Abstract
Background
Limited comparative, prospective data exist regarding cardiovascular risk factors in HIV-infected women starting antiretroviral therapy (ART) in Africa.
Methods
In 7 African countries, 741 women with CD4<200 cells/mm3 were randomized to tenofovir/emtricitabine (TDF/FTC) plus either nevirapine (NVP, n=370) or lopinavir/ritonavir (LPV/r, n=371). Lipids and blood pressure (BP) were evaluated at entry, 48, 96, and 144 weeks. Multivariable linear and logistic regression models were used to evaluate mean risk factor changes and clinically relevant risk factor changes.
Results
At entry, both NVP and LPV/r groups were similar regarding age (mean=33.5 [SD=7.1] yrs), CD4 (129 [67] cells/mm3), and HIV-1 RNA (5.1 [0.6] log10 copies/ml). Nearly all women had normal lipids and BP except for HDL. Over 144 weeks, the LPV/r compared to NVP group had significantly greater mean lipid increases (e.g. non-HDL: +29 vs. +13 mg/dL) and smaller HDL increases (+12 vs. +21 mg/dL). In contrast, the NVP compared to LPV/r group had greater mean increases in BP (e.g. diastolic BP: +5 vs. −0.5 mmHg). Significantly more women assigned LPV/r had week 144 “abnormal” lipid levels (e.g. HDL 29.7% vs. 14.8% and triglycerides 28.6% vs. 8.2%), and significantly more women assigned NVP had “abnormal” BP (e.g. diastolic BP 22.7% vs. 6.5%). Most differences remained significant when adjusted for baseline risk factor, age, CD4, and HIV-1 RNA.
Conclusions
In HIV-infected women initiating ART in Africa, LPV/r+TDF/FTC was associated with less favorable changes in lipids, and use of NVP+TDF/FTC was associated with less favorable changes in BP.
Keywords: HIV-1, women, Africa, nevirapine, lopinavir/ritonavir, cardiovascular disease risk factors
INTRODUCTION
Cardiovascular disease (CVD) is an emerging epidemic that is anticipated to lead to marked increases in morbidity and mortality in sub-Saharan Africa [1, 2]. As observed in resource rich countries where non-communicable diseases including CVD have become leading causes of morbidity and mortality in persons living with HIV/AIDS, a similar epidemiological shift is anticipated in sub-Saharan Africa as HIV care and treatment become more widely available [3–5]. While the relationship between HIV infection, antiretroviral therapy (ART), and dyslipidemias has been well described in resource rich settings [6–20], the associations among these factors in sub-Saharan Africa is less well understood, with only limited observational data subject to inherent methodological limitations [21–25].
Long-term, prospective data from resource limited settings evaluating the effect of ART regimen on CVD risk factors are currently unavailable but will be important in optimizing health outcomes in persons receiving ART, the majority of whom reside in resource limited settings. Here, we examine data on several key CVD risk factors (lipids, body mass index [BMI], and blood pressure) following initiation of non-nucleoside reverse transcriptase inhibitor-(nNRTI-) vs. protease inhibitor (PI)-based ART in women from 7 countries in sub-Saharan Africa who participated in A5208/OCTANE (Optimal Combination Therapy After Nevirapine Exposure).
METHODS
Study Design and Participants
The A5208/OCTANE study consisted of two parallel, randomized, open-label ART trials. A5208/OCTANE was approved by all relevant local and U.S. institutional review boards and ethics committees, and all participants provided written informed consent. In Trial 1, 241 women who had previously received single dose nevirapine (sdNVP) ≥6 months before enrollment for prevention of mother-to-child transmission (PMTCT) of HIV were randomized and started study treatment. In Trial 2, 500 women who had no prior NVP exposure were randomized and started study treatment. Details of primary analyses and results from Trial 1 and Trial 2 have been previously published [26, 27]. In brief, participants were HIV-1-infected adult women who were not pregnant or breastfeeding from 10 sites in 7 countries of sub-Saharan Africa (three sites in South Africa; two in Kenya; and one each in Zimbabwe, Botswana, Zambia, Malawi, and Uganda). Participants had screening CD4+ T-cell count <200 cells/mm3 and were ART-naïve. At baseline, women had estimated creatinine clearance ≥80 mL/min (changed to ≥60 mL/min one year into the study). Additionally at baseline, women had absolute neutrophil count (ANC) ≥750/mm3, hemoglobin ≥7.0 g/dL, platelet count ≥50,000/mm3, aspartate transaminase (AST) ≤2.5 × upper limit of normal (ULN), alanine transaminase (ALT) ≤2.5 × ULN, alkaline phosphatase ≤2.5 × ULN, and total bilirubin ≤2.5 × ULN. Women were followed until 48 weeks after the last participant enrolled.
Women were randomized to open-label ART with lopinavir/ritonavir (LPV/r) 400/100mg twice daily or nevirapine (NVP) 200mg twice daily after a 14-day lead-in period with NVP 200mg once daily. Both groups also received tenofovir/emtricitabine (TDF/FTC) 300mg/200mg daily. TDF/FTC was supplied as co-formulated Truvada®, and TDF and FTC were also available separately. LPV/r was initially supplied as Kaletra® capsules but was switched to heat-stable Aluvia® tablets approximately half way through the study. Women discontinuing NVP or LPV/r could switch to LPV/r or NVP, respectively. Women who developed tuberculosis (Tb) requiring rifampin-containing treatment were offered efavirenz for the duration of rifampin-containing Tb treatment and for thirty days after stopping rifampin in place of NVP or LPV/r if they could use appropriate contraception. Thereafter, women returned to NVP or LPV/r assigned prior to Tb treatment.
The primary study endpoint was time to virologic failure or death. A secondary study objective was to determine the tolerability and safety of the study drug regimens.
Data Collection and Cardiovascular Disease Risk Factor Evaluations
HIV-1 RNA and CD4+ T-cell count were evaluated at study entry and every 12 weeks. CVD risk factors evaluated included fasting or non-fasting lipids (total cholesterol [TC], high density lipoprotein-cholesterol [HDL], calculated or measured low density lipoprotein-cholesterol [LDL], and triglycerides [TG]), systolic and diastolic blood pressures (SBP and DBP), and body mass index (BMI). For results presented here, non-HDL cholesterol [non-HDL] was calculated (non-HDL = TC-HDL). Lipids were evaluated at entry and every 48 weeks. Glucose measurements were obtained as part of metabolic panels evaluated throughout the study. Additional risk factor evaluation for the metabolic syndrome was not possible given data was not collected (i.e. waist circumference) or absent in follow-up (i.e. fasting glucose).
