The holy grail of hematopoietic stem cell (HSC) biology has been to harness self-renewal to expand stem cells for greater availability of transplantation procedures in people with cancer or hematologic diseases. Despite the advances in transplantation biology, many individuals do not have a suitable allogeneic donor. In these cases, unrelated cord blood-derived HSC has shown promise as a source of cells for transplantation, but the number of HSC recovered in a single cord is generally too low for adult transplantation, and infusion of two separate cords still led to delayed hematopoietic recovery1. Thus, the development of methods to readily expand HSC in vitro would be a major leap forward.
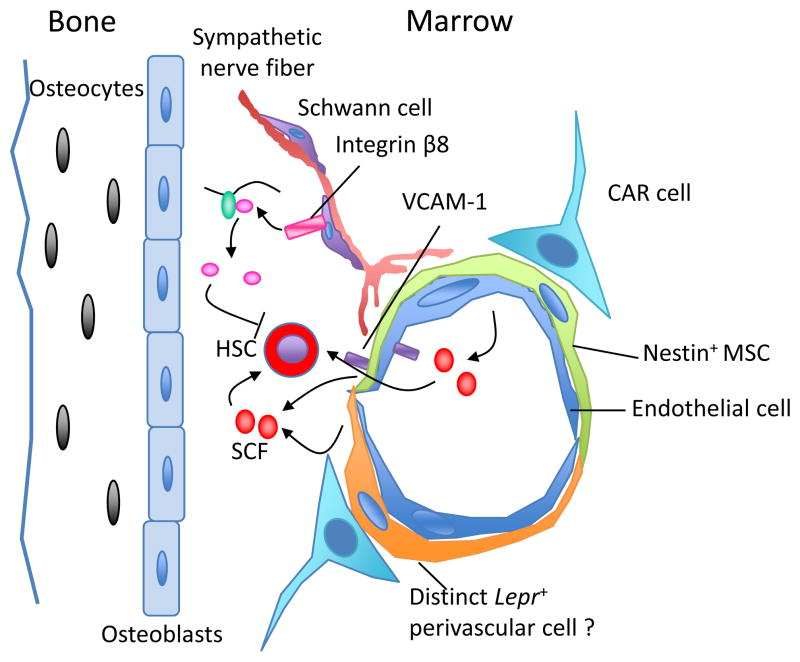
In-depth understanding of the identities and function of each component of the hematopoietic microenvironment at the cellular and molecular levels will be crucial to learn how to properly expand HSCs. This knowledge gap has closed substantially after the concept of a stem cell niche was enunciated decades ago2. It has been assumed by many, perhaps by wishful thinking, that a ‘do-it-all’ niche cell exists in the mammalian bone marrow. This idea comes from the original concept by Schofield2, and also from strong experimental work in the germline of invertebrates, where specific niche cells are thought to provide the necessary ‘goods’ to maintain germline stem cells3. In the mammalian bone marrow, candidate niche cells including osteoblasts4, Cxcl12-abundant reticular cells5 and Nestin-positive mesenchymal stem cells6 have been reported, suggesting a key role for skeletal precursor cells. Two recent studies have added further complexity to the cellular make-up of putative HSC niches in bone marrow7,8 (Fig. 1).
Figure 1.
The complex cellular and molecular make up of the HSC niche in the bone marrow. A variety of cells, including osteoblasts, Cxcl12-abundant reticular (CAR) cells, Nestin-positive mesenchymal stem cells (MSC), Lepr-expressing perivascular cells and endothelial cells have been shown as possible components of the niche. Cells from the neural system, nonmyelinating Schwann cells wrapping sympathetic nerve fibers, promote HSC quiescence through activation of TGF-β. The structural composition of these cells provides a specialized microenvironment that regulates HSC self-renewal and differentiation, either though contact-dependent signals such as vascular cell adhesion molecule-1 (VCAM-1)6, or via soluble factors such as SCF.
