Abstract
The epidemiologic information regarding international differences in bone mineral density (BMD) in women is currently insufficient. We compared BMD in older women across five racial/ethnic groups in four countries. The femoral neck, total hip, and lumbar spine BMD were measured in women (aged 65–74 years) from the Study of Osteoporotic Fractures (SOF) (5,035 Caucasian women and 256 African American women in the US), the Tobago Women’s Health Study (116 Afro-Caribbean women), the Ms Os Hong Kong Study (794 Hong Kong Chinese women) and the Namwon Study (1,377 South Korean women). BMD was corrected according to the cross-site calibration results for all scanners. When compared with US Caucasian women, the age adjusted mean BMD measurements at the hip sites were 21–31 % higher among Tobago Afro-Caribbean women and 13–23 % higher among African American women. The total hip and spine BMD values were 4–5 % lower among Hong Kong Chinese women and 4–7 % lower among South Korean women compared to US Caucasians. The femoral neck BMD was similar in Hong Kong Chinese women, but higher among South Korean women compared to US Caucasians. Current/past estrogen use was a significant contributing factor to the difference in BMD between US versus non-US women. Differences in body weight partially explained the difference in BMD between Asian versus non-Asian women. These findings show substantial racial/ethnic differences in BMD even within African or Asian origin individuals, and highlight the contributing role of body weight and estrogen use to the geographic and racial/ethnic variation in BMD.
Keywords: Bone densitometry, Bone mineral density, Epidemiology, Ethnicity, Women
Introduction
Bone mineral density (BMD) is a strong and consistent predictor of fracture risk among postmenopausal women [1, 2]. Comparisons of BMD among international groups may aid in our understanding of the ethnic/geographic variability of fracture risk in women. A wide variation in BMD and fracture incidence exists across female racial/ethnic groups [3–14]. African American women have been reported to possess a higher BMD than Caucasians in both the spine and femur [3–8], and this difference may contribute to the lower fracture rate among African American women [8]. In contrast, Asian women have a similar or lower BMD than Caucasian women [9–13]. Nevertheless, Asian women possess lower hip fracture rates compared to Caucasian women [7, 9, 14], perhaps due to their shorter hip axis length [15] and other geometric differences.
Ethnic groups differ by geographical region and cultural background across Asia. According to the Asian Osteoporosis Study (AOS) [7], the age-adjusted hip fracture rates in women differ across the four Asian countries. However, few studies have been performed in Asia to understand the wide variability in fracture rates. Excluding Chinese and Japanese women, epidemiologic information regarding the BMD of other Asian women and its determinants is scarce. Substantial variation in BMD may also occur within women of African ancestry [16]. It has been reported that [17, 18] older West African women have similar or lower BMD than white British women. Other studies have shown that [19] the degree of European ancestry in African Americans was inversely correlated with BMD.
The majority of epidemiologic studies on racial/ethnic differences in the BMD of women have been confined to a comparison of African American or Chinese women to Caucasian women. The Study of Women’s Health Across the Nation (SWAN) [20] is unique in its inclusion of four US ethnic groups (African American, Caucasian, Chinese, and Japanese women) and identified the important role of body weight on the ethnic variation in BMD. To further explore whether similar ethnic variation exists in non-US women and to explore the major factors associated with this variation, we collected datasets from one US [8] and three non-US bone health studies [21–23] and compared the mean BMD in older women across five racial/ethnic groups: US Caucasian, African American, Tobago Afro-Caribbean, Hong Kong Chinese, and South Korean.
Materials and methods
Study subjects and BMD measurements
We employed a cross-sectional design; the datasets included the Study of Osteoporotic Fractures (SOF) [8], the Ms Os Hong Kong Study (Hong Kong Study) [21], the Tobago Women’s Health Study (Tobago Study) [22], and the Namwon Study [23]. Details on the recruitment of study subjects and measurements for these studies have been published elsewhere [8, 21–24]. Briefly, by contacting women from voter-registration lists, the SOF enrolled 9,704 women aged 65 or older at four US clinical settings in Baltimore, MD; Minneapolis, MN; Portland, OR; and the Monongahela Valley near Pittsburgh, PA, from 1986 through to 1988 [8, 24]. Six hundred and sixty-two African American women were enrolled in the SOF at a later visit (1997–98). There were 8,074 Caucasian and 647 African American women who had a technically adequate hip BMD measurement. BMD was measured using Hologic QDR 1000 (Hologic Inc., Bedford, MA, USA) (for Caucasian and African American women) or 2000 scanners (for African American women). The Hong Kong Study enrolled 2,000 Chinese women aged 65 or older between 2001 and 2004 through recruitment notices placed in community centers [21]. Among these women, the BMD values for 1,226 were measured by two Hologic QDR 4500W (Hologic Inc., Bedford, MA, USA) scanners included in the calibration procedure for the current study. The Tobago Study enrolled 340 Afro-Caribbean women aged 50 or older (n = 116 women aged 65–74) from the Caribbean Island of Tobago in 2002 [22]. The methods of recruitment were flyers and word-of-mouth referral by research staff and male participants in the Tobago Prostate Cancer Survey Study [25]. The BMD values of these women were measured using a Hologic QDR 4500W (Hologic Inc., Bedford, MA, USA) scanner. In the Namwon Study, 6466 Korean women aged 45–74 were recruited from Namwon City, South Korea between 2004 and 2007 through mailings and telephone calls based on the list of officially registered residents [23]. Focusing on women aged 65–74, there were 1,969 Korean women who had a femoral neck, total hip, or lumbar spine BMD measurement by DPX Bravo (n = 584) (GE, Madison, WI, USA) or Lunar Prodigy (n = 1,385) (GE, Madison, WI, USA) scans. Only the Lunar Prodigy was available for the cross-calibration procedure. Thus, we limited our study to the 1,385 Korean women scanned on the Lunar Prodigy.
All recruited subjects were able to walk without the assistance of another person. An assistive device was permitted. All of the study subjects provided informed consent, and each study was conducted in accordance with the guidelines of the Declaration of Helsinki. Each study was approved by the appropriate institutional research ethics committee in their respective centers.
