normal concentration observed in intact or T treated macaques.
Testosterone was administered with 4 Silastic implants per animal (6-cm long; i.d. 0.132 in.; o.d. 0.183 in.; Dow Corning, Midland, MI) that were packed with crystalline T (Sigma, St. Louis, MO) and implanted subcutaneously on the back. With T-only treatment, it was expected that the brain would be exposed to T and DHT via neural 5α-reductase and neural E via neural aromatase, which in turn fully activated AR and ER in a local manner (high AR & high ER activation).
The placebo group (empty Silastic capsules) was expected to have very little androgen activity (adrenal source) and very little conversion of T to E in the brain. However, independent production of neural E from cholesterol could remain (Mukai et al., 2006, Tsutsui, 2012). Therefore, the placebo group was predicted to have virtually no AR activation and little ER activation in aromatase positive regions (low AR & reduced ER activation).
DHT and FLUT were administered with 90-day pellets (DHT=1 pellet/animal or flutamide=5 pellets/animal; Innovation Research of America, Sarasota, FL), which were implanted subcutaneously on the left side of the periscapular region. All DHT- and FLUT-treated animals also received ~2 gms ATD powder (Changzhou Harvest Chemistry, Changzhou, Jiangsu, China) in ~3 × 3 cm packets made from Silastic sheeting (0.01 in thick; AART, Inc., Reno, NV) and implanted subcutaneously on the right side of the periscapular region. ATD was shown to inhibit >90% of the aromatase activity in the amygdala (Ellinwood et al., 1984). The purity of the ATD was greater than 90% as determined with NMR (Dr. Andrew Placzek, Dept Physiology and Pharmacology, OHSU).
The DHT+ATD group was expected to have significant androgen activity and ~90% inhibition of aromatase, which led to fully activated AR and virtually no ER activation in a local manner (high AR and no ER activation). The rationale for adding FLUT to ATD was derived from the observation that ATD activated androgen receptors (AR) in castrated macaques (Resko et al., 1993). Therefore, the FLUT+ADT group was expected to have no androgen activity and inhibition of most aromatase, which in turn significantly reduced activation of both AR and ER in a local manner (no AR & no ER activation).
Blood samples were obtained prior to treatment, after 1–2 months, and after 3 months of treatment for immunoassay of T, DHT, and E. The 3-month sample for steroid assays was obtained prior to administration of a fenfluramine challenge (see below). The animals were euthanized shortly after the fenfluramine challenge.
Weights, Ages, Ranks
Each animal was weighted before treatment and at intervals during treatment when blood samples were obtained, implants were placed, prior to surgery and prior to necropsy. The ages were known from exact birth dates obtained from daily observations of the corral by ONPRC technicians. The dominance ranks were determined from win-loss recordings during focal observations after the animals were placed into group housing (supplement, (Bethea et al., 2013b).
Behavior
Behavioral data were collected with focal observations before and during the treatment period by an behavioral technician with no knowledge of treatment using Observer Software as previously described in detail (Bethea et al., 2013b). The ethogram that details the specific behaviors that were recorded was previously published and can be referred to if needed (Bethea et al., 2013b). To better compare the behavioral results with the neurobiological endpoints, we extracted the data for the 4 groups used for postmortem neurobiology studies. Initially, all purported aggressive behaviors were combined and reported (Bethea et al., 2013b). Herein, we separated the mild aggressive behavior, yawning, from the overt aggressive behaviors of threat, chase and contact. The rationale is discussed.
Fenfluramine-stimulated prolactin secretion
Fenfluramine is a potent serotonin releaser and it also blocks the re-uptake of serotonin, which in turn elevates serum prolactin (Cleare et al., 1998, Abel and Cleare, 1999, Rothman and Baumann, 2002, Bethea et al., 2005). Fenfluramine challenges were previously conducted. Results and details of the protocol can be found in Bethea et al., 2013b. The results of the fenfluramine challenges from the 4 groups used in this study were extracted and compared here for reader convenience.
Euthanasia
Monkeys were euthanized at the end of the treatment periods for collection of the brain and other tissues according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association and performed by an expert veterinary pathologist. They were transported to the necropsy suite under sedation, given an overdose of pentobarbital (30 mg/kg, i.v., Hospira, Lake Forest, IL) and exsanguinated with severance of the descending aorta.
Assays
Assays for PRL, T, DHT and E were performed on serum using a Roche Diagnostics Cobas e411 automatic clinical platform assay instrument or with RIA following chromatography as previously described. Steroid hormone concentrations, fenfluramine-stimulated and basal prolactin secretion were extracted from data previously collected and published, but are repeated here for reader convenience (Bethea et al., 2013b).
Tissue Preparation
The left ventricle of the heart was cannulated and the head was perfused with 1L of saline (made with DEPC-treated water [0.1% diethyl pyrocarbonate] to minimize RNase contamination) followed by 7 liters of 4% paraformaldehyde in 3.8% borate, pH 9.5 (Berod et al., 1981, Simmons et al., 1989). The brain was removed and dissected. Blocks of brain were post-fixed for 3 hours in 4% paraformaldehyde, then washed in 0.02 M potassium phosphate-buffered saline (KPBS) containing first 10% (overnight), then 20% glycerol (48 h), plus 2% dimethyl sulfoxide (DMSO) to cryoprotect the tissue. The blocks were frozen in precooled methylbutane (−55°C) and stored at −80°C for up to 6 months.
The midbrain blocks containing the dorsal raphe and locus ceruleus were cut on a sliding microtome at 25 μm, and half of the sections were mounted on Superfrost Plus Slides (Fisher Scientific, Pittsburgh, PA), dehydrated under vacuum overnight and then stored at −80°C until processing for in situ hybridization (ISH) assays. The rest of the sections were stored at −20°C in cryoprotectant for subsequent immunohistochemical (IHC) assays.
Riboprobes
A monkey specific partial cDNA clone of TPH2 containing 251 bp of the 5′ region (which has very little homology to TPH1) was previously constructed (Sanchez et al., 2005) and the TPH2 riboprobe incorporating digoxigenin was synthesized as previously described (Bethea et al., 2012b). New monkey specific partial cDNA clones of SERT and 5HT1A were constructed.
Based upon the rhesus monkey SERT sequence (NM_001032823), forward and reverse primers were ordered (Invitrogen, Carlsbad, CA) to amplify a 309bp sequence from 1230–1182 bp. The primer sequences were:
Forward: TGCAGAAGCGATAGCCCAACAT
Reverse: TTTCCTTCACGTCCCTGCAG
A 309bp amplicon was generated from rhesus macaque dorsal raphe mRNA. The amplicon was inserted into the pGEM-T vector and sequenced. The cRNA antisense probe was generated by linearization with SAC I digestion and T7 transcription. The cDNA sense probe was generated by linearization with SAC II digestion and SP6 transcription.
Based upon the rhesus monkey 5HT1A sequence (NM_001198700), forward and reverse primers were ordered (Invitrogen, Carlsbad, CA) to amplify a 306bp sequence from 483–788 bp. The primer sequences were:
Forward: ACCAGCGGCAACACTACTGG
Reverse: GGACGAGGTGCAACACAGC
A 306bp amplicon was generated from rhesus macaque dorsal raphe mRNA. The amplicon was inserted into the pGEM-T vector and sequenced. The cRNA antisense probe was generated by linearization with SAC II digestion and SP6 transcription. The cRNA sense probe was generated by linearization with SAC I digestion and T7 transcription.
The cRNA probes were labelled with digoxigenin according to established protocol. In-vitro transcription for cRNA was done in 500ng digested plasmid, 2mM Dig UTP (Roche Diagnostics, Indianapolis, IN), 5X Transcription buffer, NTPs (ACG) 2.5mM, DTT 10mM, RNA polymerase 20U (Promega, Madison WI) RNAse inhibitor 40U (Ambion/Invitrogen) in 10ul reaction. After DNAse treatment (5U/reaction) the quantity and quality of the digoxigenin-labelled probes was assessed with agarose gels and dot blot analysis. The cRNA probe was titrated for ISH assay as follows: TPH2 equalled 18ng/ul, SERT equalled 15ng/ul and 5HT1A equalled 20ng/ul.