All laboratories participated in external quality assurance (EQA) programs. Blood pressure measurements were taken at scheduled visits according to standard practices at each site. Height and weight were measured at time of ART initiation, and weight was measured on scheduled visits with BMI calculated.
Established guidelines [28–30] were used in establishing clinically relevant (i.e. “normal” or “desirable” vs. “abnormal” or warranting intervention) categories for CVD risk factors as follows: TC: <200 vs. ≥200 mg/dL; LDL: <130 vs. ≥130 mg/dL; HDL ≥40 vs. <40 mg/dL; non HDL <160 vs. ≥160 mg/dL; TG <150 vs. ≥150 mg/dL; SBP <140 vs. ≥140 mmHg; DBP <90 vs. ≥90 mmHg; and BMI <25 vs. ≥ 25 kg/m2.
Statistical Analysis
Data for this analysis were restricted to the period during which women remained on NVP or LPV/r (as initially randomized). The period ended when study-provided NVP or LPV/r, as initially randomized, was permanently discontinued or when a temporary discontinuation of >30 days started. Changes in NRTIs during this period were allowed. A linear regression model was used to estimate unadjusted and adjusted mean changes (95% confidence interval [CI]) 48, 96, and 144 weeks after ART initiation for women remaining on initial randomized ART. Logistic regression models were used to evaluate the relationship between treatment (LPV/r or NVP) and unadjusted and adjusted odds of abnormal CVD risk factors. SAS version 9.2 (Cary, NC) was used for statistical analysis.
RESULTS
Enrollment and Cardiovascular Risk Factor Follow-up
A total of 741 women were randomized and started study treatment: 370 to NVP-based ART, and 371 to LPV/r-based ART. Median follow-up on initial randomized treatment was 104 weeks (90 weeks for NVP and 115 weeks for LPV/r). Women were followed until 48 weeks after the last woman was randomized. Therefore, not all women completed 96 or 144 weeks of follow-up to allow CVD risk factor evaluations (Table 1). The most common reason women were not available for follow-up evaluation was study completion. Other reasons for unavailability included follow-up events (e.g. adverse event, lost to follow-up, DSMB recommended termination of randomized treatment), treatment requiring ART change (e.g. Tb treatment), and reaching study endpoint. No participants stopped initial assigned treatment due to occurrence of a CVD risk factor.
Table 1.
Number of Participants with Evaluations While on Initial Treatment and Reasons for Previous Discontinuation of Initial Treatment
| Week | Reason Category | NVP-based ART (n=370) No. (%) |
LPV/RTV-based ART n=(371) No. (%) |
|---|---|---|---|
| 48 | Evaluation Available1 | 273 (73.8) | 330 (88.9) |
| Adverse Events | 46 (12.4) | 6 (1.6) | |
| Clinical Events | 2 (0.5) | 2 (0.5) | |
| Patient-Related Issues/LFU | 12 (3.2) | 12 (3.2) | |
| TB Treatment | 8 (2.2) | 16 (4.3) | |
| Virologic Failure | 21 (5.7) | 1 (0.3) | |
| After DSMB Recommendation3 | 1 (0.3) | 0 (0) | |
| Death | 7 (1.9) | 4 (1.1) | |
| 96 | Evaluation Available1 | 182 (49.2) | 243 (65.5) |
| Completed Study Treatment | 62 (16.8) | 75 (20.2) | |
| Adverse Events | 51 (13.8) | 9 (2.4) | |
| Clinical Events | 2 (0.5) | 2 (0.5) | |
| Patient-Related Issues/LFU | 19 (5.1) | 15 (4.0) | |
| TB Treatment | 12 (3.2) | 17 (4.6) | |
| Virologic Failure | 30 (8.1) | 5 (1.3) | |
| After DSMB Recommendation3 | 5 (1.4) | 0 (0) | |
| Death | 7 (1.9) | 5 (1.3) | |
| 144 | Evaluation Available1 | 75 (20.3) | 108 (29.1) |
| Completed Study Treatment | 149 (40.3) | 201 (54.2) | |
| Adverse Events | 52 (14.1) | 12 (3.2) | |
| Clinical Events | 2 (0.5) | 2 (0.5) | |
| Patient-Related Issues/LFU | 25 (6.8) | 19 (5.1) | |
| TB Treatment | 13 (3.5) | 18 (4.9) | |
| Virologic Failure | 35 (9.5) | 6 (1.6) | |
| After DSMB Recommendation3 | 12 (3.2) | 0 (0) | |
| Death | 7 (1.9) | 5 (1.3) |
Evaluation Available: Study participant completed visit and had at least one evaluation
Abbreviations: NVP, Nevirapine; LPV/RTV, Lopinavir/ritonavir; ART, antiretroviral therapy; LFU, lost to follow-up; TB, tuberculosis; DSMB, Data and Safety Monitoring Board
DSMB recommended release of results in Trial 1 showing a higher virologic failure rate among recipients of NVP than LPV/r.
Baseline Characteristics
At entry (pre-ART), both NVP and LPV/r groups were similar with regard to age, CD4+ T-cell count, and HIV-1 RNA with overall mean (±standard deviation) values of 33.5 (±7.1) years, 129 (±67) cells/mm3 and 5.1 (±0.6) log10 copies/mL, respectively (Table 2). Mean lipid CVD risk factors (TC, HDL, non-HDL, LDL, and TG) were also similar. Nearly all women had TC <200 mg/dL (97%), non-HDL <160 mg/dL (98%), LDL <130 mg/dL (97%), and TG <150 mg/dL (87%). Only about one third of women had HDL ≥40 mg/dL (29%). SBP, DBP, and BMI were also similar at enrollment in the NVP and LPV/r groups. Nearly all women had blood pressure <140/90 mmHg (91%), and most had BMI ≥16.5 and <25 kg/m2 (69%) with few (2%) having BMI < 16.5 kg/m2, meeting criteria for being malnourished.
Table 2.
