Abstract
Background
Deficits in the capacity to reflect about the self and others (“social reflection” [SR]) have been identified in schizophrenia, as well as in people with a genetic or clinical risk for the disorder. However, the neural underpinnings of these abnormalities are incompletely understood.
Methods
Responses of a network of brain regions known to be involved in self and other processing (e.g., medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), and superior temporal gyrus (STG)) were measured during SR in 16 first-degree, non-psychotic relatives (RELS) of schizophrenia patients and 16 healthy controls (CONS). Because of prior evidence linking dysfunction in this network and delusions, associations between SR-related responses of this network and subclinical delusions (measured using the Peters et al. Delusions Inventory) were also examined.
Results
Compared with CONS, RELS showed significantly less SR-related activity of the right and left PCC and STG. Moreover, response magnitudes were negatively correlated with levels of delusional thinking across both groups.
Conclusions
These findings suggest that aberrant function of the neural circuitry underpinning SR is associated with the genetic liability to schizophrenia and confers vulnerability to delusional beliefs.
Keywords: social reflection, schizophrenia, delusions, genetic risk, relatives
1. Introduction
Disturbances of reflection about the self and others (“social reflection” [SR]) are thought to make a contribution to the symptomatology (Bosia et al., 2012; Brent et al., 2014c) and social dysfunction (van Os et al., 2010) of schizophrenia. Several lines of evidence suggest SR impairments contribute to the vulnerability to schizophrenia. First, SR disruptions (e.g., poor self-other boundary discrimination, or an altered sense of self) have been identified in children at genetic risk for schizophrenia (Keshavan et al., 2008) and are among the earliest reported symptoms in people later developing the illness (Klosterkotter et al., 2001; Poulton et al., 2000). Second, prospective studies have found deficits in taking the perspective of others (Schiffman et al., 2004) and impaired social function (Amminger et al., 1999; Tarbox and Pogue-Geile, 2008) in children and adolescents who develop schizophrenia. Third, a meta-analysis has shown that theory of mind [ToM] deficits are significantly greater in people at clinical high-risk for psychosis (i.e., “prodromal” individuals) and in unaffected relatives of schizophrenia patients (Bora and Pantelis, 2013). Furthermore, schizotypal traits (Pickup, 2006) and subclinical delusions (Galbraith et al., 2008) (e.g., mild delusional ideation that is thought in many cases to be on an etiological continuum with delusions in schizophrenia (Dominguez et al., 2011)) have been associated with SR impairments in otherwise healthy subjects. Based on these findings, one remaining question is whether SR impairment in schizophrenia is linked with a pattern of altered neural function that may represent an intermediate phenotype for the disorder.
Neuroimaging studies in healthy subjects have shown that SR tasks involving the retrieval of information about the self (Jenkins and Mitchell, 2011; Kelley et al., 2002; Schmitz et al., 2004) and/or others (Frith and Frith, 2003; Gallagher et al., 2000; Saxe and Kanwisher, 2003) consistently engage a network comprised of cortical midline structures ([CMS]; e.g., medial prefrontal cortex [MPFC] and posterior cingulate cortex [PCC]) and parts of the lateral temporal cortex ([LTC]; e.g., superior temporal gyrus [STG]). Also, these same brain areas show increased activity during resting-states (i.e., “default mode” activity (Buckner et al., 2008)).
Typically, brain areas underpinning SR show increased activation during tasks involving judgments about mental states (i.e., thoughts, feelings, intentions), or personality characteristics of the self or others, as opposed to judgments about their physical characteristics, or the semantic aspects of the stimuli (Amodio and Frith, 2006). According to simulation models of social perception, self-reflection may serve as a fundamental starting point for understanding other people (Gallese and Goldman, 1998). Consistent with this theory, a number of studies have shown considerable overlap between the pattern of neural activation observed during self-reflection and other-processing, including ToM (Happe, 2003; Lombardo et al., 2009; Tamir and Mitchell, 2010).
Several studies have reported aberrant activation in schizophrenia patients of MPFC and/or PCC (Bedford et al., 2012; Blackwood et al., 2004; Holt et al., 2011a; Lee et al., 2011; van der Meer et al., 2013) (CMS structures) and the LTC (Brune et al., 2008; Murphy et al., 2010; Pedersen et al., 2012; Walter et al., 2009) during SR tasks. A few studies have also linked altered CMS and LTC function during SR with subsyndromal psychotic symptoms (Brent et al., 2014a; Brune et al., 2011; Modinos et al., 2011). Additionally, several studies have provided evidence for similar alterations of CMS (de Achaval et al., 2012; Marjoram et al., 2006) and LTC (Walter et al., 2011) during ToM in people at genetic risk for schizophrenia. Consistent with these task-based fMRI findings, abnormal resting-state functional connectivity of CMS in relatives of schizophrenia patients (Jang et al., 2011; van Buuren et al., 2012; Whitfield-Gabrieli et al., 2009) suggest that these regional abnormalities are linked to an overall change in the coordinated functioning of this network. Taken together, these results support the possibility that impaired functioning of the neural circuitry involved in SR in schizophrenia is genetically mediated in part and contributes to the emergence of psychotic symptoms.
Here, we investigated this possibility by comparing neural responses during SR in first-degree, non-psychotic relatives of schizophrenia patients and demographically-matched controls, using a well-validated fMRI SR paradigm (Kelley et al., 2002). Given the evidence for altered CMS and LTC activity during SR in schizophrenia, we predicted that, compared to controls, relatives would show aberrant CMS and the LTC activation during SR. Additionally, because of prior evidence linking impaired neural function during SR and subclinical delusions in healthy subjects (Brent et al., 2014a), we tested for a similar association in this cohort.
