Abstract
Objective
Nutritional deficiencies are very significant to the overall health of humans at all ages and for both genders, yet in infants, children and women of childbearing age these deficiencies can seriously affect growth and development. The present work is aimed to assess homocysteine and vitamin B12 status in females with iron deficiency anemia from the Gaza Strip.
Methods
Venous blood samples were randomly collected from 240 female university students (18–22 years old) and parameters of the complete blood count, serum ferritin, homocysteine and vitamin B12 were measured. Statistical analysis included the t-test and analysis of variance (ANOVA) using the IBM SPSS software (version 18). Statistical significance was set for p-values <0.05.
Results
The results revealed that 20.4% of the students have iron deficiency anemia. The mean serum vitamin B12 level in females with iron deficiency anemia (212.9 ± 62.8 pg/mL) was significantly lower than in normal controls (286.9 ± 57.1 pg/mL) and subjects with microcytic anemia and normal ferritin (256.7 ± 71.1 pg/mL). Significantly higher serum homocysteine levels were reported in the iron deficiency anemia group (27.0 ± 4.6 μmol/L) compared to normal controls (15.5 ± 2.9 μmol/L) and in subjects with microcytic anemia and normal ferritin (18.1 ± 2.7 μmol/L). Statistically significant negative correlations were reported for serum homocysteine with serum ferritin, vitamin B12, hemoglobin, and hematocrit levels.
Conclusion
Important associations were found between serum homocysteine and markers of iron deficiency. Monitoring homocysteine levels might be essential to understand the development of different clinical conditions including anemia. It seems necessary to conduct prospective trials to determine whether treating anemia ameliorates homocysteine levels.
Keywords: Anemia, Iron-deficiency, Microcystis, Hyperhomocysteinemia
Introduction
Iron deficiency anemia (IDA) is the most common type of nutritional anemia in the world; it significantly affects individuals of all ages and economic groups in both developing and developed countries with very staggering international estimates. The World Health Organization (WHO) believes that about one-third of the world's population (more than 2 billion people) is anemic, mostly due to iron deficiency.1, 2 Despite worldwide economic and scientific development together with the international efforts and campaigns to combat the prevalence of IDA, there has been little impact on the worldwide prevalence, which remains high and challenging. The WHO reports more troubling prevalences of IDA in developing countries showing that one in every two pregnant woman and 30–40% of preschool children are likely to be anemic, mainly due to iron deficiency.3 In Palestine, IDA is recognized as an important health problem and different studies involving different age groups and both genders have underscored the magnitude of IDA as a major public health challenge for improving the health status especially in refugee camps and among vulnerable at-risk groups.4, 5, 6, 7 The consequences of anemia are not limited to poor pregnancy outcomes, impaired physical and cognitive development, and increased risk of morbidity in children8, 9, 10 but affect national productivity and economics.11, 12, 13
Vitamin B12 deficiency, which is common in wealthier countries, principally among the elderly, is even more prevalent in poorer populations worldwide.14 Hyperhomocysteinemia, an elevated circulating total homocysteine concentration, is influenced by genetic and dietary factors such as deficiencies of the enzymes or vitamin cofactors in the homocysteine metabolic pathway.15 Hyperhomocysteinemia is a strong and independent risk factor for coronary atherosclerosis and cerebrovascular disorders and strokes.16 Hyperhomocysteinemia could be attributed to low concentrations of vitamin B12.17, 18, 19 Moreover, hyperhomocysteinemia and/or low folate and vitamin B12 concentrations are potential risk factors for poor pregnancy outcomes and neurocognitive performance.17, 20, 21
The aim of the present work was to assess homocysteine and vitamin B12 status in female university students with iron deficiency anemia aged 18–22 years from the Gaza Strip. To the best of our knowledge, after searching published scientific works, the present work could be the first that directly investigates the association between the levels of body iron, vitamin B12 and homocysteine.
Methods
The present cross-sectional descriptive study enrolled 240 randomly selected female students, between 18 and 22 years old, in the Islamic university in Gaza. The sample size was calculated for a level of precision of ±7% and a 95% confidence level.22 According to these calculations at least 200 female students should be included; this number was increased to 240. About 5 mL of venous blood [2.5 mL in K3 ethylenediaminetetraacetic acid (EDTA) tubes and 2.5 mL in serum tubes] were collected from each subject to perform a complete blood count (CBC) using a Sysmex XE-2100 hematology analyzer, and to determine the levels of vitamin B12, serum ferritin, and serum homocysteine using commercially available kits. Anthropometric characteristics were measured and body mass index was calculated.