Baseline Characteristics of Women Receiving Nevirapine- and Lopinavir/ritonavir-Based Antiretroviral Therapy
| NVP-based ART (n=370) | LPV/RTV-based ART (n=371) | Total (n=741) | ||
|---|---|---|---|---|
| Age at randomization (years) | Mean±SD (No.) | 33.5±7.0 (370) | 33.4±7.2 (371) | 33.5±7.1 (741) |
| CD4 count (cells/mm3) | Mean±SD (No.) | 130±66 (370) | 128±68 (371) | 129±67 (741) |
| HIV-1 RNA (log10 copies/mL) | Mean±SD (No.) | 5.1±0.6 (370) | 5.1±0.6 (371) | 5.1±0.6 (741) |
| Total cholesterol (mg/dL) | Mean±SD (No.) | 133±32 (361) | 130±31 (368) | 132±32 (729) |
| <200 (mg/dL), No. (%) | 353 (98) | 357 (97) | 710 (97) | |
| HDL (mg/dL) | Mean±SD (No.) | 34±15 (356) | 34±13 (363) | 34±14 (719) |
| >=40 (mg/dL), No. (%) | 104 (29) | 107 (29) | 211 (29) | |
| Non-HDL (mg/dL) | Mean±SD (No.) | 99±28 (356) | 96±28 (363) | 98±28 (719) |
| <160 (mg/dL), No. (%) | 364 (98) | 361 (97) | 725 (98) | |
| LDL (mg/dL) | Mean±SD (No.) | 81±27 (356) | 80±27 (366) | 81±27 (722) |
| <130 (mg/dL), No. (%) | 346 (97) | 352 (96) | 698 (97) | |
| Triglycerides (mg/dL) | Mean±SD (No.) | 103±61 (361) | 97±51 (367) | 100±56 (728) |
| <150 (mg/dL), No. (%) | 311 (86) | 323 (88) | 634 (87) | |
| Blood pressure-systolic (mmHg) | Mean±SD (No.) | 112±16 (370) | 112±15 (371) | 112±16 (741) |
| <140 (mmHg), No. (%) | 346 (94) | 358 (96) | 704 (95) | |
| Blood pressure-diastolic (mmHg) | Mean±SD (No.) | 73±12 (370) | 73±11 (371) | 73±11 (741) |
| <90 (mmHg), No. (%) | 337 (91) | 346 (93) | 683 (92) | |
| Blood pressure (mmHg) | <140/90 (mmHg), No. (%) | 331 (89) | 342 (92) | 673 (91) |
| Body mass index (kg/m2) | Mean±SD (No.) | 23.7±4.9 (370) | 23.5±4.7 (371) | 23.6±4.8 (741) |
| <16.5 (kg/m2), No. (%) | 6 (2) | 6 (2) | 12 (2) | |
| >=16.5, <25 (kg/m2), No. (%) | 257 (69) | 251 (68) | 508 (69) | |
| >=25, <30 (kg/m2), No. (%) | 63 (17) | 78 (21) | 141 (19) | |
| >30 (kg/m2), No. (%) | 44 (12) | 36 (10) | 80 (11) | |
| Glucose (mg/dL) | Mean±SD (No.) | 82±21 (357) | 84±18 (366) | 83±20 (723) |
| <110 (mg/dL), No. (%) | 335 (94) | 338 (92) | 673 (93) |
Abbreviations: NVP, Nevirapine; LPV/RTV, Lopinavir/ritonavir; ART, antiretroviral therapy; SD, standard deviation; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Among those with fasting evaluations (63 women in each arm; all from two sites in South Africa and two sites in Kenya), most (98% in both groups) had glucose <110 mg/dL and very few (1% in both groups) had glucose ≥126 mg/dL. Similarly, the majority of subjects in the NVP and LPV/r groups had LDL <130 mg/dL (97% and 92%) and TG <150 mg/dL (94% and 89%), respectively.
Associations Between Baseline CVD Risk Factors and CD4+ T-cell Count, HIV-1 RNA, and Age
In univariate analyses, there were significant associations at baseline between higher HIV-1 RNA and lower levels of TC, HDL, LDL, BMI and SBP, but not non-HDL or DBP (Table 3). Lower baseline CD4 count was significantly associated with lower BMI and SBP. There were also significant associations between higher HIV-1 RNA and higher triglycerides, and between lower CD4 count and higher triglycerides. Except for triglycerides and HDL, all CVD risk factors were significantly worsened among older women. In multivariate analysis that evaluated the association between each CVD risk factor and HIV-1 RNA, CD4 count and age jointly, except for a weaker association between both SBP and BMI with CD4 count, all of the associations between CVD risk factors and HIV-1 RNA, CD4 count and age that were significant in univariate analysis remained significant in multivariate analysis.
Table 3.
Association Between Baseline Age, CD4 Count, HIV-1 RNA and Cardiovascular Risk Factors1
| Cardiovascular Risk Factor | N | Age (per 10 years) | CD4 count(per 100 cells/mm3) | HIV-1 RNA (per 1 log10 copies/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted1 | Unadjusted | Adjusted1 | Unadjusted | Adjusted | ||||||||
| EST±SE | P | EST±SE | P | EST±SE | P | EST±SE | P | EST±SE | P | EST±SE | P | ||
| Total cholesterol (mg/dL) | 729 | 7.4±1.6 | <.001 | 7.3±1.6 | <.001 | 0.9±1.8 | 0.606 | −0.5±1.8 | 0.795 | −6.8±1.9 | <.001 | −6.7±1.9 | <.001 |
| HDL (mg/dL) | 719 | 2.3±0.7 | 0.002 | 2.2±0.7 | 0.002 | 0.5±0.8 | 0.535 | −0.5±0.8 | 0.485 | −4.7±0.8 | <.001 | −4.8±0.8 | <.001 |
| Non-HDL (mg/dL) | 719 | 5.1±1.5 | <.001 | 5.0±1.5 | <.001 | 0.8±1.6 | 0.631 | 0.4±1.6 | 0.815 | −2.2±1.7 | 0.184 | −2.1±1.7 | 0.232 |
| LDL (mg/dL) | 722 | 5.1±1.4 | <.001 | 5.1±1.4 | <.001 | 2.9±1.5 | 0.057 | 1.7±1.5 | 0.271 | −6.3±1.6 | <.001 | −5.8±1.6 | <.001 |
| Triglycerides (mg/dL) | 728 | 3.0±2.9 | 0.303 | 3.1±2.9 | 0.289 | −10.6±3.1 | <.001 | −8.1±3.2 | 0.011 | 13.2±3.3 | <.001 | 11.2±3.4 | 0.001 |
| Body mass index (kg/m2) | 741 | 0.9±0.2 | <.001 | 0.9±0.2 | <.001 | 0.7±0.3 | 0.008 | 0.5±0.3 | 0.061 | −1.0±0.3 | <.001 | −0.9±0.3 | 0.002 |
| Blood pressure-systolic (mmHg) | 741 | 4.6±0.8 | <.001 | 4.6±0.8 | <.001 | 2.2±0.9 | 0.011 | 1.7±0.9 | 0.050 | −2.9±0.9 | 0.002 | −2.4±0.9 | 0.011 |
| Blood pressure-diastolic (mmHg) | 741 | 2.9±0.6 | <.001 | 2.9±0.6 | <.001 | 0.5±0.6 | 0.456 | 0.4±0.6 | 0.477 | −0.3±0.7 | 0.608 | −0.2±0.7 | 0.807 |
Associations were evaluated using linear regression. Adjusted results are from a model which included age, CD4 count and log10 HIV-1 RNA at baseline as continuous covariates. P-values are from Wald tests.