The total hip, femoral neck, and lumbar spine BMD (g/cm2) were measured in each racial/ethnic group excluding the spine BMD, which was not measured in African American and Afro-Caribbean women. All BMD scans were conducted using standardized procedures following the manufacturer’s recommended protocols. Longitudinal quality control was performed daily with a spine phantom and showed no shifts or drifts in each study site.
For the current study, we restricted our analyses to women aged 65–74 years, who had a BMD measurement at the femoral neck, total hip, or lumbar spine with complete age, body weight, and height data. The final dataset included 5,035 subjects for femoral neck or total hip BMD (4,998 subjects for lumbar spine BMD) among US Caucasian women; 256 African American women and 116 Afro-Caribbean women had a femoral neck and total hip BMD; 794 subjects for femoral neck or total hip BMD (786 subjects for spine BMD) among Hong Kong Chinese women; and 1,377 subjects for femoral neck or total hip BMD (1,319 subjects for spine BMD) among Korean women.
Cross-calibration of scanners
The Musculoskeletal and Quantitative Imaging Research Group at the University of California, San Francisco (UCSF) cross-calibrated all scanners using the Hologic spine, femur, and block phantoms. Details on the cross-calibration studies for each DXA scanner, excluding those for SOF, were previously described [23]. The scanners for Tobago, Hong Kong, and Korean women in the current study were identical to those of our previous study in men [23]. Briefly, for the cross-calibration study, phantom BMD results were first converted to standardized BMD (sBMD) [26]. The same phantoms were used for the cross-calibration on the scanners of SOF. To derive the linearity of each machine, linear regression was used to analyze the block phantom results. The ratio between the study site and the reference site (reference site/measurement site) for sBMD was then calculated. An analysis of variance (ANOVA) with a Dunnett’s test was applied to determine the mean sBMD differences between the study site and the reference site. If the sBMD for a study site was significantly different from the reference site, the ratio was used as the cross-calibration factor for each specific scan type. Otherwise the cross-calibration factor was set to 1.
Using these results, we corrected the participant spine, total hip and femoral neck BMD values: scanner specific raw BMD values were converted to sBMD values [26], and then the sBMD values were adjusted with the cross-calibration factors.
Other measurements
Information on the demographics, lifestyle, and reproductive and medical history were obtained by trained interviewers with questionnaires in each study.
In the SOF, Hong Kong Study, and Namwon Study, the race/ethnicity of subjects was self-declared. In the Tobago Study, the study subjects provided detailed information on the ethnic ancestry of their parents and grandparents. All of the Tobagonian women reported four African ancestry grandparents.
Smoking status was categorized as current, past, or never, and lifetime smoking levels were computed as pack-years. Current alcohol consumption was calculated as drinks per week. Dietary calcium intake was calculated by food-frequency questionnaires specific for each country, i.e., the modified versions of the Block Food Frequency Questionnaire in SOF [27], Hong Kong Study, and Tobago Study [28], and the food-frequency questionnaire developed for the Korean Genome Epidemiologic Study [29] in the Namwon Study.
All studies collected information on the age of the last menstrual period, the current and past use of oral estrogen, and physician-diagnosed medical conditions including diabetes mellitus, hypertension, coronary heart disease, and hyperthyroidism.
Body weight was measured in indoor clothing or a light gown without shoes using a calibrated Inbody 3.0 (Bio-space Co., Korea) in the Namwon Study, and calibrated balanced beam scales in the SOF, Hong Kong Study, and Tobago Studies. Standing height was measured using a wall-mounted stadiometer in each study. Body mass index (BMI) was calculated by dividing body weight (kg) by square height (m2). The grip strengths was measured in both hands using the Jamar handheld dynamometer (JA Preston Co., Jackson, MI, USA) in the SOF and Tobago Studies; the Preston grip dynamometer (Takei Kiki Kogyo, Japan) in the Hong Kong Study; and the TKK 5401 Grip-D (Takei, Japan) in the Namwon Study. The average values of left and right grip strength were calculated.
Statistical analyses
Descriptive data for the major characteristics and BMD values were expressed as the percentage or mean ± standard deviation (SD). BMD was compared across racial/ethnic groups following the adjustment for age only, for age and body weight, and for age, height, and body weight using general linear models (GLM). In addition to these variables, we examined grip strength, smoking, current alcohol consumption, dietary calcium intake, years since last menstrual period, past and current use of oral estrogen, and physician-diagnosed medical conditions as potential confounders. When these variables were separately included in the previous GLM including age, height, and body weight, the majority of variables excluding coronary heart disease and hyperthyroidism were significantly (p <0.05) associated with femoral neck BMD. We selected those significant variables as covariates for inclusion in the full model. The least square mean BMD (LSM) for each racial/ethnic group was estimated by the multivariable GLMs and the percentage differences in LSMs between US Caucasian women and other racial/ethnic groups were calculated. The analysis was performed for all subjects and sensitivity analysis in subjects with no history of estrogen use. A Tukey–Kramer adjustment was applied to correct for pairwise comparisons. The results were considered statistically significant when p ≤ 0.05. The SAS software version 9.2 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Demographic and lifestyle characteristics
Table 1 shows the demographic and lifestyle characteristics across the racial/ethnic groups. Compared with US Caucasian women, African American women were slightly older and had a greater number of years since menopause; Afro-Caribbean, Hong Kong Chinese, and South Korean women were slightly younger and had a lower or similar number of years since menopause. Afro-Caribbean women were taller and had a higher body weight than both US Caucasian and African American women, although the latter was not significant due to the smaller sample size. Both Asian ethnic groups (Hong Kong Chinese and South Korean women) weighed less and were shorter than other racial/ethnic groups. The range of BMI differed dramatically across the racial/ethnic groups. Approximately half (51.2–53.4 %) of women in the African ancestry groups (African American and Afro-Caribbean women) and a substantial proportion (20.6 %) of US Caucasian women were obese (BMI>30 kg/m2). On the contrary, few Asian women (3.6–4.8 %) were obese. The average grip strength was highest among Afro-Caribbean women and lowest among Korean women.