Digoxigenin In situ Hybridization (ISH) Raphe Nucleus
The non-isotopic in situ hybridization procedure, described in detail elsewhere (Berg-von der Emde et al., 1995, Lima et al., 2009, Bethea et al., 2012b), utilized digoxigenin-labelled TPH2, SERT and 5HT1A cRNAs. It was applied to determine if mRNA abundance differs between treatment groups. The cRNA was prepared using cDNAs as template and SP6 RNA polymerase to drive the transcription reaction as described above (Berg-von der Emde et al., 1995). Following an overnight hybridization at 55–56°C, the slides were washed and processed for digoxigenin detection of TPH2, SERT and 5HT1A as reported elsewhere (Bethea et al., 2012b). After developing the digoxigenin/antidigoxigenin-alkaline phosphatase conjugate reaction by an overnight incubation in a 4-nitrobluetetrazolium chloride/5-bromo-4-chloro-3indoyl-phosphate staining solution, the slides were extensively washed in potassium phosphate-sodium chloride buffer pH 7.4, dehydrated in graded ethanol, dried, and cover slipped for microscopic examination. Five levels of the dorsal raphe were analysed at 250 μm intervals. The sections were matched anatomically and rostral, medial and caudal sections from all animals were processed together.
Densitometric Analysis of Digoxigenin ISH
Sections (5 levels/animal) were video-captured with the Marianas Stereology Workstation (Intelligent Imaging Innovations, Denver, CO) and Slidebook 5.0 (Olympus Imaging Systems, Center Valley, PA), which created a montage of the dorsal raphe at each level. The image was exported as a Tiff file and ImageJ (NIH freeware) was used to perform the image analysis. For each anatomical level, the largest representation of the dorsal raphe was chosen from amongst all of the animals. A square outline was placed over the chosen dorsal raphe and the exact dimensions were recorded. The same size square was then used for all of the animals at that anatomical level. First, the operator outlined (boxed) the dorsal raphe nucleus. Image J enabled segmentation of the image into positive (stained) and negative (unstained) pixels with the same saturation parameters. The program then provided the area of positive pixels, and with a filter applied, provided the number of cells in the defined area. The same procedure was applied with each gene at each anatomical level. The outlined area was also measured to ensure equal areas were analysed across all animals. Therefore, for each animal the following data were obtained from all sections: average positive pixel area, average positive cell number, and average fraction of the total area that was positive.
Serotonin Immunohistochemistry (IHC) in the Locus Ceruleus Nucleus
Midbrain sections were removed from cryoprotectant and −20°C storage and washed in KPBS buffer 4 times for 15 min each, immersed in 1% hydrogen peroxide for 30 min, washed in KPBS buffer 4 times for 15 min each and then incubated with the following blocking solutions: Vector normal goat serum (NGS; Vector Laboratories, Burlingame, CA) for 60 min; 3% bovine serum albumin (BSA; Sigma, St. Louis, MO) for 60 min; Vector avidin for 20 min; Vector biotin for 20 min. Sections were then incubated for 48 h in primary antibody to serotonin (Sigma, St Louis, MO). This antibody was extensively characterized across a range of titers with positive and negative controls; and it was previously applied to primate brain (Sanchez et al., 2010, Bethea et al., 2012a). The primary serotonin antibody was diluted in 2% NGS, 0.02 M KPBS, and 0.4% Triton at 1/10,000. Sections were then rinsed in KPBS buffer 4 times for 15 min each, incubated in Vector biotinylated goat anti-rabbit serum for 60 min, washed in KPBS buffer 4 times for 15 min each, incubated with Vector ABC reagent for 60 min, washed in KPBS buffer 4 times for 15 min each, incubated with 0.05% diaminobenzidine (DAB kit, Vector Laboratories) containing 3% hydrogen peroxide for 1–10 min, and finally washed in KPBS buffer 4 times for 15 min each. Sections were mounted on gelatin-coated slides and dried overnight under vacuum. The sections were further dehydrated through a graded series of ethanols and xylene. The sections were finally mounted under glass with DPX.
Densitometric Analysis of Serotonin Varicosities in the Locus Ceruleus
Four sections processed with serotonin IHC were analyzed for each animal. The sections were morphologically matched between animals using anatomical reference points. Several landmarks were pivotal and defined the LC from a rostral to a caudal level. The mesencephalic 5 tract (me5) was most definitive. It emerged at the most rostral level, then elongated into the LC and gradually merged with the LC becoming shorter and wider at our most caudal level (Bethea et al., 2012a). The location of me5 relative to the superior cerebellar peduncle (branchium conjunctivum) was also useful. Although we cut in a slightly different plane, the sections are approximately located from Plate 90 (Interaural 00.30mm; Bregma −21.60mm) to Plate 94 (Interaural −01.50 mm; Bregma −23.40 mm) in the atlas “The Rhesus Monkey Brain in Stereotaxic Coordinates (Paxinos et al., 2000). The Marianas Stereological workstation with Slidebook 5.0 was used for image capture. A montage of the entire area of serotonin immunostaining was built by the workstation. The image was exported as a Tiff file and ImageJ was used to perform the analysis. The area containing serotonin staining was defined, further cropped and kept constant across the levels and animals for each assay. After contrast adjustment and a sharpen feature were applied, the image was segmented into positive and negative pixels with the same saturation parameters. The program provided positive pixel area, and with a filter applied, provided the number of varicosities in the defined area. Therefore, for each section the following data were obtained: positive pixel area, varicosity number, and fraction of the total area that was positive. The data were then averaged across all sections from each individual animal.
Statistical Analysis
All measurements were averaged across the sections, generating one overall average for each animal. Therefore, the variance around the mean of each group reflects the difference between animals. The data were compared with analysis of variance (ANOVA) followed by Newman-Keuls post hoc pairwise comparison. The data were tested for unequal variance and if present, a non-parametric ANOVA was applied (Kruskal-Wallace). Finally, regression analysis was applied to gene expression, serotonin axon and varicosity staining, yawning, fenfluramine-stimulated serotonin-dependent prolactin release and basal prolactin secretion. All statistical analyses were conducted using the Prism Statistic Program 5.0 (GraphPad, San Diego, CA). A confidence level of p<0.05 was considered significant.
Results
Weights, Ages, Ranks
The ages, weights and dominance ranks are presented in Tables 1 and 2. The age of the animals was that of adult reproductively competent Japanese macaques in the ONPRC troop. Following placement in social housing, focal observations commenced. Rank was assessed by determining the number of wins and losses in encounters during the treatment period (Bethea et al., 2013b). The weights and ranks remained stable throughout the experimental periods. There was no correlation between serotonin endpoints and weight, age or rank.
Table 1.
The average and range of age and weight of the male Japanese macaques used in this study.
| Age (yrs) Mean±sem (range) | Weights (kg) Mean±sem (range) | |
|---|---|---|
| Year 1 (n=10) | 7.05±0.14 (5.5–10) | |
| Castrate 5 mo | 13.1±1.3 (9.4–17.2) | |
| Treated 3 mo | 12.8±0.9 (9.3–16.2) | |
| Year 2 (n=10) | 7.7±0.4 (6–10) | |
| Castrate 7 mo | 12.9±0.5 (10.2–14.9) | |
| Treated 3 mo | 13.9±1.1 (11.3–18.3) |
Table 2.
The rank of the individual males determined by wins and losses after castration and prior to the experimental treatments.