2. Materials and Methods
2.1 Participants
Sixteen first-degree relatives (RELS) of DSM-IV diagnosed schizophrenia patients and 16 controls (CONS) were enrolled. Patients’ schizophrenia diagnoses were confirmed using the Structured Clinical Interview for DSM-IV Axis I Disorders ([SCID]; (Frist et al., 2002)). RELS had no history of taking psychotropic medications and no lifetime history of schizophrenia, Axis I psychotic disorders, major depression, bipolar disorder, or Axis I anxiety disorders based on SCID interviews. CONS were recruited via advertisement and had no first-degree relatives with schizophrenia or psychosis based on family psychiatric history screening interviews. CONS had no history of taking psychotropic medications, or of Axis I disorders as determined via the SCID. All subjects were right-handed and native English speakers. Subjects with neurological disorders, serious medical illnesses, substance abuse or dependence, or contraindications to MRI scanning (e.g., claustrophobia, metal implants) were excluded. Written informed consent was obtained from all subjects in accordance with the guidelines of Beth Israel Deaconess Medical Center’s Committee on Clinical Investigations and Partners HealthCare’s Institutional Review Board. There were no significant between-group differences in the demographic variables or across additional symptom and cognitive domains that were assessed (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Participants
| Total Sample n = 32, 19 female |
Controls n = 16, 10 female |
Relatives n = 16, 9 female |
p Value | Effect Size | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | IQR | Mean | SD | Median | IQR | Mean | SD | Median | IQR | |||
| Age* (years) | 43.8 | 13.7 | 49.0 | 27.5 | 41.4 | 14.2 | 46.0 | 27.8 | 46.1 | 13.3 | 53.5 | 26.2 | .25 | .20 |
| Education* (years) | 15.9 | 2.5 | 16.0 | 3.4 | 16.2 | 1.8 | 16.0 | 3.6 | 15.6 | 3.1 | 16.0 | 2.7 | .53 | .08 |
| MPE (years) | 14.9 | 2.7 | 15.5 | 4.4 | 14.1 | 3.4 | 13.5 | 4.0 | 16.1 | 3.5 | 16.0 | 4.6 | .06 | .58 |
| Mean Parental SESa | 1.8 | 1.0 | 2.0 | 1.0 | 2.1 | 1.1 | 2.0 | 1.7 | 1.6 | 1.0 | 1.0 | 1.0 | .23 | .47 |
| Premorbid IQb | 109.9 | 7.7 | 110.6 | 10.7 | 109.2 | 8.6 | 111.1 | 13.4 | 110.7 | 6.9 | 110.2 | 11.1 | .59 | .19 |
| PDI* Totalc | 3.4 | 4.5 | 2.5 | 3.7 | 2.3 | 2.3 | 2.0 | 2.0 | 4.5 | 5.9 | 3.0 | 4.7 | .15 | .26 |
| PDI*c Distress | 8.0 | 20.2 | 3.0 | 5.8 | 4.5 | 6.4 | 2.5 | 4.8 | 11.6 | 27.9 | 3.5 | 8.0 | .28 | .19 |
| PDI*c Preoccupation | 9.4 | 19.7 | 5.0 | 10.0 | 5.5 | 5.8 | 3.0 | 7.5 | 13.3 | 27.1 | 6.0 | 10.7 | .37 | .16 |
| PDI*c Conviction | 10.9 | 13.9 | 8.0 | 10.7 | 7.6 | 7.6 | 5.0 | 9.5 | 14.2 | 17.9 | 10.5 | 14.0 | .19 | .24 |
| State Anxietyd | 31.1 | 9.0 | 30.5 | 12.2 | 29.9 | 7.4 | 28.0 | 13.7 | 32.3 | 10.0 | 28.0 | 13.7 | .44 | .28 |
| Trait Anxietyd | 33.7 | 8.7 | 32.0 | 15.5 | 31.9 | 8.3 | 29.0 | 11.5 | 35.6 | 9.6 | 29.0 | 11.5 | .26 | .41 |
| Depression*e | 4.0 | 5.5 | 1.5 | 7.5 | 2.8 | 4.9 | 1.0 | 3.7 | 5.3 | 6.3 | 2.5 | 8.7 | .18 | .24 |
| Physical Anhedonia*f | 54.1 | 4.4 | 56.0 | 7.0 | 55.3 | 4.5 | 54.0 | 7.7 | 53.0 | 4.2 | 56.5 | 4.5 | .11 | .29 |
| ToM*g (Social Stories) | 13.8 | 1.9 | 14.0 | 2.2 | 13.9 | 1.8 | 14.0 | 2.0 | 13.7 | 2.1 | 14.0 | 3.0 | .84 | .04 |
| ToM*g (Phys. Stories) | 14.3 | 2.3 | 15.0 | 2.2 | 13.6 | 2.8 | 15.0 | 4.0 | 14.9 | 1.4 | 15.0 | 2.0 | .33 | .19 |
| WASI Vocabh | 61.3 | 8.4 | 63.0 | 9.0 | 60.2 | 7.8 | 61.0 | 13.0 | 62.3 | 9.0 | 65.5 | 8.3 | .48 | .25 |
| WASI Block Designh | 55.2 | 10.1 | 55.0 | 15.7 | 54.8 | 8.8 | 53.0 | 16.2 | 55.6 | 11.4 | 55.5 | 26.5 | .82 | .08 |
| Head Motion (mm)*i | 1.0 | 0.7 | 0.8 | 0.7 | 1.0 | 0.7 | 0.8 | 0.7 | 1.0 | 0.8 | 0.8 | 0.9 | .81 | .05 |
There were no significant differences between the relative and control groups in any of the demographic variables. Variables exhibiting a non-normal distribution are indicated with an asterisk (*). For non-parametric data, differences between the two groups were assessed using the Wilcoxon-Mann-Whitney test. For normally distributed variables, differences between the two groups were assessed using an independent two-sample Student t-test.