Descriptive statistics, frequencies, central tendencies, and statistical analysis tests such as t-test and analysis of variance test (ANOVA) were used to clarify the relationship between the research variables. The IBM SPSS software (version 18, IBM Corporation, Somers, NY) was used in the statistical analysis.
Results
The present study comprised 240 female university students aged 18–22 years. Complete blood count analysis revealed microcytic red blood cells (RBCs) with a mean corpuscular volume (MCV) <80 fl in 30.0% (72/240) of the screened subjects. On assessing iron stores using the serum ferritin test, 20.4% (49/240) of all screened females had low levels concomitant to reduced hemoglobin (Hb <12 g/dL) and thus were suffering from iron deficiency anemia while 9.6% (23/240) were microcytic but with normal iron stores. The subjects of this study were categorized into three groups: iron deficiency anemia (n = 49), microcytic with normal serum ferritin (n = 23), and 20 normocytic non-anemic female students (MCV = 80–96 fl; Hb ≥12.0 g/dL) who were randomly selected as a normal control group. The age and anthropometric characteristics of the study subjects are presented in Table 1 which shows no significant differences in the mean age and weight of the students in the three groups, while females of the iron deficiency anemia group were shorter than the females in the other groups.
Table 1.
Age and anthropometric measurements of the study subjects.
| Anthropometric measurements | IDA (n = 49) | Microcytic normal ferritin (n = 23) | Normal control (normocytic) (n = 20) | P-valuea |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 19.6 ± 1.3 | 20.1 ± 1.5 | 20.0 ± 1.7 | 0.369 |
| Weight (kg) | 59.0 ± 8.5 | 60.4 ± 9.9 | 59.2 ± 12.2 | 0.842 |
| Height (cm) | 159.6 ± 4.7 | 164.0 ± 5.9 | 161.7 ± 5.9 | 0.006 |
| Body mass index | 23.1 ± 3.0 | 22.4 ± 3.5 | 22.5 ± 4.2 | 0.674 |
IDA, iron deficiency anemia; SD, standard deviation.
ANOVA.
The serum levels of ferritin, vitamin B12 and homocysteine of the different groups are shown in Table 2. Apart from the significant reduction of serum ferritin, females in the IDA group had significantly lower levels of vitamin B12 and significantly higher levels of homocysteine compared to the other two groups. The decrements in vitamin B12 and increments in homocysteine of the IDA group were 25.8% and 74.8%, respectively compared to the control group.
Table 2.
Serum ferritin, vitamin B12 and homocysteine levels of the different study groups.
| Biochemical analysis | IDA (n = 49) | Microcytic normal ferritin (n = 23) | Normal control (normocytic) (n = 20) | P-value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Serum ferritin (ng/mL) | 5.1 ± 2.7 | 25.1 ± 10.5 | NA | <0.001a |
| Vitamin B12 (pg/mL) | 212.9 ± 62.7 | 256.7 ± 71.1 | 286.9 ± 57.1 | <0.001b |
| Homocysteine (μmol/L) | 27.0 ± 4.6 | 18.1 ± 2.7 | 15.45 ± 2.9 | <0.001b |
IDA, Iron deficiency anemia; NA, Not available; SD, Standard deviation.
Independent t-test.
ANOVA.
The CBC analysis revealed significantly lower values except for the platelet count in IDA subjects compared to the control group (Table 3). The mean reductions in hemoglobin and hematocrit (Hct) levels in the IDA group were 15.5% and 9.7%, respectively compared to controls. Some significant differences were also reported between IDA subjects and the microcytic anemia with normal ferritin group.
Table 3.