| Year 1 | |||
|---|---|---|---|
| ID | Dominance | Implant | Drug |
| 1 | Dom | Empty | 0 |
| 2 | Sub | Empty | 0 |
| 3 | Mid | Empty | 0 |
| 4 | Mid | Empty | 0 |
| 5 | Dom | Empty | 0 |
| 6 | Sub | T | 0 |
| 7 | Sub | T | 0 |
| 8 | Mid | T | 0 |
| 9 | Mid | T | 0 |
| 10 | Dom | T | 0 |
| Year 2 | |||
|---|---|---|---|
| ID | Dominance | Implant | Drug |
| 11 | Mid | DHT | ATD |
| 12 | Sub | DHT | ATD |
| 13 | Dom | DHT | ATD |
| 14 | Mid | DHT | ATD |
| 15 | Sub | DHT | ATD |
| 16 | Mid | FLUT | ATD |
| 17 | Dom | FLUT | ATD |
| 18 | Dom | FLUT | ATD |
| 19 | Mid | FLUT | ATD |
| 20 | Sub | FLUT | ATD |
Prolactin secretion, behavior and achieved hormone concentrations
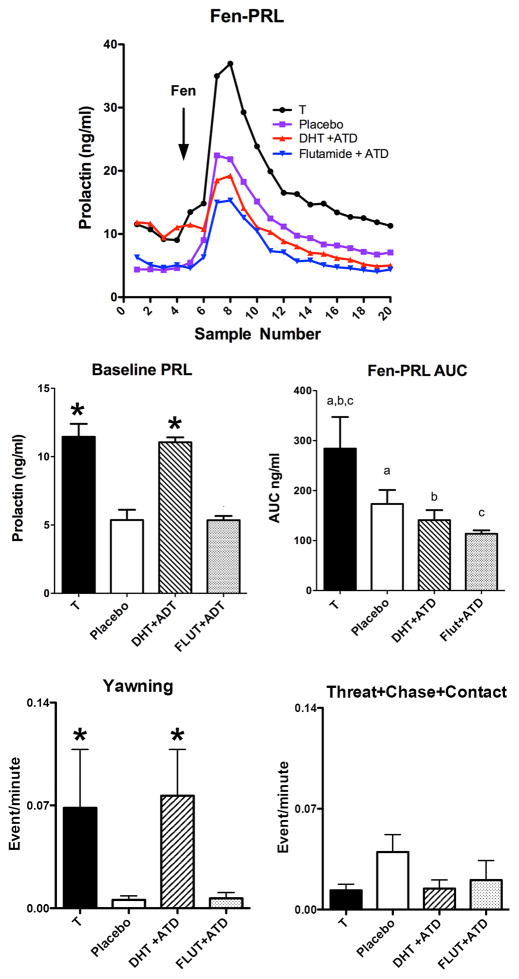
Table 3 contains steroid hormone results during the experimental period. The concentration of T achieved was significantly different between groups (F [3,16]= 37.58; p<0.0001). The concentration of DHT achieved was significantly different between groups (F [3,15]= 15.44; p<0.0001). Figure 1 shows data extracted from our previous publication for only the 4 groups used for neurobiology studies (Bethea et al., 2013b). Further statistical analysis compared only the 4 groups. All other figures contain new data. Figure 1, top panel illustrates prolactin secretion during fenfluramine challenge, which was significantly different between the groups (2 way ANOVA F[3,16] = 3.591; p = 0.04 for treatment). In Figure 1, middle panels, there was a significant difference in the area under the fenfluramine-stimulated prolactin curve or AUC (F [3,16] = 4.44; p = 0.019), and in baseline prolactin secretion (F [3,20] = 27.40; p < 0.0001) between the 4 groups. Newman Keul’s posthoc pairwise comparison on the AUC data (challenge samples 6–20) showed that the groups treated with T or DHT+ATD were significantly higher than the groups treated with placebo or FLUT+ATD (astericks). In addition, posthoc comparison on baseline prolactin secretion (challenge samples 1–5) showed that the T-treated group was significantly higher than all of the other groups. This is illustrated with letters so that groups with the same letters are different (a-a, different; b-b, different; c-c different (p < 0.05 all). Figure 1, bottom panels illustrates aggressive behavior that has been parsed into mild (yawning) and overt (threat, chase, contact). There was a significant increase in yawning with androgen administration (nonparametric ANOVA p=0.0067). Both T- and DHT+ATD groups showed elevated yawning, which indicates that yawning is an androgen specific behavior and does not depend on aromatization. Overt aggressive behavior was rare and did not change between the groups.
Table 3.
Steroid hormone levels achieved in serum during the treatment period of the 4 groups processed for neurobiology studies (reproduced from Bethea et al., 2013 for reader convenience).
| Groups Steroid concentrations (mean ± SEM) | Year 1 | Year 2 | ||
|---|---|---|---|---|
|
| ||||
| Castrate | Testosterone | Flutamide+ATD* | DHT+ATD | |
| Castrate 5–7 mo | ||||
| T (ng/ml) | 0.05±0.001 | 0.04±0.006 | 0.063±0.008 | 0.04±0.006 |
| DHT (ng/ml) | 0.11±0.02 | 0.08±0.009 | 0.178±0.007 | 0.24±0.053 |
| E (pg/ml) | <5 | <5 | <5 | <5 |
| Mid-Treatment | ||||
| T (ng/ml) | <0.025 | 7.85±1.71 | 1.31±0.52 | 1.74±0.67 |
| DHT (ng/ml) | 0.22±0.15 | 2.07±0.79 | 0.31±0.05 | 19.37±3.69 |
| E (pg/ml) | <5 | <5 | <5 | <5 |
| 3 months Treatment | ||||
| T (ng/ml) | 0.347±0.25 | 7.14±0.98 | 0.87±0.32 | 0.88±0.59 |
| DHT (ng/ml) | 0.15±0.03 | 2.93±0.83 | 0.49±0.049 | 20.52±5.32 |
| E (pg/ml) | <5 | <5 | <5 | <5 |
Absolute levels of hormones are not informative since the animal was treated with flutamide, a potent androgen antagonist.
Figure 1.
TOP. Line graph of the PRL response to fenfluramine injection in castrated male monkeys in the 4 groups treated with T, placebo, DHT+ATD and FLUT+ATD that were used in this study. Middle. Histograms illustrating the area under the fenfluramine-stimulated prolactin secretory curve and baseline prolactin across the 4 treatment groups used in this study (out of 6 groups examined for behavior and prolactin secretion in a previous publication (Bethea et al., 2013b). The data have been extracted and reprinted for ease of comparison to data collected in this study. Further statistical analysis was conducted on the 4 included groups. The area under the prolactin curve (fen-PRL AUC) showed a significant difference across the groups (F [3,16] = 4.44; p = 0.019). There was also a significant difference across the groups in baseline prolactin, obtained at 10 min intervals for 1 hour prior to fenfluramine challenge (F [3,20] = 27.40; p < 0.0001). Bottom. Histograms illustrating different components of behavior during the treatment periods. Androgen treatment increased yawning and there was no effect of aromatase inhibition. There was little overt aggressive behavior during the treatment period and no differences between the groups.
* different from placebo and FLUT+ATD with with Neuman-Keuls posthoc pairwise comparison (p < 0.05).
a,b,c AUC - groups with the same letter were different with Neuman-Keuls posthoc pairwise comparison (p < 0.05).
Digoxigenin signal and appearance of dorsal raphe
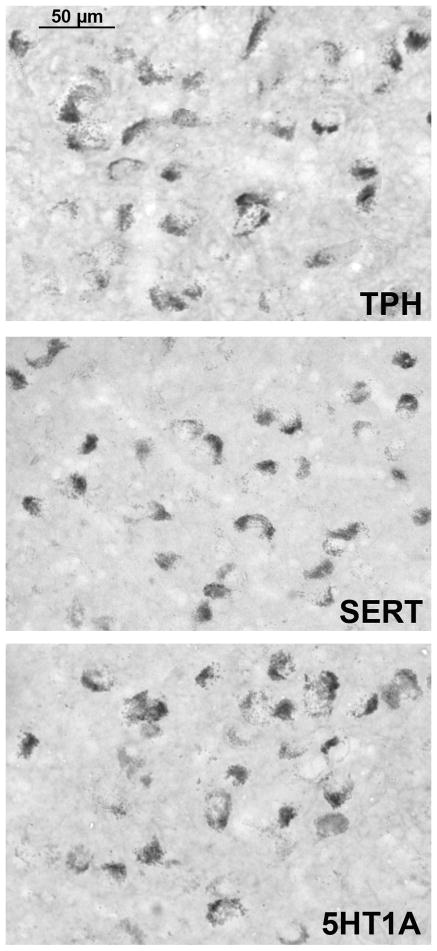
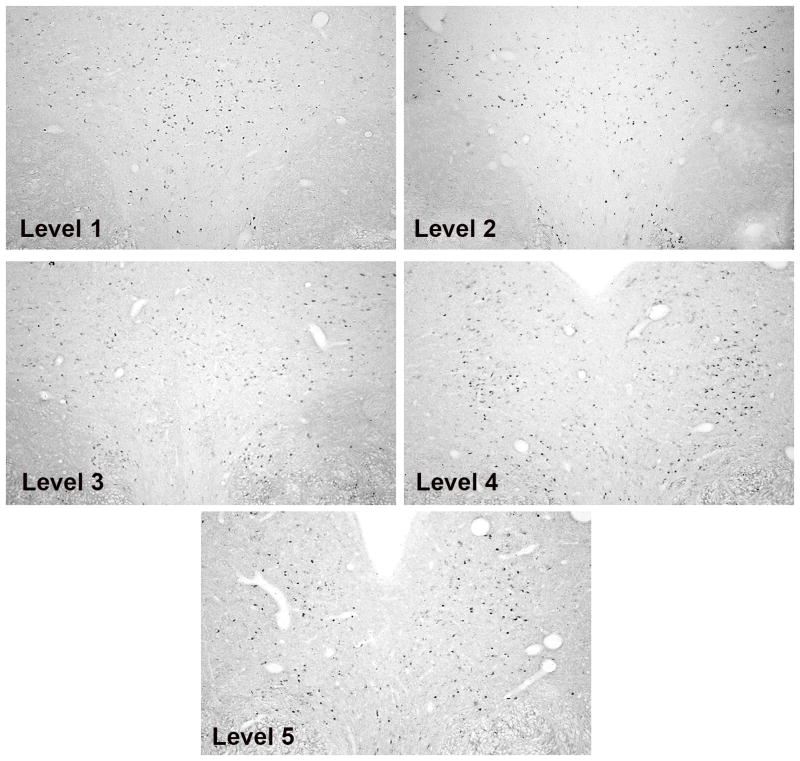
The staining for TPH2-, SERT- and 5HT1A-digoxigenin is illustrated in Figure 2. Control sections processed without probe or with sense probe showed little non-specific staining (Bethea et al., 2013a). The staining is confined to the cytoplasm with each probe, consistent with labeling of RNA. The photomicrographs were obtained with a Microbrightfield digital camera (MBF Bioscience, Williston, VT) mounted on a Zeiss microscope using Stereoinvestigator software (MBF Bioscience). The anatomy of the raphe sections from a rostral to a caudal level is illustrated in Figure 3 with TPH2 labeling. The dark dots are TPH2 positive neurons. Five representative levels are shown that illustrate the range of morphologies in a rostral to caudal direction. These low magnification photomicrographs are typical montages generated by the Marianus Workstation, which were subsequently enlarged, cropped and used for image analysis.
