Abstract
Sphingosine 1-phosphate (S1P) is a membrane-derived lysophospholipid that acts primarily as an extracellular signaling molecule. Signals initiated by S1P are transduced by five G protein-coupled receptors, named S1P1–5. Cellular and temporal expression of the S1P receptors (S1PRs) determine their specific roles in various organ systems, but they are particularly critical for regulation of the cardiovascular, immune, and nervous systems, with the most well-known contributions of S1PR signaling being modulation of vascular barrier function, vascular tone, and regulation of lymphocyte trafficking. However, our knowledge of S1PR biology is rapidly increasing as they become attractive therapeutic targets in several diseases, such as chronic inflammatory pathologies, autoimmunity, and cancer. Understanding how the S1PRs regulate interactions between biological systems will allow for greater efficacy in this novel therapeutic strategy as well as characterization of complex physiological networks. Because of the rapidly expanding body of research, this review will focus on the most recent advances in S1PRs.
Keywords: immunity, endothelium, vascular permeability, nervous system, migration, activation, immune cells, nervous system
Sphingosine 1-phosphate [2S-amino-1-(dihydrogen phosphate)-4E-octadecene-1,3R-diol] (S1P) is a simple membrane-derived lysophospholipid with regulatory roles in almost all facets of mammalian biology (1). Concentrations of S1P in blood and lymph plasmas are high, in the high nanomolar to low micromolar ranges, whereas S1P concentrations in tissues are kept low, creating an S1P gradient (2). S1P signals through five highly-specific G protein-coupled receptors with nanomolar dissociation constants (3, 4). Expression patterns of the five S1P receptors (S1PRs) vary in tissues and also during development and ageing. S1P1, S1P2, and S1P3 are essentially ubiquitously expressed, whereas expression of S1P4 and S1P5 are highly restricted to distinct cell types (4).
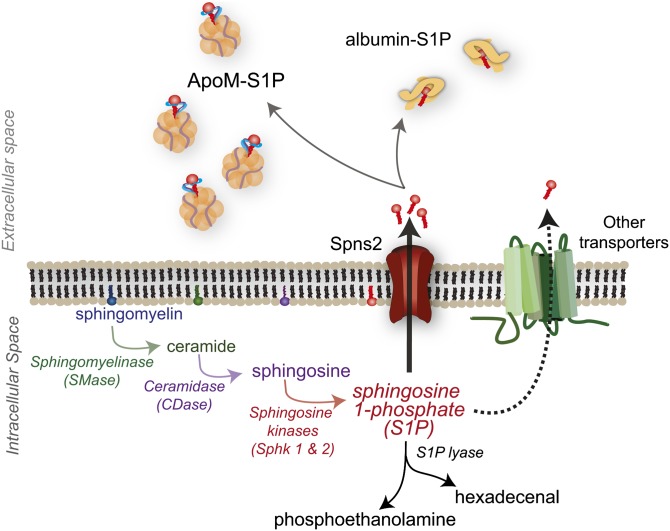
Production of S1P can be initiated by external or internal signals, which lead to activation of the biosynthetic pathway beginning with metabolism of membrane SM to ceramide by SMases (5, 6). Ceramide, an important signaling molecule itself, can be metabolized by ceramidase to sphingosine (Sph) (7). Sph is then phosphorylated by one of two Sph kinases (Sphks), Sphk1 or Sphk2, resulting in S1P genesis (8–10) (Fig. 1).
Fig. 1.
Synthesis and export of S1P. S1P synthesis primarily begins with metabolism of membrane SM. Once synthesized, S1P can be irreversibly degraded to phosphoethanolamine and hexadecenal by S1P lyase, or actively transported out of the cell. Once outside of the cell, S1P is found bound to ApoM or albumin. Spns2, spinster 2.
Although there are proposed intracellular roles for S1P, it is often transported out of the cell where it can act in an autocrine or paracrine manner on S1PRs (11, 12). Transport out of the cell may occur via several transporters; however, the only bona fide transporter to date is spinster 2, which is also capable of FTY720 (fingolimod/Gilenya; Novartis) export (13–22). Once outside of the cell, S1P can bind to two known carriers, albumin or ApoM (6, 23, 24) (Fig. 1). Approximately 35% of plasma S1P is bound to albumin and 65% to ApoM, which is found on a small percentage (∼5%) of HDL particles (24). This ApoM+HDL-bound S1P has been proposed as a primary contributor to the vasoprotective properties of HDLs (25–27). How albumin or ApoM deliver S1P to specific S1PRs has yet to be characterized.
AGONISTS AND ANTAGONISTS
There are several well-characterized agonists and antagonists of S1PRs; however, most compounds have been directed toward modulating the activity of S1P1. FTY720 is the prototypical S1PR agonist and was approved by the US Food and Drug Administration as a first line oral therapy for relapsing-remitting multiple sclerosis (MS) (18, 28). Although FTY720 acts as an agonist at picomolar to nanomolar concentrations on S1P1 and S1P3–5, it also acts as a functional antagonist for S1P1 by inducing receptor endocytosis and degradation of this receptor (29–31). This promiscuity may be responsible for adverse affects, such as acute bradycardia (decreased heart rate) and hypertension, seen in fingolimod-treated patients (32, 33). Initial results from rodent studies indicated that FTY720 phosphate activation of S1P3 was responsible for both bradycardia and hypertension; however, treatment of humans with more selective agonists indicated that S1P1 agonism was responsible for reduced heart rate, whereas S1P3 signaling contributed to the development of hypertension (34–37). The divergent utilization of S1P1 and S1P3 in rodents versus primates for the regulation of these coordinated physiological functions highlights the difficulties encountered upon extrapolation from rodent model-based characterization of S1PR function to human disease therapies.
SEW2871 is an S1P1-specific agonist that activates ERK1/2, AKT, and Rac signals at nanomolar concentrations and induces receptor internalization and recycling; however, it has a relatively short half-life in vivo (38). AUY954 is another commonly used S1P1-selective agonist with an EC50 of approximately 1 nM, which induces phosphorylation of ERK and AKT (39). At high concentrations, AUY954 also has some activity on S1P5 (39). Conversely, W146 antagonizes AKT and ERK phosphorylation and is the only widely utilized S1P1-specific antagonist (40). Administration of W146 enhances vascular leakage and induces pulmonary edema (40, 41). VPC23019 is a useful in vitro tool as a dual S1P1/3 antagonist; however, poor stability and in vivo efficacy limit its use (42–44). The only known compound with activity at S1P2 is JTE-013, an antagonist with an IC50 of approximately 20 nM, which blocks S1P2 signaling through Rho-associated protein kinase (ROCK) and phosphatase and tensin homologue (45, 46). The S1P2 specificity of JTE-013 has been called into question by several studies that indicate it may have activity at S1P4 as well as non-S1PR-mediated effects (44, 47–49).