Table 1.
Baseline characteristics of participants according to racial/ethnic group
| US Caucasian | African American | Tobago Afro-Caribbean | Hong Kong Chinese | South Korean | |
|---|---|---|---|---|---|
| Sample size (N) | 5035 | 256 | 116 | 794 | 1377 |
| Age (years) | 70.3 ± 2.2b,c,d,e | 71.4 ± 2.0a,c,d,e | 68.5 ± 2.6a,b,d,e | 69.4 ± 2.7a,b,c | 69.4 ± 2.8a,b,c |
| Years since menopause | 22.0 ± 6.1b,c,d | 26.5 ± 8.1a,c,d,e | 20.0 ± 5.9a,b,e | 20.3 ± 5.5a,b,e | 22.5 ± 7.2b,c,d |
| Standing height (cm) | 159.9 ± 5.7c,d,e | 159.5 ± 4.7c,d,e | 162.3 ± 5.5a,b,d,e | 151.7 ± 5.2a,b,c,e | 149.3 ± 5.2a,b,c,d |
| Weight (kg) | 67.6 ± 12.2b,c,d,e | 77.7 ± 12.6a,d,e | 79.5 ± 14.8a,d,e | 55.4 ± 8.4a,b,c,e | 53.6 ± 8.3a,b,c,d |
| Body mass index (kg/m2) | 26.4 ± 4.5b,c,d,e | 30.5 ± 4.8a,d,e | 30.3 ± 5.8a,d,e | 24.1 ± 3.5a,b,c | 24.0 ± 3.3a,b,c |
| >30 (%) | 20.6b,c,d,e | 51.2a,d,e | 53.4a,d,e | 4.8a,b,c | 3.6a,b,c |
| <20 (%) | 5.2b,d,e | 0.0a,c,d,e | 4.3b,d | 12.0a,b,c | 9.7a,b |
| Average of right and left grip strength (kg) | 21.8 ± 4.2b,c,d,e | 20.5 ± 4.9a,c,e | 25.3 ± 5.6a,b,d,e | 20.7 ± 4.1a,c,e | 17.2 ± 5.0a,b,c,d |
| Smoking | |||||
| Current (%) | 9.4c,d,e | 11.8c,d,e | 0.0a,b,e | 2.4a,b,e | 6.0a,b,c,d |
| Past (%) | 32.6 | 34.3 | 2.6 | 5.2 | 3.9 |
| Pack-years | 14.4 ± 27.3c,d,e | 10.8 ± 18.8c,d,e | 0.0 ± 0.4a,b | 1.0 ± 5.4a,b | 1.5 ± 6.9a,b |
| Drinking (drinks/week) | 1.9 ± 3.8b,c,d,e | 0.4 ± 1.2a | 0.0 ± 0.1a | 0.1 ± 0.6a,e | 0.6 ± 2.7a,d |
| Dietary calcium intake (mg/day) | 729.1 ± 441.9b,c,d,e | 619.9 ± 355.9a,c,e | 296.0 ± 175.7a,b,d | 581.0 ± 274.7a,c,e | 283.2 ± 189.6a,b,d |
| Oral estrogen use | |||||
| Current (%) | 17.7b,c,d,e | 20.0a,c,d,e | 1.7a,b,d,e | 0.4a,b,c | 0.9a,b,c |
| Past (%) | 30.8 | 23.3 | 9.5 | 2.8 | 3.9 |
| Self-reported medical history (%) | |||||
| Diabetes | 6.2b,c,d,e | 16.8a,c,e | 25.9a,b,d,e | 13.5a,c | 10.8a,b,c |
| Hypertension | 33.9b,c,d | 63.3a,d,e | 61.2a,d,e | 42.8a,b,c,e | 33.7b,c,d |
| Coronary heart disease | 12.7b,d,e | 19.5a,c,d,e | 8.6b,e | 8.7a,b,e | 3.3a,b,c,d |
| Hyperthyroidism | 8.7d,e | 8.6d,e | 4.3e | 5.0a,b,e | 1.6a,b,c,d |
Sample size is based on the number of women with complete total hip BMD, age, weight, and height data BMD bone mineral density
Significantly different compared to US Caucasian (p <0.05 by Tukey test for continuous variable or Chi-square test for categorical variable)
Significantly different compared to African American (p <0.05)
Significantly different compared to Tobago Afro-Caribbean (p <0.05)
Significantly different compared to Hong Kong Chinese (p <0.05)
Significantly different compared to South Korean (p <0.05)
There was a larger number of current or past smokers among US women (Caucasian and African American women) compared to non-US women, and no current smokers among Afro-Caribbean women were recorded. US Caucasian women reported more alcohol consumption compared to other groups. The dietary calcium intake was greatest in US Caucasian women, and much lower in Afro-Caribbean and Korean women compared to other groups. US women used a higher level of oral estrogen compared to the other groups. Both African ancestry groups were more likely to report diabetes and hypertension, and US Caucasian and African American women were more likely to report coronary heart disease and hyperthyroidism (Table 1).
Differences in BMD among racial/ethnic groups
Table 2 shows the mean BMD values at the femoral neck, total hip, and lumbar spine among the racial/ethnic groups. Figures 1 and 2 present the percentage differences in the mean BMD at each site among the racial/ethnic groups compared with Caucasians.
Table 2.