Abbreviations: NVP, Nevirapine; LPV/RTV, Lopinavir/ritonavir; ART, antiretroviral therapy; EST, effect estimate(for the mean cardiovascular risk factor level per change in covariate indicated in the column heading); SE, standard error (of effect estimate); HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Mean Changes in CVD Risk Factors Over Time
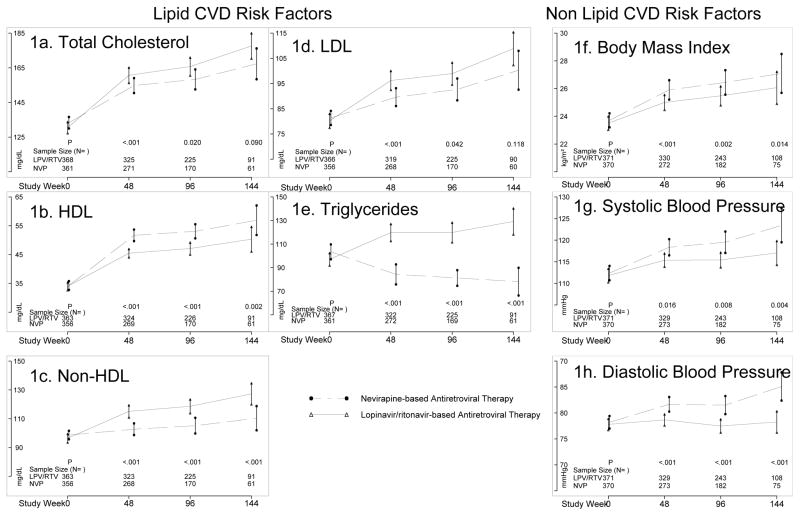
Overall, significant mean increases in all CVD risk factor values were observed for both NVP and LPV/r groups over 144 weeks of follow-up with the exception of TG which decreased significantly in the NVP group (Figure 1 and Table 4). Greater mean increases in TC in the LPV/r vs. NVP groups were observed throughout follow-up (e.g., 41mg/dL for LPV/r vs. 34 mg/dL for NVP at week 144). Similarly, greater mean increase in LDL was observed in the LPV/r compared to NVP group at week 48 but the differences were attenuated at weeks 96 and 144 and not significant. Mean increases in HDL were significantly less in the LVP/r group compared to the NVP group at all follow-up time points (12 mg/dL for LPV/r vs. 21 mg/dL at week 144). Likewise, non-HDL levels were higher in the LPV/r group throughout follow-up (29 mg/dL for LPV/r vs. 13 mg/dL for NVP at week 144). While a mean decrease in TG (−21 mg/dL) was observed in the NVP group at week 144, an increase was observed in the LPV/r group (30 mg/dL).
Figure 1.
Mean Cardiovascular Risk Factor Levels Over Time Among Women Receiving Nevirapine- and Lopinavir/Ritonavir-Based Antiretroviral Therapy
Figures A-H represent changes in lipid (A-E) and non-lipid (F-H) cardiovascular risk factors over time by Nevirapine (NVP)- and Lopinavir/ritonavir (LPV/RTV)-based ART regimens.
1Bars are 95% confidence intervals for the mean
2P-values are for comparison of treatments adjusted for age, CD4 count, log10 HIV-1 RNA and cardiovascular risk factor level at baseline
3Abbreviations: NVP, Nevirapine; LPV/RTV, Lopinavir/ritonavir; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 4.
Mean Changes from Baseline in Cardiovascular Risk Factors Among Women Receiving Nevirapine- and Lopinavir/ritonavir-Based Antiretroviral Therapy1
| Cardiovascular Risk Factor | week | NVP-based ART | LPV/RTV-based ART | Difference in Means | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | Mean±SE | No. | Mean±SE | Unadjusted (95% CI) | P | Adjusted (95% CI) | P | ||
| Total cholesterol (mg/dL) | 48 | 262 | 19.1±1.8 | 322 | 29.4±1.8 | −10.3 (−15.4, −5.3) | <.001 | −9.2 (−14.1, −4.3) | <.001 |
| 96 | 164 | 25.8±2.7 | 223 | 33.4±2.1 | −7.5 (−14.2, −0.9) | 0.027 | −7.6 (−14.0, −1.2) | 0.020 | |
| 144 | 57 | 34.1±4.0 | 90 | 41.1±3.2 | −7.1 (−17.1, 3.0) | 0.169 | −8.3 (−17.9, 1.3) | 0.090 | |
| HDL (mg/dL) | 48 | 257 | 16.8±1.0 | 318 | 11.0±0.7 | 5.7 (3.4, 8.1) | <.001 | 5.8 (3.7, 7.9) | <.001 |
| 96 | 164 | 18.5±1.3 | 224 | 12.9±0.9 | 5.7 (2.7, 8.7) | <.001 | 5.5 (2.7, 8.2) | <.001 | |
| 144 | 57 | 21.0±2.6 | 89 | 12.2±1.7 | 8.8 (3.0, 14.6) | 0.003 | 8.5 (3.2, 13.8) | 0.002 | |
| Non-HDL(mg/dL) | 48 | 256 | 2.3±1.8 | 317 | 18.2±1.7 | −15.9 (−20.8, −11.0) | <.001 | −15.0 (−19.9, −10.2) | <.001 |
| 96 | 164 | 7.3±2.6 | 223 | 20.5±1.9 | −13.2 (−19.5, −6.9) | <.001 | −13.1 (−19.3, −7.0) | <.001 | |
| 144 | 57 | 13.1±3.9 | 89 | 29.4±2.9 | −16.3 (−25.7, −6.8) | <.001 | −17.1 (−26.3, −8.0) | <.001 | |
| LDL (mg/dL) | 48 | 257 | 5.9±1.5 | 315 | 15.1±1.5 | −9.2 (−13.4, −5.0) | <.001 | −8.6 (−12.7, −4.5) | <.001 |