Measured with Hollingshead index
Measured with the Adult North American Reading List
Measured with Peters et al. Delusions Inventory
Measured with Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1970)
Measured with Beck Depression Inventory (Beck and Steer, 1987)
Measured with the Chapman Physical Anhedonia Subscale (Chapman et al., 1976)
Measured with Strange Stories (Happe, 1994)
T scores
Mean total vector translation in millimeters
MPE, Mean Parental Education; PDI, Peters et al. Delusions Inventory; Phys., Physical; SES, socioeconomic status; ToM, Theory of Mind; WASI, Wechsler: Abbreviated Scale of Intelligence (Wechsler, 1999)
2.2 Stimulus Presentation and Task
During the experiment, participants were asked to make judgments (“yes” or “no”) about 144 trait adjectives, presented one at a time, in four conditions: 1) “does this word describe you?” [Self (S)]; 2) “does this word describe your mother?” [Other (O)]; 3) “is this a desirable trait?” [Affect Labeling (AL)]; 4) “is this word printed in upper, or lower case letters?” [Perceptual (P)]. Each word was viewed for 3 seconds in eight 18-second blocks (i.e., 6 words per block and 2 blocks per condition) during three (each 6 minute 9 seconds long) functional runs. Each block was preceded by an instruction screen viewed for 3 seconds and was followed by a 21 second fixation period. Subjects registered their responses via a button box, using the index finger of either the right or left hand. Additional details regarding the stimuli and task are included in the Supplementary Materials (see also Holt et al., 2011a and Brent et al., 2014a). Behavioral responses were not recorded for two CONS and one of the RELS due to equipment failure.
2.3 MRI Data Acquisition
Functional and structural MRI data were collected using a 3.0 Tesla TIM Trio magnetic resonance scanner at Massachusetts General Hospital (Siemens Medical Systems, Iselin, New Jersey) with echo-planar capability and a twelve-channel head coil. Acquisition parameters for the functional and anatomical scans, which were identical to those of our previous study (Brent et al., 2014a), are provided in the Supplementary Materials.
2.4 fMRI Analysis
Three mm isotropic functional data were analyzed using the FreeSurfer Functional Analysis Stream (http://surfer.nmr.mgh.harvard.edu/fswiki) using standard preprocessing methods. Images were spatially smoothed with a 6-mm (full-width-half maximum) 3-dimensional spatial filter. As done previously, we compared activation during SR [0.5(S) + 0.5(O)] with activation during AL (Brent et al., 2014a). Since the P task entailed responses with objectively correct answers, it was used to assess subjects’ task engagement. Prior fMRI work using the same SR paradigm employed here has shown alterations in CMS (MPFC and/or PCC) during self-reflection (Bedford et al., 2012; Blackwood et al., 2004; Holt et al., 2011a; van der Meer et al., 2013) and in the STG during other-reflection (Murphy et al., 2010) in schizophrenia. Thus, we hypothesized that we would observe differences between the two groups in SR-related activity within three a priori, anatomically-defined regions of interest (ROIs): the MPFC, PCC, and STG. These ROIs were defined using an automated parcellation method (FreeSurfer (Fischl et al., 1999)) that uses sulcal and gyral landmarks of each subject’s anatomical scan to delineate the boundaries between cortical areas (Desikan et al., 2006). To test our hypothesis, a regions-of-interest (ROI) analysis was conducted using responses averaged across each ROI during each condition, compared to a low-level baseline (Brent et al., 2014a). A 2 (task: SR, AL) x 3 (region: MPFC, PCC, STG) x 2 (hemisphere: right, left) x 2 (group: relatives, controls) analysis of variance was performed to test for group main effects or interactions. Significant effects were followed up by planned t-tests.
2.5 Subclinical Delusions and Correlations with fMRI Data
Subclinical delusions were measured using the Peters et al. Delusions Inventory ([PDI] (Peters et al., 1999). The 40-item PDI is a validated self-report questionnaire (Peters et al., 1999) assessing delusions in the non-clinical population. Each delusional idea is rated 0 (‘no’) or 1 (‘yes’), yielding a PDI-total score ranging from 0 to 40. Every endorsed belief is also rated on three 5-point Likert subscales: Distress, Preoccupation, and Conviction. Because both subject groups were healthy and non-clinical, and to optimize our power to detect effects, our primary correlational analysis was conducted in the full cohort of subjects using Spearman’s rank correlation coefficient (r). The significance level was set at p < .017 (p < .05 with Bonferroni correction for the number of ROIs (3) examined). Exploratory analyses in the two separate groups and with PDI subscale scores were also conducted.
3. RESULTS
3.1 Symptoms
Mean PDI-total scores (Table 1) both for the total sample and each group were consistent with prior studies in non-clinical populations (Brent et al., 2014a; Peters et al., 1999). There were no significant differences between RELS and CONS in levels of delusional thinking (mean PDI-total and delusional subscale scores) or any of the other clinical or cognitive characteristics measured.
3.2 Behavior
ANOVAs revealed no main effects of group, or group by condition interactions (all ps > .28) for reaction times, and no between-group differences in response types (see Table 2).
Table 2.
