Abstract
Antiestrogens such as tamoxifen are widely used in the clinic to treat estrogen receptor–positive breast tumors. Resistance to tamoxifen can occur either de novo or develop over time in a large proportion of these tumors. Additionally, resistance is associated with enhanced motility and invasiveness in vitro. One molecule that has been implicated in tamoxifen resistance, breast cancer antiestrogen resistance-3 (BCAR3), has also been shown to regulate migration of fibroblasts. In this study, we investigated the role of BCAR3 in breast cancer cell migration and invasion. We found that BCAR3 was highly expressed in multiple breast cancer cell lines, where it associated with another protein, p130Cas (also known as breast cancer antiestrogen resistance-1; BCAR1), that plays a role in both tamoxifen resistance and cell motility. In cells with relatively low migratory potential, BCAR3 overexpression resulted in enhanced migration and colocalization with p130Cas at the cell membrane. Conversely, BCAR3 depletion from more aggressive breast cancer cell lines inhibited migration and invasion. This coincided with a relocalization of p130Cas away from the cell membrane and an attenuated response to epidermal growth factor stimulation that was characterized by a loss of membrane ruffles, decreased migration toward EGF, and disruption of p130Cas/Crk complexes. Based on these data, we propose that the spatial and temporal regulation of BCAR3/p130Cas interactions within the cell is important for controlling breast cancer cell motility.
Introduction
Breast cancer will be diagnosed in over 200,000 American women this year and a large percentage of these individuals will be treated with the antiestrogen tamoxifen. Unfortunately, a substantial number of breast tumors either do not respond to initial treatment with tamoxifen or become nonresponsive over time, despite continued expression of the estrogen receptor (ER). Clinically, tamoxifen resistance is associated with poor prognosis and outcome (1). Several molecules have been shown to regulate antiestrogen resistance, including epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2, phosphoinositol 3-kinase (PI3K), and the adapter molecule breast cancer antiestrogen resistance-1 (BCAR1; also known as p130Cas; refs. 2, 3). A second protein, BCAR3 (the human homologue of the murine protein AND-34), was identified in a genetic screen along with BCAR1 as a gene product whose overexpression conferred tamoxifen resistance in vitro (4). BCAR3 is a member of the novel Src homology 2 (SH2)–containing protein (NSP) family that includes two other members, Chat/SHEP1 and NSP1. These proteins share a common domain structure consisting of an amino-terminal SH2 domain and a carboxyl-terminal domain with sequence homology to the Cdc25-family of guanine nucleotide exchange factors (GEF). Several studies have shown that BCAR3 expression results in the activation of numerous small GTPases, including Rap1, R-Ras, RalA, Cdc42, and Rac1 (5–7). The carboxyl-terminal domain of BCAR3 has been shown to bind to the carboxyl terminus of p130Cas, providing additional support for a functional relationship between these proteins (5).
In a previous study, we showed that co-overexpression of BCAR3 and p130Cas in fibroblasts promoted cell migration, and this coincided with translocation of ectopic p130Cas to the leading edge of the cell (8). Other NSP family members have been shown to have similar functions. For example, migration of COS-7 cells toward EGF was elevated in cells that overexpressed Chat/SHEP1 and was further augmented by p130Cas overexpression. EGF stimulation of these cells resulted in colocalization of Chat and p130Cas at membrane ruffles (9).
Although BCAR3 function has been implicated in antiestrogen-resistant proliferation of breast cancer cells (4, 10), its role in breast cancer cell motility has not been established. In this study, we used a panel of breast cancer cell lines that vary in stage and aggressiveness to investigate the potential role of BCAR3 in migration and invasion. We found that BCAR3 was expressed and in complex with p130Cas in the majority of these breast cancer cells. In cells with relatively low migratory potential, BCAR3 overexpression resulted in enhanced migration and colocalization with p130Cas at the cell membrane. Conversely, BCAR3 depletion from more aggressive breast cancer cell lines inhibited migration and invasion, caused a relocalization of p130Cas away from the cell membrane, and attenuated the cellular response to EGF stimulation. Taken together, these data suggest that BCAR3 may regulate breast cancer cell migration by localizing p130Cas to the cell membrane, thereby spatially coordinating multiple signaling pathways.
Materials and Methods
Cell culture
MCF-7 and BT549 cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin. MDA-MB-231 cells were cultured in DMEM supplemented with 10% FBS, 1 mmol/L sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin. T47D cells were cultured in RPMI medium supplemented with 10% FBS, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, 4.5 g/L glucose, 0.2 units/mL bovine insulin, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cells were starved for 16 h in serum-free DMEM before EGF stimulation (100 ng/mL; Receptor Grade EGF, Sigma).
Antibodies
Polyclonal BCAR3 antisera were generated by immunizing rabbits with a 15-amino-acid BCAR3 peptide, TLPRKKKGPPPIRSC, conjugated to keyhole limpet hemocyanin (Covance Research Products). This peptide, which spans amino acids 50 to 64, is not present in other NSP family members. Antibodies recognizing phosphotyrosine (pTyr; 4G10; Upstate Biotechnology), Crk (BD/Transduction Laboratories), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnologies), and FLAG (Sigma) were obtained from the indicated sources. Texas red–phalloidin was purchased from Molecular Probes. CasB antisera and p130Cas monoclonal antibody 6G11 have been described previously (11). FITC-conjugated goat anti-mouse and Texas red–conjugated goat anti-rabbit antibodies were purchased from Jackson ImmunoResearch.
RNA interference, plasmid transfection, and protein detection
A small interfering RNA (siRNA) oligonucleotide (AAAUCAACCGGACAGUUCU) was synthesized to target human but not murine BCAR3 (Dharmacon). MDA-MB-231 and BT549 cells were plated into 60-mm or 10-cm tissue culture plates and incubated overnight. Cells were treated with H2O (vehicle), 20 μmol/L control nontargeting siRNA (Dharmacon), or 20 μmol/L BCAR3-specific siRNAs using Oligofectamine (Invitrogen) transfection reagent as per manufacturer’s specifications. BCAR3 was expressed by nucleofection of plasmids encoding full-length BCAR3 (Origene) or FLAG-AND-34 as per manufacturer’s specifications (Amaxa Corporation).
Cell lysis, immunoprecipitation, and immunoblotting
Cells were lysed in modified radioimmune precipitation assay buffer [150 mmol/L NaCl, 50 mmol/L Tris (pH 7.5), 1% Ipegal CA-630, 0.5% deoxycholate] supplemented with protease and phosphatase inhibitors (100 μmol/L leupeptin, 1 mmol/L phenylmethylsulfonyl fluoride, 0.15 unit/mL aprotinin, and 1 mmol/L sodium orthovanadate). Protein concentrations were determined using the bicinchoninic acid assay kit (Pierce). Immunoprecipitations were done as described previously (8). Proteins were resolved by SDS-PAGE, transferred to nitrocellulose, immunoblotted with the indicated antibodies, and detected by horseradish peroxidase (HRP)–conjugated anti-mouse or anti-rabbit immunoglobulin (Amersham) followed by HRP Substrate Luminol Reagent (Millipore).
