Abstract
Objectives
Aging is associated with changes in circadian rhythms. Current evidence supports a role for circadian rhythms in the pathophysiology of depression. However, little is known about the relationship between depressive symptoms and circadian activity rhythms in older adults. We examined this association in community-dwelling older women.
Methods
We performed a cross-sectional analysis of 3,020 women (mean age: 83.55 ± 3.79 years) enrolled in the Study of Osteoporotic Fractures. Depressive symptoms were assessed with the Geriatric Depression Scale categorizing participants as “normal” (0–2; referent group, N = 1,961), “some depressive symptoms” (3–5, N = 704), or “depressed” (≥6, N = 355). Circadian activity rhythm variables were measured using wrist actigraphy.
Results
In age-adjusted and Study of Osteoporotic Fractures site–adjusted models, greater levels of depressive symptoms were associated with decreased amplitude (height; df = 3,014, t = −11.31, p for linear trend <0.001), pseudo F-statistic (robustness; df =3,014, t =−8.07, p for linear trend <0.001), and mesor (mean modeled activity; df = 3014, t = −10.36, p for linear trend <0.001) of circadian activity rhythms. Greater levels of depressive symptoms were also associated with increased odds of being in the lowest quartile for amplitude (df =1, χ2 =9240, p for linear trend <0.001), pseudo F-statistic (df =1, χ2 =49.73, p for linear trend <0.001), and mesor (df =1, χ2 =81.12, p for linear trend <0.001). These associations remained significant in multivariate models. Post-hoc analyses comparing mean amplitude, mesor, and pseudo F-statistic values pair-wise between depression-level groups revealed significant differences between women with “some depressive symptoms” and the “normal” group.
Conclusion
These data suggest a graded association between greater levels of depressive symptoms and more desynchronization of circadian activity rhythms in community-dwelling older women. This association was observed even for women endorsing subthreshold levels of depressive symptoms.
Keywords: Depression, circadian rhythms, activity rhythms, actigraphy, older women, subthreshold depression
INTRODUCTION
Depressive syndromes are common in older adults and represent a major public health concern. In primary care settings, the estimated prevalence of major depressive disorder in older adults is 3%–10%,1–4 accounting for only a fraction of older adults suffering from depression. In community-dwelling adults older than 65 years, prevalence rates of “less than major” depressive syndromes (including dysthymia, minor depression, and subsyndromal depression) are significantly more common (9–24%),3,5–8 and these syndromes may be even more prevalent in long-term care settings.9 Depressive syndromes in this age group are associated with a number of adverse outcomes, including functional impairment,10 medical illnesses11 and nonpsychiatric hospitalizations,12 disability,13 and increased health services utilization.14,15 In older adults, depression has also proven to be difficult to treat, with a high rate of treatment refractoriness using first-line pharmacotherapies.16 Meanwhile, several studies suggest bright-light therapy, a chronobiologic treatment designed to correct circadian rhythm disruption, may be an effective treatment for depression in older adults.17,18 A better understanding of the relationship between depressive symptoms and circadian rhythm disruption in this age group is therefore critical and may lay the groundwork for the development of better treatment strategies.
Circadian rhythms are intrinsic physiologic cycles of approximately 24 hours, like the sleep–wake cycle, the core body temperature cycle, and the cycle of activity and rest (circadian activity rhythm). The circadian pacemaker is located in the superchiasmatic nucleus (SCN) of the anterior hypothalamus, and the sleep–wake cycle is synchronized to the time of day by a number of cues, the strongest of which is the environmental rhythm of light and darkness. Aging is associated with changes in circadian rhythms, including decreased amplitude (height of rhythm), advanced phase (timing of the peak of the rhythm), shortened natural free-running period (period of rhythm without environmental cues), and a tendency toward internal desynchronization.19–21 The disruption of circadian rhythms during aging is paralleled by decreased photic input both because of age-related losses in circadian photoreception22 and because older people tend to be exposed to less light.23 Hence, the circadian system of the average older adult receives less light input compared with the average younger adult. Animal studies suggest that aging is also associated with a number of molecular changes in the circadian system, including changes in expression patterns of clock genes and changes in the neurochemistry of the SCN.20,24–26
Considerable evidence supports a role for circadian rhythm disturbances in the pathophysiology of depression.27–29 Circadian rhythm disturbances have been observed in unipolar depression, bipolar depression, and seasonal affective disorder.30,31 In unipolar depression, multiple rhythms are disrupted including prolactin, cortisol, growth hormone, melatonin, body temperature, and the sleep–wake cycle.32 At the neuroanatomic level, depressed patients have been shown to have different patterns of regional brain glucose metabolism throughout the day compared with normal patients, and the expression of enzymes that control catecholamine metabolism may be regulated by clock genes.33,34 Although aging promotes disruption in circadian rhythms, the relationship between depression and circadian rhythm disruption in older adults remains largely unexplored.
We examined the relationship between levels of depressive symptoms and disruption of circadian activity rhythms cross-sectionally in a large group of community-dwelling older women. We hypothesized that both subthreshold levels of depressive symptoms and greater levels of depressive symptoms would be associated with disturbances in circadian activity rhythms.