Behavioral Results
| Reaction Times (Milliseconds) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Self-Reflection | Other-Reflection | Affect Labeling | Perceptual* | |||||||||||||||||
| Mean | SD | MDN | IQR | p Value | Mean | SD | MDN | IQR | p Value | Mean | SD | MDN | IQR | p Value | Mean | SD | MDN | IQR | p Value | |
| Controls | 1333 | 224 | 1263 | 300 | 0.65 | 1467 | 261 | 1422 | 347 | 0.79 | 1301 | 203 | 1304 | 329 | 0.27 | 852 | 174 | 790 | 278 | 0.38 |
| Relatives | 1369 | 204 | 1398 | 356 | 1442 | 242 | 1422 | 327 | 1378 | 165 | 1447 | 272 | 903 | 203 | 843 | 95 | ||||
| Response Types (%) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Self-Reflection (% Like Self) | Other-Reflection (% Like Other) | Affect Labeling* (% Positive) | Perceptual* (% Correct) | |||||||||||||||||
| Mean | SD | MDN | IQR | p Value | Mean | SD | MDN | IQR | p Value | Mean | SD | MDN | IQR | p Value | Mean | SD | MDN | IQR | p Value | |
| Controls | 42.4 | 13 | 45.7 | 6.3 | 0.29 | 44.0 | 15 | 48.6 | 9.0 | 0.87 | 49.0 | 6.5 | 47.2 | 6.0 | 0.20 | 96.0 | 13.3 | 100 | 0 | 0.89 |
| Relatives | 48.1 | 15 | 50.0 | 9.7 | 44.9 | 14 | 50.0 | 8.3 | 49.2 | 2.9 | 50.0 | 4.0 | 95.8 | 13.3 | 100 | 0 | ||||
Mean reaction times and percentage of response types with p values for the independent t-tests, or Wilcoxon-Mann-Whitney tests (for nonparametric data, indicated with asterisk[*]) comparing the two group means for the four experimental tasks. Both groups showed significantly greater response times during the Other condition compared to the Self or Affect Labeling conditions, but there was no significant difference in response times between the Self. and Affect Labeling conditions. There were no between-group differences in response times for any experimental condition. Both groups showed comparable percentages of words rated as “like self,” “like mom,” or “positive” in the Self, Other, and Affect Labeling conditions, respectively. The percentage of correct trails in the Perceptual condition was similarly high in each group.
3.3 Functional MRI
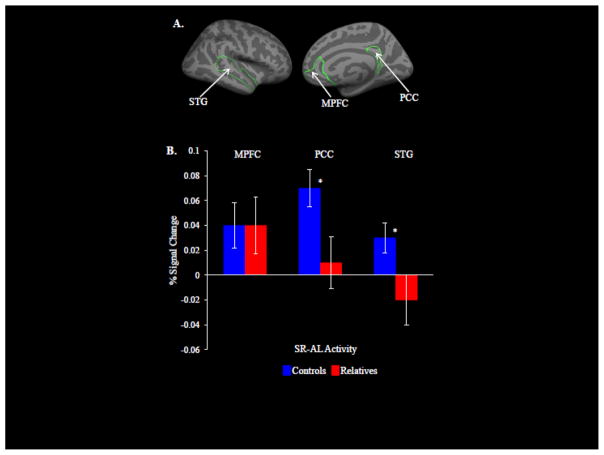
A significant Region by Task by Group interaction (F = 4.20; df = 2, 30; p = .02, ηρ2 = .12) was found, but no Region by Task by Group by Hemisphere interaction (F = .08; df = 2, 30; p = .92; ηρ2 = .003). Follow-up, within-group analyses showed that CONS exhibited greater SR responses compared with AL (i.e., SR-AL activity) in the MPFC (t = 2.2; df = 15; p = .04; d = .57), PCC (t = 4.5; df = 15; p = .0005; d = 1.16), and STG (t = 2.4; df = 15; p = .03; d = .62). The RELS showed no significant SR-AL responses in any of the three a priori ROIs. Between-group comparisons revealed that, compared to RELS, CONS showed significantly greater SR-AL responses in the PCC (t = 2.3; df = 30; p = .03; d = .84) and STG (t = 2.3; df = 30; p = .03; d = .84), but not in the MPFC (t = −.05; df = 30; p = .96; d = −.02) (Figure 1B).
Figure 1.
Results of the fMRI analysis. (A) The three ROIs (medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), and superior temporal gyrus (STG)), defined a priori using FreeSurfer, are shown in these right medial and lateral views of a representative cortical surface. (B) Between-group comparisons showed that controls exhibited significantly greater SR-AL activation in the PCC and STG (* between-group difference, p < .05) compared to relatives, but not in the MPFC.
3.4 Correlations
Total PDI score was negatively correlated with SR-AL responses (data for right and left hemispheres combined) of the PCC (r = −.42; df = 30; p = .01) and STG (r = −.49; df = 30; p = .004; [Figure 2A, 2B]). These correlations between total PDI and the SR-AL responses of the PCC and STG remained significant when controlling for anxiety, depression, and anhedonia. A negative correlation between total PDI scores and SR-AL responses of the MPFC was also found, but did not survive correction for multiple comparisons (r = −.38; df = 30; p = .03). Correlational analyses for the three PDI subscales (Distress, Preoccupation, and Conviction) showed the same pattern of effects, or trends, for each ROI (ps = .005 to .08; all rs = −.48 to −.31). Similar analyses in the two separate groups revealed that in the RELS there were effects in all three ROIs, with the strongest association in the MPFC, while CONS showed significant correlations in the STG only (Table 3). To compare these associations in the RELS and CONS, correlation coefficients were converted to z-scores (Zar, 1996) (Table 3). The association between subclinical delusions and the SR-related responses of the MPFC showed a trend toward being significantly greater in RELS compared to CONS (z = −1.84, p = .07). There were no significant differences in the correlations between subclinical delusions and SR-related activity in the PCC and STG in RELS compared to CONS. Secondary analyses showed no correlations between any of the additional symptom and cognitive domains assessed and the SR-AL responses of the MPFC, PCC, or STG. Further, the results of the main ANOVA remained unchanged when total PDI scores were entered as a covariate.