Confocal microscopy and immunofluorescence
Cells were plated onto coverslips coated with 15 μg/mL fibronectin and allowed to spread for 4 to 5 h. Cells were fixed in 3% paraformaldehyde for 20 min and permeabilized using 0.4% Triton X-100 in PBS for 6 min at room temperature. Cells were incubated in 10% bovine serum albumin (BSA)/ PBS for 30 min, primary antibodies in 2% BSA/PBS for 1 h, followed by Texas red–conjugated goat anti-rabbit, FITC-conjugated goat anti-mouse, and/or Texas red–phalloidin for 1 h. Coverslips were mounted using Vectashield (Vector Labs) or Prolong Gold Antifade with 4′,6-diamidino-2-phenylindole (Molecular Probes) for detection of nuclei. For confocal images, cells were visualized on a Zeiss LSM 510 confocal microscope and images were captured using Zeiss LSM 5 software (Carl Zeiss Micro-Imaging). For epifluorescence microscopy, cells were visualized through a Nikon TE2000-E Eclipse fluorescence microscope and photographed with an ORCA cooled charged-coupled device camera controlled by Openlab software (Improvision, Inc.).
Migration and invasion assay
MDA-MB-231 and BT549 cells were treated with H2O or siRNA oligonucleotides for 48 h, as described above. For migration toward 10% serum, the lower chamber of a modified Boyden chamber (6.5 mm, 8.0-μm Transwell Costar membrane; Corning Incorporated) was preincubated with 20% FBS in DMEM for 2 h. MDA-MB-231 (2.5 × 104) or BT549 (1.2 × 104) cells were plated in the top chamber in DMEM without serum and allowed to migrate toward 10% serum for 6 h at 37°C. For migration toward EGF, cells were plated as described above into chambers containing DMEM supplemented with 100 ng/mL EGF in the bottom chamber. For invasion, the top and bottom of Biocoat invasion chambers (24-well, 8.0 μm, growth factor reduced Matrigel matrix, BD Biosciences) were preincubated with serum-free DMEM for 2 h. Cells were then plated as described above. Cells were allowed to invade through the Matrigel for 24 h at 37°C. Following migration or invasion, the nonmigratory cells were removed from the top of the membrane with cotton swabs. The underside of the membrane was fixed, stained using the Diff-Quik staining set (Dade Behring), and mounted onto coverslips using Cytoseal-60 (Richard Allen Scientific). The total number of migrated cells was determined by light microscopy. Migration of cells cotransfected with plasmids encoding green fluorescent protein (GFP) and vector or BCAR3 was measured by determining the percentage of GFP-positive cells that migrated and dividing by the transfection efficiency (12). The log values for each group of data were analyzed by single-factor ANOVA. When significant differences were found between groups at the 5% level, they were compared using the Student’s t test assuming unequal variance.
Results
Endogenous BCAR3 is expressed and associated with p130Cas in multiple breast cancer cell lines
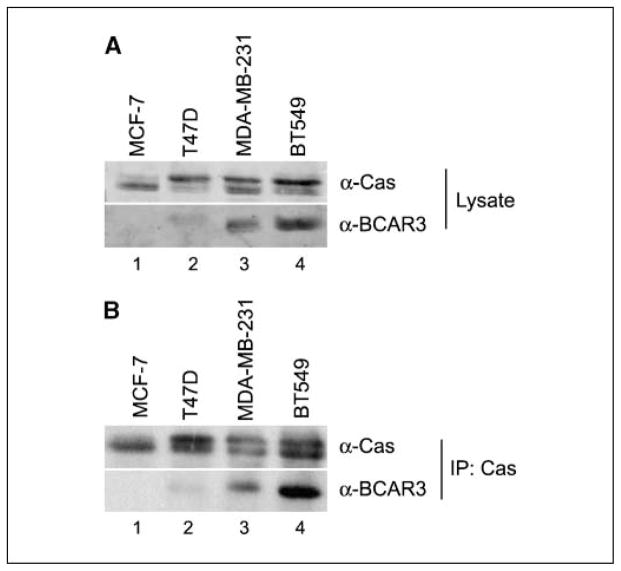
BCAR3 mRNA has been detected in various adult tissues and in a number of cancer cell lines, including breast (4). Results to date, however, have not addressed BCAR3 protein expression. Polyclonal antisera raised against a peptide unique to BCAR3 were developed to measure endogenous protein. This antisera specifically recognized FLAG-tagged AND-34 (murine BCAR3), both by immunoprecipitation and immunoblot (data not shown). BCAR3 expression was measured using this antisera in a panel of breast cancer cell lines representative of various stages of breast cancer progression. The ER-positive cell lines MCF-7 and T47D represent noninvasive and moderately invasive breast cancer, respectively, whereas the ER-negative breast cancer cell lines MDA-MB-231 and BT549 are models for more aggressive, metastatic disease (13). BCAR3 was differentially expressed in these cell lines; MCF-7 and T47D cells expressed very little BCAR3 whereas MDA-MB-231 and BT549 cells expressed moderate to high levels (Fig. 1A, bottom). p130Cas, which is a binding partner of BCAR3, was also expressed at varying levels in these cell lines (top). The relative intensity of the p130Cas-specific bands varied, indicative of differences in the level of p130Cas phosphorylation between cells.
Figure 1.
BCAR3 and p130Cas are coexpressed and in complex in multiple breast cancer cell lines. Fifty micrograms of total cell lysate (A) or p130Cas immune complexes isolated from 500 μg cell extract (B) were separated by 8% SDS-PAGE and immunoblotted with antibodies recognizing p130Cas (top) or BCAR3 (bottom). IP, immunoprecipitation.
Previous studies have shown that BCAR3 associates with p130Cas in HEK293 cells and fibroblasts (5, 6, 8). To determine if BCAR3 and p130Cas also formed a complex in breast cancer cells, endogenous p130Cas was immunoprecipitated from cell lysates and the resultant immune complexes were immunoblotted with p130Cas and BCAR3 antibodies. BCAR3 was readily evident in immune complexes isolated from MDA-MB-231 and BT549 cells (Fig. 1B).