METHODS
Participants
Participants were older women enrolled in the Study of Osteoporotic Fractures (SOF), a multisite, prospective cohort study of primarily white, community-dwelling women aged 65 years and older from four geographic areas (Portland, OR; Minneapolis, MN; Pittsburgh, PA/Monongahela Valley, PA; and Baltimore, MD). Women gave informed consent for participation in the study. Between September 1986 and October 1988, 9,704 participants were recruited via community listings and mailed announcements. Between February 1997 and February 1998, 662 African American women were enrolled. Women were excluded if they required assistance with ambulation or had history of bilateral hip replacement. Details regarding SOF have been published elsewhere.35,36
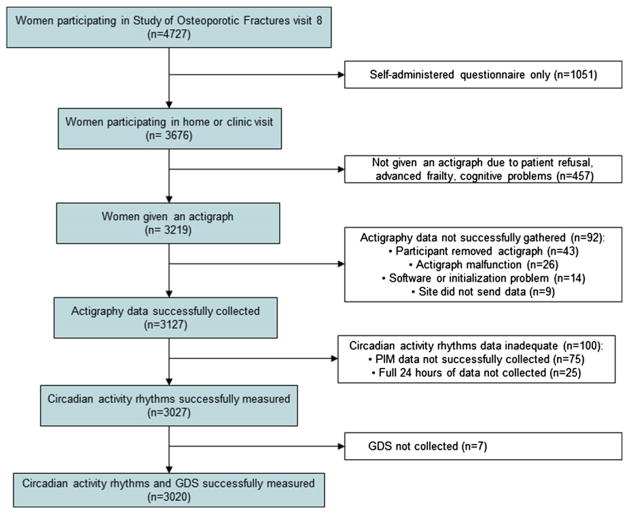
These analyses focused on women participating in SOF visit 8, approximately 15 years after the baseline assessment, between January 2002 and February 2004. There were 4,727 participants, representing 84% of active survivors (Fig. 1). Of these women, 457 women (12.4%) either refused participation or were considered either too frail or too cognitively impaired to participate in the actigraphy portion of the study. Of the remaining women, actigraphy data were successfully gathered for 3,027 (94.8%). Reasons for unsuccessful collection (92 women) included having an actigraph malfunction, software initialization problem, removing the actigraph and not replacing it, and data not being collected or saved successfully at the clinic site. One hundred women (3.3%) who had successful collection of actigraphy data had inadequate circadian activity rhythms data; for 75 women data were not recorded in the proportional integration mode, whereas 25 women did not have at least one 24-hour block of data required to calculate the variables. Of the 3,027 women with usable circadian activity rhythm data, 3,020 returned completed Geriatric Depression Scale (GDS) questionnaires. The analyses presented here were performed on this subset of 3,020 participants.
FIGURE 1. A summary of participant recruitment, inclusion, and exclusion is given.
GDS: Geriatric Depression Scale.
Subjective Measures and Demographic Information
Demographic information (birth date, ethnicity, years education) was recorded at baseline. Age at SOF visit 8 was used for these analyses. At SOF visit 8, participants completed questionnaires regarding health status, smoking, alcohol consumption, caffeine intake, exercise habits, and medical history. Women rated their health status as excellent, good, fair, poor, or very poor. Self-reported average daily intake of caffeinated beverages (coffee, soda, tea) was used to estimate average daily caffeine intake assuming the following: cup of coffee (95 mg), cup of tea (55 mg), and can of soda (45 mg). A clinic interview included an assessment of impairments in instrumental activities of daily living (IADLs) in which the patient was asked whether they had any problems with six IADLs: walking two to three blocks, climbing 10 steps, walking down 10 steps, preparing meals, heavy housework, and shopping. A physical examination included measurement of height and weight. Body mass index was calculated as weight in kilograms divided by height squared in meters. Medications taken daily or almost daily during the prior 30 days were recorded and categorized according to a computerized coding dictionary. Cognition was assessed using the Mini-Mental State Exam, and cognitive impairment was defined as ≤24.36
Depression was assessed using the GDS, a 15-item validated self-report questionnaire commonly used for assessment of depressive symptoms in older adults.37 Women were categorized into three groups based on level of depressive symptoms. Women scoring 0–2 (no or few depressive symptoms) were categorized as “normal.” Those scoring 3–5 were categorized as having “some depressive symptoms,” and those scoring ≥6 were categorized as “depressed.” This strategy was used previously.38,39 A standard cut-point of ≥6 has a sensitivity of 91% and specificity of 65% for diagnosis of a major depressive episode compared with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.40 To our knowledge a lower cut-point correlating with “less than major” depressive syndromes has not been validated.
Actigraphy
Circadian activity rhythms were computed from activity data gathered by actigraphy, a previously validated41–43 noninvasive tool. Actigraphs (SleepWatch-O, Ambulatory Monitoring, Inc., Ardsley, NY) were worn on participants’ nondominant wrist for at least three consecutive 24-hour periods. Movements were recorded and summarized in 1-minute epochs. Measuring wrist actigraphy over time produces information about daily circadian activity rhythms. Data were collected in three different modes: proportional integration mode of digital integration mode, zero crossing mode, and time above threshold mode. Proportional integration mode data were used in this study.
The proportional integration mode is a high-resolution measurement of the area under the curve (rectified conditioned transducer signal) and is therefore a measure of activity level or vigor of motion. In the SOF cohort, this mode has been shown to correlate best with polysomnography for the measurement of sleep–wake variables such as wake after sleep onset.41,42
Participant’s data were fitted to a five-parameter extended cosine model from which six outcome variables were computed (Table 1).44 Circadian activity rhythm variables included acrophase (time of day of maximum modeled activity), amplitude (difference between the maximum and minimum of the modeled activity curve), mesor (mean of the modeled activity curve), left half-deflection (time of day women’s activity pattern switched from low, below the mesor, to high, above the mesor), right half-deflection (time of day women’s activity pattern switched from high to low), and pseudo F-statistic. Pseudo F-statistic is a measure of how well the data fit the modeled extended cosine curve, with higher pseudo F-statistics indicating a more robust rhythm.
TABLE 1.
Circadian Activity Rhythm Variables Derived by Actigraphy
| Variable | Definition | Interpretation |
|---|---|---|
| Acrophase | Time of day of the maximum modeled value for activity | Lower values indicate a more advanced rhythm |
| Amplitude | Difference between the maximum and the minimum of the modeled activity curve | Lower values indicate a more dampened rhythm |
| Mesor | Mean of the modeled rhythm; half-way between the minimum and maximum of the modeled activity curve | Lower values indicate lower mean activity levels |
| Pseudo F-statistic | Measure of overall fit | Lower values indicate a less robust rhythm |
| Left half-deflection | Time of day when the women switched from low activity to high activity (i.e., from below the mesor to above the mesor) | Lower values indicate becoming active earlier in the day (i.e., getting going later in the morning) |
| Right half-deflection | Time of day when the women switched from high activity to low activity (i.e., from above the mesor to below the mesor) | Lower values indicate decreasing activity earlier in the evening (i.e., settling down for the evening later) |
Circadian activity rhythm variables were expressed both continuously and as dichotomous outcomes. Because there were no previously described clinically relevant cut-points, data for amplitude, mesor, pseudo F-statistic, right half-deflection, and left half-deflection were divided into sample-based quartiles. Specific categories included amplitude <1,556.37 (lowest quartile) versus amplitude ≥1,556.37, mesor <2,064.06 (lowest quartile) versus mesor ≥2,064.06, pseudo F-statistic <522.64 (lowest quartile) versus pseudo F-statistic ≥522.64, left half-deflection <6:37 hours (lowest quartile) versus left half-deflection ≥6:37 hours, left half-deflection >8:06 hours (highest quartile) versus left half-deflection ≤8:06 hours, right half-deflection <21:02 hours (lowest quartile) versus right half-deflection ≥21:02 hours, and right half-deflection >23:07 hours (highest quartile) versus right half-deflection ≤23:07 hours. Acrophase >1.5 standard deviation (SD) above mean (advanced; >16:14 hours) versus acrophase ≤1.5 SD above or below mean and acrophase >1.5 SD below mean (delayed; <12:35 hours) versus acrophase ≤1.5 SD above or below mean were compared.