Figure 2.
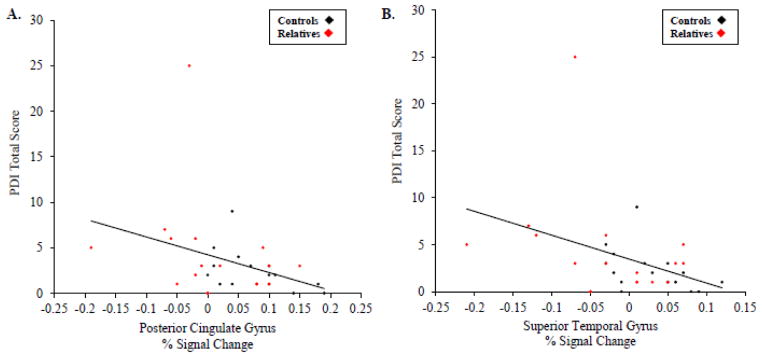
Correlations between subclinical delusions and SR-AL responses. Scatter plots display the negative correlations between PDI total score and the SR-AL responses (percent signal change) of the PCC (r = −.42; df = 30; p = .01) (A) and the STG (r = −.49; df = 30; p = −.004) (B) in the full sample (n = 32).
Table 3.
Correlations between Subclinical Delusions and Social Reflection-Related Responses in the Medial Prefrontal Cortex, Posterior Cingulate Cortex and Superior Temporal Gyrus
| A. Medial Prefrontal Cortex | ||||||
| RH | Z-Score | LH | Z-Score | RH + LH | Z-Score | |
|
| ||||||
| Total Sample | −.36 (.04) | −.38 | −.39 (.03) | −.41 | −.38 (.03) | −.40 |
| Relatives | −.64 (.007) | −.76 | −.64 (.007) | −.76 | −.67 (.005) | −.81 |
| Controls | −.04 (.88) | −.04 | −.16 (.55) | −.16 | −.09 (.74) | −.09 |
| B. Posterior Cingulate Cortex | ||||||
| RH | Z-Score | LH | Z-Score | RH + LH | Z-Score | |
| Total Sample | −.44 (.01) | −.47 | −.39 (.03) | −.41 | −.42 (.01) | −.45 |
| Relatives | −.42 (.10) | −.45 | −.38 (.14) | −.40 | −.45 (.07) | −.49 |
| Controls | −.36 (.16) | −.38 | −.35 (.19) | −.37 | −.32 (.22) | −.33 |
| C. Superior Temporal Gyrus | ||||||
| RH | Z-Score | LH | Z-Score | RH + LH | Z-Score | |
| Total Sample | −.47 (.01) | −.51 | −.48 (.01) | −.52 | −.49 (.004) | −.54 |
| Relatives | −.45 (.07) | −.49 | −.45 (.07) | −.49 | −.44 (.08) | −.47 |
| Controls | −.44 (.08) | −.47 | −.51 (.04) | −.56 | −.58 (.02) | −.66 |
Correlations between levels of subclinical delusions (Peters et al. Delusions Inventory (PDI) total scores) and the social reflection (SR) – affect labeling (AL) responses of the right hemisphere (RH), left hemisphere (LH), and right and left hemispheres combined (RH + LH) for the (A) medial prefrontal cortex (MPFC), (B) posterior cingulate cortex (PCC), and (C) superior temporal gyrus (STG) in the total sample, relatives, and controls are represented as Spearman’s r, with p values in parentheses.
LH, left hemisphere; RH, right hemisphere.
4. Discussion
Here we found that first-degree relatives of schizophrenia patients showed significantly reduced SR-related activity in the PCC and STG, compared to demographically matched controls without genetic risk for schizophrenia or psychosis. These between-group differences were due to the presence of significant SR-related activity in all three a priori ROIs (MPFC, PCC, STG) in the controls, as expected, whereas relatives failed to show this activity in all three regions. Greater levels of delusional thinking also predicted reduced SR-related activity of these same brain regions across all subjects.
Altered PCC and/or STG function has been reported in prior studies of SR in schizophrenia (Blackwood et al., 2004; Holt et al., 2011a; Lee et al., 2010). The pattern of aberrant SR-related activation we observed in relatives differs from some prior neuroimaging findings in schizophrenia (e.g., aberrant MPFC activity (for reviews see: Bosia et al., 2012; Brent et al., 2014c), or PCC hyperactivity (Blackwood et al., 2004; Holt et al., 2011a; Shad et al., 2012)). This is not inconsistent with the literature, as a risk phenotype may not entirely overlap with the pattern of disease-associated abnormalities (Kern et al., 2013). For example, medial temporal lobe hyperactivity is observed during memory tasks in mild cognitive impairment, whereas Alzheimer’s dementia is associated with reduced hippocampal and parahippocampal activation during memory task performance (Dickerson and Sperling, 2008).