Hematological parameters of the study subjects.
| Hematological parameter | IDA (n = 49) | Microcytic normal ferritin (n = 23) | Normal control (n = 20) | P-valuea |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| WBC (× 109 L–1) | 6.53 ± 1.98 | 7.73 ± 1.82 | 8.07 ± 2.1 | 0.005 |
| RBC (× 1012 L–1) | 4.45 ± 0.39 | 4.56 ± 0.41 | 4.72 ± 0.36 | 0.015 |
| Hb (g/dL) | 10.5 ± 1.13 | 11.5 ± 1.49 | 12.42 ± 0.99 | <0.001 |
| Hct (%) | 32.4 ± 2.4 | 34.4 ± 3.0 | 35.9 ± 2.4 | <0.001 |
| MCV (fl) | 73.1 ± 5.4 | 75.1 ± 5.5 | 84.8 ± 3.8 | <0.001 |
| MCH (pg) | 23.8 ± 2.8 | 25.1 ± 3.1 | 29.3 ± 1.1 | <0.001 |
| MCHC (g/dL) | 32.4 ± 1.9 | 33.1 ± 2.2 | 34.6 ± 0.91 | <0.001 |
| PLT (× 109 L–1) | 280.2 ± 70.6 | 307.0 ± 75.3 | 310.1 ± 45.2 | 0.137 |
IDA, iron deficiency anemia; WBC, white blood cell count; RBC, red blood cell count; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelet count; SD, standard deviation.
ANOVA.
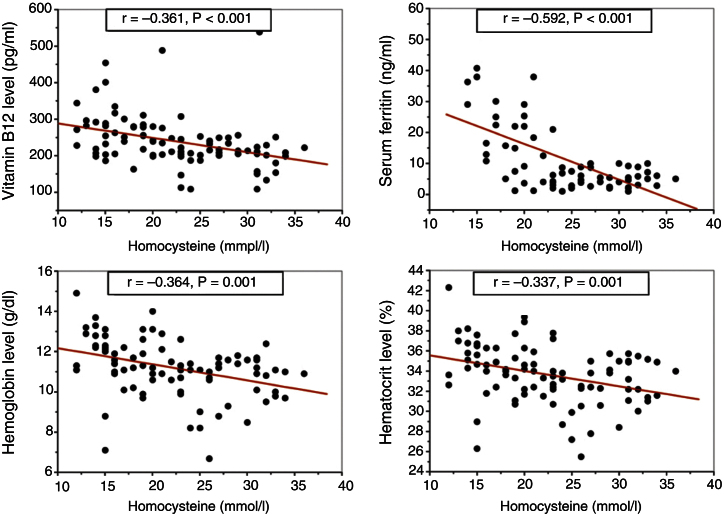
Analyses using the Pearson correlation coefficient revealed a significant correlation between the serum homocysteine level and other hematological and biochemical parameters as shown in Table 4. Among these important correlations are the negative correlation of homocysteine with vitamin B12 and the IDA biomarkers such as serum ferritin, hemoglobin and hematocrit (Figure 1).
Table 4.
Homocysteine level in relation to serum ferritin of the study population.
| Homocysteine (μmol/L) |
||
|---|---|---|
| Pearson correlation (r) | P-valuea | |
| Serum ferritin (ng/mL) | −0.592 | 0.000 |
| Vitamin B12 (pg/mL) | −0.361 | 0.000 |
| WBC (× 109 L−1) | −0.262 | 0.012 |
| RBC (× 1012 L−1) | 0.038 | 0.720 |
| Hb (g/dL) | −0.364 | 0.000 |
| Hct (%) | −0.337 | 0.001 |
| MCV (fl) | −0.352 | 0.001 |
| MCH (pg) | −0.359 | 0.000 |
| MCHC (g/dL) | −0.248 | 0.017 |
| PLT (× 109 L−1) | −0.178 | 0.090 |
WBC, white blood cell count; RBC, red blood cell count; Hb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; and PLT, platelet count.
ANOVA.
Figure 1.
Pearson correlation coefficient analysis of homocysteine with vitamin B12 and iron deficiency anemia biomarkers.
Discussion
Nutritional deficiencies are very significant to the overall health of humans at all ages and for both genders, however in some age groups (infants and children, and women of childbearing age) these deficiencies can be graver and then the growth and development may be seriously hindered by shortages of essential vitamins or nutrients.23 IDA, characterized by microcytic RBC, is the commonest nutritional anemia worldwide affecting people in developing as well as developed countries.11 Although vitamin B12 deficiency is common in rich countries, particularly among the elderly, it is much more widespread in poorer populations around the world.14 Moreover, several scientific reports have associated hyperhomocysteinemia with different medical conditions and nutritional deficiencies.16 To the best of our knowledge this work may be the first that directly investigates the association between the levels of body iron, vitamin B12 and homocysteine.