Statistical Analyses
Differences in the characteristics of participants according to level of depressive symptoms were assessed using a χ2 test for categorical variables and analysis of variance for normally distributed continuous data. Characteristics expressed as continuous variables (caffeine intake and number of reported medical conditions) were normally distributed. For age, participants were grouped by approximate quartile: 70–80 years, 81–82 years, 83–85 years, and 86–100 years. Participants were categorized based on years of education: those who had less than a high school diploma, those with a high school diploma but no college degree, and those that had a college degree or more. Groupings for self-reported health status were (1) excellent or good and (2) fair, poor, or very poor. Categories for smoking status were never smoked, former smoker, and current smoker. Categories for reported use of alcohol were 0–2 drinks per week, 3–13 drinks per week, and >13 drinks per week. Categories for IADL impairment were (1) no IADL impairments and (2) one or more IADL impairment.
Circadian activity rhythm measures were expressed as continuous variables using linear regression models, and the least-squared means were used to estimate the mean (95% confidence interval [CI]) for each measure by level of depressive symptoms. Post-hoc analyses, comparing mean activity rhythm parameter values between pairs of depression groups, were performed using the Bonferroni adjustment. Logistic regression was used to determine the odds (95% CI) of being in the lowest quartile of circadian rhythm parameters by depression group, as described above. The “normal” group served as a referent group for these comparisons. Probability values for linear trend across the categories were calculated to see if a linear relationship existed by including the three-level depression categorization as a continuous variable in models.
All models were adjusted for age and SOF site and also for multiple possible confounders. Covariates were included in the final multivariate models if they were known correlates of circadian rhythm disruption or depression or if they were found to be associated with level of depressive symptoms in this population (p ≤0.10). Multivariate models were adjusted for age, race, site, smoking status, body mass index, self-reported health status, education, exercise, number of reported medical conditions, IADLs, cognitive impairment, and use of antidepressants, benzodiazepines, nonbenzodiazepine anxiolytics, or medications for sleep.
RESULTS
Characteristics of the Study Population
Of the 3,020 women in the study sample, 1,961 scored 0–2 and were classified as “normal,” 704 women scored 3–5 and were classified as having “some depressive symptoms,” and 355 women scored ≥6 and were classified as “depressed” (Table 2). Women with greater levels of depressive symptoms were more likely to be older, less educated, and cognitively impaired. They were also more likely to report poor health, poor health-related behaviors, and use of psychotropic or sleep medications.
TABLE 2.
Characteristics of Participants According to Level of Depressive Symptoms
| Population Characteristics | Level of Depressive Symptoms
|
||||||
|---|---|---|---|---|---|---|---|
| All Participants | Normal (GDS 0–2) | Some Depressive Symptoms (GDS 3–5) | Depressed (GDS ≥ 6) | p trend | χ2 (or F) | df | |
| Total number of participants | 3,020 | 1,961 | 704 | 355 | |||
| Age group, n (%) | <0.001 | 61.2197 | 6 | ||||
| 70–80 | 527 (17.45) | 390 (19.89) | 96 (13.64) | 41 (11.55) | |||
| 81–82 | 850 (28.15) | 583 (29.73) | 191 (27.13) | 76 (21.41) | |||
| 83–85 | 827 (27.38) | 534 (27.23) | 188 (26.7) | 105 (29.58) | |||
| 86–100 | 816 (27.02) | 454 (23.15) | 229 (32.53) | 133 (37.46) | |||
| African American, n (%) | 323 (10.7) | 230 (11.73) | 59 (8.38) | 34 (9.58) | 0.037 | 6.60576 | 2 |
| Self-reported health status, n (%) | <0.001 | 392.028 | 2 | ||||
| Excellent or good | 2,275 (75.33) | 1,685 (85.93) | 441 (62.64) | 149 (41.97) | |||
| Fair, poor, or very poor | 745 (24.67) | 276 (14.07) | 263 (37.36) | 206 (58.03) | |||
| Lives alone, n (%) | 1,824 (60.40) | 1,163 (59.31) | 436 (61.93) | 225 (63.38) | 0.224 | 2.98925 | 2 |
| Education, n (%) | <0.001 | 25.7618 | 4 | ||||
| Less than high school diploma | 542 (17.96) | 324 (16.52) | 130 (18.49) | 88 (24.86) | |||
| High school diploma | 1,264 (41.88) | 800 (40.64) | 312 (44.38) | 155 (43.79) | |||
| College/graduate school | 1,212 (40.16) | 840 (42.84) | 261 (37.13) | 111 (31.36) | |||
| Alcohol use (drinks per week), n (%) | 0.034 | 10.4062 | 4 | ||||
| 0–2 drinks per week | 2,624 (86.89) | 1,678 (85.57) | 623 (88.49) | 323 (90.99) | |||
| 3–13 drinks per week | 339 (11.23) | 242 (12.34) | 71 (10.09) | 26 (7.32) | |||
| >13 drinks per week | 57 (1.89) | 41 (2.09) | 10 (1.42) | 6 (1.69) | |||
| Smoking status, n (%) | 0.001 | 17.8589 | 4 | ||||
| Never smoked | 1,917 (63.50) | 1,276 (65.07) | 435 (61.88) | 206 (58.03) | |||
| Former smoker | 1,018 (33.72) | 643 (32.79) | 246 (34.99) | 129 (36.34) | |||
| Current smoker | 84 (2.78) | 42 (2.14) | 22 (3.13) | 20 (5.63) | |||
| Caffeine intake, mean ± SD, mg/d | 151.01 ± 154.26 | 152.48 ± 156.05 | 147.34 ± 151.66 | 151.18 ± 149.7 | 0.765 | (−0.269) | (2, 3017) |
| Current antidepressant use, n (%) | 413 (13.69) | 181 (9.23) | 134 (19.09) | 98 (27.61) | <0.001 | 108.426 | 2 |
| Current benzodiazepine use, n (%) | 219 (7.26) | 110 (5.61) | 57 (8.12) | 52 (14.65) | <0.001 | 37.458 | 2 |
| Current nonbenzodiazepine anxiolytic/hypnotic use, n (%) | 33 (1.09) | 16 (0.82) | 10 (1.42) | 7 (1.97) | 0.099 | 4.63433 | 2 |
| Reported use of sleep medication, n (%) | 423 (14.02) | 228 (11.63) | 113 (16.10) | 82 (23.10) | <0.001 | 36.0506 | 2 |
| Cognitively impaired (MMSE < 24), n (%) | 157 (5.51) | 86 (4.55) | 41 (6.37) | 30 (9.55) | 0.001 | 14.1317 | 2 |