Consistent with prior findings in schizophrenia and our results, evidence for aberrant activity of the left PCC (de Achaval et al., 2012) and left temporo-parietal junction (Walter et al., 2011) (including the STG) during ToM has been found in individuals at genetic risk for schizophrenia. In contrast, one study found no differences between relatives of schizophrenia patients and controls in the magnitude of neural responses during self-reflection (van Buuren et al., 2012). This may be related to a difference in power (our study collected data over three six minute-long functional runs, compared to the single four minute run collected in the earlier study (van Buuren et al., 2010)).
The relationship between abnormal neural function during SR and subclinical psychotic symptoms is incompletely understood. Models of delusions in schizophrenia suggest impaired SR may play a role in the formation of delusional beliefs (Bentall et al., 2001; Salvatore et al., 2012b). Supporting this hypothesis, fMRI studies have demonstrated links between dysfunction of brain areas mediating SR, including the MPFC (Menon et al., 2011; Modinos et al., 2011), PCC (Holt et al., 2011b), and the STG (Backasch et al., 2013) and delusions in schizophrenia or schizotypy. Additionally, we previously found negative correlations between levels of delusional thinking in healthy individuals and 1) left LTC activation during SR and 2) left LTC-left MPFC resting-state connectivity (Brent et al., 2014a). Our current finding of a negative correlation between levels of subclinical delusions and STG and PCC SR-related activity replicates and extends this earlier work.
The association between subclinical delusions and SR-related activity may have a somewhat different pattern in people with genetic loading for schizophrenia, compared to those without this loading. Our within-group analyses showed that the relationship between subclinical delusions and SR-related activity in the PCC and STG was similar in relatives and controls. However, relatives showed a negative correlation between delusions and SR-related MPFC responses not observed in controls. Notably, relatives showed impaired SR-related PCC and STG responses compared to controls, whereas MPFC function was on average preserved in the relatives. One possible interpretation of these findings is that these regions are affected in a sequential manner in psychosis, with the STG and the PCC showing the earliest abnormalities (thus relatives showed the greatest changes in these regions, leading to significant between-group differences). The MPFC, on the other hand, may be affected later in the course of the illness, or only in those who have subclinical delusions. This model can be tested in future studies that longitudinally measure changes in SR-related functioning of this network and symptom severity in at-risk populations.
The interpretation of our study is limited by its sample size; replication of our findings in an additional, larger sample will be necessary. Also, future longitudinal studies can test if altered neural function during SR represents a stable, trait-like characteristic associated with genetic risk for schizophrenia, and whether worsening SR-related neural function is predictive of the transition to psychosis.
In summary, these results suggest that dysfunction of the neural circuitry mediating SR is related to the genetic liability to schizophrenia and the pathophysiology of delusional beliefs. Additionally, the breakdown of SR, combined with elevated stress, may lead to aberrant explanations of social experience and the emergence of psychosis (Brent et al., 2014b). Increasing evidence suggests that appropriately designed treatments (e.g., cognitive training (Eack et al., 2009; Subramaniam et al., 2012) and metacognitive psychotherapy (Lysaker et al., 2007; Salvatore et al., 2012a)) may ameliorate SR deficits in schizophrenia and improve real world functioning. Incorporating similar treatments into the care of people at risk for schizophrenia could preempt the deterioration of SR and potentially reduce the chances of transitioning to psychosis.
Supplementary Material
Acknowledgments
Role of funding source None
Funding
This work was supported by a KL2 Medical Research Investigator Training (MeRIT) award from the Harvard Catalyst and the Harvard Clinical and Translational Science Center, NIH KL2 RR 025757 (BKB), Harvard Medical School’s Stuart T. Hauser Clinical Research Training Program, NIH T32 16259 (BKB), the Commonwealth Research Center of the Massachusetts Department of Mental Health (SCDMH82101008006; BKB, LJS), and by the National Institute of Mental Health Grant No. K23MH076054 (DJH).
Footnotes
Contributors
Dr. Brent contributed to the study design, collected and analyzed the data, and wrote the first draft of the manuscript. Drs. Seidman and Keshavan contributed to the design of the study and data interpretation. Mr. Coombs assisted with data collection and contributed to data analysis. Dr. Moran contributed to data interpretation and design of the fMRI paradigm. Dr. Holt contributed to designing the study, data analysis/interpretation and the writing of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
The authors have no conflicts of interest to declare.