VASCULAR AND LYMPHATIC SYSTEMS
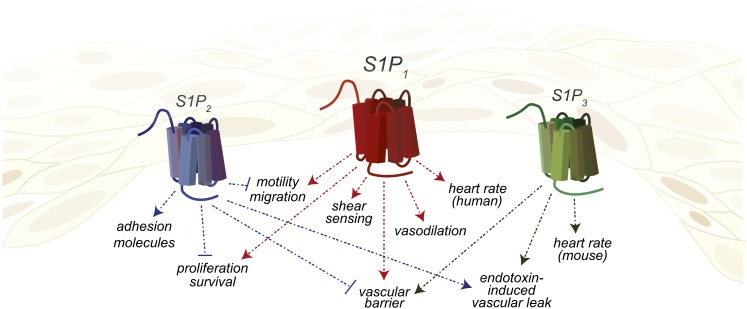
Many effects of S1P on the vasculature are due to expression of S1P1 by the endothelium. S1P1, originally named EDG1 (endothelial differentiation gene), was discovered during a search for immediate early genes regulating endothelial cell differentiation (50). Although S1pr1−/− embryos developed a vascular network, they died in utero at E12.5– E14.5 due to defective coverage of large vessels by pericytes and vascular smooth muscle cells (VSMCs) (51, 52). Specifically, the aorta exhibited severe morphological abnormalities, endothelial hypersprouting, and altered VSMC recruitment and localization (Fig. 2) (53, 54). The generation of inducible cell-specific S1P1 knockout mice has clarified the roles of endothelial cells (ECs) or VSMC S1P1 in the regulation of postnatal vascular development, maturation, and function. In the developing retinal vasculature, S1P1 expression is restricted to the ECs and increases with vessel maturity, as the lowest levels of expression are found at the vascular leading front (55). Postnatal deletion of EC S1pr1 did not affect mural cell recruitment or vessel coverage in the retina; however, angiogenic hypersprouting occurred, characterized by dilated vessels and increases in the number of branch points and tip cells. Induced over-expression of EC S1P1 suppressed vascular sprouting (55). Changes in the vascular architecture of EC S1pr1−/− mice were accompanied by increased vascular permeability, resulting from altered vascular endothelial cadherin localization at endothelial cell-cell junctions (54, 55). These data confirmed numerous earlier in vitro studies describing the necessity of EC S1P1 for the maintenance of vascular barrier function through adherens junction formation induced by activation of Rac after Gαi coupling to S1P1 (Fig. 2) (56, 57).
Fig. 2.
Expression of S1PRs and responses by endothelial cells. Endothelial cells express S1P1, S1P2, and S1P3 protein. Endothelial cells may express different S1PRs depending on activation status.
Maintenance and formation of adherens junctions was dependent on S1P1 signaling initiated not only by ligand, but also by fluid shear stress (Fig. 2). Examination of murine aortae found that areas of turbulent flow (the lesser curvature) had poor endothelial cell alignment and S1P1 relocalized from the EC surface to endocytic vesicles, whereas in the descending aorta, an area of laminar flow, S1P1 and vascular endothelial cadherin colocalized to the cell surface (55). Additionally, maintenance of vascular homeostasis by the endothelial glycocalyx, which is also susceptible to changes in flow dynamics, was dependent upon S1P1-induced inhibition of matrix metalloproteinase (58).
Mice with endothelium-specific deletion of S1P1 developed severe pathology in a model of renal ischemia/reperfusion injury, both in the kidneys and the liver, characterized by elevated plasma creatinine, alanine transferase, and tissue necrosis (59). Conversely, of the five S1PRs, S1P2 mRNA in the kidney was most increased upon renal ischemia/reperfusion, and mice deficient in S1P2 developed significantly less pathology compared with WT controls (60). When S1pr2−/− mice were treated with the S1P1 antagonist, W146, before ischemia/reperfusion, they were no longer protected from renal injury, suggesting that S1P1 and S1P2 in the renal vasculare endothelium play protective and injurious roles, respectively, in kidney injury and disease (60).
The pro-inflammatory tendency of S1P2 is supported by in vitro studies suggesting a paracrine feedback loop involving EC TNFα induction of S1P2 expression leading to activation of nuclear factor (NF)-κB and increases in intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 (61). In vivo studies utilizing S1pr2−/− mice and a model of acute inflammation, endotoxemia, further support the conclusion that S1P2 is an important regulator of vascular activation and therefore, permeability (62). Induction of endotoxemia in mice lacking S1pr2 in the stroma and not in the bone marrow (BM) compartment resulted in decreased vascular permeability, VCAM-1 and ICAM-1 expression, and more rapid resolution (62). Similarly, in vitro, S1P2 actively suppressed angiogenic sprouting through leukemia-associated RhoGEF (LARG) activation of RhoC (63). These recent studies reaffirm the conclusion that an antagonistic relationship exists between S1P1 and S1P2 in the vascular endothelium during tissue injury and disease.
Lymphatic endothelium also expresses S1PRs, although more interest has focused on the role it may play in S1P metabolism (21, 64). Examination of murine iliac collecting lymph vessels demonstrated that while S1P does not induce nitric oxide or prostaglandin release, signaling via S1P2 regulates tonic contractility of lymph vessels, as shown using S1P2 inhibition by JTE013 (65).
IMMUNE SYSTEM
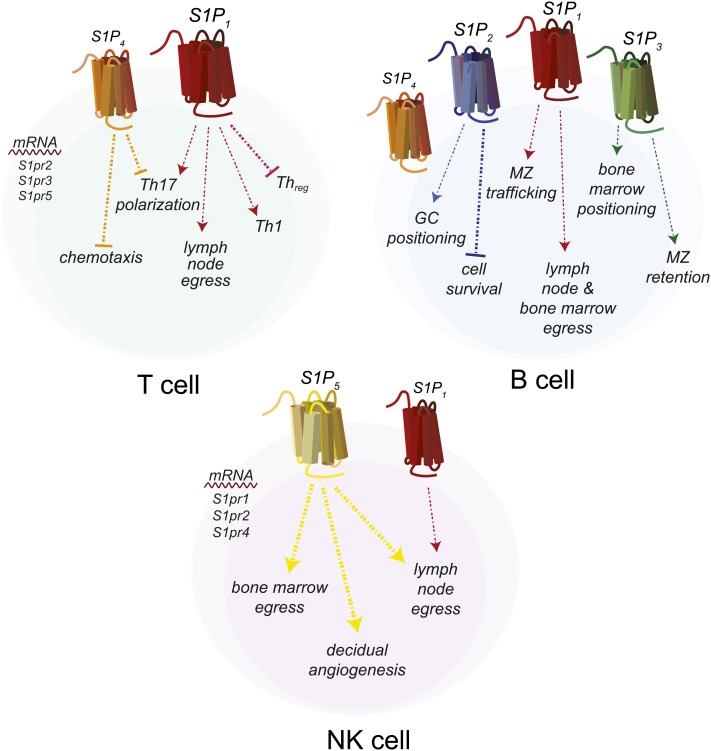
S1PRs regulate many aspects of immune cell biology. The best known is the regulation by S1P1 of lymphocyte migration out of the secondary lymphoid organs into the blood and lymph (Fig. 3) (66). Regulation of migration occurs by S1P1 counteracting the retention signals provided by the chemokine receptor CCR7 (67). However, this is not the only role for S1P1 in lymphocytes, and roles for the other four S1PRs in the immune system have recently been revealed.
Fig. 3.
Expression of S1PRs and responses by cells of the acquired immune system. T cells express S1P1 and S1P4, B cells express S1P1, S1P2, S1P3, and S1P4, and NK cells express S1P1 and S1P5. Cells do not necessarily express all of the illustrated S1PRs at one time, but may have differential expression during different stages of maturation or activation.
The contribution of S1PRs to regulation of the immune response has been studied extensively in the context of experimental autoimmune encephalomyelitis (EAE), the most commonly used animal model of MS (68). Although EAE and MS are considered to be primarily diseases of the immune system, the role of S1PRs on neural cells is also gaining an appreciation and will be discussed later. FTY720 is a Sph analog that is phosphorylated, acts on S1P1,3–5, and was the first US Food and Drug Administration approved oral therapy for MS (69). The presumed mechanism of action has been the trapping of autoreactive T and B cells in the lymphoid organs, away from the central nervous system (70, 71). However, T cell S1P1 may also regulate the activation and differentiation status of these immune cells. Deletion of T cell S1P1 significantly suppresses the ability of these cells to be polarized to T-helper (Th)17 in vitro (72). Conversely, when EAE was induced in mice expressing an internalization-defective S1P1 (S5A), this significantly increased polarization of T cells to the Th17 phenotype resulting in increased disease pathology and immune cell infiltration into the CNS (72).
