Abstract
The experimental induction of specific cell fates in related or unrelated lineages has fascinated developmental biologists for decades. The evaluation of altered cell fates in response to ectopic expression during embryonic development has been a standard assay for interrogating gene function. However, until recently examples of cell lineage conversions were limited to closely related and primitive cell types. The induction of pluripotency in fibroblasts prominently highlighted that combinations of transcription factors can be extremely powerful and are much more effective than single genes. On the basis of this conclusion we previously identified transcription factor combinations that directly induce functional neuronal cells from mesodermal and endodermal cells. This work has evoked numerous additional studies demonstrating direct lineage conversion into neural and other lineages. Here, we review the generation of neural progenitor cells from fibroblasts, which is the newest addition to the arena of induced cell types. Surprisingly, two fundamentally different approaches have been taken to induce this cell type, one direct approach and another that involves the intermediate generation of a partially reprogrammed pluripotent state.
Introduction
The understanding of molecular determinants of cell lineage identity is one of the key interests in developmental biology and stem cell biology. Certainly an important milestone in this regard was the discovery that the single factor MyoD was sufficient to convert fibroblasts to muscle cells [1••]. This finding suggested the existence of ‘master regulators’, genes at the very top of a hierarchical developmental program that act as molecular switches to initiate the differentiation into a specific cell lineage. The search began for similar master regulators for other lineages. An exciting discovery along these lines was that expression of Pax6 in various drosophila embryonic imaginal disc primordia induced the ectopic development of entire eye structures [2••]. Thus, Pax6 leads to a phenomenal transdetermination and is a master control gene of eye formation. But its function in embryonic rather than the adult context distinguishes it from MyoD. Important work in the hematopoietic lineage led to the discovery that the transcription factor Cebpα is sufficient to convert adult terminally differentiated B lymphocytes into macrophages [3]. Similarly, forced expression of various transcription factors were shown to induce ectopic hepatic and insulin-producing cells from other endodermal lineages [4,5] and again Pax6 was suggested to induce neuronal cells from neonatal glia [6]. Thus, while these studies described clearly important lineage regulators, they were not context-independent as the resulting transdifferentiation was limited to closely related cell types.
The development of induced neuronal cells
In 2006, Takahashi and Yamanaka embarked on their bold experiment to combine up to 24 different gene products, resulting in their landmark discovery of induced pluripotent stem (iPS) cells by a combination of four transcription factors [7••]. The conversion of fibroblasts to iPS cells was one of the most drastic cell fate changes observed at the time. However, in principle, de-differentiation could be fundamentally different than direct transdifferentiation since a reversion to a more primitive state could potentially be achieved more easily than the adoption of a completely foreign identity [8,9]. Another important lesson from Yamanaka’s work is that the key to success was to combine multiple transcription factors rather than relying on the assumption that a single master regulator exists for the pluripotent state. This conclusion has been quickly adopted to other reprogramming paradigms and led to the definition of 3 transcription factors that could convert exocrine to endocrine pancreatic tissue in vivo [10]. But still this lineage conversion was between two cell types sharing a direct common progenitor.
Given the clinical relevance and scientific interest in brain physiology and pathology, we set out to attempt to generate functional neural cells by direct lineage conversion from non-ectodermal cells. As a starting cell population we began with mouse embryonic fibroblasts, an ill-defined but easily accessible mesodermal cell type. We screened combinations of transcription factors from 19 candidates and indeed found a combination of three (Ascl1, Brn2, and Myt1l) that very efficiently induced neuronal cells with molecular and functional properties of postmitotic neurons [11••]. This was the first demonstration that direct lineage conversion is possible between two distantly related somatic cell types. Because of the morphological, molecular and functional resemblance to brain-derived neurons we termed these cells induced neuronal (iN) cells [11••]. Others and we then went on to show that this approach can also be extended to human fibroblasts [12–17]. Moreover, addition of subtype-specific transcription factors allowed the generation of iN cells showing traits of dopaminergic and spinal motor neurons [15–17]. We also showed that genetically defined hepatocytes, an endodermal lineage, can be efficiently converted to iN cells using the same three transcription factors suggesting that reprogramming with these transcription factors are not limited to mesodermal cell types [18].