The present work enrolled 240 female university students aged 18–22 years, and found a considerable percentage (20.4%) of anemic cases due to IDA which is almost three-fold the percentage (7.0%) reported by Sirdah et al. for the same age group and in the same city 11 years ago.4 The higher prevalence of IDA today could be due to a drop in socioeconomic status related to the local political climate and restrictions that have seriously affected the economics of the Gaza Strip over the last seven years.6, 24 Additionally, another fraction (9.6%) of study subjects were found to have microcytic anemia with normal iron stores possibly caused by genetic factors.25
A very interesting finding of the present study is the significantly increased homocysteine levels found in IDA cases compared to the other study groups: 74.5% and 49.2% higher than control and microcytic groups, respectively. It is worthwhile to mention that it seems that no previous studies have directly investigated the homocysteine level in IDA, but hyperhomocysteinemia has been reported in anemic cases due to hereditary factors26 and in vegetarians, particularly among vegans.27 Elevated homocysteine levels had been found to be associated with low hemoglobin levels in the elderly (85 years old).16 Unfortunately iron levels were not determined in these elderly individuals, and then the etiology of anemia could be different. The present study revealed a low level of vitamin B12 in IDA subjects. It is not uncommon to find iron deficiency associated with vitamin B12 deficiency or other nutritional deficiencies. However, the combined iron and vitamin B12 deficiencies could aggravate the clinical condition.28, 29
Significant correlations were found between serum homocysteine levels on one hand and different blood parameters and indices on the other. This study reports on significant correlations between homocysteine and biomarkers of IDA (Hb, Hct, serum ferritin); unluckily no published study investigated these correlations, thus, further large scale investigations should be performed to evaluate the correlations at different IDA stages and in different risk groups. Nasri and Baradaran reported a positive correlation of homocysteine with Hb and Hct in Pakistani hemodialyzed anemic patients with end-stage renal disease, however these patients had high iron stores.30
The significant correlations between the serum levels of homocysteine, serum ferritin, and vitamin B12 in the present study are very clear and stress the need for future studies with in-depth investigations to identify any associations related to the clinical picture not only of microcytic cases but related to other hematological disorders. The significant inverse correlation (r = −0.592; p-value = 0.001) reported here between serum homocysteine and serum ferritin levels was not reported before in any studies. The association between elevated homocysteine levels and reduced free vitamin B12 was previously reported by Miller et al., who assumed this elevation was an early marker for the development of vitamin B12 deficiency.31 The significant inverse correlation between serum homocysteine and vitamin B12 reported in this study is concomitant to what has been published previously.32, 33, 34
Although this study found significant associations between different variables, as with all cross-sectional descriptive designs, direct evidence of a temporal relationship between exposure and outcome is generally not provided; this should be considered a primary limitation of all cross-sectional descriptive designs.35
Conclusion