Comparison of BMD at each site among racial/ethnic groups
| US Caucasian | African American | Tobago Afro-Caribbean | Hong Kong Chinese | South Korean | |
|---|---|---|---|---|---|
| Femoral neck BMD (g/cm2) | |||||
| For all subjects (N) | 4964 | 256 | 116 | 794 | 1377 |
| Age adjusted mean (SE) | 0.686 (0.001)b,c,e | 0.841 (0.007)a,c,d,e | 0.897 (0.010)a,b,d,e | 0.692 (0.004)b,c,e | 0.739 (0.003)a,b,c,d |
| Age and Wt adjusted mean (SE) | 0.673 (0.001)b,c,d,e | 0.789 (0.006)a,c,d | 0.844 (0.009)a,b,d,e | 0.726 (0.004)a,b,c,e | 0.780 (0.003)a,c,d |
| All covariates adjusted mean (SE)f | 0.666 (0.002)b,c,d,e | 0.791 (0.007)a,c,d | 0.828 (0.012)a,b,d,e | 0.725 (0.004)a,b,c,e | 0.787 (0.003)a,c,d |
| For estrogen never users (N) | 2514 | 139 | 103 | 769 | 1311 |
| All covariates adjusted mean (SE)f | 0.650 (0.002)b,c,d,e | 0.760 (0.009)a,c,d | 0.832 (0.013)a,b,d,e | 0.719 (0.004)a,b,c,e | 0.784 (0.003)a,c,d |
| Total hip BMD (g/cm2) | |||||
| For all subjects (N) | 5035 | 256 | 116 | 794 | 1376 |
| Age adjusted mean (SE) | 0.833 (0.002)b,c,d,e | 0.943 (0.008)a,c,d,e | 1.011 (0.012)a,b,d,e | 0.801 (0.004)a,b,c,e | 0.775 (0.003)a,b,c,d |
| Age and Wt adjusted mean (SE) | 0.816 (0.002)b,c,d,e | 0.874 (0.007)a,c,d,e | 0.939 (0.011)a,b,d,e | 0.846 (0.004)a,b,c,e | 0.829 (0.003)a,b,c,d |
| All covariates adjusted mean (SE)f | 0.810 (0.002)b,c,d,e | 0.879 (0.008)a,c,d,e | 0.922 (0.014)a,b,d,e | 0.837 (0.004)a,b,c | 0.830 (0.004)a,b,c |
| For estrogen never users (N) | 2551 | 139 | 103 | 769 | 1310 |
| All covariates adjusted mean (SE)f | 0.789 (0.003)b,c,d,e | 0.849 (0.010)a,c | 0.928 (0.015)a,b,d,e | 0.832 (0.004)a,c | 0.825 (0.004)a,c |
| Lumbar spine BMD (g/cm2) | |||||
| For all subjects (N) | 4998 | 786 | 1319 | ||
| Age adjusted mean (SE) | 0.872 (0.002)d,e | 0.825 (0.006)a | 0.834 (0.004)a | ||
| Age and Wt adjusted mean (SE) | 0.849 (0.002)d,e | 0.872 (0.005)a,e | 0.892 (0.004)a,d | ||
| All covariates adjusted mean (SE)f | 0.837 (0.003)d,e | 0.871 (0.005)a,e | 0.907 (0.005)a,d | ||
| For estrogen never users (N) | 2533 | 761 | 1254 | ||
| All covariates adjusted mean (SE)f | 0.805 (0.004)d,e | 0.865 (0.005)a,e | 0.902 (0.005)a,d | ||
BMD bone mineral density, Wt weight
Significantly different compared to US Caucasian (p <0.05 by Tukey test)
Significantly different compared to African American (p <0.05)
Significantly different compared to Tobago Afro-Caribbean (p <0.05)
Significantly different compared to Hong Kong Chinese (p <0.05)
Significantly different compared to South Korean (p <0.05)
Adjusted for age, weight, height, grip strength, smoking amount, current alcohol consumption, dietary calcium intake, years since last menstrual period, diabetes mellitus and hypertension
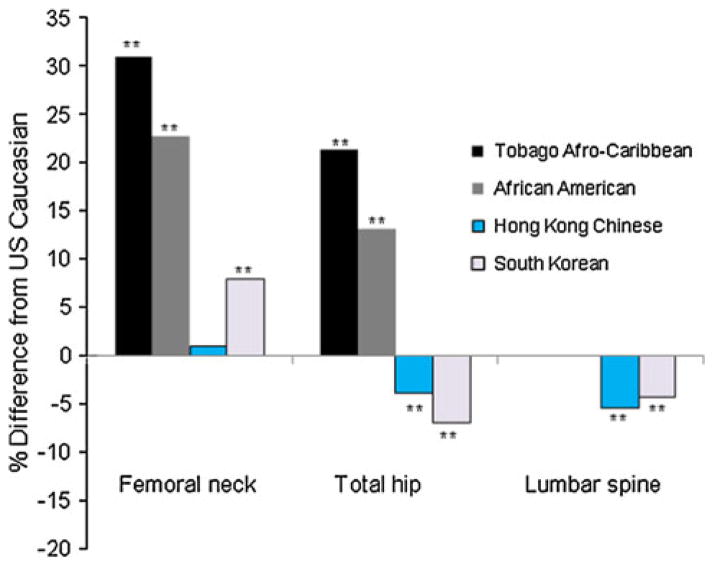
Fig. 1.
Percentage differences in age adjusted mean of BMD among Afro-Caribbean, African American, Hong Kong Chinese and South Korean women compared to US Caucasian women 65 year or older. **p <0.001 by Tukey test comparing BMD between US Caucasian women and each ethnic group
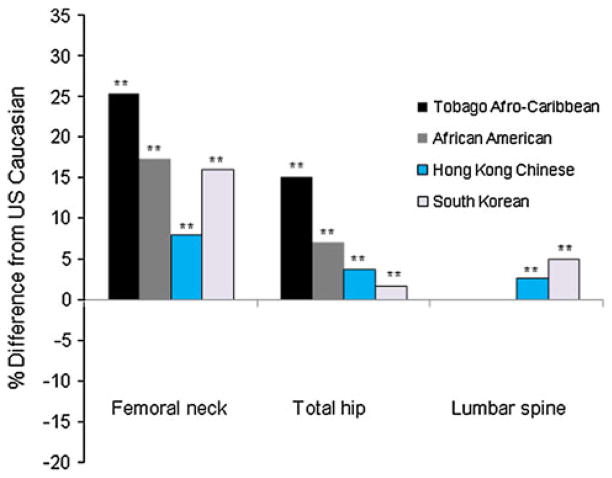
Fig. 2.