| Body mass index (BMI), n (%) | 0.003 | 15.9484 | 4 | ||||
| Underweight/normal weight (BMI < 25) | 1,094 (37.02) | 687 (35.36) | 263 (38.68) | 144 (43.37) | |||
| Overweight (BMI 25–30) | 1,126 (38.10) | 780 (40.14) | 249 (36.62) | 97 (29.22) | |||
| Obese (BMI ≥ 30) | 735 (24.87) | 476 (24.50) | 168 (24.71) | 91 (27.41) | |||
| Takes walks for exercise, n (%) | 1112 (37.27) | 828 (42.68) | 200 (28.78) | 84 (24.07) | <0.001 | 71.7508 | 2 |
| IADL impairments, n (%) | 1592 (53.00) | 812 (41.62) | 490 (70.00) | 290 (82.15) | <0.001 | 303.084 | 2 |
| Number of selected medical conditions, mean ± SD | 2.46 ± 1.61 | 2.20 ± 1.49 | 2.87 ± 1.70 | 3.08 ± 1.73 | <0.001 | (−139.112) | (2, 3017) |
Notes: Differences in the characteristics of participants according to level of depressive symptoms were assessed using a χ2 test for categorical variables and analysis of variance for normally distributed continuous data. Reported medical conditions includes stroke, diabetes, Parkinson disease, chronic obstructive pulmonary disease, congestive heart failure, myocardial infarction, thyroid disease, hypertension, other neurologic conditions, other cardiac conditions, osteoarthritis, rheumatoid arthritis, osteoporosis, and cancer. Characteristics expressed as continuous variables (caffeine intake and number of reported medical conditions) were normally distributed. MMSE: Mini Mental State Exam; p trend: p for linear trend.
Comparison of Circadian Activity Rhythm Variables by Level of Depressive Symptoms
Greater levels of depressive symptoms were associated with greater desynchronization of circadian activity rhythms in linear regression models (Table 3). After adjustment for age and SOF site, tests for linear trend revealed graded associations between greater levels of depressive symptoms and a more dampened rhythm (decreased amplitude; df = 3,014, t = −11.31, p for linear trend <0.001), decreased robustness (pseudo F-statistic; df = 3,014, t = −8.07, p for linear trend <0.001), decreased mean modeled activity of the rhythm (mesor; df = 3,014, t = 10.36, p for linear trend <0.001), and later average time becoming active in the morning (increased left half-deflection), whereas there were no associations between level of depressive symptoms and the timing of peak activity (acrophase) or average time settling down in the evening (right half-deflection). Associations between increased level of depressive symptoms and decreased amplitude, pseudo F-statistic, and mesor remained significant after adjustment for multiple potential confounders, whereas the association between level of depressive symptoms and increased left half-deflection was no longer significant. A test for linear trend also revealed a graded association between greater levels of depressive symptoms and earlier average time settling down in the evening (decreased right half-deflection) in multivariable adjusted models (df = 2,104, t = −2.19, p for linear trend = 0.028). Specifically, women with some depressive symptoms settled down an average of 7 minutes earlier and women in the depressed group settled down an average of 26 minutes earlier compared with those in the normal group.
TABLE 3.
Comparison of Circadian Activity Variables by Level of Depressive Symptoms
| Circadian Activity Rhythm Parameters | Mean (95% Confidence Interval)
|
|||||
|---|---|---|---|---|---|---|
| Normal (GDS 0–2) | Some Depressive Symptoms (GDS 3–5) | Depressed (GDS ≥ 6) | p trend | t | df | |
| Time of rhythm peak (acrophase; hours:minutes) | ||||||
| Age and site adjusted (N = 3,020) | 14.38 (14.33–14.44) | 14.44 (14.35–14.53) | 14.44 (14.31–14.57) | 0.262 | 1.12 | 3,014 |
| Multivariate (2,764) | 14.41 (14.35–14.46) | 14.37 (14.27–14.46) | 14.30 (14.16–14.44) | 0.191 | −1.31 | 2,740 |
| Height (amplitude) | ||||||
| Age and site adjusted (N = 3,020) | 2,005.28 (1,981.40–2,029.17) | 1,796.79 (1,756.96–1,836.63) | 1,714.94 (1,658.68–1,771.20) | <0.001 | −11.31 | 3,014 |
| Multivariate (2,764) | 1,980.92 (1,957.68–2,004.17) | 1,885.36 (1,845.29–1,925.43) | 1,898.08 (1,838.05–1,958.10) | <0.001 | −3.55 | 2,740 |
| Mean modeled activity (mesor) | ||||||
| Age and site adjusted (N = 3,020) | 2,520.28 (2,496.14–2,544.42) | 2,354.16 (2,313.90–2,394.42) | 2,233.77 (2,176.90–2,290.63) | <0.001 | −10.36 | 3,014 |
| Multivariate (2,764) | 2,498.15 (2,473.64–2,522.66) | 2,414.53 (2,372.28–2,456.78) | 2,384.53 (2,321.24–2,447.81) | <0.001 | −3.88 | 2,740 |
| Robustness (pseudo F-statistic) | ||||||
| Age and site adjusted (N = 3,020) | 918.88 (897.64–940.12) | 777.64 (742.22–813.07) | 740.94 (690.91–790.98) | <0.001 | −8.07 | 3,014 |
| Multivariate (2,764) | 901.13 (879.48–922.79) | 837.77 (800.45–879.09) | 834.39 (778.48–890.30) | 0.005 | −2.83 | 2,740 |
| Time becoming active in morning (hours:minutes) | ||||||
| Age and site adjusted (N = 3,020) | 7:21 (7:19–7:25) | 7:29 (7:23–7:35) | 7:33 (7:25–7:41) | 0.003 | 2.97 | 3,014 |
| Multivariate (2,764) | 7:24 (7:20–7:28) | 7:24 (7:18–7:30) | 7:23 (7:14–7:31) | 0.836 | −0.21 | 2,740 |
| Time evening settling (hours:minutes) | ||||||
| Age and site adjusted (N = 3,020) | 22:01 (21:56–22:06) | 21:58 (21:49–22:06) | 21:52 (21:40–22:03) | 0.116 | −1.57 | 3,014 |
| Multivariate (2,764) | 22:02 (21:57–22:01) | 21:55 (21:47–22:04) | 21:47 (21:33–22:00) | 0.028 | −2.19 | 2,740 |
Notes: A comparison of mean circadian activity rhythm parameters, estimated by linear regression and least-squared means, for groups of women with different levels of depressive symptoms is shown. Multivariate models adjusted for age, race, site, smoking status, body mass index, self-reported health status, education, exercise, number of reported medical conditions, impairments in activities of daily living, cognitive impairment, and use of antidepressants, benzodiazepines, nonbenzodiazepine anxiolytics, or medications for sleep. p trend: p value for linear trend.