S1P1 is also expressed on CD4 T cells isolated from human rheumatoid arthritis patients (73). S1P enhances TNFα-induced expression of the receptor activator of nuclear factor kB (RANK) ligand by these cells, an effect replicated in a synovial cell-like cell line, MH7 (73). In collagen-induced models of rheumatoid arthritis, a S1P1-specific antagonist prevented or ameliorated disease by upregulating lymphocyte CD69 expression, which downregulates S1P1 surface expression, blocking thymic egress (73–75).
S1P1 also affects other populations of T cells, such as T regulatory cells (Treg), which, as the name implies, play an important role in controlling immune responses and T memory cells (76, 77). S1P1 suppresses Treg development via the AKT/mammalian target of rapamyacin pathway and affects their migration from the thymus and out of the periphery by counteracting CCR7 retention signals, similar to the mechanism regulating the egress of effector T cells from lymph nodes (67, 77, 78). S1P1 signals may also modulate nuclear localization of the transcription factor forkhead box P3, which is necessary for Treg generation (78). In human patients, FTY720 significantly increased the number of Treg while decreasing central memory T cells (79). In a specific subset of T memory cells, nonlymphoid resident memory cells (TRM), cytokines that induce the TRM phenotype also downregulate the transcription factor Krüppel-like factor2 and its target gene, S1pr1 (80). Subsequently, TRM are unable to sense S1P in circulation and are maintained in the periphery.
Although S1P1 has been the focus of much research, not much is known of the roles of the other S1PRs. In CD8 effector T cells, S1P4 may influence their trafficking to lymph nodes (LNs), although it appears not to be a primary regulator (81). S1pr4−/− mice have decreased Th17 T cell polarization; however, reduced Th17 differentiation is likely T cell extrinsic and primarily due to functions of S1P4 in dendritic cells (DCs) (81).
S1PR expression choreographs many aspects of B cell subset localization within lymphoid organs, thereby affecting their functionality; however, there are some direct effects of S1P signaling on B cell survival (Fig. 3) (82, 83). While S1P1 has some regulatory functions in B cells, it appears that S1P2 has a greater impact on these cells. Aged S1pr2−/− mice develop diffuse large B cell lymphoma (DLBCL), characterized by increased germinal center (GC) B cells and spontaneous GC formation, which correlates with an approximate 26% mutation incidence for S1PR2 in human DLBCL (84). Under homeostatic conditions, S1P2 signals via G12/13 to activate Rho/ROCK, antagonizing activation of AKT and pro-survival signals (82). B cell S1P2 also regulates follicular positioning of B cells by directing their clustering to GC in response to follicular DC-derived S1P (82, 85). The ability of follicular B cells to exit the follicle is, however, dependent upon S1P1 expression (86). Additionally, trafficking of marginal zone (MZ) B cells between the MZ and the follicle is regulated by S1P1, which maintains these cells in the MZ in order for them to capture blood-borne antigens (86–88).
Studies of nonobese diabetic mice have shown that upregulation of S1P3 by MZ B cells and their T2 MZ precursors may also play a role in enhancing MZ retention in these mice (89, 90). S1P3 has already been shown to regulate B cell migration in vitro, but not in vivo, in WT mice (83, 87). However, it may be important for positioning of immature B cells and their progenitors within the BM, whereas S1P1 participates in directing their migration from the BM parenchyma into sinusoids and subsequently into circulation (83).
Natural killer (NK) cells are considered innate lymphoid cells that develop from lymphoid progenitors in the BM, but do not undergo genomic changes that occur in the B or T cell receptor genes (91, 92). They are important for anti-tumor immunity and are prolific producers of IFNγ (92). Mouse NK cells have low levels of transcript for S1pr1, S1pr2, and S1pr4 and high S1pr5 mRNA levels (Fig. 3) (93, 94). S1P5 normally antagonizes NK CXCR4 BM retention signals, and S1pr5−/− mice have decreased numbers of NK cells in the periphery and increased numbers in lymph nodes and BM due to defective migration (93, 95). This phenotype is also observed in the mouse model of Niemann-Pick disease type C, a lysosomal storage disorder presenting as an accumulation of cholesterol and sphingolipids in the lysosome and decreased concentrations of circulating S1P in human patients (96, 97). Studies utilizing FTY720 indicated that S1P1 also contributes to NK cell migration from LN to lymph, but the contribution is relatively minor compared with that of S1P5, which is not subject to CD69 regulation (75, 94). Decidual NK (dNK) cells are a specialized NK cell subset that regulates trophoblast invasion during early pregnancy by secreting pro-angiogenic and growth factors, including vascular endothelial growth factor (VEGF) (98). S1P1 and S1P5 are increased in human dNK cells compared with circulating NK cells, and S1P5 expression decreases after the first trimester (99). FTY720 treatment decreased dNK S1P5 expression, VEGF production, and trophoblast invasion in vitro (99).
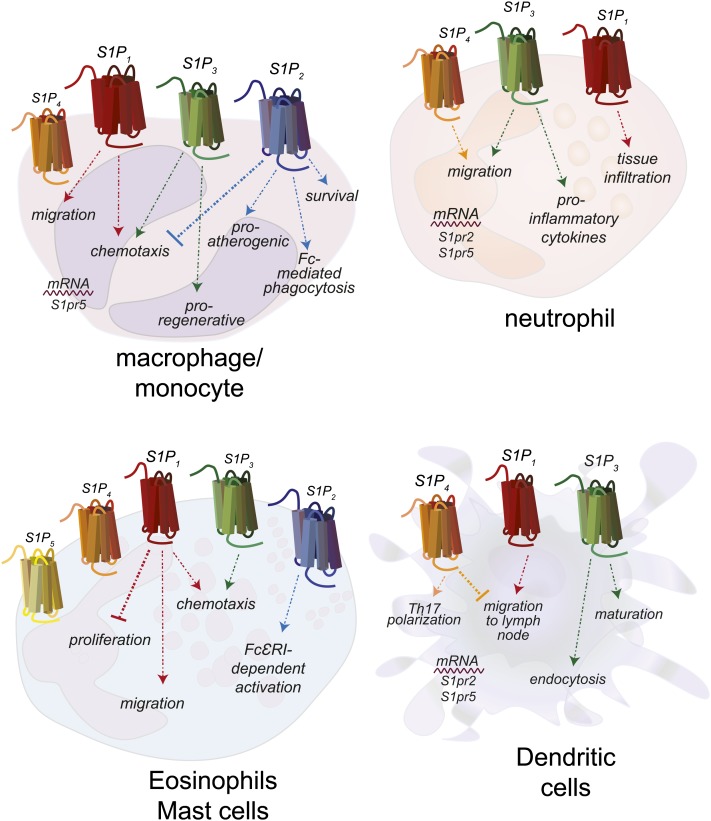
Macrophages are important sentinel cells that develop from monocytes to fight infection and repair damaged tissue (100). S1PRs expressed by monocytes and macrophages regulate their migration and activation, and the receptors responsible are cell subtype- and situation-specific (Fig. 4). In general, S1P1 and S1P3 appear to induce migration toward S1P, whereas S1P2 expression repulses macrophages from S1P (101, 102). S1pr2−/− mice on a pro-atherogenic genetic background (Apoe−/−) developed significantly less atherosclerosis, accompanied by decreased macrophage and monocyte retention in atherosclerotic plaques, indicating effects on migration, tissue retention, and activation (103). In comparison, S1pr3−/− mice on the same Apoe−/− background do not have altered development of atherosclerosis, but do have decreased monocytes and macrophages with atherosclerotic lesions (101). In WT mice, treatment with FTY720 results in decreased circulating monocytes; however, use of the S1P1/4/5 agonist, BAF312, yielded similar results, both at homeostasis and during EAE, indicating that S1P3 is not the sole regulator of monocyte circulation (104). This could be a cell subtype-specific effect, or dependent on environment, as local administration of FTY720 appeared to enhance recruitment of anti-inflammatory pro-angiogenic monocytes (105). This supports an earlier report that macrophage S1P3 induces a pro-regenerative phenotype in a model of renal ischemia/reperfusion (106).