Percentage differences in age and weight adjusted mean of BMD among Afro-Caribbean, African American, Hong Kong Chinese and South Korean women compared to US Caucasian women 65 year or older. **p <0.001 by Tukey test comparing BMD between US Caucasian women and each ethnic group
When compared with US Caucasian women, the age adjusted mean BMD values at the femoral neck and total hip were 21.3–30.9 % higher among Afro-Caribbean woman and 13.1–22.7 % higher among African American women, respectively. The total hip and spine BMD values were 3.9–5.4 % lower between Hong Kong Chinese woman and 4.3–7.0 % lower among South Korean women compared to US Caucasians. The femoral neck BMD values were similar among Hong Kong Chinese women and higher among South Korean women, compared to US Caucasians (Table 2; Fig. 1).
The additional adjustment for body weight attenuated the differences in the mean BMD (5.4–6.2 % point change) between US Caucasian women and African American or Afro-Caribbean women, but the differences remained significant. The body weight adjustment strengthened the differences in the femoral neck BMD values (7.0–8.1 % point change) between US Caucasian women and Asian ethnic groups, and reversed the differences in the total hip or spine BMD values (7.6–9.3 % point change) (Table 2; Fig. 2). Afro-Caribbean women had a higher adjusted BMD at all sites compared to African American women regardless of the adjustment for body weight. Compared to Hong Kong Chinese women, South Korean women displayed higher femoral neck and spine BMD and lower total hip BMD. The adjustment for age, weight, and height also displayed similar findings (Table 2).
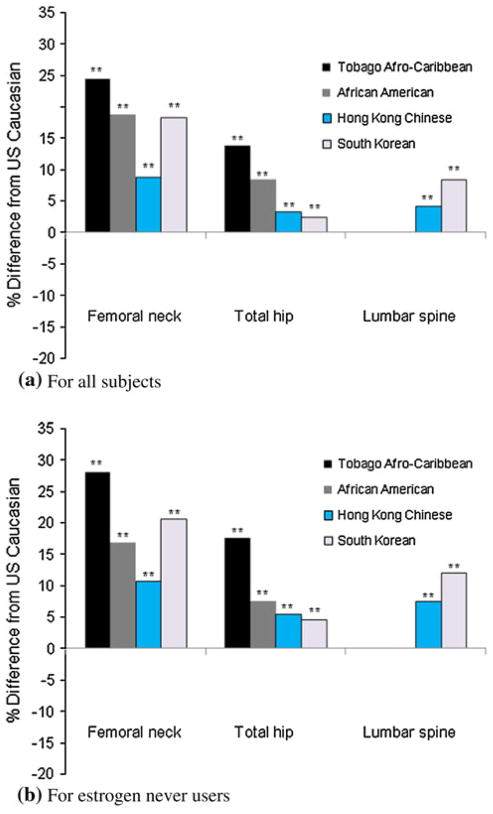
The additional adjustment for other factors (grip strength, smoking levels, current alcohol consumption, dietary calcium intake, years since last menstrual period, diabetes mellitus, and hypertension) showed relatively minor changes (−1.3 to 1.4 % point) to the differences in BMD between Caucasian women and each racial/ethnic group (Table 2; Fig. 3). When excluding current or past oral estrogen users, the percentage difference in the mean BMD from US Caucasian women was generally larger at all sites measured in both Afro-Caribbean and Asian women (1.8–3.8 % point change) (Table 2; Fig. 3).
Fig. 3.
Percentage differences in all covariates (age, weight, height, grip strength, smoking amount, current alcohol consumption, dietary calcium intake, years since last menstrual period, diabetes mellitus and hypertension) adjusted mean of BMD among Afro-Caribbean, African American, Hong Kong Chinese and South Korean women compared to US Caucasian women 65 year or older: a for all subjects, b for estrogen never users. **p <0.001 by Tukey test comparing BMD between US Caucasian women and each ethnic group
Discussion
Bone mineral density is a strong and consistent predictor of fracture risk among postmenopausal women [1, 2]. Therefore, current findings on the racial/ethnic and geographic differences in BMD have important implications for improving our understanding of the ethnic/geographic variability for fracture risk. When using the FRAX® tool [30] to calculate the major osteoporotic fracture risk with a mean BMD at the femoral neck in each ethnic group, if all subjects are 160 cm in height, 67.6 kg in weight, and 70 years old without other risk factors, the 10-year probability of fracture is 11.0 % in US Caucasian women, 3.7 % in African American women, 8.4 % in Hong Kong women, and 6.2 % in South Korean women. A probability of 3.3 % exists for Tobago Afro-Caribbean women using the FRAX® tool for African Americans. The large differences (23–31 %) in the age adjusted femoral neck BMD between African and Caucasian ancestry may contribute to the lower fracture risk among women of African ancestry. However, a paradox in the relationship of BMD and fracture risk between Asian and Caucasian women exists: lower or similar BMD values, but lower fracture rates among Asian women have been reported. Reported hip fracture rates among Hong Kong [7] and South Korean women [31] aged 60 to 79 are lower than US Caucasian women in the same age group. This paradox may in part be attributable to a more favorable hip geometry [32, 33], greater cortical thickness, higher trabecular volumetric BMD [34], and less frequent falls [35, 36] among Asian compared to Caucasian women. In addition to these findings, this study demonstrates that a higher femoral neck BMD adjusted for age in Korean women compared to Caucasian women may explain, in part, the lower fracture rates among Korean women.
Many studies [10–12, 37] have reported that the hip or spine BMD is similar between Caucasian and Asian women when adjusting for body size. In particular, the National Osteoporosis Risk Assessment (NORA) study [9] and SWAN [20] demonstrated the important role of body weight on the differences in BMD between US Caucasian and Asian women. In this study, similar to previous findings, adjustment for body weight decreased or reversed the differences in the total hip or spine BMD between Asian and non-Asian women. This phenomenon could be explained by the weight-bearing effect. A large difference in the range of body weight exists between Asian and non-Asian groups. The racial/ethnic variation in the mechanical stress of muscle mass may also explain the phenomenon. In general, Asian women display a lower lean mass compared to Caucasian women [38].