In general, differences between the “normal” and “some depressive symptoms” groups were greater than that between the “some depressive symptoms” and “depressed” groups (Table 3). Pair-wise analyses were done post-hoc, to see if there were differences between the group with “some depressive symptoms” and either the “normal” or the “depressed” groups. After adjustment for multiple confounders, significant differences were found between women in the “normal group” and those with “some depressive symptoms” for amplitude (df = 2,737, t = 4.09, p <0.001), mesor (df = 2,737, t = 3.31, p = 0.002), and robustness (pseudo F-statistic; df = 2,737, t = 2.94, p = 0.010). Post-hoc analyses showed no significant differences between depressed women and those with some depressive symptoms or for average times becoming active in the morning (left half-deflection) or settling down in the evening (right half-deflection) (data not shown).
Odds of Circadian Activity Rhythm Abnormalities According to Level of Depressive Symptoms
In logistic regression models adjusted for age and SOF site, tests for linear trend suggested graded associations between greater levels of depressive symptoms and increased odds of being in the lowest quartile for amplitude (more dampened rhythms), mesor (mean modeled activity), pseudo F-statistic (less robust rhythms), and right half-deflection (earliest time settling down in the evening) as well as being in the highest quartile for left half-deflection (latest time becoming active in the morning) (Table 4). In models adjusted for multiple potential confounders, associations between greater levels of depressive symptoms and lowest quartile for amplitude, mesor, pseudo F-statistic, and right half-deflection remained significant, whereas the association with highest quartile left half-deflection was no longer significant. In age- and site-adjusted models, the odds of being in the lowest quartile for amplitude, mesor, and pseudo F-statistic were increased by 1.78–1.98-fold for women in the “some depressive symptoms” group and 2.08–2.91 for those in the “depressed” group compared with the “normal” group. In models adjusted for multiple potential confounders, the specific association between the “depressed” group and lowest quartile pseudo F-statistic was no longer observed, whereas other associations persisted. The odds of being in quartile that settled down earliest in the evening (right half-deflection) were not specifically increased for women in either depressive symptom group compared with the “normal” group. There was no association between level of depressive symptoms and acrophase (time of peak activity).
TABLE 4.
Association Between Depressive Symptoms and Circadian Activity Rhythm Disturbances
| Circadian Activity Rhythm Disturbance | Odds Ratio (95% Confidence Interval)
|
|||||
|---|---|---|---|---|---|---|
| Normal (GDS 0–2) | Some Depressive Symptoms (GDS 3–5) | Depressed (GDS ≥ 6) | p trend | χ2 | df | |
| Time of activity peak (acrophase) delayed (>1.5 SD above mean) | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.28 (0.90–1.81) | 1.22 (0.77–1.94) | 0.202 | 1.63 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 0.92 (0.61–1.37) | 0.77 (0.44–1.33) | 0.345 | 0.89 | 1 |
| Time of activity peak (acrophase) advanced (>1.5 SD below mean) | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.15 (0.79–1.66) | 1.24 (0.77–2.00) | 0.303 | 1.06 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 1.10 (0.71–1.69) | 1.37 (0.77–2.44) | 0.309 | 1.03 | 1 |
| Dampened rhythm (amplitude) lowest quartile | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.94 (1.59–2.36) | 2.91 (2.28–3.72) | <0.001 | 92.40 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 1.32 (1.04–1.67) | 1.51 (1.11–2.05) | 0.003 | 8.78 | 1 |
| Decreased mean activity (mesor) lowest quartile | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.98 (1.63–2.40) | 2.62 (2.05–3.35) | <0.001 | 81.12 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 1.55 (1.24–1.94) | 1.62 (1.20–2.18) | <0.001 | 15.63 | 1 |
| Less robust (pseudo F-statistic) lowest quartile | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.78 (1.47–2.16) | 2.08 (1.62–2.66) | 0.001 | 49.73 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 1.30 (1.03–1.63) | 1.26 (0.93–1.71) | 0.045 | 4.01 | 1 |
| Latest time becoming active morning (highest quartile left half-deflection) | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.30 (1.07–1.58) | 1.28 (0.99–1.66) | 0.008 | 6.98 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 1.08 (0.86–1.36) | 0.93 (0.68–1.26) | 0.890 | 0.02 | 1 |
| Earliest time becoming active morning (lowest quartile left half-deflection) | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.00 (0.82–1.22) | 0.97 (0.74–1.27) | 0.839 | 0.04 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 1.10 (0.88–1.38) | 1.15 (0.83–1.59) | 0.304 | 1.06 | 1 |
| Latest time evening settling (highest quartile right half-deflection) | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 0.93 (0.76–1.14)) | 0.87 (0.66–1.14) | 0.266 | 1.24 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 0.89 (0.71–1.12) | 0.79 (0.57–1.10) | 0.123 | 2.37 | 1 |
| Earliest time evening settling (lowest quartile right half-deflection) | ||||||
| Age and site adjusted (N = 3,020) | 1.0 (referent) | 1.14 (0.93–1.39) | 1.29 (1.00–1.66) | 0.035 | 4.46 | 1 |
| Multivariate (2,764) | 1.0 (referent) | 1.19 (0.95–1.49) | 1.33 (0.98–1.81) | 0.036 | 4.38 | 1 |
Notes: Odds ratios and 95% confidence intervals estimated by logistic regression for disturbances in circadian activity rhythms are shown for groups of women with differing levels of depressive symptoms compared with the “normal” (referent) group. Multivariate models adjusted for age, race, site, smoking status, body mass index, self-reported health status, education, exercise, number of reported medical conditions, impairments in activities of daily living, cognitive impairment, and use of antidepressants, benzodiazepines, non-benzodiazepine anxiolytics, or medications for sleep. p trend: p for linear trend.