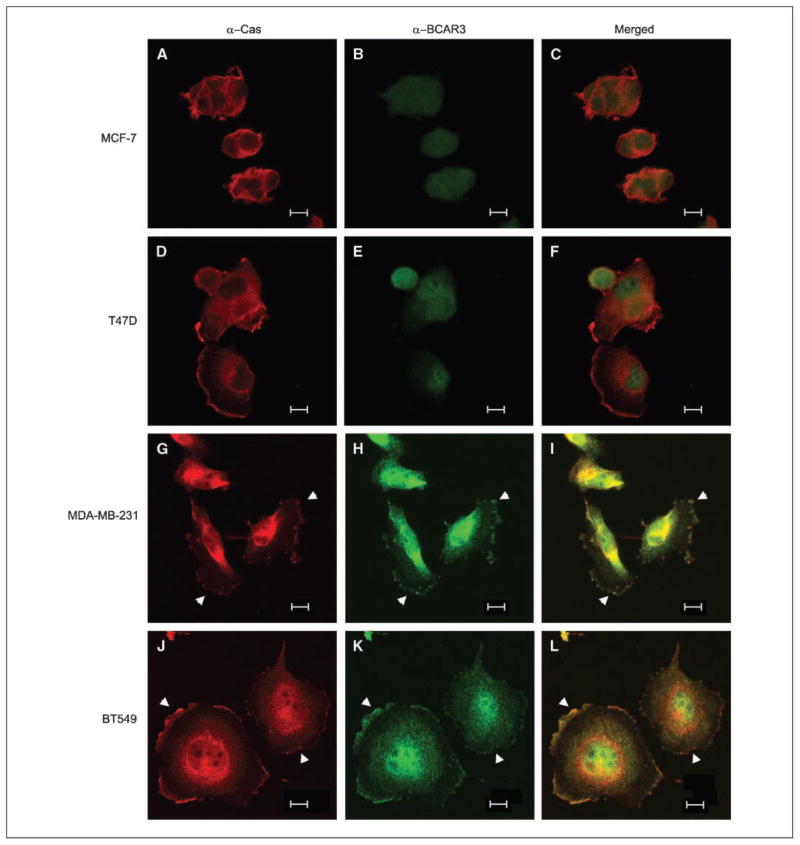
To investigate the spatial relationship between BCAR3 and p130Cas in breast cancer cells, the four cell lines analyzed above were examined by confocal microscopy. Consistent with biochemical analysis, BCAR3 was expressed at low levels in MCF-7 and T47D cells, whereas p130Cas expression was observed throughout the cytoplasm and at the edges of the cells (Fig. 2A–F). In the more aggressive cell lines (MDA-MB-231 and BT549), p130Cas and BCAR3 were colocalized both in the cytoplasm and at the cell periphery (G–L, see arrowheads). Together, these data show that p130Cas and BCAR3 colocalize and are associated with one another in invasive MDA-MB-231 and BT549 breast cancer cell lines.
Figure 2.
BCAR3 and p130Cas colocalize to membrane ruffles in aggressive breast cancer cell lines. MCF-7, T47D, MDA-MB-231, and BT549 cells were plated onto fibronectin-coated coverslips in serum-containing medium, allowed to spread for 6 h, and processed for immunofluorescence. Cells were stained with antibodies recognizing p130Cas (A, D, G, and J) or BCAR3 (B, E, H, and K) and visualized by confocal microscopy as described in Materials and Methods. Merged images (C, F, I, and L). Arrowheads, sites of BCAR3 and p130Cas colocalization. Bar, 10 μm.
Overexpression of BCAR3 promotes migration and p130Cas interactions in MCF-7 and T47D cells
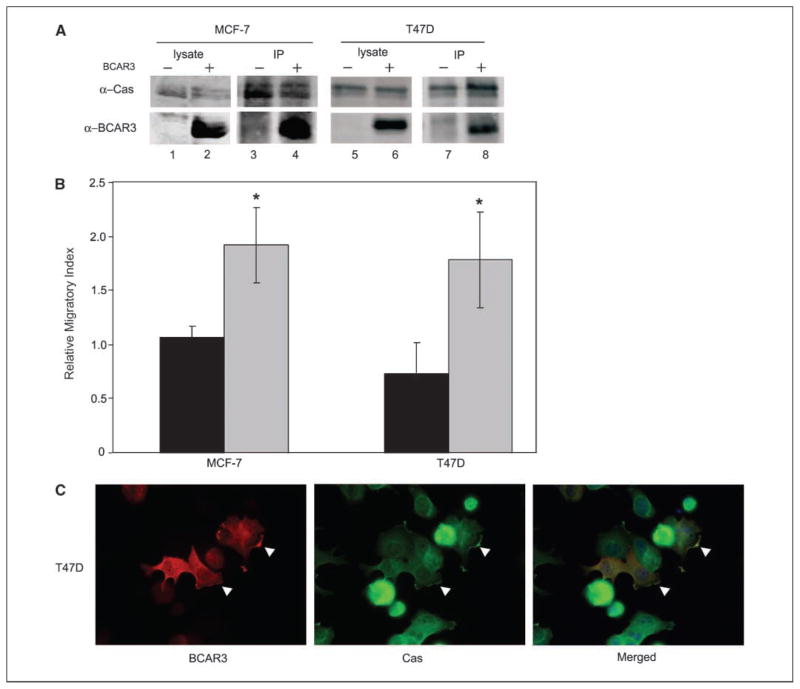
Previous data from our laboratory showed that co-overexpression of p130Cas and BCAR3 synergistically promoted the migration of fibroblasts (8). Likewise, expression of the BCAR3 family member, Chat/SHEP1, also increased migration in COS-7 cells (14). To investigate whether BCAR3 expression influences the migratory potential of breast cancer cells, BCAR3 was overexpressed in MCF-7 and T47D breast cancer cells, which are relatively noninvasive and nonmotile. Transfection of plasmids encoding BCAR3 resulted in significant overexpression (Fig. 3A, bottom panels, compare lane 1 with 2 and lane 5 with 6). This coincided with an ~2-fold increase in migration of both cell lines toward serum, as measured in Boyden chamber assays (Fig. 3B). Increased proliferation of BCAR3-overexpressing cells could not account for the differences in migration because these cells exhibited no growth advantage over control cells (data not shown). In both MCF-7 and T47D cells, BCAR3-p130Cas association was also increased under conditions of BCAR3 over-expression (Fig. 3A, compare lane 3 with 4 and lane 7 with 8). In T47D cells, this increased association coincided with colocalization of BCAR3 with p130Cas at the cell periphery (Fig. 3C, arrowheads). These data indicate that BCAR3 overexpression in MCF-7 and T47D breast cancer cells can promote cell migration, and that this may be due to increased BCAR3-p130Cas interactions at the cell membrane.
Figure 3.