Generation of neural precursor cells using Yamanaka factors
Human iN cells may develop into an interesting tool for modeling brain diseases affecting neurons. However, iN cells would have limitations for diseases affecting glia. Moreover, iN cells are postmitotic — just like neurons —and therefore cannot be easily expanded. Especially for transplantation-based treatments presumably large cell numbers will be required. Both of these limitations would be overcome if neural stem or progenitor cells, here referred to as neural precursor cells (NPCs), could be generated directly from fibroblasts. Therefore, several groups have set out to reprogram fibroblasts into cells with NPC-like properties. Clinically relevant NPCs would have two important properties: (i) tri-potency (i.e. potential to differentiate into neurons, astrocytes and oligodendrocytes) and (ii) self-renewal (maintaining a full differentiation potential after cell division on the single cell level).
Kim et al. were the first to report the generation of NPC-like cells without going through an iPS cell-state by expanding on their previous work where they generated cardiomyocytes from fibroblasts after transient expression of the Yamanaka factors and subsequent addition of extracellular signals [19•]. Indeed, upon transient expression of the Yamanaka factors for 3–6 days using doxycy-cline-inducible lentiviruses and subsequent culture in neural media, which contained the growth factors FGF2, FGF4, and EGF, colonies appeared with rosette-like structures somewhat akin to neural differentiation of pluripotent stem cells [19•]. These cells expressed neural and typical rosette-cell markers like ZO-1 and PLZF and were negative for endodermal or mesodermal gene products. Surprisingly, c-Myc was required for reprogramming and the efficiency of colony formation of 0.5% was similar to the efficiency of iPS cell reprogramming using the same reprogramming approach [20]. Further analysis showed that these cells readily gave rise to fully functional neurons as well as to GFAP-positive astrocytic cells but no cells with oligodendrocytic markers were found suggesting at least a bipotent state. The differentiation analysis was not performed on a single cell level though and self-renewal of the presumed NPCs has not been demonstrated. No elevated transcripts specific to pluripotent stem cells such as Oct4 were detected on the population level, which could suggest that no pluripotent state was induced before neural differentiation. But it remains possible that endogenous pluripotency genes including Oct4 in 0.5% of the cells were transiently activated.
Taking a similar approach, Thier et al. expanded upon this finding by limiting the expression of Oct4 to 5 days while maintaining constitutive retroviral expression of Sox2, Klf4, and c-Myc [21•]. Initial attempts to regulate Oct4 by using a tetracycline inducible lentiviral system proved inadequate since iPS cell colonies that had reactivated the endogenous Oct4 locus evolved when cells were treated with doxycycline for 5 days only. The authors therefore resorted to deliver Oct4 as protein. Under this regimen 5 days of Oct4 transduction in combination with retroviral delivery of the other 3 factors was sufficient to generate colonies with neural appearance 18 days after transduction without Oct4-positive iPS cell formation [21•]. The efficiency was much lower than reported by Kim et al. and standard iPSC reprogramming (7–11 colonies induced from 130 000 fibroblasts). These cells expressed many markers commonly used to characterize brain-derived NPCs and could be expanded using standard NPC media containing the growth factors EGF and FGF2. Importantly, when differentiated in media favoring either neuronal, astroglial, or oligodendroglial differentiation, cells with markers of all three cell types could be observed. This demonstrated the multilineage potential of these clonally derived iNPCs. Despite their expandability to over 50 passages and their capacity to generate single cell-derived neurospheres, their self-renewal capacity was not formally demonstrated because their differentiation potential was not tested at those higher passage numbers. Importantly, though, the retro-viral transgenes were effectively silenced, demonstrating that the induced NPC state is not dependent at least on high levels of exogenous reprogramming factors and most likely stable even in their complete absence. Finally, transplantation experiments showed survival and tri-lineage differentiation of iNPCs in vivo (Table 1).
Table 1.