Fig. 4.
Expression of S1PRs and responses by cells of the innate immune system. Monocytes and/or macrophages express S1P1–4, neutrophils express S1P1, S1P3, and S1P4, eosinophils and MCs express all S1PRs, and DCs express S1P1, S1P3, and S1P4. Cells do not necessarily express all of the illustrated S1PRs at one time, but may have differential expression during different stages of maturation or activation.
A report utilizing the zymosan peritonitis model proposed that the resulting apoptotic neutrophils induced S1P1 expression on recruited macrophages and that S1P1 is necessary for emigration from the inflamed peritoneum, but has no role in efferocytosis or activation (107). S1P2 on alveolar macrophages may regulate their phagocytic capacity, as S1pr2−/− alveolar macrophages displayed decreased phagocytosis of the fungus Cryptococcus neoformans due to decreased expression of Fc receptors necessary for phagocytosis of antibody-opsonized fungus (108).
Neutrophils are the first immune cell line of defense and can shape the immune response (109). Neutrophils express mRNA for all S1PRs; however, the level of expression and the ability of S1P to affect changes in their responses depend upon their activation status (Fig. 4) (110). More recently, it was reported that S1P lyase (Sgpl)−/− mice are unable to degrade S1P and have neutrophilia (111). Although S1P4 deficiency in Sgpl knockouts resulted in circulating neutrophil numbers that were close to WT, S1P4 was not specifically deleted in neutrophils, raising the possibility that multiple cell types were responsible for the effect. Specific deletion of neutrophil S1P1 did not normalize neutrophil numbers in Sgpl−/− mice. However, in rat models of hyperalgesia dependent upon neutrophil infiltration, S1P1 was necessary for neutrophil recruitment (112). Specific S1P1 antagonism blocked neutrophil infiltration, whereas agonism increased sensitivity.
Eosinophils and mast cells (MCs) are both involved in anti-parasite immune responses and allergic immunity (113). Eosinophils from mice over-expressing interleukin-5, an eosinophil growth factor, express high levels of S1P3 and demonstrate increased chemotactic responses to S1P in vitro (Fig. 4) (114). In a model of allergic rhinitis, FTY720 treatment significantly decreased the numbers of infiltrating MCs and eosinophils, resulting in resolution (115). In vitro, FTY720 induced MC apoptosis in a dose-dependent manner (115). Similar to lymphocytes, S1P1 regulates MC migration toward the antigen, whereas S1P2 regulates their activation status upon FcεRI ligation, inducing degranulation and CCL2 secretion (116).
DCs are professional antigen-presenting cells and as such, are required for proper induction and direction of the acquired immune response (117). Both human and mouse DCs express mRNA for S1P1–5 and exhibit varied responses to S1P stimulation in vitro and in vivo (Fig. 4) (118, 119, 120). Langerhans cells, skin resident DCs, require S1P1 for migration to LN, whereas kidney resident DCs require S1P3 for maturation in ischemia/reperfusion (121, 122). This is also the case in models of sepsis, where DC S1P3 is required for interleukin-1β production (123). In EAE, although S1P1 agonism decreased disease pathology, it did not affect entry into the CNS of a subset of DCs (plasmacytoid DCs). However, plasmacytoid DCs in the CNS were necessary for the efficacy of S1P1 agonist treatment (124).
S1P4 was cloned from mature human DCs, yet not much is known about the role this receptor plays in these cells (125). In models of autoimmune disease, Th2-type immune responses such as allergic airway inflammation and cutaneous hypersensitivity, S1pr4−/− mice had increased pathology and up to 50% increase in DCs in draining LN after topical antigen application (81). This implies that S1P4 may antagonize S1P1 in DCs, regulating their ability to migrate from the periphery after antigen uptake.
NERVOUS SYSTEM
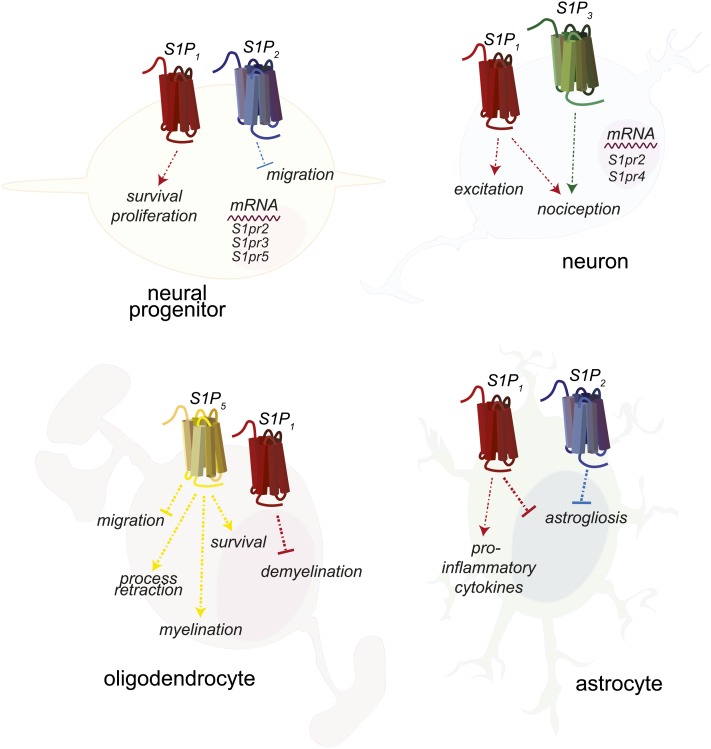
Neural progenitors express mRNA for S1P1–5 and respond to S1P stimulation with induction of Ca2+ mobilization (Fig. 5) (126). S1P regulates embryonic nervous system development, as the neuroepithelial layers of the developing telencephalon in S1pr1−/− embryos have significantly increased apoptosis and decreased mitosis (127). S1P2 may also play a role in regulating neural progenitors, as postischemic administration of the S1P2 antagonist JTE-013 or short hairpin RNA against S1P2 significantly increased progenitor migration to the ischemic region (128). This indicates that S1P2 may repel neural progenitors from areas of high S1P concentration in the same manner as it regulates macrophage migration (102). Indirectly, S1P signaling on astrocytes affects neural progenitors by increasing lamin production, thereby encouraging maturation and neurite outgrowth by progenitors (129). Interestingly, neural stem cells were protected from radiation-induced apoptosis by nanomolar FTY720 treatment in vitro, although it is unknown which receptor is involved in this protection (130).
Fig. 5.
Expression of S1PRs and responses by neural cells. Neural progenitors express S1P1 and S1P2, neurons express S1P1 and S1P3, oligodendrocytes express S1P1 and S1P5, and astrocytes express S1P1 and S1P2. S1P1 couples exclusively to Gαi. S1P2 and S1P3 can couple to Gαi, Gα12/13, or Gαq, and S1P5 can couple to Gαi or Gα12/13. Cells do not necessarily express all of the illustrated S1PRs at one time, but may have differential expression during different stages of maturation or activation.