BCAR3 overexpression in MCF-7 and T47D cells increases BCAR3-p130Cas interactions and promotes cell migration. A, MCF-7 and T47D cells were transiently transfected with vector (lanes 1, 3, 5, and 7) or BCAR3-encoding plasmids (lanes 2, 4, 6, and 8). Twenty micrograms of total cell lysate (lanes 1, 2, 5, and 6) or p130Cas immune complexes generated from 200 μg of total protein (lanes 3, 4, 7, and 8) were immunoblotted for p130Cas (top) or BCAR3 (bottom). B, MCF-7 and T47D cells were cotransfected with plasmids encoding GFP plus vector or BCAR3. Twenty-four hours later, cells were seeded onto Boyden chambers and allowed to migrate toward serum for 24 h at 37°C (see Materials and Methods for calculation of relative migratory index). Columns, mean for five or seven independent experiments, respectively; bars, SD. Black columns, vector-transfected cells; gray columns, BCAR3-transfected cells. *, P < 0.05 relative to vector-transfected cells. C, T47D cells transfected with BCAR3-expressing plasmids were plated onto fibronectin-coated coverslips 24 h posttransfection and processed for immunofluorescence 24 h later. BCAR3 and p130Cas were visualized by epifluorescence microscopy as described in Materials and Methods. Overexpressed BCAR3 colocalized with p130Cas at the periphery of the cell (arrowheads).
Cell migration and invasion are inhibited by BCAR3 depletion
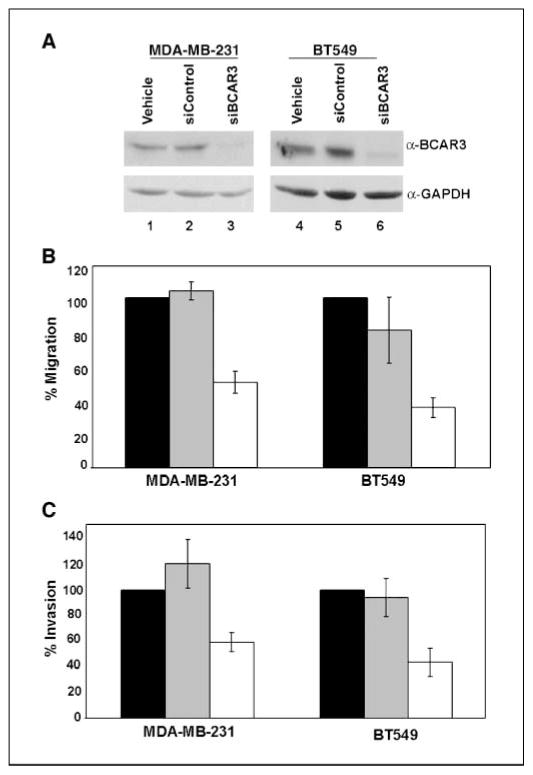
To further investigate the role of BCAR3 in migration and invasion of breast cancer cells, BCAR3 expression was reduced by BCAR3-specific siRNA oligonucleotides in MDA-MB-231 and BT549 cells, which are highly migratory and invasive. In both cell lines, BCAR3 expression was reduced by ~90% (Fig. 4A, lanes 3 and 6) compared with mock-transfected cells (lanes 1 and 4) or cells treated with nontargeting control siRNA oligonucleotides (lanes 2 and 5).
Figure 4.
Depletion of BCAR3 in MDA-MB-231 or BT549 cells results in reduced migration and invasion. A, MDA-MB-231 and BT549 cells were treated with vehicle (H2O; lanes 1 and 4 ), nontargeting siRNAs (lanes 2 and 5 ), or BCAR3-specific siRNAs (lanes 3 and 6). Forty-eight hours later, cells were lysed and 50 μg total cell lysate were immunoblotted for BCAR3 (top panels) and GAPDH (bottom panels). B, vehicle-treated (black columns), control siRNA–treated (gray columns), or BCAR3-specific siRNA–treated (white columns) MDA-MB-231 and BT549 cells were seeded onto Boyden chambers 48 h posttransfection and allowed to migrate toward 10% serum for 6 h. C, vehicle-treated (black columns), control siRNA–treated (gray columns), or BCAR3-specific siRNA–treated (white columns) MDA-MB-231 and BT549 cells were seeded onto Matrigel-coated Boyden chambers 48 h posttransfection and allowed to migrate toward 10% serum for 24 h. Columns (B and C), mean for eight independent experiments; bars, SD. *, P < 0.05 relative to both vehicle siRNA and control siRNA treatment of cells.
To test if BCAR3 expression in MDA-MB-231 and BT549 cells was important for migration and invasion, siRNA-transfected cells were analyzed for their ability to migrate toward serum or invade through Matrigel. BCAR3 depletion was found to inhibit migration of MDA-MB-231 and BT549 cells by 50% and 65%, respectively (Fig. 4B). Similarly, BCAR3 depletion inhibited invasion of MDA-MB-231 and BT549 cells through Matrigel by 41% and 57% (Fig. 4C). The differences in migration and invasion observed between control and BCAR3-depeleted cells were not due to decreases in cell viability, proliferation, spreading, or adherence (data not shown). Furthermore, this inhibition of migration and invasion was specifically due to the loss of BCAR3 expression because reexpression of the nontargeted murine BCAR3 homologue, AND-34, in siRNA-treated BT549 cells resulted in rescue of migration to levels seen in control cells (data not shown).
BCAR3 depletion in BT549 cells alters p130Cas localization
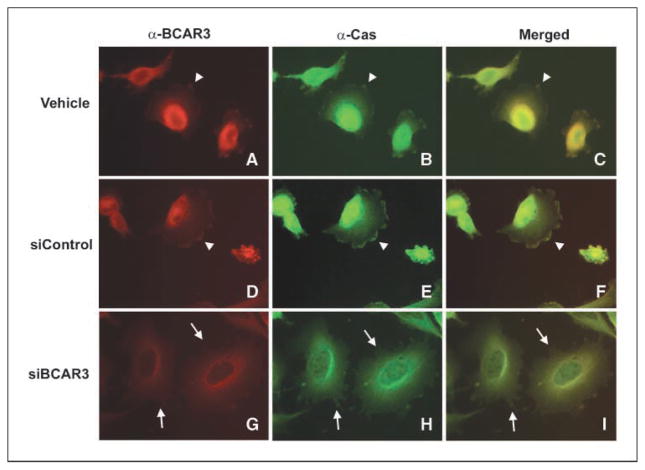
In our previous work, the p130Cas-binding region of BCAR3 was found to be necessary for the synergistic enhancement of migration caused by co-overexpression of p130Cas and BCAR3. Enhanced migration was coincident with p130Cas relocalization to the leading edge, where BCAR3 was located (8). We hypothesized that BCAR3 depletion might have the opposite effect on p130Cas localization in breast cancer cells, resulting in a diminution of p130Cas at membrane ruffles coincident with the decrease in cell migration shown in Fig. 4. To test this hypothesis, the localization of BCAR3 and p130Cas was examined by immunofluorescence in BT549 cells treated with control or BCAR3-specific siRNAs. As was the case for untreated cells (Fig. 2J–L), BCAR3 and p130Cas colocalized at the periphery in BT549 cells treated under control conditions (Fig. 5A–F, arrowheads). Cells treated with BCAR3-targeted siRNAs showed reduced overall BCAR3 staining (G). Residual staining was confined predominantly to the perinuclear region of the cell, where the highest density of BCAR3 was observed in control cells (A and D). The overall intensity of BCAR3 staining in these images is likely to overestimate the amount of BCAR3 in the cell due to an attempt to enhance the detection of low amounts of BCAR3 at the cell periphery. Even under these staining conditions, however, BCAR3 was not evident at the cell periphery (G, see arrows). Coincidentally, p130Cas was absent from these sites despite the fact that membrane protrusions were still present (G–I, see arrows). These data raise the possibility that depletion of p130Cas and BCAR3 from the membrane may contribute at least in part to reduced migration and invasion independently of the ability of the cell to form membrane protrusions.