Additional Analyses
To determine whether associations observed between greater level of depressive symptoms and circadian rhythms measures were mediated by sleep, total sleep time and sleep efficiency were added separately to the multivariable linear regression models. The significant associations between greater level of depressive symptoms and decreased amplitude, mesor, pseudo F-statistic, and right half-deflection persisted after adjusting for these sleep measures (data not shown). To further assess the effect of antidepressants, benzodiazepines, nonbenzodiazepine anxiolytic medications, and medications for sleep on these analyses, the multivariate linear regression analyses were repeated excluding 946 participants who reported use of these medications. The significant associations between increased level of depressive symptoms and decreased amplitude, mesor, pseudo F-statistic, and right half-deflection remained (data not shown).
To examine whether the association between greater levels of depressive symptoms and reduced amplitude was related to differences in overall locomotor activity, we repeated both the linear regression models and logistic regression models using normalized amplitudes (i.e., dividing amplitude by mesor). In the age- and site-adjusted linear regression model, a test for linear trend suggested a graded association between greater levels of depressive symptoms and decreased amplitude/mesor (df = 3,014, t = −5.32, p for linear trend <0.001), whereas this association was no longer significant in multivariate models. In the age- and site-adjusted logistic regression model, odds falling into the lowest quartile for amplitude/mesor were increased by 1.66-fold (1.27–2.02) for the “some depressive symptoms” and by 1.61 (1.25–2.07) for the “depressed” group compared with the “normal” group (df = 1, χ2 = 0.30, p for linear trend <0.001). Again, this relationship was greatly attenuated and no longer significant in multivariate models.
Because GDS cut-off scores for “normal” and “some depressive symptoms” groups have not been previously validated, the multivariate linear regression analyses were repeated defining the “some depressive symptoms” group as GDS = 1–5 and the “normal” group as GDS = 0. The significant associations between greater level of depressive symptoms and decreased amplitude, mesor, pseudo F-statistic, and right half-deflection remained.
DISCUSSION
The results presented here suggest that greater levels of depressive symptoms are associated with more desynchronization of circadian activity rhythms in community-dwelling older women. Specifically, in multivariate models, greater levels of depressive symptoms were associated with decreased robustness of circadian activity rhythms. Greater levels of depressive symptoms were also associated with decreased amplitude. One possible interpretation of this finding is that greater levels of depressive symptoms are associated with reductions in overall locomotor activity. Alternatively, there may be a relationship between greater levels of depressive symptoms and a dampening of circadian activity rhythms. The latter interpretation is supported by the association between greater levels of depressive symptoms and decreased normalized amplitude in age- and site-adjusted models where amplitude was divided by mean activity level, although in the multivariate normalized models the association was no longer significant. These findings also suggest that covariates play an important role and contribute to a growing epidemiologic support for an association between disrupted circadian rhythms and health problems in older adults.
We also found a graded association between greater levels of depressive symptoms and an earlier evening settling time in multivariate models. This is unlikely to represent an advance in circadian phase because there was no corresponding association between level of depressive symptoms and acrophase. One possible interpretation is there may be an overall increase in the total time of restful behavior with increasing levels of depressive symptoms. In fact, there was also an association between greater level of depressive symptoms and later time becoming active in the morning in age- and site-adjusted models that was not significant in multivariate models. The impact of covariates on this association again underscores the complexity of the relationship, and additional studies are therefore required to better understand it.
Few studies have examined the relationship between depression and circadian rhythms in older adults. One study examining the relationship between circadian activity rhythms and well-being in 87 demented older women found that depressive symptoms were inversely related to the amplitude of circadian activity rhythms as well as their interdaily stability.45 To our knowledge, this is the first study examining the relationship between depressive symptoms and circadian activity rhythms in a population of older adults that was not selected on the basis of dementia. Indeed, in our analyses significant cognitive impairment was included in multivariate models, suggesting that the associations reported here exist, at least in part, independently of cognitive status.
In this study, post-hoc analyses suggested significant associations between depressive symptoms and circadian activity rhythm variables both for women in the “depressed” group and for those with “some depressive symptoms” compared with the “normal” group. This suggests that subthreshold depressive syndromes may be associated with desynchronization of circadian activity rhythms. In older adults, “less than major” depressive syndromes, which include dysthymia, minor depression and sub-syndromal depression, are almost twice as common as major depression3,5–7 and therefore represent most depressive syndromes experienced in this age group. Similar to major depression, these “less than major” depressive syndromes have been shown to be associated with a number of adverse outcomes in older adults,46 including functional impairment,47 increased disability burden,48 increased risk of physical decline,49 and increased risk for developing major depression.5 It is therefore critical that we increase knowledge about these syndromes and develop treatment interventions for them.50 No significant differences were found between the “depressed” group and those with “some depressive symptoms” when compared pair-wise in post-hoc analyses. It is notable, however, that this analysis had less power due to the smaller sizes of the groups being compared.
Because this study was cross-sectional in design, the associations reported provide no information regarding whether disturbances in circadian activity rhythms are part of a pathophysiologic pathway leading to depressive symptoms. Several groups have investigated whether chronobiologic treatments can improve depressive symptoms in older adults. The literature regarding the use of bright-light treatment for depression in patients with dementia was reviewed and found to be both limited and inconclusive.51 Several studies examining the efficacy of bright light for treatment of depressive symptoms in older adults without dementia have yielded promising results,17,18 although at least one has found no benefit.52 The efficacy of bright-light therapy for the treatment of subthreshold depressive symptoms in older adults remains unexplored.
This study has several important limitations. The study is cross-sectional, and it is therefore not possible to determine whether circadian activity rhythm disturbances are a risk factor for depression. The sample is made up of community-dwelling women ages 70 years and older, the vast majority of whom were white and over 80 years old, and the findings may not be applicable to other populations. Actigraphy does not provide information about differences in stages of sleep or rapid-eye-movement latency. Finally, our assessment of depression was based on the GDS score rather than diagnostic interview, so we were not able to look at associations with specific diagnoses. The study also has several strengths. The sample size is large and is collected from four separate U.S. locations and the women were not selected based on depressive symptoms or on the basis of disturbances in circadian activity rhythms.
