Abstract
Objective
To present the initial results of first three years of implementation of a genetic evaluation test for bone marrow-derived mesenchymal stem cells in a Cell Technology Center.
Methods
A retrospective study was carried out of 21 candidates for cell therapy. After the isolation of bone marrow mononuclear cells by density gradient, mesenchymal stem cells were cultivated and expanded at least until the second passage. Cytogenetic analyses were performed before and after cell expansion (62 samples) using G-banded karyotyping.
Results
All the samples analyzed, before and after cell expansion, had normal karyotypes, showing no clonal chromosomal changes. Signs of chromosomal instability were observed in 11 out of 21 patients (52%). From a total of 910 analyzed metaphases, five chromatid gaps, six chromatid breaks and 14 tetraploid cells were detected giving as total of 25 metaphases with chromosome damage (2.75%).
Conclusion
The absence of clonal chromosomal aberrations in our results for G-banded karyotyping shows the maintenance of chromosomal stability of bone marrow-derived mesenchymal stem cells until the second passage; however, signs of chromosomal instability such as chromatid gaps, chromosome breaks and tetraploidy indicate that the long-term cultivation of these cells can provide an intermediate step for tumorigenesis.
Keywords: Cytogenetics, Mesenchymal stromal cells, Chromosomal instability
Introduction
Cultivated stem cells (SC) have shown great potential for use in several areas of cell therapy, requiring, for their applicability, strict quality control, safety and traceability. However, ex vivo expansion of these cells can lead to an accumulation of genetic and epigenetic alterations, featuring a genetic instability that may explain, at least in part, their tumorigenic potential.1, 2
Differently from embryonic SC, where there have been several reports of genetic abnormalities and tumor formation in mice,3, 4, 5, 6 adult human SC, especially mesenchymal SC (MSC), seem to maintain their genetic stability during cultivation,7 and therefore would not be susceptible to malignant transformation.8, 9
However, the observation that all cultivated cells develop chromosomal aberrations over time, even those that are not SC,10 suggests that these cells, even adult cells, may have a potential tumorigenic transformation after in vitro expansion.11, 12
Wang et al.13 developed two hypotheses for the presence of transformed MSCs in a culture: a population of MSCs that have undergone transformation during cell culture, or the presence of low frequencies of abnormal cells in the donor's bone marrow (BM) that have expanded during the culture. These explanations could also be extended to MSC from other sources because, in essence, the main fact is that cultivation can create a favorable environment for transformed cells to expand and propagate in vitro.
The evaluation of the propensity for malignant transformation can and should be addressed by cytogenetic studies, especially karyotyping, since the maintenance of a normal karyotype is a reliable indicator of genetic stability of MSCs13 and must be considered as a release criterion for the clinical use of these cells.14
Although technical difficulties encourage researchers to complement their studies with techniques of molecular cytogenetics, conventional cytogenetics (karyotyping through G-banding) is the most informative and considered the gold standard in the genetic evaluation of cell lines, allowing the identification of both numerical and structural chromosomal abnormalities.10, 15, 16
In Brazil, eight Cell Technology Centers (CTCs) were created in 2008 with support from the Ministry of Health, contributing to the development of these lines of research, ensuring quality and safety in new technologies related to cell therapy.
There is a widely reported concern in the literature, that in vitro cultivation of SC may be a risk factor for tumor formation. Thus, the Brazilian National Health Surveillance Agency (ANVISA), through a decree governing the operation of CTCs (RDC09/2011), requires the implementation of genetic control as a release criterion for the use of these cells.
The aim of this study was to describe how the genetic evaluation test by G-banded karyotyping is performed at the Pontifícia Universidade Católica do Paraná (PUCPR). This type 2 CTC is authorized to culture and expand SC with its sanitary permit being granted on May 17th, 2012. In addition, the initial results of the first three years of implementation of this test for BM-derived MSC are presented.
Methods
Patients and samples
A retrospective study was carried out of 21 candidates for cell therapy selected between December 2009 and December 2012. All patients enrolled in this study provided signed informed consent. This study was approved by national and local Ethics Committees (CONEP 03780084000-09, CEP 0005710/22 and CONEP n197205, CEP 1370, 1371) and followed the criteria of the Helsinki convention. BM samples and the respective MSCs were cultivated and evaluated at the CTC-PUCPR.