Although analyses of entire mouse dorsal root ganglion found that S1P3 was the most highly expressed S1PR, single cell mRNA analysis of individual neurons found that S1P1 was most highly expressed, regardless of neuronal subtype, indicating that high expression of S1P3 occurs in ganglion cell types other than neurons (Fig. 5) (131, 132). One group found that pain responses induced by intradermal S1P injection or models of postoperative pain were significantly decreased in S1pr3−/− mice, whereas minimal differences were seen in S1pr1−/− mice (131); however, another group found that mice lacking S1P1 specifically in nociceptor neurons were protected from S1P-induced pain (133). Finally, in the murine model of the neurodevelopmental disease, Rett syndrome, FTY720 or S1P1-specific agonist SEW2871 in vivo treatment increased neuron production of brain-derived neurotrophic factor and decreased neurological symptoms (134).
Oligodendrocytes are the myelinating cells of the CNS and the primary cell type affected in MS and in the mouse EAE model (135). Process retraction, Rho/ROCK-mediated inhibition of immature oligodentrocyte precursor migration, and Gi/AKT-mediated survival in mature oligodendrocytes occurs via S1P5 (Fig. 5) (136, 137). Ex vivo studies using cerebellar slice cultures indicated that S1PR agonism, particularly S1P1, could prevent or reverse demyelination, explaining the ability of FTY720 to induce remyelination and process extension in the same system (138, 139). Data from a different in vitro system, myelinated neurospheres, indicated that FTY720 decreased microglial activation and oligodenrocyte apoptosis, and induced remyelination primarily by S1P5 agonism (140). An in vivo study provides conflicting evidence to these in vitro studies, reporting no effects on myelin repair with FTY720 treatment; however, the models of demyelination utilized in both the in vitro and in vivo studies were induced chemically and were meant to exclude possible effects of immune or vascular cells (141). As such, they cannot model complex neuroinflammatory disease and care must therefore be taken when attempting to extrapolate results to in vivo disease, such as EAE or MS.
The resident immune cells of the CNS, microglia, express all S1PRs (142). In vitro studies indicated that FTY720 downregulated production of pro-inflammatory molecules by microglia while increasing neurotrophic factor production, resulting in an overall neuroprotective phenotype (142). FTY720 also inhibited secretory vesicle mobility and exocytic release by astroglia, thus inhibiting the release of pro-inflammatory mediators by this cell type, as well (143). Astrocytic gliosis also occurs in EAE and MS (Fig. 5) (71). In vitro treatment of a human astrocyte cell line with FTY720 suppressed S1P-induced production of pro-inflammatory cytokines (144). In vivo, specific deletion of astrocyte S1P1 resulted in decreased EAE pathology and a loss of FTY720 efficacy, indicating that the primary target of FTY720 during EAE was S1P1 specifically on astrocytes (145). Additionally, in a model of spinal cord injury, FTY720 affected the later stages of vascular permeability and astrogliosis, partially through agonism of S1P1 (146). Another target of FTY720, S1P3, was also found on reactive astrocytes in human MS lesions and upregulated by lipopolysaccharide stimulation of astrocytes in vitro, although it is unknown if expression of S1P3 is protective or pathogenic in the context of MS/EAE (147). Mice deficient in the one S1PR not targeted by FTY720, S1P2, are prone to seizures resulting in 40% mortality and have enhanced hippocampal gliosis accompanied by behavioral defects (148). Importantly, MS patients treated with fingolimod show reduced brain volume loss and lesional activity, suggesting the importance of S1PR pathways in neuroprotection (149–151).
The blood brain barrier (BBB) forms through unique interactions between brain endothelial cells, astrocyte foot-processes, and pericytes, and regulates interactions between the immune and nervous systems (152). Alterations in the BBB are implicated or present in numerous neurological diseases, including MS, stroke, and dementias (153). S1P5 was highly expressed by human brain capillary endothelial cells, and antagonism of S1P5 in an in vitro model of BBB decreased vascular permeability and monocytic transmigration (154). Studies of FTY720 treatment in the context of transient cerebral ischemia and reperfusion have demonstrated neuroprotection in mouse and rat models; however, these effects may be due to effects on interactions between the neurovasculature and immune cells (155, 156). FTY720 treatment reduced brain edema as well as expression of the vascular adhesion molecule, ICAM-1, resulting in decreased neutrophil infiltration (155). Additionally, when transient cerebral ischemia was induced in lymphocyte-deficient Rag1−/− mice, the protective effect of FTY720 was lost, further implying that FTY720-mediated protection is due to effects on the neurovasculature and its interactions with immune cells (156). Conversely, a study utilizing a model of permanent cerebral ischemia demonstrated no effect on pathology with FTY720 treatment, whereas another group demonstrated efficacy after delaying FTY720 treatment for 3 days after photothrombosis induction, with increased functional capacity and decreased astrogliosis (157, 158). Thus, protection by FTY720 may be dependent on the method of ischemia induction and temporal regulation of cell activation and recruitment.
INVOLVEMENT OF S1PRs IN CANCER AND ONCOGENESIS
S1PRs have also been implicated in cancer pathogenesis, playing roles in tumor maintenance similar to their roles in maintenance of homeostasis, such as modulation of survival and proliferation (159–161). WT hamster lung fibroblasts were protected from nutrient deprivation-induced apoptosis by expression of S1P1, which induced the anti-apoptotic protein Mcl1 via the phosphoinositide 3-kinase and PKC pathways (162). Lung adenocarcinoma cell lines respond to S1P with increased proliferation and invasion through S1P3-mediated expression of epidermal growth factor receptor (EGFR) (163).
Estrogen receptor positive (ER+) breast cancer cells also responded to S1P via S1P3 to coordinately regulate EGFR localization and signaling (164). High expression of S1P1 or S1P3 by ER+ breast cancer cells correlated with poor prognosis and high S1P1 expression induced decreased expression of pro-apoptotic markers (165, 166). In ER− breast cancer cells, S1P4 expression activated the ERK1/2 pathway and correlated with poor prognosis (167). In vitro, several breast cancer cell lines respond to S1P or S1P1 agonist SEW2871 with increased proliferation (168).
Another malignancy that S1P signaling may play a prominent role in is colonic inflammation and the resultant cancer (169). In a model of ulcerative colitis, considered a possible precursor for colon cancer, increased colonic bleeding and mortality resulted from S1P1 deletion (170). In a model of colitis-associated cancer, S1P1 signaling was necessary for persistent activation of nuclear factor-kB and signal transducer and activator of transcription3 transcription factors needed for maintaining the chronic inflammatory state and could be blocked by FTY720 treatment (171). In human colon cancer cells, expression of the chemotherapeutic resistance and cancer stem cell marker CD44 was regulated by S1P2-induced ERK phosphorylation (172). Interestingly, FTY720 treatment impaired the mucosal immune response to the extracellular bacterium, Citrobacter rodentium, including decreased DC numbers, as well as macrophages and T cells in the colon, while increasing bacterial burden (173). These data suggest that FTY720 or other S1PR modulators could be beneficial or detrimental, depending upon how they influence the immune response.
In prostate adenocarcinoma, Sphk1-derived S1P activated AKT pro-survival pathways through activation of S1P2 (174). AKT and Bcl-associated death promoter pro-survival pathways were also reduced by FTY720 administration to neuroblastoma cells in an in vitro and an in vivo xenograft model, resulting in decreased cancer cell viability (175).