It is well known that women using postmenopausal hormone therapy have a higher BMD and experience slower rates of bone loss and fewer fractures compared to non-users [39–41]. In the current study, US women reported a higher prevalence of estrogen use compared to non-US women. The inclusion of estrogen users tended to modestly underestimate the ethnic differences in BMD between US and non-US women, and thus may be a confounder in the ethnic comparison of BMD among post-menopausal women. To our knowledge, this is the first study to demonstrate the contribution of the prevalence of estrogen use to differences in BMD between US and other women.
Substantial racial/ethnic differences in the BMD values within African or Asian ancestry groups were observed. The relatively higher hip BMD among Afro-Caribbean women compared to African American women could be explained by several factors. Afro-Caribbean women all reported that their parents and four grandparents were of African ancestry and thus possessed little or no European admixture. The higher weight-bearing activity due to the lower industrialization in Tobago compared with African American women in the US may additionally contribute [42]. In addition, Afro-Caribbean women are more likely to experience greater sun-exposure due to the lower latitude and higher levels of outdoor activity. Indeed, we published higher 25(OH)D among Tobago men [43]. However, factors that underlie the difference in BMD between Hong Kong Chinese women and South Korean women are not clear. As the magnitude of BMD differences between both groups only slightly differed after adjustment for lifestyle factors; therefore, genetic factors rather than lifestyle may play a more significant role.
We previously reported ethnic differences in men’s BMD [23] with a design similar to the current study. Results are similar in men and women; however, the magnitude of difference between Asian women and Caucasian women is smaller than that observed in men. The magnitude of BMD differences between African American women and Caucasian women is larger. Finally, in the analysis we demonstrated the contributing role of estrogen use to the difference in BMD between US and non-US women.
Several potential limitations exist in this study. Our findings were limited to women aged 65–75 and may not be generalizable to other age groups. However, our results are consistent with the SWAN ethnic comparisons of BMD in women aged 42–50 [20]. Subjects in the SOF and Namwon Study were recruited from population-based listings, but the Tobago Study and Hong Kong Study relied on word-of-mouth and posters. Hence, Tobago and Hong Kong women may over-represent healthy subjects. We could not adjust BMD for physical activity as the questionnaires differed across the studies. The comparison of dietary calcium intake between ethnic groups may be inaccurate as the food frequency questionnaires are specific for each country. We were unable to directly correct for the possible confounding effects of bone size, although the adjustment for height and weight may in part correct this effect. The values of BMD may have been underestimated in Asian, Afro-Caribbean, and African American women compared to Caucasian women. In several studies [44, 45], bone mineral apparent density (BMAD) measurements have been used to correct for differences in bone size. However, recent evidence [46] suggests that the BMAD may not address bone size differences appropriately when the race/ethnic groups differ in body size. Finally, the manufacturer of the DXA scanner for Korean women differed from that of the other racial/ethnic groups. Lunar scanners are more likely to overestimate BMD, while Hologic scanners underestimate the values [47, 48]. To remove this inherent bias, we used sBMD [26] in the cross-calibration procedure.
Our findings display substantial racial/ethnic differences in BMD within African or Asian ancestry individuals, and highlight the contributing role of body weight and estrogen use on the geographic and racial/ethnic variations in BMD.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2008-013-E00011). The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, R01 AG005407, R01 AG027576-22, 2 R01 AG005394-22A1, and 2 R01 AG027574-22A1. The Tobago Women’s Health Study was supported, in part, by funding or in-kind services from the Division of Health and Social Services, Tobago House of Assembly and grant R01 AR049747 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The Ms Os Hong Kong Study was supported by grants from the National Institute of Health, USA, with grant no. 1R01 AR049439-01A1, and the Research Grant Council of Hong Kong.
Footnotes
Conflict of interest All authors have no conflicts of interest.
Contributor Information
Hae-Sung Nam, Department of Preventive Medicine and Public Health, Research Institute for Medical Sciences, Chungnam National University School of Medicine, Daejeon, South Korea. Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, Crabtree Hall A524, Pittsburgh, PA 15261, USA.
Sun-Seog Kweon, Jeonnam Regional Cancer Center, Chonnam National University Hwasun Hospital, Hwasun, Jeollanam-do, South Korea. Department of Preventive Medicine, Chonnam National University Medical School, Gwangju, South Korea.
Jin-Su Choi, Department of Preventive Medicine, Chonnam National University Medical School, Gwangju, South Korea.
Joseph M. Zmuda, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, Crabtree Hall A524, Pittsburgh, PA 15261, USA
P. C. Leung, Jockey Club Centre for Osteoporosis Care and Control, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China
Li-Yung Lui, San Francisco Coordinating Center, California Pacific Medical Center, San Francisco, CA, USA.