Culture of MSCs
After the isolation of BM mononuclear cells by density gradient and loading onto 1.077 g/mL Histopaque® (Sigma–Aldrich), MSCs were cultivated in Iscove's Modified Dulbecco's Medium (IMDM – Gibco™) supplemented with 15% fetal bovine serum (FBS – Gibco™) in a humidified incubator with 5% carbon dioxide at 37 °C. When the MSCs reached approximately 80% of confluence, they were dissociated using 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA – Sigma–Aldrich) and continually expanded at least until the second passage (P2). Samples were taken for cytogenetic studies, immunophenotyping and osteogenic, adipogenic and chondrogenic differentiation assays,17, 18 as recommended by the International Society for Cellular Therapy.19
G-banded karyotyping protocol
Cytogenetic analyses were performed before and after cell expansion. Standard cytogenetic procedures were used in the cytogenetic analysis of BM samples. To evaluate the MSC, a literature-based protocol for fibroblasts20 and SC from many sources10, 21 was developed in the CTC with modifications as described below. When the culture reached a confluence of 80%, Colcemid® (10 mg/mL) was added to each flask to a final dilution of 0.1 μg/mL and then incubated at 37 °C for 2–6 h. Changes in cell morphology were monitored using an inverted microscope. The MSCs were detached using 2 mL of heated 0.25% trypsin-EDTA. After 3 min of monitored detachment, two drops of FBS were added and the cells were transferred to a centrifuge tube containing the medium. Samples were centrifuged at 400 × g for 10 min. For the hypotonic treatment, 5 mL of 0.075 M KCl with Hepes was slowly and carefully added followed by incubation at 37 °C for 30 min and fixation using methanol:acetic acid (3:1) solution. To improve the quality of metaphases, the samples were washed twice in a cold solution (5 °C) of fresh methanol:acetic acid (2:1). Prior to slide preparation, clean slides were placed in steam while the cells were resuspended in fresh fixative solution and dropped onto the surface of the slide. In order to obtain G-bands, the slides were aged at 60 °C for at least 16 h. Then, they were immersed in trypsin solution (0.002 g/mL) for 5 s, washed in saline solution and finally quickly rinsed in distilled water. The staining procedure was carried out using Giemsa (1:20) or Wright (1:6) solution, producing trypsin and Giemsa (GTG) or trypsin and Wright (GTW) bands, respectively. The band quality was evaluated under the microscope (magnification: 100×) and the trypsin and staining times were adjusted to produce clear well stained bands.
Analysis and interpretation
Whenever possible, 20 metaphases were analyzed. To be considered a clone, the same structural alteration or the gain of a particular chromosome had to be present in at least two different metaphase cells, while the loss of a chromosome should be detected in at least three cells. In the International System for Human Cytogenetic Nomenclature (2013), the recommendations related to abnormalities in neoplasia, non-clonal aberrations should not be part of the description of the karyotype.22
Results
A total of 21 patients with a mean age 53 ± 9.25 years were evaluated, of which 16 were male. The total number of samples was 62, with 21 being BM samples and 41 MSC cultivated until the second passage and, in some cases, until the fourth passage (P4) (Table 1).
Table 1.
Cytogenetic results detected in bone marrow cells (BM) and mesenchymal stem cells (MSC).
| Patient | Before cell expansion (BM) |
After cell expansion (MSC) |
|||
|---|---|---|---|---|---|
| Karyotype | Chromosomal instability signs | Samples analyzed (passages) | Number of metaphases analyzed | Chromosomal instability signs | |
| 1 | 46,XX [20] | – | P0,P1, P2 | 19 | 4n [1] |
| 2 | 46,XY [35] | – | P0,P1, P2 | 51 | – |
| 3 | 46,XY [20] | – | P1, P2,P3 | 22 | – |
| 4 | 46,XY [12] | – | P0,P1, P2,P3 | 64 | 4n [1], chtg [3] |
| 5 | 46,XY,inv(9)(p12q13) [15] | – | P0,P1, P2,P3 | 15 | 4n [2] |
| 6 | 46,XY [20] | – | P2 | 20 | 4n [1] |
| 7 | 46,XY [20] | – | P2 | 57 | – |
| 8 | 46,XY [20] | – | P2 | 32 | – |
| 9 | 46,XY [10] | – | P2,P3 | 11 | – |
| 10 | 46,XX [8] | – | P0,P1, P2,P3 | 11 | – |
| 11 | 46,XY [18] | – | P1, P2,P3 | 21 | – |
| 12 | 46,XY [20] | – | P3,P4 | 42 | – |
| 13 | 46,XY [30] | chtb (9q) [1], chtg [1] | P2 | 22 | |
| 14 | 46,XY [20] | 4n [1], chtb (3q)[1] | P2, P3 | 20 | – |
| 15 | 46,XX [20] | – | P2 | 15 | – |
| 16 | 46,XY [20] | 4n[3] | P2 | 20 | – |
| 17 | 46,XY [20] | 4n [1] | P2 | 20 | 4n [1], chtb (10q) [1] |
| 18 | 46,XX [20] | – | P2 | 20 | 4n [2] |
| 19 | 46,XY [20] | – | P2 | 20 | – |
| 20 | 46,XY [20] | chtb [1], chtg [1] | P2 | 20 | 4n [1] |
| 21 | 46,XX [20] | – | P2 | 20 | chtb (1q)[1], chtb(22q)[1] |
| Total metaphases | 408 | 10 | 502 | 15 | |
chtg: chromatid gap; chtb: chromatid break; 4n: tetraploid cell.