Summary of approaches to iNPC generation
| Genes | Vector | Time | Efficiencies (method) | Multipotency | Self-renewal | Transgene dependence | Ref |
|---|---|---|---|---|---|---|---|
| Oct4, Sox2, Kl4, c-Myc | Endogenous induction/lentivirus | 11 days | 0.69% (Colonies) | Neuronal astrocytic | N | N | [19] |
| Oct4, Sox2, Klf4, c-Myc | Oct4 protein transduction, retrovirus | 18 days | 0.005–0.008% (Colonies) | Tripotent | ? | N | [21•] |
| Ascl1, Ngn2, Hes1, Id1, Pax6, Brn2, Sox2, Klf4, c-Myc | Retrovirus | 3 days | 0.001–0.002% (Colonies) | Tripotent | ? | Y | [23] |
| FoxG1, Sox2 | Lentivirus | 16 days | 5% (FACS) | Neuronal astrocytic | Y | Y | [24•] |
| Brn2, FoxG1, Sox2 | Lentivirus | 21 days | 11.5% (FACS) | Tripotent | Y | Y | [24•] |
| Brn4, Sox2, Klf4, c-Myc | Retrovirus | 4–5 weeks | 0.002–0.006% (Colonies) | Tripotent | ? | N | [25•] |
More recently, yet another group has shown that a similar tripotent neural precursor state could also be obtained after retroviral infection and constitutive expression of the four Yamanaka factors from mouse and human fibroblasts [22]. In contrast to the papers described above, the authors relied on retroviral silencing of all 4 vectors. Accordingly, a large subset of the colonies that gave rise to NPCs also transiently expressed the pluripotency gene Nanog suggesting that the majority of these iNPCs had not bypassed a pluripotent state. Again compatible with this interpretation, the efficiency of iNPC colony formation was also similar to iPSC production and was largely dependent on LIF and FGF2 treatment.
Induced neural precursor cells using lineage-specific transcription factors
In contrast to the papers described in the previous section, the generation of iN cells was achieved by expression of lineage-specific transcription factors, not factors inducing pluripotency [11••]. We therefore hypothesized that a similar approach may also be possible for the induction of NPC-like cells from fibroblasts. This may yield different kind of NPCs and perhaps could be achieved without the use of outright oncogenes like c-Myc, which would not be desired for transplantation-based approaches.
A first report along those lines showed that combined forced expression of 9 transcription factors (Ascl1, Ngn2, Hes1, Id1, Pax6, Brn2, Sox2, c-Myc, and Klf4) in mesoderm-derived Sertoli cells was able to induce an expandable neural precursor population which could differentiate into the three neural lineages and engraft into the mouse brain [23]. Surprisingly, colonies could be generated in as little as 3 days upon expression of these factors, but another month of expansion appeared necessary before cells acquired neural character. Only 1/10th of these colonies were able to generate cells resembling NPCs, suggesting that the large number of factors used may produce other neural or even non-neural lineages. Of note, 2 of those 9 factors were known before to be sufficient to induce immature postmitotic iN cells [11••]. Additionally, even in expanded lines, retroviral expression of these factors was never silenced; therefore, the transgene dependence could not be assessed. Importantly, though, the cells were shown to differentiate under defined conditions.
At the same time we had also set out to achieve iNPC reprogramming with lineage-specific transcription factors. We began with a similar list of 11 transcription factors expressed in NPCs but in contrast to the study above used mouse fibroblasts as a starting cell population [24•]. We observed Sox2-positive colonies with NPC-like properties after infection of the pooled transcription factors and went on to show that Sox2 and FoxG1 were the most critical factors to generate iNPCs. Strikingly, just those 2 factors alone were sufficient to induce clonal iNPC cultures that expressed a subset of NPC markers tested and showed neuronal-restricted or neuronal/astroglial-restricted differentiation potential. These cells could differentiate at multiple passages and were thus self-renewing precursor cells but without oligodendrocytic differentiation potential. Moreover, these iNPCs failed to silence fibroblast marker genes. We therefore screened the remaining candidate factors in combination with Sox2 and FoxG1 and found that Brn2 robustly conferred an oligodendrocytic differentiation potential in these iNPCs while still entertaining an efficient neuronal and astroglial potential when tested at several passages. Thus, we concluded that Sox2, Brn2, FoxG1-iNPCs represented a self-renewing, tri-potent NPC state. Accordingly, many more NPC markers were induced and all tested fibroblast-specific markers were downregulated.