| 96 | 164 | 10.6±2.1 | 223 | 15.9±1.8 | −5.3 (−10.7, 0.1) | 0.054 | −5.4 (−10.5, −0.2) | 0.042 | |
| 144 | 56 | 16.5±3.7 | 89 | 23.2±2.7 | −6.8 (−15.7, 2.2) | 0.136 | −6.8 (−15.5, 1.8) | 0.118 | |
| Triglycerides (mg/dL) | 48 | 263 | −17.8±4.5 | 318 | 22.2±3.9 | −40.1 (−51.8, −28.3) | <.001 | −37.2 (−47.8, −26.6) | <.001 |
| 96 | 163 | −17.4±4.0 | 223 | 21.9±4.2 | −39.3 (−51.1, −27.5) | <.001 | −39.3 (−49.5, −29.1) | <.001 | |
| 144 | 57 | −20.6±8.3 | 90 | 30.1±6.3 | −50.7 (−71.0, −30.4) | <.001 | −50.4 (−65.8, −35.0) | <.001 | |
| Body mass index (kg/m2) | 48 | 272 | 2.0±0.2 | 330 | 1.3±0.1 | 0.7 (0.3, 1.1) | <.001 | 0.7 (0.3, 1.0) | <.001 |
| 96 | 182 | 2.6±0.2 | 243 | 1.8±0.2 | 0.9 (0.3, 1.4) | 0.002 | 0.9 (0.3, 1.5) | 0.002 | |
| 144 | 75 | 3.3±0.4 | 108 | 2.1±0.3 | 1.2 (0.2, 2.1) | 0.018 | 1.2 (0.2, 2.1) | 0.014 | |
| Blood pressure-systolic(mmHg) | 48 | 273 | 5.0±0.9 | 329 | 3.0±0.8 | 2.0 (−0.3, 4.4) | 0.087 | 2.5 (0.5, 4.5) | 0.016 |
| 96 | 182 | 6.1±1.2 | 243 | 3.3±0.9 | 2.8 (−0.1, 5.8) | 0.059 | 3.5 (0.9, 6.1) | 0.008 | |
| 144 | 75 | 7.0±1.7 | 108 | 2.3±1.6 | 4.8 (0.1, 9.4) | 0.044 | 5.7 (1.8, 9.7) | 0.004 | |
| Blood pressure-diastolic(mmHg) | 48 | 273 | 2.9±0.7 | 329 | 0.4±0.6 | 2.5 (0.8, 4.2) | 0.005 | 2.8 (1.3, 4.2) | <.001 |
| 96 | 182 | 3.6±0.9 | 243 | 0.0±0.7 | 3.6 (1.5, 5.8) | <.001 | 3.8 (2.0, 5.7) | <.001 | |
| 144 | 75 | 5.3±1.3 | 108 | −0.5±1.0 | 5.8 (2.6, 9.0) | <.001 | 6.2 (3.4, 9.1) | <.001 | |
Differences in means were evaluated using linear regression. Adjusted results are with adjustment for age, CD4 count, log10 HIV-1 RNA and cardiovascular risk factor at baseline included as continuous covariates. P-values are from Wald tests.
Abbreviations: NVP, Nevirapine; LPV/RTV, Lopinavir/ritonavir; ART, antiretroviral therapy; SE, standard error (of the estimated mean); CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
In considering non-lipid CVD risk factors, week 144 mean changes were significantly higher in the NVP vs. LPV/r group for both SBP (7.0 mmHg vs. 2.3 mmHg) and DBP (5.3mmHg vs. −0.5 mmHg). Finally, significantly larger mean increases in BMI were observed in the NVP (3.3 kg/m2) compared to the LPV/r (2.1 kg/m2) group. For those CVD risk factors with significantly different mean changes according to treatment group (above), the differences remained significant after adjusting for baseline risk factor, age, CD4+ T-cell count, and HIV-1 RNA. Furthermore, the differences between treatments in change in DBP and SBP persisted when also adjusted for change in BMI during follow-up
Categorical CVD Risk Factors Over Time
In an attempt to assess the clinical relevance of the mean quantitative changes in risk factor levels observed, we examined changes in the proportion of women with CVD risk factors that exceeded “normal” values or thresholds for intervention. The LPV/r group had less favorable changes in the proportion of women with abnormal lipid levels, with significant differences from the NVP group for HDL and TG at all follow-up weeks, and for non-HDL and LDL at week 48 but not at later weeks (in part due to the smaller sample size) (Table 5). In multivariate analyses, treatment group was significantly associated with the differences observed in HDL and TG at all follow-up visits. Additionally, treatment group was significant for TC, non-HDL, and LDL at week 48 but not later weeks, again likely in part due to smaller number of women at later follow-ups.
Table 5.