Metaphases were successfully obtained in all cases and all were identified as normal diploid karyotypes, although many metaphases presented monosomy (non-clonal) in different chromosomes.
The samples analyzed before cell expansion, with BM cells, had a normal karyotype, showing no clonal chromosomal changes. One case showed inv(9)(p12q13), considered a normal variation in the population. All samples had cells with random chromosome losses.
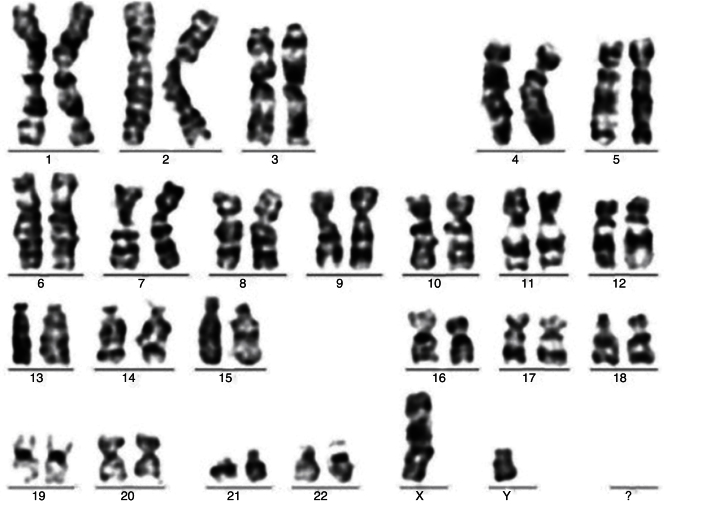
Samples analyzed after cell expansion maintained normal karyotypes without the appearance of acquired clonal chromosomal abnormalities (Figure 1). All samples had cells with random chromosome losses.
Figure 1.
Case 20; mesenchymal stem cells – Passage 2. Normal complete karyogram (46,XY).
Signs of chromosomal instability were observed in 11 (52%) out of 21 patients. From a total of 910 analyzed metaphases, five chromatid gaps, six chromatid breaks (Figure 2) and 14 tetraploid cells were detected giving a total of 25 metaphases with chromosome damage (2.75%).
Figure 2.

Case 20; bone marrow. Partial karyogram showing chromatid breaks in the long arm of chromosome 8.
A small fraction of these cells showed diplochromosomes (Figure 3).
Figure 3.

Case 05; mesenchymal stem cells – Passage 1. Metaphase showing diplochromosome.
No clonal chromosomal rearrangements were detected. All the samples were approved by the cytogenetic quality control for autologous therapeutic use.
Discussion
Standardization for stem cell studies
The presence of aneuploid cells with non-clonal chromosomal losses should be related to the adjustment of hypotonic solution exposure: short hypotonic exposure may not be sufficient for a good dispersion of metaphasic chromosomes, resulting in many overlapping chromosomes, whereas overexposure can weaken the plasma membrane, eventually resulting in disruption and loss of some chromosomes.20 The hypotonic treatment, therefore, is critical for the karyotyping test, and its standardization is crucial to correctly interpret results. In arresting mitotic division, unlike BM cells or peripheral blood (stimulated), cultures of adherent cells require longer periods of colchicine to compensate for the slower growth rate. However, there is a dose-dependent effect that may change the quality of chromosomes, such as their length, shape and distribution of chromatids. In the current study, less than 1 h of mitotic inhibition was not sufficient to deliver a reasonable number of metaphases, while more than 6 h affected the quality of chromosomes. In order to optimize metaphase quality, MSCs were obtained using a 3:1 methanol:acetic acid solution, followed by additional washes with higher concentrations of acetic acid (2:1). For G-band staining (GTG or GTW), a higher than usual trypsin concentration was required, which should be of at least 0.002 g/mL, with an exposure time ranging from 5 to 20 s, depending on the situation and conditions during the preparation of the slides.