Surprisingly, a similar population could be generated with just FoxG1 and Brn2 alone (and without Sox2) but in contrast colony generation was not an indicator of transdifferentiation — rather the entire fibroblast population gradually morphed into a homogenous Sox2+ iNPC population that could be expanded and differentiated. Therefore, the true reprogramming efficiency of these iNPCs was difficult to assess. After 21 days, about 10% had activated the endogenous Sox2 locus and after some passages the entire population homogenously expressed Pax6, Nestin, and BLBP. While the cells produced with FoxG1 and Brn2 alone were also tripotent and could self-renew, the neurons differentiated from these cells were not as mature as neurons differentiated from Brn2/FoxG1/Sox2-iNPCs. Importantly, these iNPCs could engraft into the shiverer mouse brain where they differentiated into oligodendrocytes ensheathing host axons. Also, while fibroblast-specific genes were repressed in the FoxG1/Brn2 and FoxG1/Brn2/Sox2-iNPCs, both populations were transgene-dependent and differentiated to neural lineages within several passages upon removal of doxycycline, suggesting incomplete epigenetic reprogramming of the proliferative state while the differentiated progeny were transgene independent.
Soon following our publication, Han et al. reported the generation of an expandable, tripotent population from mouse fibroblasts with forced expression of four NPC transcription factors (Sox2, Klf4, c-Myc, and Brn4) [25•]. Remarkably, three of these factors are in fact Yamanaka factors, but those 3 alone (without Oct4) are not sufficient to generate iPS cells. Initially the pool also contained Tcf3, but this factor proved dispensable albeit at the price of somewhat lower efficiencies (1–3 colonies compared to 2–5 colonies from 50 000 fibroblasts 4–5 weeks post infection). Both 4 factor and 5 factor iNPCs could produce similar amounts of neuronal and astrocytic cells when compared to control NPCs, though both were inferior to regular NPCs at producing O4-positive oligodendrocytic cells which displayed only immature morphologies. The iNPC-derived neuronal cells could generate action potentials, an important neuronal function, but their shape was not typical of neurons suggesting that the full set of ionotropic membrane channels responsible for active membrane properties was not properly reprogrammed. Surprisingly, while retroviral silencing was not observed in 5 factor-iNPCs, all retroviral transgenes were silenced in 4 factor-iNPCs and endogenous levels of Sox2 and Brn4 were similar to control NPCs, suggesting that an endogenous transcriptional network was activated that could maintain the 4 factor-iNPC population state.
Conclusions
Direct lineage reprogramming is emerging as a new and rapidly developing field. One of the most recent achievements is the generation of iNPCs by multiple independent groups. Since there are many different kinds of naturally occurring NPCs (adult and embryonic, each of various regional identities and lineage potential) it is perhaps not surprising that several different combinations of transcription factors were found to induce an NPC state. Presumably, the various factor combinations induce NPC-like cells of different identities. For example the oligodendrocyte differentiation potential is high in the FoxG1/Brn2-iNPCs, low in Brn4/Sox2/Klf4/c-Myc-iNPCs and totally absent in FoxG1/Sox2-iNPCs. Different FoxG1/Sox2-iNPC clones can be either neuron-restricted or neuron/astroglial-restricted. A common feature between all approaches described to date appears to be the limited capacity to differentiate into fully functional neurons as none of the reports demonstrated either presynaptic or postsynaptic competence and in some cases endogenous membrane properties were immature or atypical (e.g. in FoxG1/Brn2 and Brn4/Sox2/Klf4/c-Myc cells).