Figure 2.
Photomicrographs of typical digoxigenin ISH signals in the male monkey raphe for TPH2, SERT and 5HT1A. Pictures were obtained from DHT+ATD-treated animals on a Zeiss brightfield microscope with a Microbrightfield Neurolucida camera and Stereoinvestigator software. The DHT+ATD group had robust signals for each gene.
Figure 3.
TPH2 digoxigenin ISH staining is shown to illustrate the morphology of the raphe at the 5 levels that were subjected to analysis. Levels 1–3 were obtained from a DHT+ATD-treated animal and levels 4–5 were obtained from a T-treated animal because these groups exhibited the most robust signals. These images were obtained on the Marianas Stereology Workstation with Slidebook 5.0 and represent the typical areas that were analyzed.
TPH2, SERT, and 5HT1A expression
The overall average positive pixel area for TPH2, SERT and 5HT1A in each group is illustrated in Figure 4. There was a significant difference in the average TPH2 positive pixel area (F [3,14] =6.2; ANOVA p= 0.0067). The DHT+ATD- treated group was significantly higher than the placebo- and FLUT+ATD-treated groups (Newman-Keuls posthoc p< 0.05). T- and DHT+ATD-treated groups were not different from each other. The average TPH2 positive cell numbers reflected the average positive pixel area (data not shown; F [3,14] = 6.4; ANOVA p=0.0059).
Figure 4.
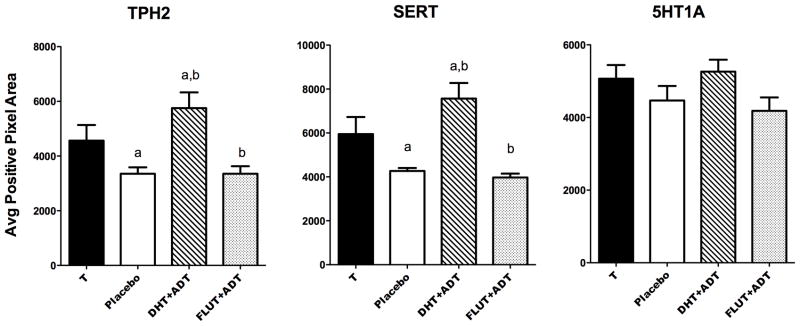
Histograms illustrating gene expression for TPH2, SERT and 5HT1A in castrated male macaques treated with T, placebo, DHT+ATD and FLUT+ATD. There was a difference between the groups in TPH2 expression (F [3,14] =6.2; ANOVA p= 0.0067) and in SERT expression (F [3,14] = 8.6; ANOVA p=0.0018). There was no difference post-hoc between T- and DHT+ATD-treated groups. There was no difference between the groups in 5HT1A autoreceptor expression after 3 months of treatment. Groups with the same letter are different by Newman-Keuls posthoc pairwise comparison (p< 0.05).
There was a significant difference in the average SERT-positive pixel area between the groups (F [3,14] = 8.6; ANOVA p=0.0018). The DHT+ATD- treated group was significantly higher than the placebo- and FLUT+ATD-treated groups (Newman-Keuls posthoc p< 0.05). T- and DHT+ATD-treated groups were not different from each other. The average SERT positive cell numbers reflected the average positive pixel area (data not shown; F [3,14] = 7.6; ANOVA p=0.003).
There was no difference in the average 5HT1A-positive pixel area or positive cell number across the 4 groups after 3 months of treatment. The lack of a difference in 5HT1A cell number indicates that serotonin neuron number did not change with treatment. Therefore, the changes in TPH2 and SERT expression are due to intercellular regulation and not a change in overall neuron number. Hence, in the dig-ISH assay, TPH2 and SERT expression fall below the level of detection in some cells.
Serotonin Innervation of the Locus Ceruleus (LC)
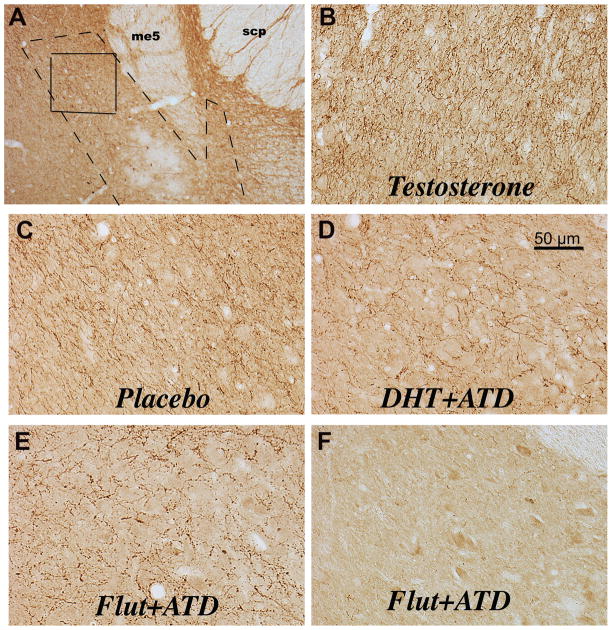
The serotonin innervation of the LC is illustrated in Figure 5, with a representative section from each group. The photomicrographs were obtained on a Zeiss brightfield scope with a Microbrightfield camera and Stereoinvestigator software (MBF Bioscience). Serotonin axon density was determined by analysis of the positive pixel area (axons and varicosities) and determination of serotonin varicosity number. In panel A, a low magnification photomicrograph illustrates the LC and surrounding structures. A dashed line outlines the location of the NE neurons of the LC. A box outlines the area in which serotonin axon and varicosity density were examined (panel A). The area is medial to the mesencephalic tract 5 (me5). The rostral to caudal morphology of the LC in macaques was illustrated with tyrosine hydroxylase (TH) immunostaining in a previous publication (Bethea et al., 2012a). Panels B, C, D, and E, illustrate representative serotonin axon and varicosity density in each treatment group. There is a graded reduction in axon density such that T>placebo>DHT+ATD=FLUT+ATD treatment. Panel F illustrates the LC from one animal in which serotonin axons were nearly absent. In panels D and E, the serotonin axons are coursing around large unstained neurons (blank areas). It is difficult to see the neurons under the dense serotonin axons in panels B and C.
Figure 5.
Photomicrographs of the serotonergic innervation of the locus ceruleus. Panel A indicates the location of the LC noradrenergic neurons (dashed line) and the area examined for serotonin axons and varicosities (solid line). The landmarks in this area include the mesencephalic 5 tract (me5) and the superior cerebellar peduncles (scp). This photograph is representative of level 3 in our analysis and corresponds to level 3 in a previous publication of tyrosine hydroxylasae immunostaining in the locus ceruleus (Bethea et al., 2012a). Panels B, C and D illustrate representative serotonin immunostaining of axons and varicosities over and around the noradrenergic neurons of the locus ceruleus. The serotonin axons are coursing around large unstained neurons (blank areas) in panels D and E. It is difficult to see the neurons under the dense serotonin axons in panels B and C. Panel F illustrates the LC from one animal in which serotonin axons were nearly absent. The serotonin axons are extremely dense in the T-treated male and decrease somewhat in the placebo-treated male. Administration of ATD markedly decreases serotonin axon density in the presence (DHT) or absence (FLUT) of androgens.