Chromosomal instability and tumorigenic potential
The high frequency of chromosomal alterations may be a prerequisite for an oncogenetic process that involves multiple genetic steps, such as the inactivation of tumor suppressor genes and amplification of oncogenes,23 although the occurrence of aneuploid cells (with numerical chromosomal anomalies) is not necessarily associated with the transformation: MSCs, with or without chromosomal abnormalities, show growth arrest and enter senescence.24
In this study, no clonal chromosomal aberrations were identified in any of the analyzed cases; however, several signs of chromosomal instability were observed including chromatid gaps, chromosomal breaks and tetraploid metaphases.
It is well known that the presence of gaps and breaks, a characteristic of syndromes of chromosomal instability, increases the risk for neoplastic diseases. Therefore, the presence of these signs in cultured SC is evidence of their tumorigenic potential. On comparing before and after cultivation, there was no evidence of an increase in the signs of instability. However, increases in genetic instability probably depend on the time of cultivation. Binato et al.25 demonstrated, for example, the maintenance of genetic stability up to P4, but several genetic changes were seen from P5 onward underscoring the fact that, for the use of cells from higher passages, it is necessary to analyze case by case. Tetraploidy, i.e. cells with duplicated genetic material, is a well-documented phenomenon in cultivated cells: it occurs at a frequency of 3–5% in cultured human fibroblasts. A small fraction of these cells may show diplochromosomes, which appear only in the first division after endoreduplication26 as was detected in this study (Figure 3). This phenomenon has also been reported in cultured SC: Grimes et al.27 detected a frequency of 11.6% of tetraploids in chorionic villous-derived cells. In our sample, this frequency was 1.78%. The problem of the appearance of these cells is that they are related to an error during mitosis (regression of the cleavage furrow), which, in turn, may lead in future divisions and to the emergence of aneuploid cells. Therefore, these cells are genetically unstable and there is evidence that they can act as an intermediate step for tumorigenesis.28, 29
Interestingly, these abnormal mitotic mechanisms are described in the progression of malignant mesenchymal tumors, where the increased frequency of chromosomal aberrations can be explained by a process initiated by telomere dysfunction and anaphasic bridges, which, in turn, can determine an increased frequency of multinucleated cells through cytokinesis failure.30
The absence of clonal chromosomal aberrations in BM-derived MSC had already been described in a sample of ten healthy hematopoietic SC donors with a median age of 18 years.7 Our results confirm the maintenance of the stability of these cells until P2 in a representative sample of candidates for cell therapy with a mean age of 53 years.
Conclusions
Our results confirm the importance of the G-band cytogenetic study, since this technique is able to detect both numeric and structural alterations, including balanced rearrangements and mosaicism, besides evidencing signs of ‘instability’.
The absence of clonal chromosomal aberrations among our results for G-banded karyotyping shows the maintenance of chromosomal stability of the BM-derived MSC until the second passage; however, signs of chromosomal instability such as chromatid gaps, chromosome breaks and tetraploidy indicate that the long-term cultivation of these cells can provide an intermediate step for tumorigenesis.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the Banco Nacional de Desenvolvimento Econômico e Social (BNDES), Ministério da Ciência e Tecnologia (MCT), Financiadora de Estudos e Projetos (FINEP), Ministério da saúde (MS), Secretaria de Ciência e Tecnologia e Insumos Estratégicos (SCTIE), Departamento de Ciência e Tecnologia (DECIT), and Conselho Nacional de Pesquisa (CNPq) (Grant 06/2008, Al 11/2009, 380628/2010-4).