Proportion of Women Having Abnormal1 Cardiovascular Risk Factor Levels Among Women Receiving Nevirapine- or Lopinavir/ritonavir-Based Antiretroviral Therapy
| Cardiovascular Risk Factor | Week | NVP-based ART | LPV/RTV-based ART | Odds Ratio2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. | No. (%) Abnormal | No. | No. (%) Abnormal | Unadjusted (95% CI) | P | Adjusted (95% CI) | P | ||
| Total cholesterol (mg/dL) | 48 | 271 | 27(10.0) | 325 | 47(14.5) | 0.65(0.40,1.08) | 0.099 | 0.44(0.24,0.80) | 0.008 |
| 96 | 170 | 21(12.4) | 225 | 40(17.8) | 0.65(0.37,1.15) | 0.142 | 0.63(0.33,1.22) | 0.174 | |
| 144 | 61 | 12(19.7) | 91 | 23(25.3) | 0.72(0.33,1.59) | 0.422 | 0.88(0.36,2.15) | 0.779 | |
| HDL (mg/dL) | 48 | 269 | 65(24.2) | 324 | 113(34.9) | 0.60(0.41,0.85) | 0.005 | 0.51(0.34,0.78) | 0.002 |
| 96 | 170 | 30(17.6) | 226 | 72(31.9) | 0.46(0.28,0.74) | 0.002 | 0.42(0.25,0.72) | 0.001 | |
| 144 | 61 | 9(14.8) | 91 | 27(29.7) | 0.41(0.18,0.95) | 0.037 | 0.31(0.12,0.81) | 0.017 | |
| Non-HDL(mg/dL) | 48 | 268 | 13(4.9) | 323 | 35(10.8) | 0.42(0.22,0.81) | 0.010 | 0.32(0.15,0.67) | 0.003 |
| 96 | 170 | 10(5.9) | 225 | 25(11.1) | 0.50(0.23,1.07) | 0.075 | 0.51(0.22,1.21) | 0.129 | |
| 144 | 61 | 4(6.6) | 91 | 14(15.4) | 0.39(0.12,1.23) | 0.109 | 0.39(0.10,1.54) | 0.180 | |
| LDL (mg/dL) | 48 | 268 | 24(9.0) | 319 | 49(15.4) | 0.54(0.32,0.91) | 0.020 | 0.43(0.24,0.78) | 0.006 |
| 96 | 170 | 15(8.8) | 225 | 38(16.9) | 0.48(0.25,0.90) | 0.022 | 0.49(0.24,1.00) | 0.050 | |
| 144 | 60 | 10(16.7) | 90 | 20(22.2) | 0.70(0.30,1.62) | 0.406 | 0.92(0.34,2.49) | 0.874 | |
| Triglycerides (mg/dL) | 48 | 272 | 16(5.9) | 322 | 78(24.2) | 0.20(0.11,0.34) | <.001 | 0.16(0.09,0.30) | <.001 |
| 96 | 169 | 15(8.9) | 225 | 51(22.7) | 0.33(0.18,0.61) | <.001 | 0.26(0.13,0.52) | <.001 | |
| 144 | 61 | 5(8.2) | 91 | 26(28.6) | 0.22(0.08,0.62) | 0.004 | 0.17(0.05,0.54) | 0.003 | |
| Body mass index (kg/m2) | 48 | 272 | 122(44.9) | 330 | 152(46.1) | 0.95(0.69,1.31) | 0.767 | 0.92(0.56,1.51) | 0.730 |
| 96 | 182 | 87(47.8) | 243 | 114(46.9) | 1.04(0.71,1.52) | 0.856 | 1.23(0.72,2.12) | 0.444 | |
| 144 | 75 | 37(49.3) | 108 | 54(50.0) | 0.97(0.54,1.76) | 0.929 | 1.23(0.53,2.86) | 0.629 | |
| Blood pressure-systolic(mmHg) | 48 | 273 | 29(10.6) | 329 | 24(7.3) | 1.51(0.86,2.66) | 0.154 | 1.45(0.78,2.72) | 0.242 |
| 96 | 182 | 22(12.1) | 243 | 13(5.3) | 2.43(1.19,4.97) | 0.015 | 2.41(1.14,5.13) | 0.022 | |
| 144 | 75 | 10(13.3) | 108 | 6(5.6) | 2.62(0.91,7.54) | 0.075 | 2.93(0.88,9.73) | 0.079 | |
| Blood pressure-diastolic(mmHg) | 48 | 273 | 42(15.4) | 329 | 28(8.5) | 1.95(1.18,3.25) | 0.010 | 2.09(1.18,3.71) | 0.012 |
| 96 | 182 | 26(14.3) | 243 | 16(6.6) | 2.36(1.23,4.55) | 0.010 | 2.37(1.18,4.73) | 0.015 | |
| 144 | 75 | 17(22.7) | 108 | 7(6.5) | 4.23(1.66,10.80) | 0.003 | 4.94(1.81,13.50) | 0.002 | |
Abnormal Level for Cardiovascular Risk Factors: Total Cholesterol>=200 mg/dL; HDL<40 mg/dL; non-HDL >=160 mg/dL; LDL >=130 mg/dL; Triglycerides>=150 mg/dL; Body mass index >=25 kg/m2; Blood pressure-systolic >=140 mm/Hg; Blood pressure – diastolic >=90 mm/Hg.
Odds ratios are for having an abnormal level for the cardiovascular risk factor for NVP-based ART versus LPV/r-based ART. Odds ratios were evaluated using logistic regression. Adjusted results are with adjustment for age, CD4 count, log10 HIV-1 RNA and cardiovascular risk factor level at baseline included as continuous covariates. P-values are from Wald tests.
Abbreviations: NVP, Nevirapine; LPV/RTV, Lopinavir/ritonavir; ART, antiretroviral therapy; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Significantly greater proportions of women in the NVP compared to LPV/r group surpassed “normal” DBP thresholds with the greatest difference (22.7% vs. 6.5%, respectively) at week 144. Differences in proportion of women surpassing normal SBP thresholds were smaller than for DBP and were significant only at week 96. In multivariate analyses, differences between treatments in these proportions remained significant for DBP at all follow-up weeks as well as for SBP at week 96. Treatment group was not associated with differences in the proportion of women with BMI ≥25 kg/m2 at any visit.
DISCUSSION
In our analysis of a group of relatively young women from 7 countries in sub-Saharan Africa with advanced HIV-1 disease, nearly all women had normal TC, LDL, non-HDL, TG, SBP, and DBP, and glucose at time of ART initiation. The majority of women had low HDL consistent with their advanced HIV disease. Decreases in HDL during the course of untreated HIV infection have been well described in resource-rich settings and appear to be related to HIV disease stage and activity as evidenced by the associations between low HDL cholesterol and higher HIV RNA levels and lower CD4 cell counts [6, 31–33]. While we observed a strong inverse relationship between HIV-1 RNA and HDL at baseline alone and after adjusting for age and CD4+ T-cell count, we found no significant relationship between CD4+ T-cell count and HDL at time of ART initiation in this group who all had relatively low CD4+ T-cell counts.
The baseline CVD risk factors we observed are consistent with other limited data from smaller and/or retrospective studies in sub-Saharan Africa [21, 23]. We found mean increases in all CVD risk factors at nearly all follow-up visits with the exception of TGs, which decreased in the NVP group by 48 weeks and remained below the value at time of ART initiation thereafter. Decreases in TGs have been described in patients with advanced HIV who received zidovudine monotherapy early in the epidemic and more recently in patients on long-term NVP-based ART [6, 34, 35]. However, NVP-based ART has also been associated with increases in TGs, although to a lesser extent compared with efavirenz (EFV) [36, 37]. We observed an initial decrease in mean TG that remained low through 144 weeks at the same time TGs increased in LPV/r-based ART. While such a decrease may be associated with viral suppression, high rates of viral suppression were observed in both groups throughout the study and, if due to viral suppression alone, decreases in TGs would have been expected in both groups [26].