Yamazaki et al.7 were searching for microenvironments enabling HSC dormancy in the bone marrow. Cell cycle quiescence is a hallmark of somatic stem cells and a key behavior to protect them from hematopoietic stresses and exhaustion9. Transforming growth factor-β (TGF-β) is a powerful molecule capable of inducing HSC quiescence through inhibition of lipid raft clustering that assembles growth factor signaling microdomains10. As it is secreted as an inactive form, active TGF-β is generated locally through local protease activity. If TGF-β was crucial in promoting HSC quiescence, we would expect that the putative niche would provide a specific environment for its activation. Yamazaki et al.7 have found that glial fibrillary acidic protein (GFAP)-positive Schwann cells (that ensheath sympathetic nerve fibers) express β8 integrin, which promotes metalloproteinase-mediated TGF-β activation. Afferent sympathetic nerves form a complex with Nestin-positive cells within the bone marrow, in which the nerve itself may regulate HSC quiescence (Fig. 1). Unexpectedly, a reduction of HSC frequency in the bone marrow occurred when mice were surgically sympathectomized by ligation of the sympathetic trunk. The authors have suggested that the loss of Schwann cells led to HSC differentiation. Other less invasive, but also less specific, methods of sympathectomy (such as femoral and sciatic neurectomy), however, have not led to such reductions in HSC activity11, suggesting the presence of other signals that may collaborate with those provided by the Schwann cells of sympathetic nerves. Although the latter issue still needs to be clarified, the findings support the crucial function of local sympathetic nerves in the regulation of hematopoiesis.
Another study in mice evaluated the cellular source of stem cell factor (SCF), also called kit ligand, a growth factor required for HSC maintenance in a non-cell autonomous manner10. SCF exists in two forms, membrane-bound and soluble, due to differential splicing and proteolytic cleavage; however, the membrane-bound form appears to play a prominent role in HSC maintenance 12. When expressed under the Scf locus, green fluorescence protein (GFP) expression was mostly localized in cells associated with sinusoids, the fenestrated blood vessels within the bone marrow7. The authors made tissue-restricted deletions of Scf or knock-in expression of reporter genes including GFP in osteoblasts, perivascular cells expressing Nestin or Leptin receptor, and endothelial cells. A reduction of HSC frequency was only found in Lepr-expressing perivascular cells and endothelial cells lacking Scf, suggesting that SCF was provided by both cell types, although the contribution from the former type may be much greater. This study proposed that Lepr-expressing and Nestin-expressing cells are distinct as the deletion of Scf using transgenic lines producing Cre-mediated deletion under the Nestin promoter did not elicit the same phenotype. It is important to note, however, that Lepr was among the top 1% most highly expressed gene in sorted Nestin-positive cells by microarray analysis6, suggesting a potential overlap between the two niche cells, which will require further investigation. Thus, cells associated with the vasculature are the major source of SCF in the bone marrow under steady state. It will be important to evaluate whether the same stromal cells produce the cytokine in situation of regenerative stress, which may shed light on the requirements for building an artificial niche dedicated for HSC expansion.
These two studies bring new exciting angles that add further complexity to bone marrow niche(s); at the same time, they soberly remind us about the significant hurdles ahead of us to provide expanded HSC for clinical use. HSCs require an array of secreted and contact factors—most of which still remain unknown—for their maintenance and proliferation. If these factors are indeed provided by distinct stromal cell types, the challenge to concoct the right ratios of niche constituents to support HSC for clinical use becomes very significant. By the same token, one can imagine the translational difficulty to obtain a renewable source of good manufacturing practice (GMP) grade cellular cocktail that would meet the safety thresholds for clinical cell therapy. Further studies will aim at characterizing stromal cell types and, most important, the molecular constituents and signals that allow HSC proliferation while maintaining self-renewal.
A major obstacle for future translational HSC-based therapeutics, highlighted by the study of Yamazaki et al.7, concerns the propensity of adult HSC to remain quiescent. This natural preference was repeatedly shown in several studies where genetic mutations leading to HSC proliferation invariably produce HSC exhaustion9. How can we unlock HSC from their quiescent behavior for the purpose of expansion without reaching exhaustion? It is likely that the coupling of self-renewal and quiescence is not absolute given that it is not the case for all stem cells; for example, embryonic stem cells can both proliferate and self-renew indefinitely. The ultimate solution, through a greater understanding of the molecular basis of the niche, may lie in some sort of direct or indirect ‘reprogramming’ of adult (quiescent) HSCs into fetal-like (proliferative) stem cells, a feat that will have to be achieved without increasing the risk of malignancies. Every scientific hurdle, almost by definition, can be overcome. We are just one breakthrough away from surmounting this one.
References
- 1.Brunstein CG, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 3.Byrd DT, Kimble J. Scratching the niche that controls Caenorhabditis elegans germline stem cells. Semin Cell Dev Biol. 2009;20:1107–1113. doi: 10.1016/j.semcdb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147:1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Pietras EM, Warr MR, Passegue E. Cell cycle regulation in hematopoietic stem cells. J Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki S, et al. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113:1250–1256. doi: 10.1182/blood-2008-04-146480. [DOI] [PubMed] [Google Scholar]
- 11.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]