References
- 1.Catalina P., Montes R., Ligero G. Human ESCs predisposition to karyotypic instability: is a matter of culture adaptation or differential vulnerability among hESC lines due to inherent properties? Mol Cancer. 2008;7:76. doi: 10.1186/1476-4598-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sensebé L., Bourin P. Mesenchymal stem cells for therapeutic purposes. Transplantation. 2009;15(Suppl. 9):S49–S53. doi: 10.1097/TP.0b013e3181a28635. [DOI] [PubMed] [Google Scholar]
- 3.Buzzard J.J., Gough N.M., Crook J.M., Colman A. Karyotype of human ES cells during extended culture. Nat Biotechnol. 2004;22:381–382. doi: 10.1038/nbt0404-381. author reply 382. [DOI] [PubMed] [Google Scholar]
- 4.Inzunza J., Sahlén S., Holmberg K. Comparative genomic hybridization and karyotyping of human embryonic stem cells reveals the occurrence of an isodicentric X chromosome after long-term cultivation. Mol Hum Reprod. 2004;10:461–466. doi: 10.1093/molehr/gah051. [DOI] [PubMed] [Google Scholar]
- 5.Närvä E., Autio R., Rahkonen N. High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol. 2010;28:371–377. doi: 10.1038/nbt.1615. [DOI] [PubMed] [Google Scholar]
- 6.Laurent L.C., Ulitsky I., Slavin I. Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell. 2011;8:106–118. doi: 10.1016/j.stem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernardo M.E., Zaffaroni N., Novara F. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 8.Poloni A., Maurizi G., Babini L. Human mesenchymal stem cells from chorionic villi and amniotic fluid are not susceptible to transformation after extensive in vitro expansion. Cell Transplant. 2011;20:643–654. doi: 10.3727/096368910X536518. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y., Han Z., Song Y., Han Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;2012:6520–6534. doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meisner L.F., Johnson J.A. Protocols for cytogenetic studies of human embryonic stem cells. Methods. 2008;45:133–141. doi: 10.1016/j.ymeth.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Herberts C.A., Kwa M.S., Hermens H.P. Risk factors in the development of stem cell therapy. J Transl Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-David U., Mayshar Y., Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell. 2011;9:97–102. doi: 10.1016/j.stem.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Huso D.L., Harrington J., Kellner J., Jeong D.K., Turney J. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Z.X., Guan L.X., Zhang K. Cytogenetic analysis of human bone marrow-derived mesenchymal stem cells passaged in vitro. Cell Biol Int. 2007;31:645–648. doi: 10.1016/j.cellbi.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Catalina P., Cobo F., Cortés J.L. Conventional and molecular cytogenetic diagnostic methods in stem cell research: a concise review. Cell Biol Int. 2007;31:861–869. doi: 10.1016/j.cellbi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Payão S.L., Segato R., Santos R.R. Genetic control of cultivated human stem cells. Rev Bras Hematol Hemoter. 2009;31(Suppl. 1):15–18. [Google Scholar]
- 17.Dominici M., Le Blanc K., Mueller I. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 18.Rebelatto C.L., Aguiar A.M., Moretão M.P. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233:901–913. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz E.M., Le Blanc K., Dominici M. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 20.Lawce H.J., Brown M.G. Cytogenetics. In: Barch M.J., Knutsen T., Spurbeck J., editors. The AGT cytogenetic laboratory manual. 3rd ed. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 19–366. [Google Scholar]
- 21.Duarte D.M., Cornelio D.A., Corado C. Chromosomal characterization of cryopreserved mesenchymal stem cells from the human subendothelium umbilical cord vein. Regen Med. 2012;7:1–11. doi: 10.2217/rme.11.113. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer L.G., McGowan-Jordan J., Schmid M., editors. ISCN: an international system for human cytogenetic nomenclature. Karger; Basel: 2013. p. 140. [Google Scholar]
- 23.Lengauer C., Kinzler K.W., Vogelstein B. Genetic instabilities in human cancer. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 24.Tarte K., Gaillard J., Lataillade J.J. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115:1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 25.Binato R., de Souza Fernandez T., Lazarotto-Silva C. Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy. Cell Prolif. 2012;46:10–22. doi: 10.1111/cpr.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therman E., Susman M. 3rd ed. Sociedade Brasileira de Genética; Ribeirão Preto: 1996. Cromosomas Humanos; p. 404. [Google Scholar]
- 27.Grimes B.R., Steiner C.M., Merfeld-Clauss S. Interphase FISH demonstrates that human adipose stromal cells maintain a high level of genomic stability in long-term culture. Stem Cell Dev. 2009;18:717–724. doi: 10.1089/scd.2008.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Q., King R.W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature. 2005;437:1038–1042. doi: 10.1038/nature03958. [DOI] [PubMed] [Google Scholar]
- 29.Pampalona J., Frias C., Genesca A., Tusell L. Progressive Telomere dysfunction causes cytokinesis failure and leads to the accumulation of polyploid cells. PLoS Genet. 2012;8:1–10. doi: 10.1371/journal.pgen.1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gisselsson D., Palsson E., Yu C., Mertens F., Mandahl N. Mitotic instability associated with late genomic changes in bone and soft tissue tumours. Cancer Lett. 2004;206:69–76. doi: 10.1016/j.canlet.2003.10.022. [DOI] [PubMed] [Google Scholar]