Figure 5.
Depletion of BCAR3 from BT549 cells results in a loss of both BCAR3 and p130Cas from membrane ruffles. BT549 cells were treated with vehicle (H2O), control, or BCAR3-targeted siRNAs as described in Fig. 4. Forty-eight hours posttransfection, cells were replated onto fibronectin-coated coverslips in serum-containing medium, allowed to spread for 4 h, and processed for epifluorescence. BCAR3 and p130Cas were visualized as described in Materials and Methods, maintaining equal exposure lengths for each antibody. Merged images (C, F, and I). Arrowheads, sites of BCAR3-p130Cas colocalization at membrane ruffles. Arrows, sites of membrane ruffles devoid of BCAR3 and p130Cas in cells treated with BCAR3-targeted siRNA (G–I).
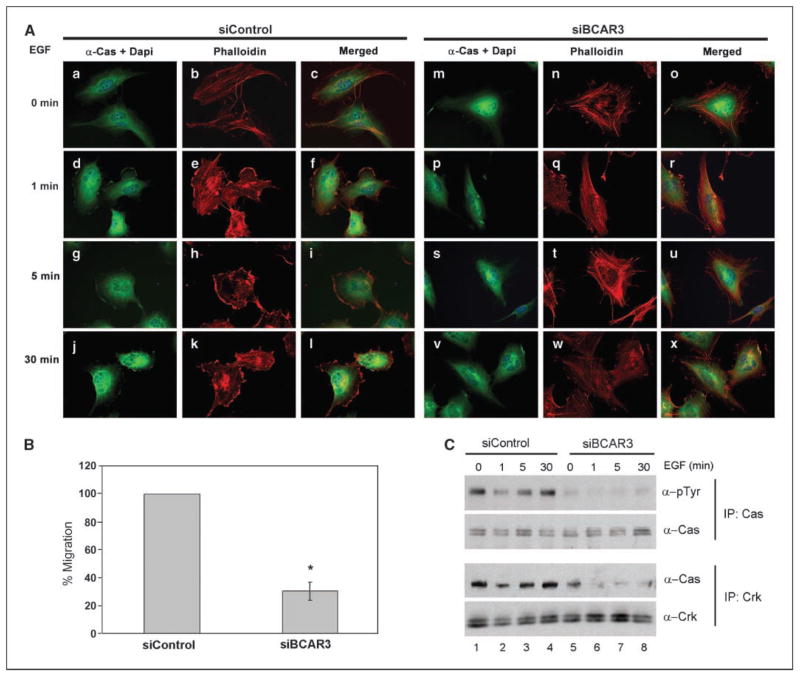
p130Cas localization to the membrane has been shown to correlate with tyrosine phosphorylation (15). Phosphorylation of p130Cas is increased upon EGF stimulation, which also results in transient membrane ruffling (16). Because p130Cas was found to be largely absent from peripheral membrane ruffles under conditions of BCAR3 depletion, we sought to determine if BT549 cells responded normally to EGF stimulation under conditions of reduced BCAR3 expression. Control and BCAR3 siRNA-treated BT549 cells were serum starved for 16 h and then stimulated with EGF for 1, 5, or 30 min to induce membrane ruffles. Before EGF stimulation, p130Cas was located throughout the cytoplasm in both control and BCAR3 siRNA-treated cells, and actin was organized into stress fibers (Fig. 6A, a–c and m–o). It is important to note that, unlike the cells shown in Fig. 2K, these cells were serum starved and therefore had few membrane ruffles under basal conditions. EGF stimulation resulted in a dissolution of stress fibers and a relocalization of p130Cas to actin-rich membrane ruffles in cells expressing endogenous levels of BCAR3 (d–l). This response was observed as early as 1 min poststimulation and persisted through 30 min. In contrast, BT549 cells with depleted BCAR3 showed little morphologic change after EGF stimulation. Prominent actin stress fibers were present throughout the 30-min time course and membrane ruffles were rare (q, t, and w). Moreover, p130Cas was not enriched at the edges of these cells after EGF stimulation (p, s, and v).
Figure 6.
Depletion of BCAR3 in BT549 cells results in an attenuated EGF response. A, BT549 cells were transfected with control or BCAR3-specific siRNAs and incubated for 48 h. Cells were then serum starved for 16 h and stimulated with 100 ng/mL of EGF for 0, 1, 5, or 30 min. p130Cas localization was visualized by epifluorescence with p130Cas antibodies (green) and filamentous actin was visualized by Texas red–phalloidin (red). Merged images are shown. B, control siRNA or BCAR3 siRNA-treated BT549 cells were seeded onto Boyden chambers 48 h posttransfection and allowed to migrate toward 100 ng/mL EGF for 6 h. *, P < 0.05 relative to control siRNA treatment of cells. C, BT549 cells were siRNA treated and stimulated with EGF as described in (A). p130Cas immune complexes were generated from 200 μg total cell lysates, separated by 8% SDS-PAGE, and immunoblotted with pTyr (top) or p130Cas (second panel) antibodies. Crk immune complexes were generated from 650 μg total cell lysates, separated by 8% SDS-PAGE, and immunoblotted with p130Cas (third panel) and Crk (bottom) antibodies.