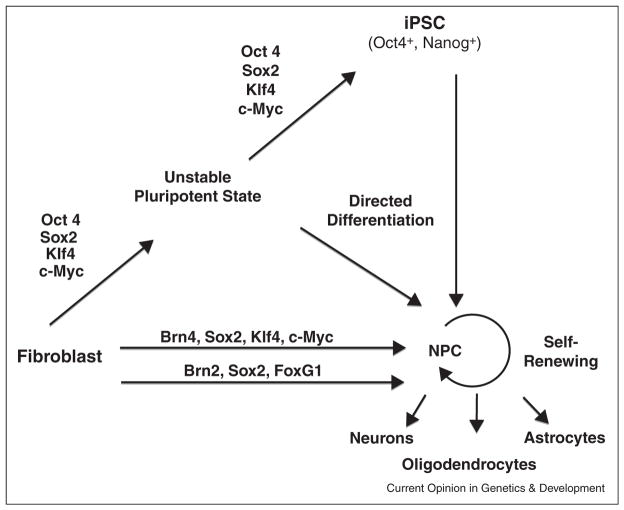
The generation of iNPCs from fibroblasts by transient expression of the Yamanaka factors has also been termed ‘direct’ reprogramming. While it appears that this relatively short and transient expression is not sufficient to induce fully reprogrammed iPS cells we hypothesize that this kind of reprogramming is not direct but involves 2 steps: (1) the induction of a partially reprogrammed pluripotent intermediate state followed by (2) a spontaneous differentiation into NPCs favored by the choice of media (Figure 1). Several lines of evidence are compatible with this interpretation. First, Oct4 is one of the most critical and specific factors of pluripotency with no detectable somatic function [26]. Second, a similar transient Yamanaka factor expression can generate blood progenitor cells or cardiomyocytes depending on the media suggesting a pluripotent nature of the induced state [27,28]. Third, the lack of Oct4 expression has been interpreted as proof of absence of a pluripotent state; however, it is well documented that, for example, Fbx15-selected iPS cells are pluripotent without activation of endogenous Oct4 [7••]. Fourth, this partially reprogrammed state could involve the expression of other pluripotency genes such as Nanog [22,30]. And finally, the efficient retroviral silencing could also be an indication that the cells underwent an intermediate state of at least some pluripotent character since Moloney viruses were shown to be silenced early in the iPS cell reprogramming process and are well known to be silenced in pluripotent cells but less so in regular NPCs [29–35].
Figure 1.
Roads to induced neural precursor cells (NPCs) from fibroblasts. NPCs capable of self-renewal and differentiation into the three neural lineages can be induced by two conceptually separate mechanisms: (1) ectopic expression of the four Yamanaka factors to induce a pluripotent state which can be differentiated to NPCs, or (2) by direct conversion with forced expression of lineage specific transcription factors.
Future studies will have to clarify the exact differences between the various kinds of iNPCs described to date and most importantly which approach will be applicable to adult human fibroblasts. The ultimate evaluation of the quality and potential utilization of iNPCs will have to include the generation of fully functional neurons and glia and the therapeutic effect in animal models of disease.
Acknowledgments
Our work is generously supported by the National Institutes of Health (NIH grants RC4 NS073015-01, R01MH092931, AG010770-18A1) and the California Institute for Regenerative Medicine (CIRM grants DR1-01454, RT2-02061), and the Department of Defense (PR100175P1) as well as the Ellison Medical Foundation, the Stinehart-Reed Foundation, and the Baxter Foundation. M.W. is a New York Stem Cell Foundation-Robertson Investigator and E.L. the recipient of predoctoral fellowships from CIRM (TG2-01159) and the National Science Foundation.
Footnotes
Note added in brief
While this article was in press, a group reported the induction of iNPCs from mouse and human fibroblasts with Sox2 alone [36].