None of the authors have any conflicts of interest to declare in relation to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amminger GP, Pape S, Rock D, Roberts SA, Ott SL, Squires-Wheeler E, Kestenbaum C, Erlenmeyer-Kimling L. Relationship between childhood behavioral disturbance and later schizophrenia in the New York High-Risk Project. Am J Psychiatry. 1999;156(4):525–530. doi: 10.1176/ajp.156.4.525. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Backasch B, Straube B, Pyka M, Klohn-Saghatolislam F, Muller MJ, Kircher TT, Leube DT. Hyperintentionality during automatic perception of naturalistic cooperative behavior in patients with schizophrenia. Soc Neurosci. 2013;8(5):489–504. doi: 10.1080/17470919.2013.820666. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Depression Inventroy: Manual. The Psychiatric Corporation; San Antonio, TX: 1987. [Google Scholar]
- Bedford NJ, Surguladze S, Giampietro V, Brammer MJ, David AS. Self-evaluation in schizophrenia: an fMRI study with implications for the understanding of insight. BMC Psychiatry. 2012;12:106. doi: 10.1186/1471-244X-12-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentall RP, Corcoran R, Howard R, Blackwood N, Kinderman P. Persecutory delusions: a review and theoretical integration. Clin Psychol Rev. 2001;21(8):1143–1192. doi: 10.1016/s0272-7358(01)00106-4. [DOI] [PubMed] [Google Scholar]
- Blackwood NJ, Bentall RP, Ffytche DH, Simmons A, Murray RM, Howard RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychol Med. 2004;34(4):591–596. doi: 10.1017/S0033291703008997. [DOI] [PubMed] [Google Scholar]
- Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: Systematic review and meta-analysis. Schizophr Res. 2013;144(1–3):31–36. doi: 10.1016/j.schres.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Bosia M, Riccaboni R, Poletti S. Neurofunctional correlates of theory of mind deficits in schizophrenia. Curr Top Med Chem. 2012;12(21):2284–2302. doi: 10.2174/156802612805289917. [DOI] [PubMed] [Google Scholar]
- Brent BK, Coombs G, Keshavan MS, Seidman LJ, Moran JM, Holt DJ. Subclinical delusional thinking predicts lateral temporal cortex responses during social reflection. Soc Cogn Affect Neurosci. 2014a;9(3):273–282. doi: 10.1093/scan/nss129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent BK, Holt DJ, Keshavan MS, Seidman LJ, Fonagy P. Mentalization-based treatment for psychosis: linking an attachment-based model to the psychotherapy for impaired mental state understanding in people with psychotic disorders. Isr J Psychiatry Relat Sci. 2014b;50(4):239–246. [PubMed] [Google Scholar]
- Brent BK, Seidman LJ, Thermenos HW, Holt DJ, Keshavan MS. Self-disturbances as a possible premorbid indicator of schizophrenia risk: a neurodevelopmental perspective. Schizophr Res. 2014c;152(1):73–80. doi: 10.1016/j.schres.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M, Lissek S, Fuchs N, Witthaus H, Peters S, Nicolas V, Juckel G, Tegenthoff M. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46(7):1992–2001. doi: 10.1016/j.neuropsychologia.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Brune M, Ozgurdal S, Ansorge N, von Reventlow HG, Peters S, Nicolas V, Tegenthoff M, Juckel G, Lissek S. An fMRI study of “theory of mind” in at-risk states of psychosis: comparison with manifest schizophrenia and healthy controls. Neuroimage. 2011;55(1):329–337. doi: 10.1016/j.neuroimage.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- de Achaval D, Villarreal MF, Costanzo EY, Douer J, Castro MN, Mora MC, Nemeroff CB, Chu E, Bar KJ, Guinjoan SM. Decreased activity in right-hemisphere structures involved in social cognition in siblings discordant for schizophrenia. Schizophr Res. 2012;134(2–3):171–179. doi: 10.1016/j.schres.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia. 2008;46(6):1624–1635. doi: 10.1016/j.neuropsychologia.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez MD, Wichers M, Lieb R, Wittchen HU, van Os J. Evidence that onset of clinical psychosis is an outcome of progressively more persistent subclinical psychotic experiences: an 8-year cohort study. Schizophr Bull. 2011;37(1):84–93. doi: 10.1093/schbul/sbp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack S, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan M. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatric Services. 2009;60:1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frist MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition. York State Psychiatric Institute; New York: Biometrics Research; 2002. [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith N, Manktelow K, Morris N. Subclinical delusional ideation and a self-reference bias in everyday reasoning. Br J Psychol. 2008;99(Pt 1):29–44. doi: 10.1348/000712607X204317. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- Happe F. Theory of mind and the self. Ann N Y Acad Sci. 2003;1001:134–144. doi: 10.1196/annals.1279.008. [DOI] [PubMed] [Google Scholar]
- Happe FGE. An advanced test of theory of mind: Understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped and normal children and adults. Journal of Autism and Developmental Disorders. 1994;24(2):129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Holt DJ, Cassidy BS, Andrews-Hanna JR, Lee SM, Coombs G, Goff DC, Gabrieli JD, Moran JM. An anterior-to-posterior shift in midline cortical activity in schizophrenia during self-reflection. Biol Psychiatry. 2011a;69(5):415–423. doi: 10.1016/j.biopsych.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lakshmanan B, Freudenreich O, Goff DC, Rauch SL, Kuperberg GR. Dysfunction of a cortical midline network during emotional appraisals in schizophrenia. Schizophr Bull. 2011b;37(1):164–176. doi: 10.1093/schbul/sbp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JH, Jung WH, Choi JS, Choi CH, Kang DH, Shin NY, Hong KS, Kwon JS. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophr Res. 2011;127(1–3):58–65. doi: 10.1016/j.schres.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Jenkins AC, Mitchell JP. Medial prefrontal cortex subserves diverse forms of self-reflection. Soc Neurosci. 2011;6(3):211–218. doi: 10.1080/17470919.2010.507948. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kern RS, Horan WP, Barch DM. On altered patterns of brain activation in at risk adolescents and young adults. Am J Psychiatry. 2013;170(11):1226–1231. doi: 10.1176/appi.ajp.2013.13081089. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: relationship to premorbid impairments. Schizophr Res. 2008;103(1–3):114–120. doi: 10.1016/j.schres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosterkotter J, Hellmich M, Steinmeyer EM, Schultze-Lutter F. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry. 2001;58(2):158–164. doi: 10.1001/archpsyc.58.2.158. [DOI] [PubMed] [Google Scholar]