The results of the serotonin axon density analysis are illustrated in Figure 6. The serotonin varicosity number and positive pixel area were significantly different between the groups (F [3,13] = 8.675; p = 0.002, and F [3,13] = 8.255; p = 0.0025, respectively). The T-treated group was significantly higher than the DHT+ATD and FLUT+ATD groups (Newman Keuls p< 0.05) and the castrate placebo-treated group was significantly higher than the FLUT+ATD group (Newman Keuls p<0.05). The average varicosity size did not change.
Figure 6.
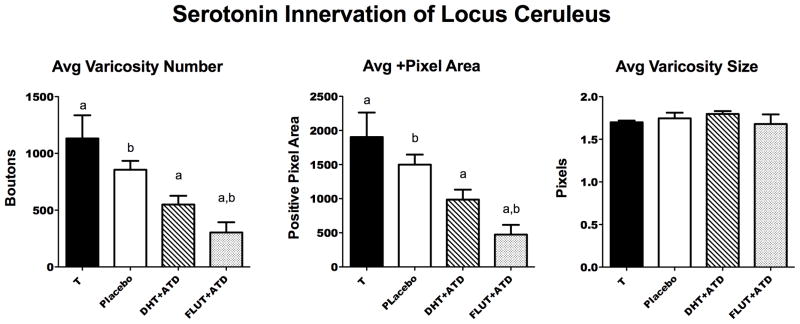
Histograms illustrating 3 important parameters of the serotonin innervation of the LC in the 4 treatment groups. There was a significant difference across the groups in the average varicosity number (F [3,13] = 8.675; p = 0.002) and in the average positive pixel area of axons and varicosities (F [3,13] = 8.255; p = 0.0025). Groups with the same letter were different in a posthoc pairwise comparison (Newman Keuls p< 0.05). The average varicosity size did not change. Rather, there were fewer of them across the groups.
Endpoint comparisons
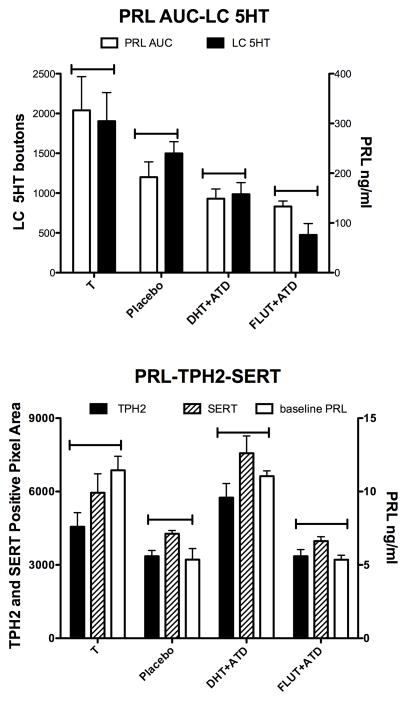
Figure 7 illustrates several different endpoints graphed together. Bars were placed above the columns to visually facilitate pattern recognition. The top panel illustrates the similarity in regulation of fenfluramine-stimulated PRL secretion and serotonin-positive pixel area in the LC. Both of these endpoints decreased as the neural production of E decreased. There was a trend toward correlation between the area under the fenfluramine-stimulated serotonin-dependent prolactin curve (AUC) and the serotonin innervation of the LC (F [1,4] = 10.71; r2 = 0.821; p = 0.08). The bottom panel illustrates the similarity in regulation of basal PRL, TPH2 expression and SERT expression. Again, bars were placed above the columns to visually facilitate pattern recognition. All 4 of these endpoints were stimulated by an androgen, either T or DHT. There was a trend toward correlation between basal prolactin secretion and TPH2 or SERT gene expression (F [1,4]; r2=0.8; p~0.1). Not shown on the graph, there was a a trend toward correlation between TPH2 and yawning (F[1,2]=15.17; r2=0.88; p=0.06) and between SERT and yawning (F[1,2]=16.91; r2=0.88; p=0.054). The small ‘n’ likely prevented the trends from reaching statistical significance, but we consider the similarity between the endpoints to be physiologically relevant.
Figure 7.
Comparisons of endpoints obtained in this study. Top. Both fenfluramine-stimulated PRL secretion (PRL AUC) and the density of serotonin pixels in the LC (LC 5HT) declined with decreased E in the brain due to castration or aromatase block. There was a trend toward correlation between the AUC of fenfluramine-stimulated prolactin secretion and the serotonergic innervation of the LC (F [1,4] = 10.71; r2 = 0.821; p = 0.08). Bottom. Basal PRL, TPH2 gene expression and SERT gene expression all showed stimulation with androgen treatment, either with T or DHT. Aromatase block had no effect on basal PRL, TPH2 expression or SERT expression. Regression analysis of prolactin secretion and TPH2 or SERT gene expression exhibited a positive trend (F [1,4]; r2=0.8; p~0.1). Regression analysis of TPH2 and yawning (F[1,2]=15.17; r2=0.88; p=0.06) and between SERT and yawning (F[1,2]=16.91; r2=0.88; p=0.054) also showed a positive trend (data not shown on graph).
A summary illustration of the treatment effects and results is presented in Figure 8. The treatment is presented at the top of each panel with arrows leading to the predicted steroid concentration as indicated by the size of the font. The relative extent of the respective activity of each steroid is indicated by the size of the font for the cognate receptor. With T treatment, there is an increase in both androgen activity (AR) and E activity (ER), which produced elevated yawning, elevated TPH2 and SERT expression, robust serotonin axon density, and robust fenfluramine-stimulated prolactin secretion. With placebo treatment, there is very little androgen activity and minor E activity from de novo synthesis, which yielded almost no yawning, low TPH2 and SERT expression, somewhat decreased axon density and reduced fenfluramine-stimulated prolactin secretion relative to T-treatment. With DHT+ATD there is high androgenic activity, but no aromatase activity and hence no E activity. This produced elevated yawning, elevated TPH2 and SERT expression, markedly reduced axon density and a further reduction in fenfluramine–stimulated prolactin secretion. With FLUT+ATD, all androgen and E activity was eliminated yielding rare yawning, low TPH2 and SERT expression, markedly reduced axon density, and a reduction in fenfluramine–stimulated prolactin secretion equal to DHT+ATD.
Discussion
Previous studies in humans and animal models showed increased aggression and reductions in serotonin in various brain areas or its metabolite, 5HIAA in CSF, that correlated with elevated levels of androgens or anabolic steroids (Brown et al., 1982, Bonson et al., 1994, Higley et al., 1996, Coccaro et al., 1997, Stanley et al., 2000, McGinnis, 2004). Our preliminary measurements of CSF 5HIAA confirm these observations, but the gene expression and serotonin innervations results contest the interpretation. In men, castration reduced fenfluramine-induced prolactin secretion (Foresta et al., 1987), which agrees with our data in macaques. Indeed, Foresta and colleagues suggested that in men, E and aromatizable androgens influence fenfluramine-induced prolactin secretion at least in part by involving the ‘activation’ of the hypothalamic serotonin system. Our data suggest that ‘activation’ may actually be ‘transport’.
Androgens increase yawning (Deputte et al., 1994), which we verified, and which in turn, confirmed that our androgen treatments were acting in the brain. Yawning has been considered a kind of low level threat in Old World macaques, and yawning has been observed in tense situations in adult males who engage in ‘canine contests’ (Anderson, 2010). Yawning has also been interpreted as an anxiety behavior in female baboons (Castles et al., 1999). However, a more recent study suggested there are different types of yawns that are either linked to affiliative-social or agonistic-tense behaviors in macaques (Leone et al., 2014). We hypothesize that yawning in our males may have been an affiliative-social behavior that normally serves to decrease tension when conflicts are prevalent during mating season.
Lately, the interpretation of NHP behavior is undergoing revision. For example, previously labeled ‘fear grimace’ is now thought to be a social signal of rank acquiescence that contributes to social adhesion (Beisner and McCowan, 2014). Individuals in our male-only social housing exhibited little overt aggressive behavior similar to bachelor groups in the wild (Barrett et al., 2002); and rank remained stable regardless of treatment. This may have been due to lack of females and unexplored mechanisms for social and affiliative behaviors in male-only social groups. Further experiments are planned to probe these hypotheses in greater depth.
In a previous study (Bethea et al., 2013b), T-treated animals exhibited the greatest release of serotonin/prolactin in response to fenfluramine compared to placebo controls, which is consistent with the data of Foresta and colleagues (Foresta et al., 1987). However, androgen + aromatase block (DHT+ATD) or androgen block +aromatase block (FLUT+ATD) exhibited the lowest fenfluramine-stimulated serotonin-dependent prolactin secretion. Clearly, there was more serotonin available for release in T animals than DHT+ATD animals.