S1PR expression in several hematological malignancies has also been described, including S1P1 expression by classical Hodgkin’s lymphoma (CHL) cells, B cell chronic lymphocytic leukemia (B-CLL) cells, and activated B cell-like DLBCL cells (176–178). Chronic myeloid leukemia (CML) cells expressed S1P2, which resulted in increased stability of the B cell receptor-Abl1 fusion protein and subsequently, increased proliferation (179). Expression of S1PRs by blood cancer cells may directly regulate their survival or by controlling the localization of cells within permissive environments such as the lymph nodes.
CONCLUDING REMARKS
S1PRs are gaining appreciation as powerful modulators of homeostasis and pathogenesis. In all biological systems, S1PRs play some role in regulating cell survival, migration, phenotype, activation status, and proliferation. In the current review, we have attempted to summarize the most recent advances in the field of S1PR biology and to provide novel insights into the biological responses regulated. As more cell-specific animal models of gene deletion or over-expression are created, and agonists and antagonists with greater S1PR subtype specificity are developed, further studies with such tools will clarify the contributions of specific S1PRs in each physiological or pathological context. This is especially true of the less explored members of the S1PR family, S1P4 and S1P5. Additionally, we anticipate that the development of more compounds for clinical use will expand our understanding of the complex signaling networks regulated by S1PRs and their role in human homeostasis and disease.
Footnotes
Abbreviations:
- BBB
- blood brain barrier
- BM
- bone marrow
- DC
- dendritic cell
- DLBCL
- diffuse large B cell lymphoma
- dNK
- decidual natural killer
- EAE
- experimental autoimmune encephalomyelitis
- EC
- endothelial cell
- ER
- estrogen receptor
- GC
- germinal center
- ICAM
- intracellular adhesion molecule
- LN
- lymph node
- MC
- mast cell
- MS
- multiple sclerosis
- MZ
- marginal zone
- NK
- natural killer
- ROCK
- Rho-associated protein kinase
- Sgpl
- sphingosine 1-phosphate lyase
- S1P
- sphingosine 1-phosphate
- Sph
- sphingosine
- Sphk
- sphingosine kinase
- Sph
- sphingosine
- S1PR
- sphingosine 1-phosphate receptor
- Th
- T helper cell
- Treg
- T regulatory cell
- TRM
- T resident memory cell
- VSMC
- vascular smooth muscle cell
This work was supported by National Institutes of Health Grants HL67330, HL70694, and HL89934 to T.H.
REFERENCES
- 1.Blaho V. A., Hla T. 2011. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 111: 6299–6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. 2005. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 309: 1735–1739. [DOI] [PubMed] [Google Scholar]
- 3.Pham T-C. T., Fells J. I., Osborne D. A., North E. J., Naor M. M., Parrill A. L. 2008. Molecular recognition in the sphingosine 1-phosphate receptor family. J. Mol. Graph. Model. 26: 1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun J., Hla T., Lynch K. R., Spiegel S., Moolenaar W. H. 2010. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 62: 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmelz E. M., Crall K. J., Larocque R., Dillehay D. L., Merrill A. H. 1994. Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J. Nutr. 124: 702–712. [DOI] [PubMed] [Google Scholar]
- 6.Yatomi Y. 2008. Plasma sphingosine 1-phosphate metabolism and analysis. Biochim. Biophys. Acta. 1780: 606–611. [DOI] [PubMed] [Google Scholar]
- 7.Hannun Y. A., Obeid L. M. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 8.Igarashi Y., Yatomi Y. 1998. Sphingosine 1-phosphate is a blood constituent released from activated platelets, possibly playing a variety of physiological and pathophysiological roles. Acta Biochim. Pol. 45: 299–309. [PubMed] [Google Scholar]
- 9.Kohama T., Olivera A., Edsall L., Nagiec M. M., Dickson R., Spiegel S. 1998. Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 273: 23722–23728. [DOI] [PubMed] [Google Scholar]
- 10.Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. 2000. Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275: 19513–19520. [DOI] [PubMed] [Google Scholar]
- 11.Olivera A., Buckley N. E., Spiegel S. 1992. Sphingomyelinase and cell-permeable ceramide analogs stimulate cellular proliferation in quiescent Swiss 3T3 fibroblasts. J. Biol. Chem. 267: 26121–26127. [PubMed] [Google Scholar]
- 12.Van Brocklyn J. R., Lee M. J., Menzeleev R., Olivera A., Edsall L., Cuvillier O., Thomas D. M., Coopman P. J., Thangada S., Liu C. H., et al. 1998. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 142: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato K., Malchinkhuu E., Horiuchi Y., Mogi C., Tomura H., Tosaka M., Yoshimoto Y., Kuwabara A., Okajima F. 2007. Critical role of ABCA1 transporter in sphingosine 1-phosphate release from astrocytes. J. Neurochem. 103: 2610–2619. [DOI] [PubMed] [Google Scholar]
- 14.Mitra P., Oskeritzian C. A., Payne S. G., Beaven M. A., Milstien S., Spiegel S. 2006. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. USA. 103: 16394–16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takabe K., Kim R. H., Allegood J. C., Mitra P., Ramachandran S., Nagahashi M., Harikumar K. B., Hait N. C., Milstien S., Spiegel S. 2010. Estradiol induces export of sphingosine 1-phosphate from breast cancer cells via ABCC1 and ABCG2. J. Biol. Chem. 285: 10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., Mochizuki N. 2009. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 323: 524–527. [DOI] [PubMed] [Google Scholar]
- 17.Hisano Y., Kobayashi N., Kawahara A., Yamaguchi A., Nishi T. 2011. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J. Biol. Chem. 286: 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun J., Hartung H-P. 2010. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T., Sunden Y., Arai Y., Moriwaki K., Ishida J., et al. 2012. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nijnik A., Clare S., Hale C., Chen J., Raisen C., Mottram L., Lucas M., Estabel J., Ryder E., Adissu H., et al. 2012. The role of sphingosine-1-phosphate transporter spns2 in immune system function. J. Immunol. 189: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendoza A., Bréart B., Ramos-Perez W. D., Pitt L. A., Gobert M., Sunkara M., Lafaille J. J., Morris A. J., Schwab S. R. 2012. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagahashi M., Kim E. Y., Yamada A., Ramachandran S., Allegood J. C., Hait N. C., Maceyka M., Milstien S., Takabe K., Spiegel S. 2013. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 27: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murata N., Sato K., Kon J., Tomura H., Yanagita M., Kuwabara A., Ui M., Okajima F. 2000. Interaction of sphingosine 1-phosphate with plasma components, including lipoproteins, regulates the lipid receptor-mediated actions. Biochem. J. 352: 809–815. [PMC free article] [PubMed] [Google Scholar]
- 24.Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnström J., Sevvana M., Egerer-Sieber C., Muller Y. A., Hla T., Nielsen L. B., et al. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 108: 9613–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura T., Sato K., Malchinkhuu E., Tomura H., Tamama K., Kuwabara A., Murakami M., Okajima F. 2003. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler. Thromb. Vasc. Biol. 23: 1283–1288. [DOI] [PubMed] [Google Scholar]
- 26.Argraves K. M., Argraves W. S. 2007. HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid Res. 48: 2325–2333. [DOI] [PubMed] [Google Scholar]
- 27.Tran-Dinh A., Diallo D., Delbosc S., Varela-Perez L. M., Dang Q., Lapergue B., Burillo E., Michel J., Levoye A., Martin-Ventura J., et al. 2013. HDL and endothelial protection. Br. J. Pharmacol. 169: 493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. 2010. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9: 883–897. [DOI] [PubMed] [Google Scholar]