Interactions between p130Cas and the adapter molecule Crk have been reported to regulate EGF-dependent cell migration (17). To assess whether the attenuated EGF responses observed under conditions of depleted BCAR3 expression were associated with altered migration toward EGF, siRNA-treated BT549 cells were serum starved for 16 h and then plated in Boyden chambers containing 100 ng/mL EGF in the bottom chamber. BCAR3 depletion inhibited migration toward EGF by 67% compared with control cells (Fig. 6B). This decrease in migration coincided with a concomitant decrease in basal and EGF-dependent p130Cas phosphorylation (Fig. 6C, top, compare lanes 5–8 with lanes 1–4) and loss of p130Cas/Crk interactions (third panel). Together, these results show that BCAR3 serves as a regulator of EGF-stimulated membrane ruffling, p130Cas localization to the ruffles, promotion of p130Cas/Crk interactions, and migration.
Discussion
Tamoxifen resistance is associated with a poor clinical prognosis, frequently occurring in patients with metastatic disease (18). Overexpression of BCAR3 has been shown to promote resistance to tamoxifen in vitro, and studies using rat embryo fibroblasts have shown that BCAR3 can function as a regulator of cell migration (4, 8). Previous studies have characterized the structural components of BCAR3 necessary for promotion of tamoxifen resistance, migration, and interaction with p130Cas (5, 7, 8). The aim of this study was to investigate the functional interactions between BCAR3 and p130Cas that regulate breast cancer cell migration and invasion. Based on our findings, we propose that BCAR3 functions in breast cancer cells to translocate p130Cas from the cytoplasm to the cell membrane, where it can become phosphorylated. Phosphorylation of p130Cas then stimulates interactions with SH2 domain–containing proteins such as Crk, leading to the formation of signaling complexes at the cell membrane that promote cell migration and invasion.
Support for this model comes from three lines of evidence. First, BCAR3/p130Cas complexes were readily detected in two invasive breast cancer cell lines (MDA-MB-231 and BT549 cells), whereas less invasive cell lines exhibited greatly reduced BCAR3/p130Cas interactions (T47D and MCF-7 cells; Fig. 1). Moreover, both molecules colocalized at the cell periphery in MDA-MB-231 and BT549 cells cultured in the presence of serum, whereas BCAR3 was not detected at these sites in the less migratory T47D cells (Fig. 2). Second, overexpression of BCAR3 in MCF-7 and T47D cells enhanced cell migration concomitant with an increase in BCAR3/p130Cas complexes and the appearance of BCAR3 in p130Cas-enriched sites at the cell periphery in T47D cells (Fig. 3). Third, knockdown of BCAR3 expression in MDA-MB-231 and BT549 cells reduced cell migration and invasion toward serum (Fig. 4). Reduced BCAR3 expression in BT549 cells also caused a decrease in migration toward EGF, coincident with a loss of p130Cas from EGF-induced membrane ruffles and a reduction in p130Cas/ Crk complexes (Fig. 6). Together, these data suggest that both the spatial and temporal regulation of BCAR3/p130Cas interactions within the cell may be critical for controlling cell motility. p130Cas has been shown to play a key role in the migration of several cell types through its function as a scaffolding molecule (19, 20). However, a role for p130Cas in breast cancer cell migration and invasion has not been established, despite the fact that p130Cas is frequently expressed at high levels in both breast cancer cell lines and tumors (Fig. 1; refs. 21, 22). Tyrosine phosphorylation of
p130Cas, which is mediated predominantly by Src family kinases (23), plays a critical role in its function as a regulator of cell migration. Phosphorylated forms of p130Cas preferentially localize to the membrane as well as nascent and mature focal complexes (15, 24). p130Cas also localizes to membrane ruffles in migrating COS cells but not in nonmigrating cells (17). Although membrane targeting of p130Cas seems to be important, the mechanism by which p130Cas is translocated to the membrane has not been established. Our results suggest that BCAR3 may be a key mediator of this localization. Interestingly, previous results showed that, in fibroblasts, BCAR3 and p130Cas colocalization at the membrane was not dependent on their direct association. It remains to be determined whether this is also the case in breast cancer cells. These studies are currently under way.
Phosphorylation of the substrate-binding domain of p130Cas can result in the generation of multiple binding sites for the small adapter molecule Crk (17). p130Cas/Crk coupling figures prominently in the process of migration of numerous cell types, including pancreatic and prostate cancer cells (25–28). We show in this report that BCAR3 expression regulates EGF-dependent p130Cas phosphorylation and association with Crk in BT549 breast cancer cells. Overexpression of membrane-targeted Chat/SHEP1 in COS cells was also found to increase p130Cas phosphorylation and p130Cas/Crk association, and inhibition of these interactions dissipated membrane ruffles (14). Nonetheless, there are cases where p130Cas/Crk interactions can be uncoupled from p130Cas phosphorylation (29). It is interesting to note that BCAR3 has recently been reported to associate with Crk in glomerular mesangial cells of the kidney, although it remains to be determined whether this interaction is direct or has an effect on cell motility (30).
Several lines of evidence point to a role for BCAR3 in regulating actin cytoskeletal structures involved in cell migration. Both our group and others have shown that fibroblasts engineered to over-express BCAR3 exhibit increases in membrane ruffles, decreases in actin stress fibers, and adoption of a polarized phenotype marked by classic leading and trailing edges (6, 8). We found in this study that reduction of BCAR3 in BT549 cells had the reverse effect, characterized by diminished EGF-dependent membrane ruffling and a failure to undergo dissolution of stress fibers after EGF stimulation. Other NSP proteins seem to play similar roles in actin regulation. For example, Chat/SHEP1 has been reported to colocalize with p130Cas in actin-rich membrane ruffles in COS-7 cells (9). Moreover, expression of a myristoylated form of this molecule that was constitutively localized to the plasma membrane increased membrane ruffling, cell spreading, and adhesion of NIH3T3 cells through a p130Cas-dependent process (14, 31).
Small GTPases are key regulators of actin dynamics. Interestingly, BCAR3 has been shown to increase the activation state of several small GTPases, including Rac1, Cdc42, R-Ras, RalA, and Rap1 (5–7). Although the carboxyl terminus of BCAR3 has limited homology to the GEF domain of Cdc25, it remains to be determined whether this molecule has intrinsic GEF activity. It is likely, however, that BCAR3 activates several of these GTPases indirectly through other GEFs (32). Based on our findings, we suggest that this may occur at least in part through p130Cas and its binding partner Crk, which can bind to DOCK180 and C3G to activate Rac1 and Rap1, respectively (33). In fibroblasts, Rap1 has been shown to play an important role in the promotion of migration by BCAR3 and signals downstream of p130Cas/Crk binding were shown to be partly responsible for Chat-induced migration (8, 31). BCAR3 may also activate these GTPases independently of p130Cas, thus contributing to the defects in actin reorganization and cell motility observed under conditions of BCAR3 depletion in response to EGF (Fig. 6).