Deanna D. Hill, Worldwide Epidemiology, GlaxoSmithKline Research & Development, Collegeville, PA, USA
Alan L. Patrick, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, Crabtree Hall A524, Pittsburgh, PA 15261, USA. Tobago Health Studies Office, Scarborough, Tobago, Trinidad and Tobago
Jane A. Cauley, Email: jcauley@edc.pitt.edu, Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, 130 DeSoto Street, Crabtree Hall A524, Pittsburgh, PA 15261, USA
References
- 1.Cummings SR, Black DM, Nevitt MC, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–668. [PubMed] [Google Scholar]
- 2.Mackey DC, Eby JG, Harris F, Taaffe DR, Cauley JA, Tylavsky FA, Harris TB, Lang TF, Cummings SR. Prediction of clinical non-spine fractures in older black and white men and women with volumetric BMD of the spine and areal BMD of the hip: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2007;22:1862–1868. doi: 10.1359/jbmr.070807. [DOI] [PubMed] [Google Scholar]
- 3.Liel Y, Edwards J, Shary J, Spicer KM, Gordon L, Bell NH. The effects of race and body habitus on bone mineral density of the radius, hip, and spine in premenopausal women. J Clin Endocrinol Metab. 1988;66:1247–1250. doi: 10.1210/jcem-66-6-1247. [DOI] [PubMed] [Google Scholar]
- 4.Luckey MM, Meier DE, Mandeli JP, DaCosta MC, Hubbard ML, Goldsmith SJ. Radial and vertebral bone density in white and black women: evidence for racial differences in premenopausal bone homeostasis. J Clin Endocrinol Metab. 1989;69:762–770. doi: 10.1210/jcem-69-4-762. [DOI] [PubMed] [Google Scholar]
- 5.Pollitzer WS, Anderson JJ. Ethnic and genetic differences in bone mass: a review with a hereditary vs environmental perspective. Am J Clin Nutr. 1989;50:1244–1259. doi: 10.1093/ajcn/50.6.1244. [DOI] [PubMed] [Google Scholar]
- 6.Kleerekoper M, Nelson DA, Peterson EL, Flynn MJ, Pawluszka AS, Jacobsen G, Wilson P. Reference data for bone mass, calciotropic hormones, and biochemical markers of bone remodeling in older (55–75) postmenopausal white and black women. J Bone Miner Res. 1994;9:1267–1276. doi: 10.1002/jbmr.5650090817. [DOI] [PubMed] [Google Scholar]
- 7.Lau EM, Lee JK, Suriwongpaisal P, Saw SM, De Das S, Khir A, Sambrook P. The incidence of hip fracture in four Asian countries: the Asian Osteoporosis Study (AOS) Osteoporos Int. 2001;12:239–243. doi: 10.1007/s001980170135. [DOI] [PubMed] [Google Scholar]
- 8.Cauley JA, Lui LY, Ensrud KE, Zmuda JM, Stone KL, Hochberg MC, Cummings SR. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293:2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 9.Barrett-Connor E, Siris ES, Wehren LE, Miller PD, Abbott TA, Berger ML, Santora AC, Sherwood LM. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20:185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 10.Russell-Aulet M, Wang J, Thornton JC, Colt EW, Pierson RN., Jr Bone mineral density and mass in a cross-sectional study of white and Asian women. J Bone Miner Res. 1993;8:575–582. doi: 10.1002/jbmr.5650080508. [DOI] [PubMed] [Google Scholar]
- 11.Ross PD, He Y, Yates AJ, Coupland C, Ravn P, McClung M, Thompson D, Wasnich RD. Body size accounts for most differences in bone density between Asian and Caucasian women. The EPIC (Early Postmenopausal Interventional Cohort) Study Group. Calcif Tissue Int. 1996;59:339–343. doi: 10.1007/s002239900137. [DOI] [PubMed] [Google Scholar]
- 12.Davis JW, Novotny R, Ross PD, Wasnich RD. The peak bone mass of Hawaiian, Filipino, Japanese, and white women living in Hawaii. Calcif Tissue Int. 1994;55:249–252. doi: 10.1007/BF00310400. [DOI] [PubMed] [Google Scholar]
- 13.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 14.Cauley JA, Wu L, Wampler NS, Barnhart JM, Allison M, Chen Z, Jackson R, Robbins J. Clinical risk factors for fractures in multi-ethnic women: the Women’s Health Initiative. J Bone Miner Res. 2007;22:1816–1826. doi: 10.1359/jbmr.070713. [DOI] [PubMed] [Google Scholar]
- 15.Cummings SR, Cauley JA, Palermo L, Ross PD, Wasnich RD, Black D, Faulkner KG. Racial differences in hip axis lengths might explain racial differences in rates of hip fracture. Study of Osteoporotic Fractures Research Group. Osteoporos Int. 1994;4:226–229. doi: 10.1007/BF01623243. [DOI] [PubMed] [Google Scholar]
- 16.Micklesfield LK, Norris SA, Pettifor JM. Ethnicity and bone: a South African perspective. J Bone Miner Metab. 2011;29:257–267. doi: 10.1007/s00774-011-0269-5. [DOI] [PubMed] [Google Scholar]
- 17.Aspray TJ, Prentice A, Cole TJ, Sawo Y, Reeve J, Francis RM. Low bone mineral content is common but osteoporotic fractures are rare in elderly rural Gambian women. J Bone Miner Res. 1996;11:1019–1025. doi: 10.1002/jbmr.5650110720. [DOI] [PubMed] [Google Scholar]
- 18.Dibba B, Prentice A, Laskey MA, Stirling DM, Cole TJ. An investigation of ethnic differences in bone mineral, hip axis length, calcium metabolism and bone turnover between West African and Caucasian adults living in the United Kingdom. Ann Hum Biol. 1999;26:229–242. doi: 10.1080/030144699282732. [DOI] [PubMed] [Google Scholar]
- 19.Shaffer JR, Kammerer CM, Reich D, et al. Genetic markers for ancestry are correlated with body composition traits in older African Americans. Osteoporos Int. 2007;18:733–741. doi: 10.1007/s00198-006-0316-6. [DOI] [PubMed] [Google Scholar]
- 20.Finkelstein JS, Lee ML, Sowers M, Ettinger B, Neer RM, Kelsey JL, Cauley JA, Huang MH, Greendale GA. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87:3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 21.Wong SY, Kwok T, Woo J, Lynn H, Griffith JF, Leung J, Tang YY, Leung PC. Bone mineral density and the risk of peripheral arterial disease in men and women: results from Mr. and Ms Os, Hong Kong. Osteoporos Int. 2005;16:1933–1938. doi: 10.1007/s00198-005-1968-3. [DOI] [PubMed] [Google Scholar]