- 29.Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G-J., Card D., Keohane C., et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296: 346–349. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann V., Davis M. D., Heise C. E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277: 21453–21457. [DOI] [PubMed] [Google Scholar]
- 31.Oo M. L., Thangada S., Wu M-T., Liu C. H., Macdonald T. L., Lynch K. R., Lin C-Y., Hla T. 2007. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J. Biol. Chem. 282: 9082–9089. [DOI] [PubMed] [Google Scholar]
- 32.Budde K., Schmouder R. L., Brunkhorst R., Nashan B., Lücker P. W., Mayer T., Choudhury S., Skerjanec A., Kraus G., Neumayer H. H. 2002. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J. Am. Soc. Nephrol. 13: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J. A., Barkhof F., Comi G., Hartung H-P., Khatri B. O., Montalban X., Pelletier J., Capra R., Gallo P., Izquierdo G., et al. 2010. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 34.Forrest M., Sun S-Y., Hajdu R., Bergstrom J., Card D., Doherty G., Hale J., Keohane C., Meyers C., Milligan J., et al. 2004. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor subtypes. J. Pharmacol. Exp. Ther. 309: 758–768. [DOI] [PubMed] [Google Scholar]
- 35.Sanna M. G., Liao J., Jo E., Alfonso C., Ahn M-Y., Peterson M. S., Webb B., Lefebvre S., Chun J., Gray N., et al. 2004. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 279: 13839–13848. [DOI] [PubMed] [Google Scholar]
- 36.Fryer R. M., Muthukumarana A., Harrison P. C., Nodop Mazurek S., Chen R. R., Harrington K. E., Dinallo R. M., Horan J. C., Patnaude L., Modis L. K., et al. 2012. The clinically-tested S1P receptor agonists, FTY720 and BAF312, demonstrate subtype-specific bradycardia (S1P1) and hypertension (S1P3) in rat. PLoS ONE. 7: e52985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moberly J. B., Ford D. M., Zahir H., Chen S., Mochizuki T., Truitt K. E., Vollmer T. L. 2012. Pharmacological effects of CS-0777, a selective sphingosine 1-phosphate receptor-1 modulator: results from a 12-week, open-label pilot study in multiple sclerosis patients. J. Neuroimmunol. 246: 100–107. [DOI] [PubMed] [Google Scholar]
- 38.Jo E., Sanna M. G., Gonzalez-Cabrera P. J., Thangada S., Tigyi G., Osborne D. A., Hla T., Parrill A. L., Rosen H. 2005. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem. Biol. 12: 703–715. [DOI] [PubMed] [Google Scholar]
- 39.Pan S., Mi Y., Pally C., Beerli C., Chen A., Guerini D., Hinterding K., Nuesslein-Hildesheim B., Tuntland T., Lefebvre S. 2006. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 13: 1227–1234. [DOI] [PubMed] [Google Scholar]
- 40.Sanna M. G., Wang S-K., Gonzalez-Cabrera P. J., Don A., Marsolais D., Matheu M. P., Wei S. H., Parker I., Jo E., Cheng W-C., et al. 2006. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat. Chem. Biol. 2: 434–441. [DOI] [PubMed] [Google Scholar]
- 41.Oo M. L., Chang S-H., Thangada S., Wu M. T., Rezaul K., Blaho V., Hwang S-I., Han D. K., Hla T. 2011. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest. 121: 2290–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis M. D., Clemens J. J., Macdonald T. L., Lynch K. R. 2005. Sphingosine 1-phosphate analogs as receptor antagonists. J. Biol. Chem. 280: 9833–9841. [DOI] [PubMed] [Google Scholar]
- 43.Awad A. S., Ye H., Huang L., Li L., Foss F. W., Macdonald T. L., Lynch K. R., Okusa M. D. 2006. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am. J. Physiol. Renal Physiol. 290: F1516–F1524. [DOI] [PubMed] [Google Scholar]
- 44.Salomone S., Waeber C. 2011. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol. 2: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osada M., Yatomi Y., Ohmori T., Ikeda H., Ozaki Y. 2002. Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem. Biophys. Res. Commun. 299: 483–487. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez T., Skoura A., Wu M. T., Casserly B., Harrington E. O., Hla T. 2007. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol. 27: 1312–1318. [DOI] [PubMed] [Google Scholar]
- 47.Pyne N. J., Pyne S. 2011. Selectivity and specificity of sphingosine 1-phosphate receptor ligands: “off-targets” or complex pharmacology? Front Pharmacol. 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salomone S., Potts E. M., Tyndall S., Ip P. C., Chun J., Brinkmann V., Waeber C. 2008. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor-null mice and pharmacological tools. Br. J. Pharmacol. 153: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long J. S., Fujiwara Y., Edwards J., Tannahill C. L., Tigyi G., Pyne S., Pyne N. J. 2010. Sphingosine 1-phosphate receptor 4 uses HER2 (ERBB2) to regulate extracellular signal regulated kinase-1/2 in MDA-MB-453 breast cancer cells. J. Biol. Chem. 285: 35957–35966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hla T., Maciag T. 1990. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 265: 9308–9313. [PubMed] [Google Scholar]
- 51.Liu Y., Wada R., Yamashita T., Mi Y., Deng C. X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S. S., Lee M. J., et al. 2000. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kono M., Mi Y., Liu Y., Sasaki T., Allende M. L., Wu Y-P., Yamashita T., Proia R. L. 2004. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 279: 29367–29373. [DOI] [PubMed] [Google Scholar]
- 53.Allende M. L., Yamashita T., Proia R. L. 2003. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 102: 3665–3667. [DOI] [PubMed] [Google Scholar]
- 54.Gaengel K., Niaudet C., Hagikura K., Laviña B., Siemsen B. L., Muhl L., Hofmann J. J., Ebarasi L., Nyström S., Rymo S., et al. 2012. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell. 23: 587–599. [Erratum. 2012. Dev. Cell. 23: 1264.] [DOI] [PubMed] [Google Scholar]
- 55.Jung B., Obinata H., Galvani S., Mendelson K., Ding B-s., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., et al. 2012. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell. 23: 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee M. J., Thangada S., Claffey K. P., Ancellin N., Liu C. H., Kluk M., Volpi M., Sha’afi R. I., Hla T. 1999. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 99: 301–312. [DOI] [PubMed] [Google Scholar]
- 57.Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. 2001. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108: 689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zeng Y., Adamson R. H., Curry F. R., Tarbell J. M. 2014. Sphingosine-1-phosphate protects endothelial glycocalyx by inhibiting syndecan-1 shedding. Am. J. Physiol. Heart Circ. Physiol. 306: H363–H372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ham A., Kim M., Kim J. Y., Brown K. M., Fruttiger M., D’Agati V. D., Lee H. T. Selective deletion of the endothelial sphingosine-1-phosphate 1 receptor exacerbates kidney ischemia-reperfusion injury. Kidney Int. Epub ahead of print. September 11, 2013; 10.1038/ki.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S. W., Kim M., Brown K. M., D’Agati V. D., Lee H. T. 2012. Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J. Am. Soc. Nephrol. 23: 266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W., An J., Jawadi H., Siow D. L., Lee J-F., Zhao J., Gartung A., Maddipati K. R., Honn K. V., Wattenberg B. W., et al. 2013. Sphingosine-1-phosphate receptor-2 mediated NFκB activation contributes to tumor necrosis factor-α induced VCAM-1 and ICAM-1 expression in endothelial cells. Prostaglandins Other Lipid Mediat. 106: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang G., Yang L., Kim G. S., Ryan K., Lu S., O’Donnell R. K., Spokes K., Shapiro N., Aird W. C., Kluk M. J., et al. 2013. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood. 122: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Del Galdo S., Vettel C., Heringdorf D. M., Wieland T. 2013. The activation of RhoC in vascular endothelial cells is required for the S1P receptor type 2-induced inhibition of angiogenesis. Cell. Signal. 25: 2478–2484. [DOI] [PubMed] [Google Scholar]