This study examined gene expression that governs serotonin neurotransmission as well as serotonergic innervation of the LC in 4 of the 6 groups of animals previously characterized for behavior and fenfluramine-stimulated serotonin-dependent prolactin secretion (Bethea et al., 2013b). TPH2 and SERT gene expression were highest in the T and DHT+ATD groups, which respectively had the highest and lowest fenfluramine-stimulated serotonin-dependent prolactin release. Thus, serotonin-related gene expression did not correlate with fenfluramine-stimulated serotonin-dependent prolactin secretion. Moreover, TPH2 and SERT expression were significantly elevated in groups with androgen replacement in contrast to accepted notions of ‘increased androgens cause decreased serotonin function’ (Clark and Henderson, 2003).
Upon showing that androgens increase serotonin-related gene expression in Japanese macaques, we then showed that the concentration of serotonin in a terminal field could be dissociated from serotonin gene expression with aromatase inhibition. The LC plays a crucial role in vigilance and provoked aggression (Aston-Jones et al., 1999, Rainer et al., 2014). We found that compared to T-treated animals, serotonin axon and varicosity density in the LC decreased with placebo and decreased further with aromatase block (DHT+ATD or FLUT+ATD). Even though serum concentrations of DHT were several fold higher than T, TPH2 and SERT gene expression were elevated to the same extent in both groups. However, the serotonin axon and varicosity density in the terminal field was significantly lower in the DHT+ATD group than the T-treated group, implicating aromatase inhibition. Moreover, serotonin axon density correlated with fenfluramine-stimulated prolactin secretion, which provides morphological support for the pharmacological fenfluramine results. We have other data to support the concept that the serotonin innervation of the LC reflects the serotonin innervation to the hypothalamus (in press, Neuroendocrinology). Continuing this line of reasoning, we propose that serotonin in terminal fields constitutes the fenfluramine-releasable pool. In summary, fenfluramine action depends on serotonin ‘transport’ and transport can be uncoupled from gene expression.
Altogether, the data suggest that measurements of serotonin or its metabolites are not necessarily indicative of gene expression. Of particular importance is the decreased density of serotonin axons that was present with aromatase block. This observation implies that for animals treated with non-aromatizable anabolic steroids, serotonin in the hypothalamus and elsewhere could be quite low, but the serotonin neurons per se may be actively transcribing genes for serotonin synthesis (TPH2) and recycling (SERT).
The idea that aromatase inhibition severely curtails axonal transport is supported by other work in female rhesus monkeys. E administration to ovariectomized monkeys significantly elevated gene transcription underlying RhoGTPases and actin remodeling in serotonin neurons (Bethea and Reddy, 2010). Our recent data show that E increased gene expression encoding protein transport motors, kinesin, dynein and dynactin (2013 Annual Meeting of the Society for Neuroscience). An important caveat is that serotonin is not packaged in vesicles until prior to release; and it is thought to diffuse through the cytoplasm. However, the movement of serotonin over long distances is not compatible with diffusion. Classical fast axonal transport involves moving cargo vesicles along actin fibers, whereas slow axonal transport moves hundreds of soluble or cytoplasmic proteins. The slow transport may involve highly kinetic association/disassociation of soluble factors with a mobile carrier (Henry et al., 1998). However, studies have been limited to soluble proteins and not monoamines. Nonetheless, VMAT, the vescicular monoamine transporter, is moved by fast axonal transport in vesicles and serotonin might associate with this cargo. Thus, it appears that androgens increase TPH2 and SERT gene expression, while E supports serotonin transport, but exactly how this occurs is unknown.
Preliminary data in our laboratory indicates that in male macaques, serotonin neurons contain ERβ, but not AR; and ERα is widely expressed in the pontine midbrain. This leads to the speculation that activated AR in neighboring neurons, possibly of glutamate or excitatory amino acid phenotype, stimulates serotonin neurons, whereas ERs within serotonin neurons respond to E and promote transport.
The measurement of 5HIAA, the main metabolite of serotonin, as a reflection of serotonin production is based upon several assumptions related to turnover and degradation. Degradation of serotonin is dependent on monoamine-oxidase A (MAO-A; (Saura et al., 1982). Newman and colleagues showed that the low transcription allele of MAO-A decreased 5HIAA concentrations in CSF (Newman et al., 2005). Therefore, the effect of androgens on MAO-A gene expression is of great importance; and we suggest that the concentration of 5HIAA reflects MAO-A activity rather than synthesized or transported serotonin. We showed that MAO-A expression was suppressed by E administration to ovariectomized female rhesus macaques (Gundlah et al., 2001). Moreover, we have preliminary data showing very low MAO-A expression in the raphe of T-treated males and marked elevation of MAO-A expression with inhibition of aromatase. The low MAO-A expression in T-treated males would produce low CSF concentrations of 5HIAA in T-treated animals, and high MAO-A in ATD treated animals would produce elevated CSF 5HIAA in animals with aromatase inhibition. Indeed, our recently obtained data show that CSF 5HIAA concentrations in the groups treated with ATD were significantly higher than the non-ATD treated groups. These preliminary data strongly indicate that there is a positive correlation between MAO-A expression and CSF 5HIAA. Thus, our data agree with previous studies, but our interpretation differs.
If a low concentration of CSF 5HIAA were interpreted as lower global serotonin production or availability, then it stands to reason that decades of research have concluded that androgens reduce serotonin when in fact androgens, via E, reduce MAO-A expression. Moreover, this would likely contribute to less serotonin degradation and higher available serotonin, if all else were equal. We suggest that one component of serotonin function cannot be widely interpreted to reflect the function of the whole system. In another example, administration of T to rodents significantly decreased 5HIAA in the hypothalamus, but increased serotonin in the frontal cortex (Kubala et al., 2008). This result could be explained by localized MAO-A activity.
There was no overt regulation of 5HT1A auto-receptor gene expression in the 3-month treatment period although longer treatment may have led to differences between the groups. Current understanding of the 5HT1A autoreceptor indicates that it acts as a brake on serotonin neural activity and it is downregulated by dendritic release of serotonin (Blier, 2001). Androgens reportedly decreased 5HT1A activity in rodents (Bonson and Winter, 1992, Bell and Hobson, 1994), but pharmacological studies, such as these, cannot discriminate between auto- and postsynaptic receptors. However, anabolic steroids also significantly reduced postsynaptic 5HT1A immunostaining in the hypothalamus of rodents (Ricci et al., 2006). In another rodent study, castration increased 5HT1A autoreceptor expression in the raphe (Zhang et al., 1999), but this observation was not duplicated in our protocol with monkeys.
Higher basal prolactin was present in animals with the highest androgenic activity. Also, basal prolactin correlated with TPH2 and SERT gene expression, but correlations do not necessarily indicate a similar cause and effect. In an early report, T increased serum prolactin in male monkeys similar to our results, but DHT was not examined (Aidara and Robyn, 1984). Based upon a review of the literature, it is highly unlikely that the androgen-induced increase in basal prolactin is due to a direct effect at the level of the pituitary or hypothalamus (Herbert et al., 1981, Giguere et al., 1982, Tong et al., 1992, Herbison et al., 1996, Blache et al., 1997, Weiss et al., 2007). Thus, it is not exactly clear how androgens increase basal prolactin in vivo.
In summary, the various components of the serotonin system can vary independently and one component does not indicate overall function. Androgens stimulated expression of TPH2 and SERT genes that encode proteins for serotonin synthesis and reuptake; in turn elevated serotonin-related gene expression did not always predict serotonin delivery to a target area, the LC. Rather, serotonin delivery to the LC, an important target area that regulates vigilance and anxiety, reflected aromatase activity. We believe that serotonin transport to the LC is likely similar in all forebrain areas and further propose that the resulting serotonin content in terminal fields constitutes the serotonin pool underlying fenfluramine-stimulated prolactin release. Altogether, this study provides provocative new information on the regulation of serotonin by androgens and on the different compartments supported by the androgenic and estrogenic metabolites of testosterone. These data have led to a new interpretation of the effect of androgens on the serotonin system than what was previously derived from measurements of 5HIAA and serotonin content in the brain.
Highlights.
Androgens increase TPH2 and SERT gene expression in male monkeys.
Aromatase inhibition prevents serotonin transport through axons.
Estrogen governs serotonin transport to the locus ceruleus.
Acknowledgments
Supported by NIH grants:
MH86542 to CLB and P51 OD011092 for the operation of ONPRC
We are very grateful to Kevin Muller for training the animals, administering drugs and monitoring the health and wellbeing of the animals. We greatly appreciate Dr. Kris Coleman and Nicola Robertson for the behavioral observations and analysis. We thank the Primate Genetics Program at the Oregon National Primate Research Center for calculations of the relatedness of our animals. We are also grateful to Dr. Jay Welch and the technicians of the Division of Comparative Medicine, DCM, for the management and care of our animals. We thank the Surgery and Pathology Sections of DCM for their expertise and handling of our needed surgeries and necropsies.