Although our data support a function for BCAR3 in translocating p130Cas from the cytoplasm to the plasma membrane, we do not yet know the mechanism by which BCAR3 is targeted to the membrane, nor do we know whether this is a regulated event. In fibroblasts, membrane localization of BCAR3 could be uncoupled from the promotion of migration because a mutant lacking the carboxyl terminus was still targeted to the membrane but did not induce migration (8). Interestingly, BCAR3 mutants containing a substitution of the conserved arginine residue present in the SH2 domain (R171V) were not targeted to the membrane in REF-52 fibroblasts,3 suggesting that the SH2 domain may play a role in BCAR3 localization. Activated Eph receptors can bind to the SH2 domain of Chat/SHEP1 and the EGFR has been reported to bind to NSP1 (14, 34). Based on these data, it is reasonable to hypothesize that BCAR3 membrane localization may occur through binding to tyrosine phosphorylated receptors. However, we were unable to detect BCAR3/EGFR interactions in BT549 cells after EGF stimulation (data not shown).
The initial discovery of BCAR3 as a gene capable of inducing tamoxifen resistance fits well with its emerging role as a modulator of breast cancer cell migration. Tamoxifen-resistant breast cancer cell lines have been shown to exhibit increased motility and invasiveness (1). Several recent studies of BCAR3-mediated resistance to antiestrogens have implicated promigratory pathways. Yu and Feig (10) showed that overexpression of constitutively active R-Ras, but not Ral or Rap1, conferred estrogen independence in MCF-7 cells through signaling to PI3K and AKT. Independently, constitutively active R-Ras has been shown to promote migration of T47D cells, increase focal adhesion complexes in a p130Cas-dependent manner, and, most recently, contribute to signaling crosstalk between estrogen and insulin in breast cancer cells (35–37). Others have shown that overexpression of full length BCAR3, but not variants lacking the SH2 or carboxy terminal domain, allowed growth of ZR-75-1 breast cancer cells in the presence of ICI 182,780 in a process that was dependent on PI3K-mediated Rac1 activation (6, 7). Rac1 activation, which is important for lamellipodia and membrane ruffle formation, is often coupled to p130Cas/Crk interactions (17). p130Cas/Crk–dependent Rac1 activation has also been shown to be a potent activator of c-Jun NH2-terminal kinase (38), which can promote cell proliferation and migration (39, 40). Finally, we have shown that phosphorylated p130Cas can bind to the p85 regulatory subunit of PI3K, resulting in enhanced PI3K activity and cell proliferation (41). Numerous studies have shown that PI3K has dual functions as a regulator of both proliferation and cell motility, raising the possibility that p130Cas/PI3K interactions may also contribute to BCAR3-dependent regulation of cell migration (42–44). Collectively, our data suggest that, like PI3K, BCAR3 and p130Cas may have dual functions involving both the promotion of antiestrogen-resistant proliferation as well as increased cell migration. Future studies will explore these functions further in tissue culture, mouse models of tumor growth and metastasis, and in human tumors.
Acknowledgments
Grant support: National Science Foundation MCB-0315927 (A.H. Bouton), Department of Defense Breast Cancer Research Program BC050339 (R.S. Schrecengost), NIH Institutional Cancer Training grant CA009109-32 (R.S. Schrecengost and M.S. Guerrero), and in part CA096846 (A.H. Bouton).
We thank the past and present members of the laboratory and Drs. Timothy Bender, Theresa Guise, Alan R Horwitz, and Ian Macara for helpful suggestions and comments.
Footnotes
R. Riggins and A. Bouton, unpublished data.
References
- 1.Hiscox S, Morgan L, Barrow D, et al. Tamoxifen resistance in breast cancer cells is accompanied by an enhanced motile and invasive phenotype: inhibition by gefitinib (“Iressa”, ZD1839) Clin Exp Metastasis. 2004;21:201–12. doi: 10.1023/b:clin.0000037697.76011.1d. [DOI] [PubMed] [Google Scholar]
- 2.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–58. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 3.Brinkman A, van der Flier S, Kok EM, Dorssers LC. BCAR1, a human homologue of the adapter protein p130Cas, and antiestrogen resistance in breast cancer cells. J Natl Cancer Inst. 2000;92:112–20. doi: 10.1093/jnci/92.2.112. [DOI] [PubMed] [Google Scholar]
- 4.van Agthoven T, van Agthoven TL, Dekker A, et al. Identification of BCAR3 by a random search for genes involved in antiestrogen resistance of human breast cancer cells. EMBO J. 1998;17:2799–808. doi: 10.1093/emboj/17.10.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gotoh T, Cai D, Tian X, Feig LA, Lerner A. p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J Biol Chem. 2000;275:30118–23. doi: 10.1074/jbc.M003074200. [DOI] [PubMed] [Google Scholar]
- 6.Cai D, Iyer A, Felekkis KN, et al. AND-34/BCAR3, a GDP exchange factor whose overexpression confers antiestrogen resistance, activates Rac, PAK1, and the cyclin D1 promoter. Cancer Res. 2003;63:6802–8. [PubMed] [Google Scholar]
- 7.Felekkis KN, Narsimhan RP, Near R, et al. AND-34 activates phosphatidylinositol 3-kinase and induces anti-estrogen resistance in a SH2 and GDP exchange factor-like domain-dependent manner. Mol Cancer Res. 2005;3:32–41. [PubMed] [Google Scholar]
- 8.Riggins RB, Quilliam LA, Bouton AH. Synergistic promotion of c-Src activation and cell migration by Cas and AND-34/BCAR3. J Biol Chem. 2003;278:28264–73. doi: 10.1074/jbc.M303535200. [DOI] [PubMed] [Google Scholar]
- 9.Sakakibara A, Hattori S. Chat, a Cas/HEF1-associated adaptor protein that integrates multiple signaling pathways. J Biol Chem. 2000;275:6404–10. doi: 10.1074/jbc.275.9.6404. [DOI] [PubMed] [Google Scholar]
- 10.Yu Y, Feig LA. Involvement of R-Ras and Ral GTPases in estrogen-independent proliferation of breast cancer cells. Oncogene. 2002;21:7557–68. doi: 10.1038/sj.onc.1205961. [DOI] [PubMed] [Google Scholar]
- 11.Bouton AH, Burnham MR. Detection of distinct pools of the adapter protein p130CAS using a panel of monoclonal antibodies. Hybridoma. 1997;16:403–11. doi: 10.1089/hyb.1997.16.403. [DOI] [PubMed] [Google Scholar]
- 12.Slack JK, Catling AD, Eblen ST, Weber MJ, Parsons JT. c-Raf-mediated inhibition of epidermal growth factor-stimulated cell migration. J Biol Chem. 1999;274:27177–84. doi: 10.1074/jbc.274.38.27177. [DOI] [PubMed] [Google Scholar]