These data suggest that greater levels of depressive symptoms are associated with more desynchronization of circadian activity rhythms in community-dwelling older women. These associations exist not only for women with many depressive symptoms but also for women with subthreshold levels of depressive symptoms. Longitudinal analyses are indicated to further explore the relationship between depressive symptoms and circadian rhythm disturbances.
Acknowledgments
This was not an industry supported study. Dr. Ancoli-Israel has been a consultant or on the advisory board of Ferring Pharmaceuticals, GlaxoSmithKline, Merck, Neurocrine Biosciences, Pfizer, Respironics/Philips, sanofi-aventis, Somaxon, and had a grant from Lite-book, Inc.
Investigators in the Study of Osteoporotic Fractures Research Group: San Francisco Coordinating Center (California Pacific Medical Center Research Institute and University of California San Francisco): S.R. Cummings (principal investigator), M.C. Nevitt (co-investigator), D.C. Bauer (co-investigator), D.M. Black (co-investigator), K.L. Stone (co-investigator), W. Browner (co-investigator), R. Benard, T. Blackwell, P.M. Cawthon, L. Concepcion, M. Dockrell, S. Ewing, M. Farrell, C. Fox, R. Fullman, S.L. Harrison, M. Jaime-Chavez, W. Liu, L. Lui, L. Palermo, N. Parimi, M. Rahorst, D. Kriesel, C. Schambach, R Scott, J. Ziarno. University of Maryland: M.C. Hochberg (principal investigator), R. Nichols (clinic coordinator), S. Link. University of Minnesota: K.E. Ensrud (principal investigator), S. Diem (co-investigator), M. Homan (co-investigator), P. Van Coevering (program coordinator), S. Fillhouer (clinic director), N. Nelson (clinic coordinator), K. Moen (assistant program coordinator), F. Imker-Witte, K. Jacobson, M. Slindee, R. Gran, M. Forseth, R. Andrews, C. Bowie, N. Muehlbauer, S. Luthi, K. Atchison. University of Pittsburgh: J.A. Cauley (principal investigator), L.H. Kuller (co-principal investigator), J.M. Zmuda (co-investigator), L. Harper (project director), L. Buck (clinic coordinator), M. Danielson (project administrator), C. Bashada, D. Cusick, A. Flaugh, M. Gorecki, M. Nasim, C. Newman, N. Watson. The Kaiser Permanente Center for Health Research, Portland, OR: T. Hillier (principal investigator), K. Vesco (co-investigator), K. Pedula (co-investigator), J. Van Marter (project director), M. Summer (clinic coordinator), A. MacFarlane, J. Rizzo, K. Snider, J. Wallace.
Dr. Maglione is supported by NIH R25MH7450 and a Mini-Grant in Aging provided by the UCSD Academic Geriatric Resource Center (09SD-A7-1-24). Dr. Ancoli-Israel is supported by National Institute on Aging grant AG08415. The Study of Osteoporotic Fractures (SOF) is also supported by National Institutes of Health funding. The National Institute on Aging provides support under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01AG005394-22A1, 2 R01 AG027574-22A1, AG05407, AR35582, AG05394, AR35584, AR35583, AG026720.
Footnotes
Presented in part at the 24th annual meeting of the American Association for Geriatric Psychiatry, San Antonio, TX, March 18–21 2011, and the 25th annual meeting of the Association of Professional Sleep Societies, Minneapolis, June 11–15 2011.
The other authors have indicated no financial conflicts of interest.
References
- 1.Borson S, Barnes RA, Kukull WA, et al. Symptomatic depression in elderly medical outpatients. I. Prevalence, demography, and health service utilization. J Am Geriatr Soc. 1986;34:341–347. doi: 10.1111/j.1532-5415.1986.tb04316.x. [DOI] [PubMed] [Google Scholar]
- 2.Schulberg HC, Mulsant B, Schulz R, et al. Characteristics and course of major depression in older primary care patients. Int J Psychiatry Med. 1998;28:421–436. doi: 10.2190/G23R-NGGN-K1P1-MQ8N. [DOI] [PubMed] [Google Scholar]
- 3.Lyness JM, King DA, Cox C, et al. The importance of subsyndromal depression in older primary care patients: prevalence and associated functional disability. J Am Geriatr Soc. 1999;47:647–652. doi: 10.1111/j.1532-5415.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 4.Steffens DC, Skoog I, Norton MC, et al. Prevalence of depression and its treatment in an elderly population: the Cache County study. Arch Gen Psychiatry. 2000;57:601–607. doi: 10.1001/archpsyc.57.6.601. [DOI] [PubMed] [Google Scholar]
- 5.Judd LL, Akiskal HS. The clinical and public health relevance of current research on subthreshold depressive symptoms to elderly patients. Am J Geriatr Psychiatry. 2002;10:233–238. [PubMed] [Google Scholar]
- 6.Judd LL, Akiskal HS, Maser JD, et al. A prospective 12-year study of subsyndromal and syndromal depressive symptoms in unipolar major depressive disorders. Arch Gen Psychiatry. 1998;55:694–700. doi: 10.1001/archpsyc.55.8.694. [DOI] [PubMed] [Google Scholar]
- 7.Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 8.Olfson M, Broadhead WE, Weissman MM, et al. Subthreshold psychiatric symptoms in a primary care group practice. Arch Gen Psychiatry. 1996;53:880–886. doi: 10.1001/archpsyc.1996.01830100026004. [DOI] [PubMed] [Google Scholar]
- 9.Adams KB, Moon H. Subthreshold depression: characteristics and risk factors among vulnerable elders. Aging Ment Health. 2009;13:682–692. doi: 10.1080/13607860902774501. [DOI] [PubMed] [Google Scholar]
- 10.Bruce ML. Depression and disability in late life: directions for future research. Am J Geriatr Psychiatry. 2001;9:102–112. [PubMed] [Google Scholar]
- 11.Frasure-Smith N, Lesperance F. Depression—a cardiac risk factor in search of a treatment. JAMA. 2003;289:3171–3173. doi: 10.1001/jama.289.23.3171. [DOI] [PubMed] [Google Scholar]
- 12.Prina AM, Deeg D, Brayne C, et al. The Association between depressive symptoms and non-psychiatric hospitalisation in older adults. PLoS One. 2012;7:e34821. doi: 10.1371/journal.pone.0034821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexopoulos GS, Vrontou C, Kakuma T, et al. Disability in geriatric depression. Am J Psychiatry. 1996;153:877–885. doi: 10.1176/ajp.153.7.877. [DOI] [PubMed] [Google Scholar]
- 14.Unutzer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA. 1997;277:1618–1623. doi: 10.1001/jama.1997.03540440052032. [DOI] [PubMed] [Google Scholar]
- 15.Vasiliadis HM, Dionne PA, Preville M, et al. The excess healthcare costs associated with depression and anxiety in elderly living in the community. Am J Geriatr Psychiatry. 2012 doi: 10.1016/j.jagp.2012.12.016. (ahead of print) [DOI] [PubMed] [Google Scholar]