Footnotes
Disclosure
Dr. Cynthia L Bethea has nothing to disclose.
Dr. Arubala P Reddy has nothing to disclose.
Kenny Phu has nothing to disclose
Andrew Phu has nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abel KM, Cleare AJ. Peripheral hormonal responses to D-fenfluramine as a probe of central serotonergic function in humans. Psychopharmacology. 1999;142:68–72. doi: 10.1007/s002130050863. [DOI] [PubMed] [Google Scholar]
- Aidara D, Robyn C. The effect of testosterone on the secretion of prolactin. Journal de pharmacologie. 1984;15:309–318. [PubMed] [Google Scholar]
- Anderson JR. Non-human primates: a comparative developmental perspective on yawning. Front Neurol Neurosci. 2010;28:63–76. doi: 10.1159/000307082. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Akaoka H, Charlety P, Chouvet G. Serotonin selectively attenuates glutamate-evoked activation of noradrenergic locus coeruleus neurons. J Neurosci. 1991a;11:760–769. doi: 10.1523/JNEUROSCI.11-03-00760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991b;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Ann Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry. 1999;46:1309–1320. doi: 10.1016/s0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B, et al. Afferent regulation of locus coeruleus neurons: anatomy, physiology and pharmacology. Prog Brain Res. 1991c;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Barrett GM, Shimizu K, Bardi M, Asaba S, Mori A. Endocrine correlates of rank, reproduction, and female-directed aggression in male Japanese macaques (Macaca fuscata) Horm Behav. 2002;42:85–96. doi: 10.1006/hbeh.2002.1804. [DOI] [PubMed] [Google Scholar]
- Beisner BA, McCowan B. Signaling context modulates social function of silent bared-teeth displays in rhesus macaques (Macaca mulatta) Am J Primatol. 2014;76:111–121. doi: 10.1002/ajp.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Hobson H. 5-HT1A receptor influences on rodent social and agonistic behavior: a review and empirical study. Neurosci Biobehav Rev. 1994;18:325–338. doi: 10.1016/0149-7634(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Berg-von der Emde K, Dees WL, Hiney JK, Hill DF, Dissen GA, Costa ME, Moholt-Siebert M, Ojeda SR. Neurotrophins and the neuroendocrine brain: different neurotrophins sustain anatomically and functionally segregated subsets of hypothalamic dopaminergic neurons. J Neurosci. 1995;15:4223–4237. doi: 10.1523/JNEUROSCI.15-06-04223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod A, Hartman BK, Pujol JF. Importance of fixation in immunohistochemisty: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981;29:844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Kim A, Cameron JL. Function and innervation of the locus ceruleus in a macaque model of Functional Hypothalamic Amenorrhea. Neurobiol Dis. 2012a;50C:96–106. doi: 10.1016/j.nbd.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005;83:148–155. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Phu K, Reddy AP, Cameron JL. The effect of short-term stress on serotonin gene expression in high and low resilient macaques. Prog Neuropsychopharmacol Biol Psychiatry. 2013a doi: 10.1016/j.pnpbp.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Effect of ovarian hormones on genes promoting dendritic spines in laser-captured serotonin neurons from macaques. Mol Psychiatry. 2010;15:1034–1044. doi: 10.1038/mp.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Robertson N, Coleman K. Effects of aromatase inhibition and androgen activity on serotonin and behavior in male macaques. Behav Neurosci. 2013b;127:400–414. doi: 10.1037/a0032016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Smith AW, Centeno ML, Reddy AP. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience. 2012b;49:251–262. doi: 10.1016/j.neuroscience.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blache D, Tjondronegoro S, Blackberry MA, Anderson ST, Curlewis JD, Martin GB. Gonadotrophin and prolactin secretion in castrated male sheep following subcutaneous or intracranial treatment with testicular hormones. Endocrine. 1997;7:235–243. doi: 10.1007/BF02778146. [DOI] [PubMed] [Google Scholar]
- Blier P. Pharmacology of rapid-onset antidepressant treatment strategies. J Clin Psychiatry. 2001;62 (suppl 15):12–17. [PubMed] [Google Scholar]
- Bonson KR, Johnson RG, Fiorella D, Rabin RA, Winter JC. Serotonergic control of androgen-induced dominance. Pharmacol Biochem Behav. 1994;49:313–322. doi: 10.1016/0091-3057(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Winter JC. Reversal of testosterone-induced dominance by the serotonergic agonist quipazine. Pharmacol Biochem Behav. 1992;42:809–813. doi: 10.1016/0091-3057(92)90034-d. [DOI] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK. Aggression, suicide, and serotonin: relationships to CSF amine metabolites. Am J Psychiatry. 1982;139:741–746. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- Brown GL, LInnoila MI. CSF serotonin metabolite (5HIAA) studies in depression, impulsivity and violence. J Clin Psychiatry. 1990;51:31–41. [PubMed] [Google Scholar]
- Castles DL, Whiten A, Aureli F. Social anxiety, relationships and self-directed behaviour among wild female olive baboons. Anim Behav. 1999;58:1207–1215. doi: 10.1006/anbe.1999.1250. [DOI] [PubMed] [Google Scholar]
- Charlety PJ, Chergui K, Akaoka H, Saunier CF, Buda M, Aston-Jones G, Chouvet G. Serotonin differentially modulates responses mediated by specific excitatory amino acid receptors in the rat locus coeruleus. Eur J Neurosci. 1993;5:1024–1028. doi: 10.1111/j.1460-9568.1993.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Clark AS, Henderson LP. Behavioral and physiological responses to anabolic-androgenic steroids. Neurosci Biobehav Rev. 2003;27:413–436. doi: 10.1016/s0149-7634(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Cleare AJ, Murray RM, O’Keane V. Assessment of sertonergic function in major depression using d-fenfluramine: relation to clinical variables and antidepressant response. Biol Psychiatry. 1998;44:555–561. doi: 10.1016/s0006-3223(98)00018-3. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ, Cooper TB, Hauger RL. Central serotonin activity and aggression: inverse relationship with prolactin response to d-fenfluramine, but not CSF 5-HIAA concentration, in human subjects. Am J Psychiatry. 1997;154:1430–1435. doi: 10.1176/ajp.154.10.1430. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Leiser SC, Snyder K, Valentino RJ. Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology. 2012;62:1737–1745. doi: 10.1016/j.neuropharm.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deputte BL, Johnson J, Hempel M, Scheffler G. Behavioral effects of an antiandrogen in adult male rhesus macaques (Macaca mulatta) Horm Behav. 1994;28:155–164. doi: 10.1006/hbeh.1994.1013. [DOI] [PubMed] [Google Scholar]
- Eaton GG. Male dominance and aggression in Japanese macaque reproduction. Adv Behav Biol. 1974;11:287–297. doi: 10.1007/978-1-4684-3069-1_13. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Resko JA. Plasma testosterone and male dominance in a Japanese macaque (Macaca fuscata) troop compared with repeated measures of testosterone in laboratory males. Horm Behav. 1974;5:251–259. doi: 10.1016/0018-506x(74)90033-6. [DOI] [PubMed] [Google Scholar]
- Eaton GG, Worlein JM, Glick BB. Sex differences in Japanese macaques (Macaca fuscata): effects of prenatal testosterone on juvenile social behavior. Horm Behav. 1990;24:270–283. doi: 10.1016/0018-506x(90)90009-m. [DOI] [PubMed] [Google Scholar]
- Ellinwood WE, Hess DL, Roselli CE, Spies HG, Resko JA. Inhibition of aromatization stimulates luteinizing hormone and testosterone secretion in adult male rhesus monkeys. J Clin Endocrinol Metab. 1984;59:1088–1096. doi: 10.1210/jcem-59-6-1088. [DOI] [PubMed] [Google Scholar]
- Foresta C, Indino M, Scandellari C. Role of gonadal steroids in the serotoninergic control of prolactin secretion in men. Clin Endocrinol (Oxf) 1987;26:601–607. doi: 10.1111/j.1365-2265.1987.tb00816.x. [DOI] [PubMed] [Google Scholar]
- Giguere V, Meunier H, Veilleux R, Labrie F. Direct effects of sex steroids on prolactin release at the anterior pituitary level: interactions with dopamine, thyrotropin-releasing hormone, and isobutylmethylxanthine. Endocrinology. 1982;111:857–862. doi: 10.1210/endo-111-3-857. [DOI] [PubMed] [Google Scholar]
- Giltay EJ, Tishova YA, Mskhalaya GJ, Gooren LJ, Saad F, Kalinchenko SY. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J Sex Med. 2010;7:2572–2582. doi: 10.1111/j.1743-6109.2010.01859.x. [DOI] [PubMed] [Google Scholar]