- 13.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83:249–89. doi: 10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 14.Dail M, Kalo MS, Seddon JA, et al. SHEP1 function in cell migration is impaired by a single amino acid mutation that disrupts association with the scaffolding protein Cas but not with Ras GTPases. J Biol Chem. 2004;279:41892–902. doi: 10.1074/jbc.M402929200. [DOI] [PubMed] [Google Scholar]
- 15.Sakai R, Iwamatsu A, Hirano N, et al. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994;13:3748–56. doi: 10.1002/j.1460-2075.1994.tb06684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojaniemi M, Vuori K. Epidermal growth factor modulates tyrosine phosphorylation of p130Cas. Involvement of phosphatidylinositol 3′-kinase and actin cytoskeleton. J Biol Chem. 1997;272:25993–8. doi: 10.1074/jbc.272.41.25993. [DOI] [PubMed] [Google Scholar]
- 17.Klemke RL, Leng J, Molander R, et al. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J Cell Biol. 1998;140:961–72. doi: 10.1083/jcb.140.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101–12. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 19.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–58. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 20.Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–63. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.van der Flier S, Brinkman A, Look MP, et al. BCAR1/ p130Cas protein and primary breast cancer: prognosis and response to tamoxifen treatment. J Natl Cancer Inst. 2000;92:120–7. doi: 10.1093/jnci/92.2.120. [DOI] [PubMed] [Google Scholar]
- 22.van der Flier S, Chan CM, Brinkman A, et al. BCAR1/ p130Cas expression in untreated and acquired tamoxifen-resistant human breast carcinomas. Int J Cancer. 2000;89:465–8. doi: 10.1002/1097-0215(20000920)89:5<465::aid-ijc11>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Brabek J, Constancio SS, Siesser PF, et al. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol Cancer Res. 2005;3:307–15. doi: 10.1158/1541-7786.MCR-05-0015. [DOI] [PubMed] [Google Scholar]
- 24.Fonseca PM, Shin NY, Brabek J, et al. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell Signal. 2004;16:621–9. doi: 10.1016/j.cellsig.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Stupack DG, Cho SY, Klemke RL. Molecular signaling mechanisms of cell migration and invasion. Immunol Res. 2000;21:83–8. doi: 10.1385/IR:21:2-3:83. [DOI] [PubMed] [Google Scholar]
- 26.Kain KH, Gooch S, Klemke RL. Cytoplasmic c-Abl provides a molecular “Rheostat” controlling carcinoma cell survival and invasion. Oncogene. 2003;22:6071–80. doi: 10.1038/sj.onc.1206930. [DOI] [PubMed] [Google Scholar]
- 27.Chodniewicz D, Klemke RL. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim Biophys Acta. 2004;1692:63–76. doi: 10.1016/j.bbamcr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhou B, Liu L, Reddivari M, Zhang XA. The palmitoylation of metastasis suppressor KAI1/CD82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res. 2004;64:7455–63. doi: 10.1158/0008-5472.CAN-04-1574. [DOI] [PubMed] [Google Scholar]
- 29.Cho SY, Klemke RL. Purification of pseudopodia from polarized cells reveals redistribution and activation of Rac through assembly of a CAS/Crk scaffold. J Cell Biol. 2002;156:725–36. doi: 10.1083/jcb.200111032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rufanova VA, Sorokin A. CrkII associates with BCAR3 in response to endothelin-1 in human glomerular mesangial cells. Exp Biol Med (Maywood) 2006;231:752–6. [PubMed] [Google Scholar]
- 31.Sakakibara A, Ohba Y, Kurokawa K, Matsuda M, Hattori S. Novel function of Chat in controlling cell adhesion via Cas-Crk-C3G-pathway-mediated Rap1 activation. J Cell Sci. 2002;115:4915–24. doi: 10.1242/jcs.00207. [DOI] [PubMed] [Google Scholar]
- 32.Bos JL, de Rooij J, Reedquist KA. Rap1 signalling: adhering to new models. Nat Rev Mol Cell Biol. 2001;2:369–77. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 33.Feller SM. Crk family adaptors-signalling complex formation and biological roles. Oncogene. 2001;20:6348–71. doi: 10.1038/sj.onc.1204779. [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Brush J, Stewart TA. NSP1 defines a novel family of adaptor proteins linking integrin and tyrosine kinase receptors to the c-Jun N-terminal kinase/stress-activated protein kinase signaling pathway. J Biol Chem. 1999;274:10047–52. doi: 10.1074/jbc.274.15.10047. [DOI] [PubMed] [Google Scholar]
- 35.Keely PJ, Rusyn EV, Cox AD, Parise LV. R-Ras signals through specific integrin α cytoplasmic domains to promote migration and invasion of breast epithelial cells. J Cell Biol. 1999;145:1077–88. doi: 10.1083/jcb.145.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwong L, Wozniak MA, Collins AS, Wilson SD, Keely PJ. R-Ras promotes focal adhesion formation through focal adhesion kinase and p130Cas by a novel mechanism that differs from integrins. Mol Cell Biol. 2003;23:933–49. doi: 10.1128/MCB.23.3.933-949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Y, Hao Y, Feig LA. The R-Ras GTPase mediates cross talk between estrogen and insulin signaling in breast cancer cells. Mol Cell Biol. 2006;26:6372–80. doi: 10.1128/MCB.00509-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dolfi F, Garcia-Guzman M, Ojaniemi M, et al. The adaptor protein Crk connects multiple cellular stimuli to the JNK signaling pathway. Proc Natl Acad Sci U S A. 1998;95:15394–9. doi: 10.1073/pnas.95.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barr RK, Bogoyevitch MA. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs) Int J Biochem Cell Biol. 2001;33:1047–63. doi: 10.1016/s1357-2725(01)00093-0. [DOI] [PubMed] [Google Scholar]
- 40.Huang C, Rajfur Z, Borchers C, Schaller MD, Jacobson K. JNK phosphorylates paxillin and regulates cell migration. Nature. 2003;424:219–23. doi: 10.1038/nature01745. [DOI] [PubMed] [Google Scholar]
- 41.Riggins RB, DeBerry RM, Toosarvandani MD, Bouton AH. Src-dependent association of Cas and p85 phosphatidylinositol 3′-kinase in v-crk-transformed cells. Mol Cancer Res. 2003;1:428–37. [PubMed] [Google Scholar]
- 42.Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–6. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 43.Roymans D, Slegers H. Phosphatidylinositol 3-kinases in tumor progression. Eur J Biochem. 2001;268:487–98. doi: 10.1046/j.1432-1327.2001.01936.x. [DOI] [PubMed] [Google Scholar]
- 44.Samuels Y, Ericson K. Oncogenic PI3K and its role in cancer. Curr Opin Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]