In contrast to the expected, less favorable changes in lipid values observed with LPV/r ART, we observed an unexpected, significantly greater increase in both SBP and DBP throughout follow-up in the NVP ART group. These increases in blood pressure persisted after adjusting for baseline blood pressure, age, CD4+ T-cell count, and HIV-1 RNA. Maggi and colleagues observed in a limited retrospective study of NVP-based ART a significant increase in hypertension at 5 years of follow-up, which was not observed in those receiving EFV [35]. Of note, no significant changes in pathology were observed in color-Doppler ultrasonography of the peri-aortic vasculature in the NVP group in that report [35]. This is the first time, to our knowledge, that continuous elevations in SBP and DBP have been described in women taking NVP-based ART in sub-Saharan Africa, with elevations being greater in NVP-based ART compared to LPV/r-based ART.
The changes in lipid values we observed may represent in part a return to pre-seroconversion levels as described in a subgroup of male HIV seroconverters of the US Multicenter AIDS Cohort Study.[38] Given the well described decreases in lipid values associated with HIV seroconversion, such a scenario seems possible here. A parallel scenario for the blood pressure changes we observed, however, may be less likely. It will be important to evaluate other prospective clinical trial data in sub-Saharan Africa to see if treatment-associated blood pressure changes are seen and mechanistic hypotheses may be generated.
The short and long-term clinical significance of changes in CVD risk factors are important to consider as life expectancies increase with HIV care and treatment more widely available in sub-Saharan Africa. Within the 144 week follow-up period, significantly more women in the LPV/r groups had clinically relevant dyslipidemia evidenced by changes in/worsening of TC, HDL, non-HDL, LDL, and TG. Likewise, more women in the NVP-based ART group developed hypertension. Treatment regimen (LPV/r vs. NVP) was found to be associated with lipid and blood pressure categorical changes, with SBP being significant at week 96 but not at week 144, possibly due to attenuated sample size. While BMI changes (mean and categorical) varied between groups and throughout follow-up, differences between treatments in mean or categorical changes in blood pressure persisted after adjusting for BMI changes (data not shown).
The primary strengths of our analysis are the long-term follow-up and relatively large number of women where comparison of randomized nNRTI (NVP) and PI (LPV/r)-based ART regimens may be evaluated concurrently. The most important limitation to consider is the fact that only a small proportion of women were fasting at baseline and during follow-up. We note, however, that the changes in lipids when considering only TC, HDL and, non-HDL (which may be interpreted in the non-fasting state) are significant [39]. Furthermore, studies have recognized the role of non-fasting TGs in predicting CVD [, 40, 41]. Blood pressure measurements were obtained at each site according to local practices and non-standardized across the entire study, and our findings are based upon one blood pressure reading, which may overestimate the prevalence of hypertension [29]. Such differences, however, were likely distributed equally between the 2 treatment groups by randomization. The numbers of women participating in the later visits (96 and 144 weeks) dropped off as they changed therapies and exited the study. This limited our ability to detect differences between the two groups.
Last and importantly, our analysis provides detailed information regarding CVD risk factors in young women with advanced HIV starting first line ART in 7 countries of sub-Saharan Africa. It is important to note we cannot draw conclusions on the clinical relevance of the observed changes in CVD risk factors and that our results may not be generalizable to other populations.
In conclusion, we found LPV/r-based ART to be associated with less favorable changes in lipids and NVP-based ART unexpectedly associated in less favorable changes in blood pressure in young women with advanced HIV initiating ART in 7 countries of sub-Saharan Africa. These changes resulted in clinically relevant changes in lipid and blood pressure CVD risk factors during up to 144 weeks of follow-up in a proportion of women. The long term significance of these changes in CVD risk factors remains uncertain. It is appreciated that these CVD risk factor changes are occurring during a period of “urbanization” (including increasing presence of lipid rich diets) and in a setting where dyslipidemia therapies have notable ART drug interactions and/or are often unavailable. Therefore, attention to these CVD risk factors and overall risk factor modification will play an increasing role in mitigating CVD-related morbidity and mortality in persons living with HIV/AIDS in sub-Saharan Africa.
Acknowledgments
Funding/Sources of support: This project was supported in part by grants (U01AI068636, AI38838, and Statistical and Data Management Center AI68634) from the National Institute of Allergy and Infectious Diseases to the AIDS Clinical Trials Group. It also was supported in part by the General Clinical Research Center Units funded by the National Center for Research. Drs. Shaffer and Sawe as part of the US Military HIV Research Unit Clinical Trials Unit are supported through the a National Institute of Allergy and Infectious Diseases-US Army Medical Research and Material Command IAA (#IAAY1AI8374). Dr. Moses is supported through the UNC Project, Kamuzu Central Hospital; Lilongwe (Site 12001) CTU Grant #5 U01 AI069518. Dr. Currier is supported in part by K24 AI56933. These funding bodies played no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The following companies donated study drug: Gilead (tenofovir/emtricitabine), Boehringer-Ingelheim (nevirapine), Abbott (lopinavir/ritonavir), GlaxoSmithKline (zidovudine), Bristol-Myers Squibb (didanosine). Pharmaceutical supporters provided study product but did not fund any other aspect of study conduct nor data analysis. Pharmaceutical supporters also participated as protocol team members and provided feedback (which the authors could include or exclude at their discretion) on the manuscript, but did not participate in writing the manuscript, in analyzing the data, nor in the decision to publish the manuscript.
Abbott Laboratories: William C. Woodward
Boehringer Ingelheim Pharmaceuticals, Inc.
Gilead: Audrey L. Shaw, Marianne Poblenz, Sabina Pfister, Farideh Said, and Howard Jaffe
Bristol-Myers Squibb: Awny Farajallah, Kristy Grimm
GlaxoSmithKline: Navdeep Thoofer, Wendy Snowden
The authors would like to acknowledge the following for the contributions to the OCTANE study and data for this manuscript: Maureen Mohata, Lerato Mohapi, Noah Tarus, Hellen Ngeno, Margret Chibowa, Elizabeth Stringer, James Tutko, Ann Walawander, Agnes Nzioka, Kipruto Kirwa, Evelyn Zheng, Beverly Alston-Smith, Cecilia Kanyama and Charity Potani.
Footnotes
Disclaimers: The views expressed are those of the authors and should not be construed to represent the positions of authors’ institutions.