- Grant SJ, Aston-Jones G, Redmond DE., Jr Responses of primate locus coeruleus neurons to simple and complex sensory stimuli. Brain Res Bull. 1988;21:401–410. doi: 10.1016/0361-9230(88)90152-9. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology. 2001;160:271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- Henry JP, Sagne C, Bedet C, Gasnier B. The vesicular monoamine transporter: from chromaffin granule to brain. Neurochem Int. 1998;32:227–246. doi: 10.1016/s0197-0186(97)00092-2. [DOI] [PubMed] [Google Scholar]
- Herbert DC, Weaker FJ, Sheridan PL. Localization of 3H-dihydrotestosterone in the pituitary gland of the rhesus monkey. Cell Tissue Res. 1981;215:499–504. doi: 10.1007/BF00233526. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology. 1996;63:120–131. doi: 10.1159/000126948. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaehler ST, Singewald N, Philippu A. Dependence of serotonin release in the locus coeruleus on dorsal raphe neuronal activity. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:386–393. doi: 10.1007/pl00005365. [DOI] [PubMed] [Google Scholar]
- Kim MA, Lee HS, Lee BY, Waterhouse BD. Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res. 2004;1026:56–67. doi: 10.1016/j.brainres.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Kubala KH, McGinnis MY, Anderson GM, Lumia AR. The effects of an anabolic androgenic steroid and low serotonin on social and non-social behaviors in male rats. Brain Res. 2008;1232:21–29. doi: 10.1016/j.brainres.2008.07.065. [DOI] [PubMed] [Google Scholar]
- Leone A, Ferrari PF, Palagi E. Different yawns, different functions? Testing social hypotheses on spontaneous yawning in Theropithecus gelada. Scientific Reports. 2014;4:4010. doi: 10.1038/srep04010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima FB, Centeno ML, Costa ME, Reddy AP, Cameron JL, Bethea CL. Stress sensitive female macaques have decreased fifth Ewing variant (Fev) and serotonin-related gene expression that is not reversed by citalopram. Neuroscience. 2009;164:676–691. doi: 10.1016/j.neuroscience.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu NZ, Eshleman AJ, Janowsky A, Bethea CL. Ovarian steroid regulation of serotonin reuptake transporter (SERT) binding, distribution and function in female macaques. Mol Psychiatry. 2003;8:353–360. doi: 10.1038/sj.mp.4001243. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde E, Leret ML, Diaz S. The influence of testosterone in the brain of the male rat on levels of serotonin (5-HT) and hydroxyindole-acetic acid (5-HIAA) Comp Biochem Physiol C, Comp Pharmacol Toxicol. 1985;80:411–414. doi: 10.1016/0742-8413(85)90077-5. [DOI] [PubMed] [Google Scholar]
- Maruhashi T. An ecological study of troop fissions of Japanese monkeys (Macaca fuscata yaku) on Yakushima Island, Japan. Primates. 1982;23:317–337. [Google Scholar]
- McGinnis MY. Anabolic androgenic steroids and aggression: studies using animal models. Ann N Y Acad Sci. 2004;1036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M. CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatry Res. 1997;72:89–102. doi: 10.1016/s0165-1781(97)00084-x. [DOI] [PubMed] [Google Scholar]
- Miller RL, Stein MK, Loewy AD. Serotonergic inputs to FoxP2 neurons of the pre-locus coeruleus and parabrachial nuclei that project to the ventral tegmental area. Neuroscience. 2011;193:229–240. doi: 10.1016/j.neuroscience.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. New York: Academic Press; 2000. [Google Scholar]
- Ponholzer A, Madersbacher S, Rauchenwald M, Jungwirth S, Fischer P, Tragl KH. Serum androgen levels and their association to depression and Alzheimer dementia in a cohort of 75-year-old men over 5 years: results of the VITA study. Int J Impot Res. 2009;21:187–191. doi: 10.1038/ijir.2009.10. [DOI] [PubMed] [Google Scholar]
- Popova NK. From genes to aggressive behavior: the role of serotonergic system. Bioessays. 2006;28:495–503. doi: 10.1002/bies.20412. [DOI] [PubMed] [Google Scholar]
- Popova NK, Voitenko NN, Kulikov AV, Avgustinovich DF. Evidence for the involvement of central serotonin in mechanism of domestication of silver foxes. Pharmacol Biochem Behav. 1991;40:751–756. doi: 10.1016/0091-3057(91)90080-l. [DOI] [PubMed] [Google Scholar]
- Rainer Q, Speziali S, Rubino T, Dominguez-Lopez S, Bambico FR, Gobbi G, Parolaro D. Chronic nandrolone decanoate exposure during adolescence affects emotional behavior and monoaminergic neurotransmission in adulthood. Neuropharmacology. 2014;83C:79–88. doi: 10.1016/j.neuropharm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res Bull. 1994;35:607–616. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Resko JA, Connolly PB, Roselli CE, Abdelgadir SE, Choate JV. Differential effects of aromatase inhibition on luteinizing hormone secretion in intact and castrated male cynomolgus macaques. J Clin Endocrinol Metab. 1993;77:1529–1534. doi: 10.1210/jcem.77.6.8263136. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- Ricci LA, Rasakham K, Grimes JM, Melloni RH., Jr Serotonin-1A receptor activity and expression modulate adolescent anabolic/androgenic steroid-induced aggression in hamsters. Pharmacol Biochem Behav. 2006;85:1–11. doi: 10.1016/j.pbb.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Rostal DC, Glick BB, Eaton GG, Resko JA. Seasonality of adult male Japanese macaques (Macaca fuscata): androgens and behavior in a confined troop. Horm Behav. 1986;20:452–462. doi: 10.1016/0018-506x(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Serotonin releasing agents. Neurochemical, therapeutic and adverse effects. Pharmacology, Biochemistry and Behavior. 2002;71:825–836. doi: 10.1016/s0091-3057(01)00669-4. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Bethea CL. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;171:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with [3H]-Ro 41-1049 and [3H]-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1982;12:1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DM, Arriza JL, Swanson LW. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radio-labeled single-stranded RNA probes. J Histotechnol. 1989;12:169–181. [Google Scholar]
- Stanley B, Molcho A, Stanley M, Winchel R, Gameroff MJ, Parsons B, Mann JJ. Association of aggressive behavior with altered serotonergic function in patients who are not suicidal. Am J Psychiatry. 2000;157:609–614. doi: 10.1176/appi.ajp.157.4.609. [DOI] [PubMed] [Google Scholar]
- Thiblin I, Finn A, Ross SB, Stenfors C. Increased dopaminergic and 5-hydroxytryptaminergic activities in male rat brain following long-term treatment with anabolic androgenic steroids. Brit J Pharmacol. 1999;126:1301–1306. doi: 10.1038/sj.bjp.0702412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Velilleux R, Pelletier G. Regulation of prolactin messenger ribonucleic acid levels by estradiol and dihydrotestosterone as evaluated by in situ hybridization performed on implanted pituitary glands and anterior pituitary cells in culture in the male rat. J Neuroendocrinol. 1992;4:359–361. doi: 10.1111/j.1365-2826.1992.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Tsutsui K. Neurosteroid biosynthesis and action during cerebellar development. Cerebellum. 2012;11:414–415. doi: 10.1007/s12311-011-0341-7. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann NY Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Rudoy C, Saunders A, Liu XB, Van Bockstaele EJ. Corticotropin-releasing factor is preferentially colocalized with excitatory rather than inhibitory amino acids in axon terminals in the peri-locus coeruleus region. Neuroscience. 2001;106:375–384. doi: 10.1016/s0306-4522(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Goldman D, Nielsen DA, Linnoila M. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20:271–275. [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Stojilkovic SS, Diedrich K, Ortmann O. Effects of testosterone on hormonal content and calcium-dependent basal secretion in female rat pituitary cells. J Steroid Biochem Mol Biol. 2007;103:149–157. doi: 10.1016/j.jsbmb.2006.09.038. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ma W, Barker JL, Rubinow DR. Sex differences in expression of serotonin receptors (subtypes 1A and 2A) in rat brain: a possible role of testosterone. Neuroscience. 1999;94:251–259. doi: 10.1016/s0306-4522(99)00234-1. [DOI] [PubMed] [Google Scholar]