Iron deficiency anemia is a serious health problem among females of childbearing age, with the prevalence of IDA being almost three-fold higher in this study than in one with a similar population of the same region 11 years ago. Increased homocysteine levels are associated with serum ferritin, IDA biomarkers and vitamin B12, and so monitoring homocysteine levels might be essential to understand the development of different clinical conditions including anemia. It is highly recommended to start a national intervention program to screen females of childbearing age for IDA, especially secondary school and university students and consequently correct anemia by replenishing body iron stores. Further studies should be conducted to identify any correlations of serum homocysteine as a biomarker of the early stages of iron deficiency in different high-risk IDA groups and to determine whether treating anemia ameliorates the homocysteine level.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.International Nutritional Anemia Consultative Group . ILSI Research Foundation; Washington, DC: 2000. INACG Symposium. 12 March 1999, Durban South Africa; pp. 1–60. [Google Scholar]
- 2.Stoltzfus R. Defining iron-deficiency anemia in public health terms: a time for reflection. J Nutr. 2001;131:565S–567S. doi: 10.1093/jn/131.2.565S. [DOI] [PubMed] [Google Scholar]
- 3.FAO/WHO Preventing micronutrient deficiencies. ICN Fact Sheet No. 1. Supporting paper of the International Conference on Nutrition; December, Rome, Italy; 1992. [Google Scholar]
- 4.Sirdah M.M., El-Agouza I.M., Abu Shahla A.N. Possible ameliorative effect of taurine in the treatment of iron-deficiency anaemia in female university students of Gaza. Palest Eur J Haematol. 2002;69:236–242. doi: 10.1034/j.1600-0609.2002.02722.x. [DOI] [PubMed] [Google Scholar]
- 5.Abdeen Z., Greenough G., Shahin M., Tayback M. Al-Quds University Publication; Jerusalem, Palestine: 2003. Nutritional assessment of the West Bank and Gaza Strip. [Google Scholar]
- 6.Radi S.M., El-Sayed N.A., Nofal L.M., Abdeen Z.A. Ongoing deterioration of the nutritional status of Palestinian preschool children in Gaza under the Israeli siege. East Mediterr Health J. 2013;19:234–241. [PubMed] [Google Scholar]
- 7.Selmi A., Al-Hindi A. Anaemia among school children aged 6–11 years old in Gaza Strip. Palest Ann Alquds Med. 2011;7:27–32. [Google Scholar]
- 8.Gangopadhyay R., Karoshi M., Keith L. Anemia and pregnancy: a link to maternal chronic diseases. Int J Gynaecol Obstet. 2011;115(Suppl. 1):S11–S15. doi: 10.1016/S0020-7292(11)60005-2. [DOI] [PubMed] [Google Scholar]
- 9.Pasricha S.R. Anaemia in pregnancy – not just iron deficiency. Acta Haematol. 2013;130:279–280. doi: 10.1159/000353162. [DOI] [PubMed] [Google Scholar]
- 10.Pasricha S.R., Drakesmith H., Black J., Hipgrave D., Biggs B.A. Control of iron deficiency anemia in low- and middle-income countries. Blood. 2013;121:2607–2617. doi: 10.1182/blood-2012-09-453522. [DOI] [PubMed] [Google Scholar]
- 11.WHO . World Health Organization; Geneva, Switzerland: 2001. Iron deficiency anemia assessment prevention and control: a guide for program managers. [Google Scholar]
- 12.Food and Agriculture Organization of the United Nations (FAO) 6th ed. Rome; Italy: 2004. The state of food insecurity in the world. [Google Scholar]
- 13.Stein A.J., Qaim M. The human and economic cost of hidden hunger. Food Nutr Bull. 2007;28:125–134. doi: 10.1177/156482650702800201. [DOI] [PubMed] [Google Scholar]
- 14.Allen L.H. How common is vitamin B-12 deficiency? Am J Clin Nutr. 2009;89:693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- 15.Katre P., Bhat D., Lubree H., Otiv S., Joshi S., Joglekar C. Vitamin B12 and folic acid supplementation and plasma total homocysteine concentrations in pregnant Indian women with low B12 and high folate status. Asia Pac J Clin Nutr. 2010;19:335–343. [PubMed] [Google Scholar]
- 16.den Elzen W.P., Westendorp R.G., Frölich M., de Ruijter W., Assendelft W.J., Gussekloo J. Vitamin B12 and folate and the risk of anemia in old age: the Leiden 85-Plus Study. Arch Intern Med. 2008;168:2238–2244. doi: 10.1001/archinte.168.20.2238. [DOI] [PubMed] [Google Scholar]
- 17.Yajnik C.S., Deshpande S.S., Lubree H.G., Naik S.S., Bhat D.S., Uradey B.S. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J Assoc Physicians India. 2006;54:775–782. [PubMed] [Google Scholar]
- 18.Dhonukshe-Rutten R.A., Pluijm S.M., de Groot L.C., Lips P., Smit J.H., van Staveren W.A. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. 2005;20:921–929. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann W., Schorr H., Obeid R., Geisel J. Vitamin B-12 status, particularly holotranscobalamin II and methylmalonic acid concentrations, and hyperhomocysteinemia in vegetarians. Am J Clin Nutr. 2003;78:131–136. doi: 10.1093/ajcn/78.1.131. [DOI] [PubMed] [Google Scholar]