Clinicaltrials.gov Identifier: NCT00089505
References
- 1.Dalal S, et al. Non-communicable diseases in sub-Saharan Africa: what we know now. International Journal of Epidemiology. 2011;40(4):885–901. doi: 10.1093/ije/dyr050. [DOI] [PubMed] [Google Scholar]
- 2.Ikem I, Sumpio BE. Cardiovascular disease: the new epidemic in sub-Saharan Africa. Vascular. 2011;19(6):301–7. doi: 10.1258/vasc.2011.ra0049. [DOI] [PubMed] [Google Scholar]
- 3.de-Graft Aikins A, et al. Tackling Africa’s chronic disease burden: from the local to the global. Globalization and health. 2010;6:5. doi: 10.1186/1744-8603-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafar TH. Commentary: cardiovascular risk factors--the next epidemic in Uganda: findings from the population-based HIV/AIDS rural surveillance cohort. International Journal of Epidemiology. 2011;40(1):171–3. doi: 10.1093/ije/dyq169. [DOI] [PubMed] [Google Scholar]
- 5.Wester CW, et al. Non-AIDS-defining events among HIV-1-infected adults receiving combination antiretroviral therapy in resource-replete versus resource-limited urban setting. AIDS. 2011;25(12):1471–9. doi: 10.1097/QAD.0b013e328347f9d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunfeld C, et al. Contribution of metabolic and anthropometric abnormalities to cardiovascular disease risk factors. CIrculation. 2008;118(2):e20–8. doi: 10.1161/CIRCULATIONAHA.107.189623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currier JS, et al. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. CIrculation. 2008;118(2):e29–35. doi: 10.1161/CIRCULATIONAHA.107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currier JS, et al. Coronary heart disease in HIV-infected individuals. Journal of Acquired Immune Deficiency Syndromes. 2003;33(4):506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Friis-Moller N, et al. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17(8):1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 10.Friis-Moller N, et al. Class of antiretroviral drugs and the risk of myocardial infarction. The New England Journal of Medicine. 2007;356(17):1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 11.Triant VA, et al. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mary-Krause M, et al. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17(17):2479–86. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 13.Bozzette SA, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. The New England Journal of Medicine. 2003;348(8):702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 14.Iloeje UH, et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Medicine. 2005;6(1):37–44. doi: 10.1111/j.1468-1293.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 15.Obel N, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44(12):1625–31. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 16.Klein D, et al. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? Journal of Acquired Immune Deficiency Syndromes. 2002;30(5):471–7. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 17.Coplan PM, et al. Incidence of myocardial infarction in randomized clinical trials of protease inhibitor-based antiretroviral therapy: an analysis of four different protease inhibitors. AIDS Research and Human Retroviruses. 2003;19(6):449–55. doi: 10.1089/088922203766774487. [DOI] [PubMed] [Google Scholar]
- 18.Phillips AN, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antiviral Therapy. 2008;13(2):177–87. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 19.Holmberg SD, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360(9347):1747–8. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 20.Rickerts V, et al. Incidence of myocardial infarctions in HIV-infected patients between 1983 and 1998: the Frankfurt HIV-cohort study. European Journal of Medical Research. 2000;5(8):329–33. [PubMed] [Google Scholar]
- 21.Pefura Yone EW, et al. First-line antiretroviral therapy and dyslipidemia in people living with HIV-1 in Cameroon: a cross-sectional study. AIDS research and therapy. 2011;8:33. doi: 10.1186/1742-6405-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo-Mbenza B, et al. Relationship between Younger Age, Autoimmunity, Cardiometabolic Risk, Oxidative Stress, HAART, and Ischemic Stroke in Africans with HIV/AIDS. ISRN cardiology. 2011;2011:897908. doi: 10.5402/2011/897908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bloomfield GS, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One. 2011;6(7):e22288. doi: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fourie CM, van Rooyen JM, Schutte AE. HIV infection and cardiovascular risk in black South Africans. Cardiovascular journal of Africa. 2011;22(3):117–9. [PMC free article] [PubMed] [Google Scholar]
- 25.Buchacz K, et al. Changes in lipid profile over 24 months among adults on first-line highly active antiretroviral therapy in the home-based AIDS care program in rural Uganda. Journal of Acquired Immune Deficiency Syndromes. 2008;47(3):304–11. doi: 10.1097/qai.0b013e31815e7453. [DOI] [PubMed] [Google Scholar]
- 26.Lockman S, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. The New England Journal of Medicine. 2010;363(16):1499–509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockman S, et al. Nevirapine-versus lopinavir/ritonavir-based initial therapy for HIV-1 infection among women in Africa: a randomized trial. PLoS Med. 2012;9(6):e1001236. doi: 10.1371/journal.pmed.1001236. Epub 2012 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. Journal of hypertension. 2003;21(11):1983–92. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 29.The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Bethesda (MD): 2004. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. [Google Scholar]
- 30.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 31.Grunfeld C, et al. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. The Journal of clinical endocrinology and metabolism. 1992;74(5):1045–52. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 32.Constans J, et al. Plasma lipids in HIV-infected patients: a prospective study in 95 patients. European Journal of Clinical Investigation. 1994;24(6):416–20. doi: 10.1111/j.1365-2362.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 33.El-Sadr WM, et al. Effects of HIV disease on lipid, glucose and insulin levels: results from a large antiretroviral-naive cohort. HIV Medicine. 2005;6(2):114–21. doi: 10.1111/j.1468-1293.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 34.Mildvan D, et al. Endogenous interferon and triglyceride concentrations to assess response to zidovudine in AIDS and advanced AIDS-related complex. Lancet. 1992;339(8791):453–6. doi: 10.1016/0140-6736(92)91058-g. [DOI] [PubMed] [Google Scholar]
- 35.Maggi P, et al. Cardiovascular risk factors in patients on long-term treatment with nevirapine- or efavirenz-based regimens. The Journal of antimicrobial chemotherapy. 2011;66(4):896–900. doi: 10.1093/jac/dkq507. [DOI] [PubMed] [Google Scholar]
- 36.Fontas E, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? The Journal of infectious diseases. 2004;189(6):1056–74. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 37.van Leth F, et al. Nevirapine and efavirenz elicit different changes in lipid profiles in antiretroviral-therapy-naive patients infected with HIV-1. PLoS medicine. 2004;1(1):e19. doi: 10.1371/journal.pmed.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riddler SA, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA : the journal of the American Medical Association. 2003;289(22):2978–82. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 39.Sidhu D, Naugler C. Fasting Time and Lipid Levels in a Community-Based Population. Arch Intern Med. 2012;172(22):1707–1710. doi: 10.1001/archinternmed.2012.3708. [DOI] [PubMed] [Google Scholar]
- 40.Bansal S, et al. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA : the journal of the American Medical Association. 2007;298(3):309–16. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 41.Nordestgaard BG, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA : the journal of the American Medical Association. 2007;298(3):299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]