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1••.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cdna converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. First to show transdifferentation of fibroblasts to myoblasts by ectopic expression of a single transcription factor, MyoD. [DOI] [PubMed] [Google Scholar]
- 2••.Halder G, Callaerts P, Gehring WJ. Induction of ectopic eyes by targeted expression of the eyeless gene in drosophila. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. Demonstrated that ectopic expression of the single paired and homeobox domain transcription factor eyeless can transdetermine imaginal discs in vivo into complete eye structures. [DOI] [PubMed] [Google Scholar]
- 3.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 4.Shen CN, Slack JM, Tosh D. Molecular basis of transdifferentiation of pancreas to liver. Nat Cell Biol. 2000;2:879–887. doi: 10.1038/35046522. [DOI] [PubMed] [Google Scholar]
- 5.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 6.Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Gotz M. Glial cells generate neurons: the role of the transcription factor pax6. Nat Neurosci. 2002;5:308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 7••.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. Demonstrated that a combination of the four transcription factors Oct-3/4, Sox2, Klf4, and c-Myc could convert mouse fibroblasts to pluripotent embryonic-like stem cells. [DOI] [PubMed] [Google Scholar]
- 8.Silva J, Smith A. Capturing pluripotency. Cell. 2008;132:532–536. doi: 10.1016/j.cell.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. First demonstration of transdifferentiation to the neural lineage from mouse fibroblasts with the forced expression of Brn2, Ascl1, and Myt1L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. Microrna-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228–231. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA, Ding S. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9:113–118. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, et al. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ, Eggan K. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 17.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Bjorklund A, Lindvall O, Jakobsson J, Parmar M. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108:10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marro S, Pang ZP, Yang N, Tsai MC, Qu K, Chang HY, Sudhof TC, Wernig M. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9:374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A. 2011;108:7838–7843. doi: 10.1073/pnas.1103113108. First to generate bipotent iNPCs from mouse fibroblasts by transient expression of the pluripotency factors Oct-3/4, Sox2, Kfl4, and c-Myc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Thier M, Worsdorfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nothen MM, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012 doi: 10.1016/j.stem.2012.03.003. Generated tripotent, transgene independent iNPCs by transient expression of the pluripotency factor Oct-3/4 and constitutive expression of the pluripotency factors Sox2, Klf4, and c-Myc. [DOI] [PubMed] [Google Scholar]
- 22.Matsui T, Takano M, Yoshida K, Ono S, Fujisaki C, Matsuzaki Y, Toyama Y, Nakamura M, Okano H, Akamatsu W. Neural stem cells directly differentiated from partially reprogrammed fibroblasts rapidly acquire gliogenic competency. Stem Cells. 2012;30:1109–1119. doi: 10.1002/stem.1091. [DOI] [PubMed] [Google Scholar]
- 23.Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W, Zhang H, Zhao XY, Liu L, Wang Z, et al. Direct reprogramming of sertoli cells into multipotent neural stem cells by defined factors. Cell research. 2012;22:208–218. doi: 10.1038/cr.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. Generated tripotent, self-renewing iNPCs from mouse fibroblasts with the lineage specific transcription factors FoxG1, Brn2 and Sox2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Han DW, Tapia N, Hermann A, Hemmer K, Hoing S, Arauzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. Generated tripotent iNPCs from mouse fibroblasts with the pluripotency factors Sox2, Klf4, c-Myc and the lineage specific transcription factor Brn4. [DOI] [PubMed] [Google Scholar]
- 26.Lengner CJ, Camargo FD, Hochedlinger K, Welstead GG, Zaidi S, Gokhale S, Scholer HR, Tomilin A, Jaenisch R. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1:403–415. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo E, Rampalli S, Risueno RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- 28.Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol. 2011;13:215–222. doi: 10.1038/ncb2164. [DOI] [PubMed] [Google Scholar]
- 29.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to ips cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent es-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 31.Vroemen M, Weidner N, Blesch A. Loss of gene expression in lentivirus- and retrovirus-transduced neural progenitor cells is correlated to migration and differentiation in the adult spinal cord. Exp Neurol. 2005;195:127–139. doi: 10.1016/j.expneurol.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 32.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JB, Sebastiano V, Wu G, Arauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous sox2 expression. Stem Cells. 2008;26:2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 35.Stewart CL, Stuhlmann H, Jahner D, Jaenisch R. De novo methylation, expression, and infectivity of retroviral genomes introduced into embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1982;79:4098–4102. doi: 10.1073/pnas.79.13.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling YA, Li G, Walker D, Zhang WR, Kreitzer AC, Huang Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell. 2012;11:100–109. doi: 10.1016/j.stem.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]