- Lee J, Quintana J, Nori P, Green MF. Theory of mind in schizophrenia: exploring neural mechanisms of belief attribution. Soc Neurosci. 2011;6(5–6):569–581. doi: 10.1080/17470919.2011.620774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Kang do H, Kim CW, Gu BM, Park JY, Choi CH, Shin NY, Lee JM, Kwon JS. Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Res. 2010;181(2):121–129. doi: 10.1016/j.pscychresns.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, Wheelwright SJ, Sadek SA, Suckling J, Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J Cogn Neurosci. 2009;22(7):1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Lysaker PH, Buck KD, Ringer J. The recovery of metacognitive capacity in schizophrenia across 32 months of individual psychotherapy: a case study. Psychotherapy Research. 2007;17(6):713–720. [Google Scholar]
- Marjoram D, Job DE, Whalley HC, Gountouna VE, McIntosh AM, Simonotto E, Cunningham-Owens D, Johnstone EC, Lawrie S. A visual joke fMRI investigation into Theory of Mind and enhanced risk of schizophrenia. Neuroimage. 2006;31(4):1850–1858. doi: 10.1016/j.neuroimage.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Menon M, Schmitz TW, Anderson AK, Graff A, Korostil M, Mamo D, Gerretsen P, Addington J, Remington G, Kapur S. Exploring the neural correlates of delusions of reference. Biol Psychiatry. 2011;70(12):1127–1133. doi: 10.1016/j.biopsych.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Modinos G, Renken R, Ormel J, Aleman A. Self-reflection and the psychosis-prone brain: an fMRI study. Neuropsychology. 2011;25(3):295–305. doi: 10.1037/a0021747. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Brent BK, Benton M, Pruitt P, Diwadkar V, Rajarethinam RP, Keshavan MS. Differential processing of metacognitive evaluation and the neural circuitry of the self and others in schizophrenia: a pilot study. Schizophr Res. 2010;116(2–3):252–258. doi: 10.1016/j.schres.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Pedersen A, Koelkebeck K, Brandt M, Wee M, Kueppers KA, Kugel H, Kohl W, Bauer J, Ohrmann P. Theory of mind in patients with schizophrenia: is mentalizing delayed? Schizophr Res. 2012;137(1–3):224–229. doi: 10.1016/j.schres.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory) Schizophr Bull. 1999;25(3):553–576. doi: 10.1093/oxfordjournals.schbul.a033401. [DOI] [PubMed] [Google Scholar]
- Pickup GJ. Theory of mind and its relation to schizotypy. Cogn Neuropsychiatry. 2006;11(2):177–192. doi: 10.1080/13546800444000236. [DOI] [PubMed] [Google Scholar]
- Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57(11):1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- Salvatore G, Lysaker PH, Gumley A, Popolo R, Mari J, Dimaggio G. Out of illness experience: metacognition-oriented therapy for promoting self-awareness in individuals with psychosis. American Journal of Psychotherapy. 2012a;66:85–106. doi: 10.1176/appi.psychotherapy.2012.66.1.85. [DOI] [PubMed] [Google Scholar]
- Salvatore G, Lysaker PH, Popolo R, Procacci M, Carcione A, Dimaggio G. Vulnerable self, poor understanding of others’ minds, threat anticipation and cognitive biases as triggers for delusional experience in schizophrenia: a theoretical model. Clin Psychol Psychother. 2012b;19(3):247–259. doi: 10.1002/cpp.746. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schiffman J, Lam CW, Jiwatram T, Ekstrom M, Sorensen H, Mednick S. Perspective-taking deficits in people with schizophrenia spectrum disorders: a prospective investigation. Psychol Med. 2004;34(8):1581–1586. doi: 10.1017/s0033291704002703. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Shad MU, Keshavan MS, Steinberg JL, Mihalakos P, Thomas BP, Motes MA, Soares JC, Tamminga CA. Neurobiology of self-awareness in schizophrenia: an fMRI study. Schizophr Res. 2012;138(2–3):113–119. doi: 10.1016/j.schres.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RC, Lushene RE. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1970. [Google Scholar]
- Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Neural correlates of anchoring-and-adjustment during mentalizing. Proc Natl Acad Sci U S A. 2010;107(24):10827–10832. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbox SI, Pogue-Geile MF. Development of social functioning in preschizophrenia children and adolescents: a systematic review. Psychol Bull. 2008;134(4):561–583. doi: 10.1037/0033-2909.34.4.561. [DOI] [PubMed] [Google Scholar]
- van Buuren M, Gladwin TE, Zandbelt BB, Kahn RS, Vink M. Reduced functional coupling in the default mode network during self-referential processing. Hum Brain Mapp. 2010;31:1117–1127. doi: 10.1002/hbm.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren M, Vink M, Kahn RS. Default-mode network dysfunction and self-referential processing in healthy siblings of schizophrenia patients. Schizophr Res. 2012;142(1–3):237–243. doi: 10.1016/j.schres.2012.09.017. [DOI] [PubMed] [Google Scholar]
- van der Meer L, de Vos AE, Stiekema AP, Pijnenborg GH, van Tol MJ, Nolen WA, David AS, Aleman A. Insight in schizophrenia: involvement of self-reflection networks? Schizophr Bull. 2013;39(6):1288–1295. doi: 10.1093/schbul/sbs122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468(7321):203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- Walter H, Ciaramidaro A, Adenzato M, Vasic N, Ardito RB, Erk S, Bara BG. Dysfunction of the social brain in schizophrenia is modulated by intention type: an fMRI study. Soc Cogn Affect Neurosci. 2009;4(2):166–176. doi: 10.1093/scan/nsn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H, Schnell K, Erk S, Arnold C, Kirsch P, Esslinger C, Mier D, Schmitgen MM, Rietschel M, Witt SH, Nothen MM, Cichon S, Meyer-Lindenberg A. Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Mol Psychiatry. 2011;16(4):462–470. doi: 10.1038/mp.2010.18. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Harcourt Assessment: 1999. San Antonio, TX: 1999. Wechsler: Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. Prentice Hall; Upper Saddle River, NJ: 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.