- 16.Lenze EJ, Sheffrin M, Driscoll HC, et al. Incomplete response in late-life depression: getting to remission. Dialog Clin Neurosci. 2008;10:419–430. doi: 10.31887/DCNS.2008.10.4/jlenze. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumaya IC, Rienzi BM, Deegan JF, 2nd, et al. Bright light treatment decreases depression in institutionalized older adults: a placebo-controlled crossover study. J Gerontol A Biol Sci Med Sci. 2001;56:M356–M360. doi: 10.1093/gerona/56.6.m356. [DOI] [PubMed] [Google Scholar]
- 18.Lieverse R, Van Someren EJ, Nielen MM, et al. Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2011;68:61–70. doi: 10.1001/archgenpsychiatry.2010.183. [DOI] [PubMed] [Google Scholar]
- 19.Carrier J, Monk TH, Buysse DJ, et al. Amplitude reduction of the circadian temperature and sleep rhythms in the elderly. Chronobiol Int. 1996;13:373–386. doi: 10.3109/07420529609012661. [DOI] [PubMed] [Google Scholar]
- 20.Hofman MA, Swaab DF. Living by the clock: the circadian pacemaker in older people. Ageing Res Rev. 2006;5:33–51. doi: 10.1016/j.arr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Munch M, Cajochen C, Wirz-Justice A. Sleep and circadian rhythms in ageing. Z Gerontol Geriatr. 2005;38(Suppl 1):I21–I23. doi: 10.1007/s00391-005-1106-z. [DOI] [PubMed] [Google Scholar]
- 22.Turner PL, Mainster MA. Circadian photoreception: ageing and the eye’s important role in systemic health. Br J Ophthalmol. 2008;92:1439–1444. doi: 10.1136/bjo.2008.141747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell SS, Kripke DF, Gillin JC, et al. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol Behav. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- 24.Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- 25.Hofman MA, Swaab DF. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human supra-chiasmatic nucleus (SCN) with aging. Brain Res. 1994;651:134–142. doi: 10.1016/0006-8993(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 26.Kolker DE, Fukuyama H, Huang DS, et al. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J Biol Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- 27.Germain A, Kupfer DJ. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–585. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClung CA. Circadian rhythms and mood regulation: insights from pre-clinical models. Eur Neuropsychopharmacol. 2012;21(Suppl 4):S683–S693. doi: 10.1016/j.euroneuro.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monteleone P, Martiadis V, Maj M. Circadian rhythms and treatment implications in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1569–1574. doi: 10.1016/j.pnpbp.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 30.Kasper S, Hamon M. Beyond the monoaminergic hypothesis: agomelatine, a new antidepressant with an innovative mechanism of action. World J Biol Psychiatry. 2009;10:117–126. doi: 10.1080/15622970902717024. [DOI] [PubMed] [Google Scholar]
- 31.Lamont EW, Legault-Coutu D, Cermakian N, et al. The role of circadian clock genes in mental disorders. Dialog Clin Neurosci. 2007;9:333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linkowski P, Mendlewicz J, Leclercq R, et al. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985;61:429–438. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- 33.Hampp G, Albrecht U. The circadian clock and mood-related behavior. Commun Integr Biol. 2008;1:1–3. doi: 10.4161/cib.1.1.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hampp G, Ripperger JA, Houben T, et al. Regulation of mono-amine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Brink TL. Clinical Gerontology: A Guide to Assessment and Intervention. New York: Howarth Press; 1986. [Google Scholar]
- 38.Maglione JE, Ancoli-Israel S, Peters KW, et al. Depressive symptoms are associated with subjective and objective disturbances in sleep in community-dwelling older women. J Am Geriatr Soc. 2012;60:635–643. doi: 10.1111/j.1532-5415.2012.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paudel ML, Taylor BC, Diem SJ, et al. Association between depressive symptoms and sleep disturbances in community-dwelling older men. J Am Geriatr Soc. 2008;56:1228–1235. doi: 10.1111/j.1532-5415.2008.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–865. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 41.Blackwell T, Ancoli-Israel S, Gehrman PR, et al. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 42.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–291. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 44.Marler MR, Gehrman P, Martin JL, et al. The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 2006;25:3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 45.Carvalho-Bos SS, Riemersma-van der Lek RF, Waterhouse J, et al. Strong association of the rest-activity rhythm with well-being in demented elderly women. Am J Geriatr Psychiatry. 2007;15:92–100. doi: 10.1097/01.JGP.0000236584.03432.dc. [DOI] [PubMed] [Google Scholar]
- 46.Grabovich A, Lu N, Tang W, et al. Outcomes of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2010;18:227–235. doi: 10.1097/JGP.0b013e3181cb87d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyness JM, Kim J, Tang W, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry. 2007;15:214–223. doi: 10.1097/01.JGP.0000235763.50230.83. [DOI] [PubMed] [Google Scholar]
- 48.Cronin-Stubbs D, de Leon CF, Beckett LA, et al. Six-year effect of depressive symptoms on the course of physical disability in community-living older adults. Arch Intern Med. 2000;160:3074–3080. doi: 10.1001/archinte.160.20.3074. [DOI] [PubMed] [Google Scholar]
- 49.Penninx BW, Guralnik JM, Ferrucci L, et al. Depressive symptoms and physical decline in community-dwelling older persons. JAMA. 1998;279:1720–1726. doi: 10.1001/jama.279.21.1720. [DOI] [PubMed] [Google Scholar]
- 50.Meeks TW, Vahia IV, Lavretsky H, et al. A tune in “a minor” can “b major”: a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011;129:126–142. doi: 10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forbes D, Culum I, Lischka AR, et al. Light therapy for managing cognitive, sleep, functional, behavioural, or psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2009;(4):CD003946. doi: 10.1002/14651858.CD003946.pub3. [DOI] [PubMed] [Google Scholar]
- 52.Loving RT, Kripke DF, Elliott JA, et al. Bright light treatment of depression for older adults. BMC Psychiatry. 2005;5:41. doi: 10.1186/1471-244X-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]