- 22.Hill DD, Cauley JA, Bunker CH, Baker CE, Patrick AL, Beckles GL, Wheeler VW, Zmuda JM. Correlates of bone mineral density among postmenopausal women of African Caribbean ancestry: Tobago women’s health study. Bone. 2008;43:156–161. doi: 10.1016/j.bone.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nam HS, Shin MH, Zmuda JM, Leung PC, Barrett-Connor E, Orwoll ES, Cauley JA. Race/ethnic differences in bone mineral densities in older men. Osteoporos Int. 2010;21:2115–2123. doi: 10.1007/s00198-010-1188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 25.Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, Becich MJ, Trump DL, Kuller LH. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002;11:726–729. [PubMed] [Google Scholar]
- 26.Hui SL, Gao S, Zhou XH, Johnston CC, Jr, Lu Y, Gluer CC, Grampp S, Genant H. Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res. 1997;12:1463–1470. doi: 10.1359/jbmr.1997.12.9.1463. [DOI] [PubMed] [Google Scholar]
- 27.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92:969–977. [PubMed] [Google Scholar]
- 28.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 29.Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 30.Kanis JA on behalf of the WHO Scientific Group. WHO Collaborating Centre for Metabolic Bone Diseases. WHO Collaborating Centre for Metabolic Bone Diseases, University of Sheffield; 2007. Assessment of osteoporosis at the primary health care level. [Google Scholar]
- 31.Lim S, Koo BK, Lee EJ, Park JH, Kim MH, Shin KH, Ha YC, Cho NH, Shin CS. Incidence of hip fractures in Korea. J Bone Miner Metab. 2008;26:400–405. doi: 10.1007/s00774-007-0835-z. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Turner CH, Yoshikawa T, Slemenda CW, Peacock M, Burr DB, Mizuno Y, Orimo H, Ouchi Y, Johnston CC., Jr Do variations in hip geometry explain differences in hip fracture risk between Japanese and white Americans? J Bone Miner Res. 1994;9:1071–1076. doi: 10.1002/jbmr.5650090715. [DOI] [PubMed] [Google Scholar]
- 33.Brownbill RA, Ilich JZ. Hip geometry and its role in fracture: what do we know so far? Curr Osteoporos Rep. 2003;1:25–31. doi: 10.1007/s11914-003-0005-8. [DOI] [PubMed] [Google Scholar]
- 34.Marshall LM, Zmuda JM, Chan BK, Barrett-Connor E, Cauley JA, Ensrud KE, Lang TF, Orwoll ES. Race and ethnic variation in proximal femur structure and BMD among older men. J Bone Miner Res. 2008;23:121–130. doi: 10.1359/JBMR.070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aoyagi K, Ross PD, Davis JW, Wasnich RD, Hayashi T, Takemoto T. Falls among community-dwelling elderly in Japan. J Bone Miner Res. 1998;13:1468–1474. doi: 10.1359/jbmr.1998.13.9.1468. [DOI] [PubMed] [Google Scholar]
- 36.Davis JW, Nevitt MC, Wasnich RD, Ross PD. A cross-cultural comparison of neuromuscular performance, functional status, and falls between Japanese and white women. J Gerontol A Biol Sci Med Sci. 1999;54:M288–M292. doi: 10.1093/gerona/54.6.m288. [DOI] [PubMed] [Google Scholar]
- 37.Roy D, Swarbrick C, King Y, Pye S, Adams J, Berry J, Silman A, O’Neill T. Differences in peak bone mass in women of European and South Asian origin can be explained by differences in body size. Osteoporos Int. 2005;16:1254–1262. doi: 10.1007/s00198-005-1837-0. [DOI] [PubMed] [Google Scholar]
- 38.Liang MT, Bassin S, Dutto D, Braun W, Wong N, Pontello AM, Cooper DM, Arnaud SB. Bone mineral density and leg muscle strength in young Caucasian, Hispanic, and Asian women. J Clin Densitom. 2007;10:157–164. doi: 10.1016/j.jocd.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ensrud KE, Palermo L, Black DM, Cauley J, Jergas M, Orwoll ES, Nevitt MC, Fox KM, Cummings SR. Hip and calcaneal bone loss increase with advancing age: longitudinal results from the study of osteoporotic fractures. J Bone Miner Res. 1995;10:1778–1787. doi: 10.1002/jbmr.5650101122. [DOI] [PubMed] [Google Scholar]
- 40.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 41.Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, Beck TJ, Ensrud KE. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53:183–189. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 42.Hill DD, Cauley JA, Sheu Y, Bunker CH, Patrick AL, Baker CE, Beckles GL, Wheeler VW, Zmuda JM. Correlates of bone mineral density in men of African ancestry: the Tobago bone health study. Osteoporos Int. 2008;19:227–234. doi: 10.1007/s00198-007-0450-9. [DOI] [PubMed] [Google Scholar]
- 43.Miljkovic I, Bodnar LM, Cauley JA, Bunker CH, Patrick AL, Wheeler VW, Kuller LH, Zmuda JM. Low prevalence of vitamin D deficiency in elderly Afro-Caribbean men. Ethn Dis. 2011;21:79–84. [PMC free article] [PubMed] [Google Scholar]
- 44.Lau EM, Lynn H, Woo J, Melton LJ., 3rd Areal and volumetric bone density in Hong Kong Chinese: a comparison with Caucasians living in the United States. Osteoporos Int. 2003;14:583–588. doi: 10.1007/s00198-003-1402-7. [DOI] [PubMed] [Google Scholar]
- 45.Wang XF, Duan Y, Beck TJ, Seeman E. Varying contributions of growth and ageing to racial and sex differences in femoral neck structure and strength in old age. Bone. 2005;36:978–986. doi: 10.1016/j.bone.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Nevill AM, Holder RL, Maffulli N, Cheng JC, Leung SS, Lee WT, Lau JT. Adjusting bone mass for differences in projected bone area and other confounding variables: an allometric perspective. J Bone Miner Res. 2002;17:703–708. doi: 10.1359/jbmr.2002.17.4.703. [DOI] [PubMed] [Google Scholar]
- 47.Reid DM, Mackay I, Wilkinson S, et al. Cross-calibration of dual-energy X-ray densitometers for a large, multi-center genetic study of osteoporosis. Osteoporos Int. 2006;17:125–132. doi: 10.1007/s00198-005-1936-y. [DOI] [PubMed] [Google Scholar]
- 48.Pearson D, Horton B, Green DJ. Cross calibration of DXA as part of an equipment replacement program. J Clin Densitom. 2006;9:287–294. doi: 10.1016/j.jocd.2006.02.006. [DOI] [PubMed] [Google Scholar]