- 20.Veena S.R., Krishnaveni G.V., Srinivasan K., Wills A.K., Muthayya S., Kurpad A.V. Higher maternal plasma folate but not vitamin B-12 concentrations during pregnancy are associated with better cognitive function scores in 9- to 10- year-old children in South India. J Nutr. 2010;140:1014–1022. doi: 10.3945/jn.109.118075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Folate and vitamin B12 deficiencies: proceedings of a WHO technical consultation held 18–21 October, 2005, in Geneva, Switzerland. Introduction. Food Nutr Bull. 2008;29(2 Suppl.):S1–S246. [PubMed] [Google Scholar]
- 22.Glenn I.D. IFAS, University of Florida; 2013. Sampling the evidence of extension program impact. Program evaluation and organizational development. PEOD-5. Available from: http://edis.ifas.ufl.edu/pd005 [cited 04.10.13] [Google Scholar]
- 23.Tulchinsky H. Micronutrient deficiency conditions: global health issues. Pub Health Rev. 2010;32:243–255. doi: 10.1186/s40985-017-0071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO . WHO Regional Office for the Eastern Mediterranean; 2012. Initial health assessment report: Gaza strip. Available from: http://www.emro.who.int/palestine [cited 04.10.13] [Google Scholar]
- 25.Camaschella C. How I manage patients with atypical microcytic anaemia. Br J Haematol. 2013;160:12–24. doi: 10.1111/bjh.12081. [DOI] [PubMed] [Google Scholar]
- 26.Pandey K., Dubay P., Bhagoliwal A., Gupta N., Tyagi G. Hyperhomocysteinemia as a risk factor for IUGR. J Obstet Gynaecol Ind. 2012;62:406–408. doi: 10.1007/s13224-012-0287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmadfa I., Singer I. Vitamin B-12 and homocysteine status among vegetarians: a global perspective. Am J Clin Nutr. 2009;89:1693S–1698S. doi: 10.3945/ajcn.2009.26736Y. [DOI] [PubMed] [Google Scholar]
- 28.Beyan C., Kaptan K., Beyan E., Turan M. The platelet count/mean corpuscular hemoglobin ratio distinguishes combined iron and vitamin B12 deficiency from uncomplicated iron deficiency. Int J Hematol. 2005;81:301–303. doi: 10.1532/IJH97.E0311. [DOI] [PubMed] [Google Scholar]
- 29.Song S.M., Bae K.W., Yoon H.S., Im H.J., Seo J.J. A case of anemia caused by combined vitamin B12 and iron deficiency manifesting as short stature and delayed puberty. Kor J Pediatr. 2010;53:661–665. doi: 10.3345/kjp.2010.53.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasri H., Baradaran A. Association of serum homocysteine with anemia in maintenance hemodialysis patients. Pak J Nutr. 2005;4:414–417. [Google Scholar]
- 31.Miller A., Slingerland D.W., Hall C.A., Chu R.C. Food-bound B12 absorption and serum total homocysteine in patients with low serum B12 levels. Am J Hematol. 1998;59:42–45. doi: 10.1002/(sici)1096-8652(199809)59:1<42::aid-ajh8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Garcia A.A., Day A.G., Zanibbi K., Zunzunegui M.V. Long-term effects of folic acid fortification and B-vitamin supplementation on total folate, homocysteine, methylmalonic acid and cobalamin in older adults. Can J Public Health. 2008;99:428–433. doi: 10.1007/BF03405255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall M.N., Liu X., Slavkovich V., Ilievski V., Pilsner J.R., Alam S. Folate, cobalamin, cysteine, homocysteine, and arsenic metabolism among children in Bangladesh. Environ Health Perspect. 2009;117:825–831. doi: 10.1289/ehp.0800164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moghaddasi M., Mamarabadi M., Mohebi N., Razjouyan H., Aghaei M. Homocysteine, vitamin B12 and folate levels in Iranian patients with multiple sclerosis: a case control study. Clin Neurol Neurosurg. 2013;115:1802–1805. doi: 10.1016/j.clineuro.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Carlson M.D., Morrison R.S. Study design, precision, and validity in observational studies. J Palliat Med. 2009;12:77–82. doi: 10.1089/jpm.2008.9690. [DOI] [PMC free article] [PubMed] [Google Scholar]