- 64.Pham T. H. M., Baluk P., Xu Y., Grigorova I., Bankovich A. J., Pappu R., Coughlin S. R., McDonald D. M., Schwab S. R., Cyster J. G. 2010. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 207: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimizuka K., Kawai Y., Maejima D., Ajima K., Kaidoh M., Ohhashi T. 2013. Sphingosine 1-phosphate (S1P) induces S1P2 receptor-dependent tonic contraction in murine iliac lymph vessels. Microcirculation. 20: 1–16. [DOI] [PubMed] [Google Scholar]
- 66.Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427: 355–360. [DOI] [PubMed] [Google Scholar]
- 67.Pham T. H. M., Okada T., Matloubian M., Lo C. G., Cyster J. G. 2008. S1P1 receptor signaling overrides retention mediated by G alpha i–coupled receptors to promote T cell egress. Immunity. 28: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCarthy D. P., Richards M. H., Miller S. D. 2012. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler's virus-induced demyelinating disease. Methods Mol. Biol. 900: 381–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chun J., Brinkmann V. 2011. A mechanistically novel, first oral therapy for multiple sclerosis: the development of fingolimod (FTY720, Gilenya). Discov. Med. 12: 213–228. [PMC free article] [PubMed] [Google Scholar]
- 70.Cohen J. A., Chun J. 2011. Mechanisms of fingolimod: efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 69: 759–777. [DOI] [PubMed] [Google Scholar]
- 71.Brinkmann V. 2009. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 158: 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garris C. S., Wu L., Acharya S., Arac A., Blaho V. A., Huang Y., Moon B. S., Axtell R. C., Ho P. P., Steinberg G. K., et al. 2013. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 14: 1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takeshita H., Kitano M., Iwasaki T., Kitano S., Tsunemi S., Sato C., Sekiguchi M., Azuma N., Miyazawa K., Hla T., et al. 2012. Sphingosine 1-phosphate (S1P)/S1P receptor 1 signaling regulates receptor activator of NF-κB ligand (RANKL) expression in rheumatoid arthritis. Biochem. Biophys. Res. Commun. 419: 154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bankovich A. J., Shiow L. R., Cyster J. G. 2010. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 285: 22328–22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiow L. R., Rosen D. B., Brdičková N., Xu Y., An J., Lanier L. L., Cyster J. G., Matloubian M. 2006. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 440: 540–544. [DOI] [PubMed] [Google Scholar]
- 76.Campbell D. J., Koch M. A. 2011. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu G., Yang K., Burns S., Shrestha S., Chi H. 2010. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat. Immunol. 11: 1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishimaru N., Yamada A., Nitta T., Arakaki R., Lipp M., Takahama Y., Hayashi Y. 2012. CCR7 with S1P1 signaling through AP-1 for migration of Foxp3+ regulatory T-cells controls autoimmune exocrinopathy. Am. J. Pathol. 180: 199–208. [DOI] [PubMed] [Google Scholar]
- 79.Serpero L. D., Filaci G., Parodi A., Battaglia F., Kalli F., Brogi D., Mancardi G. L., Uccelli A., Fenoglio D. 2013. Fingolimod modulates peripheral effector and regulatory T cells in MS patients. J. Neuroimmune Pharmacol. 8: 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Skon C. N., Lee J-Y., Anderson K. G., Masopust D., Hogquist K. A., Jameson S. C. 2013. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat. Immunol. 14: 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schulze T., Golfier S., Tabeling C., Räbel K., Gräler M. H., Witzenrath M., Lipp M. 2011. Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 25: 4024–4036. [DOI] [PubMed] [Google Scholar]
- 82.Green J. A., Suzuki K., Cho B., Willison L. D., Palmer D., Allen C. D. C., Schmidt T. H., Xu Y., Proia R. L., Coughlin S. R., et al. 2011. The sphingosine 1-phosphate receptor S1P2 maintains the homeostasis of germinal center B cells and promotes niche confinement. Nat. Immunol. 12: 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pereira J. P., Xu Y., Cyster J. G. 2010. A role for S1P and S1P1 in immature-B cell egress from mouse bone marrow. PLoS ONE. 5: e9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cattoretti G., Mandelbaum J., Lee N., Chaves A. H., Mahler A. M., Chadburn A., Dalla-Favera R., Pasqualucci L., MacLennan A. J. 2009. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res. 69: 8686–8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Cho B., Suzuki K., Xu Y., Green J. A., An J., Cyster J. G. 2011. Follicular dendritic cells help establish follicle identity and promote B cell retention in germinal centers. J. Exp. Med. 208: 2497–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arnon T. I., Horton R. M., Grigorova I. L., Cyster J. G. 2013. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress. Nature. 493: 684–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cinamon G., Matloubian M., Lesneski M. J., Xu Y., Low C., Lu T., Proia R. L., Cyster J. G. 2004. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat. Immunol. 5: 713–720. [DOI] [PubMed] [Google Scholar]
- 88.Cinamon G., Zachariah M. A., Lam O. M., Foss F. W., Cyster J. G. 2008. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat. Immunol. 9: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mariño E., Batten M., Groom J., Walters S., Liuwantara D., Mackay F., Grey S. T. 2008. Marginal-zone B-cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-cells. Diabetes. 57: 395–404. [DOI] [PubMed] [Google Scholar]
- 90.Stolp J., Mariño E., Batten M., Sierro F., Cox S. L., Grey S. T., Silveira P. A. 2013. Intrinsic molecular factors cause aberrant expansion of the splenic marginal zone B cell population in nonobese diabetic mice. J. Immunol. 191: 97–109. [DOI] [PubMed] [Google Scholar]
- 91.Kondo M., Weissman I. L., Akashi K. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 91: 661–672. [DOI] [PubMed] [Google Scholar]
- 92.Yu J., Freud A. G., Caligiuri M. A. 2013. Location and cellular stages of natural killer cell development. Trends Immunol. 34: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walzer T., Chiossone L., Chaix J., Calver A., Carozzo C., Garrigue-Antar L., Jacques Y., Baratin M., Tomasello E., Vivier E. 2007. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 8: 1337–1344. [DOI] [PubMed] [Google Scholar]
- 94.Jenne C. N., Enders A., Rivera R., Watson S. R., Bankovich A. J., Pereira J. P., Xu Y., Roots C. M., Beilke J. N., Banerjee A., et al. 2009. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 206: 2469–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mayol K., Biajoux V., Marvel J., Balabanian K., Walzer T. 2011. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood. 118: 4863–4871. [DOI] [PubMed] [Google Scholar]
- 96.Speak A. O., Te Vruchte D., Davis L. C., Morgan A. J., Smith D. A., Yanjanin N. M., Simmons L., Hartung R., Runz H., Mengel E., et al. 2014. Altered distribution and function of natural killer cells in murine and human Niemann-Pick disease type C1. Blood. 123: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan M., Sidhu R., Fujiwara H., Tortelli B., Zhang J. 2013. Identification of Niemann-Pick C1 disease biomarkers through sphingolipid profiling. J. Lipid Res. 54: 2800–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T. I., Manaster I., et al. 2006. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12: 1065–1074. [DOI] [PubMed] [Google Scholar]
- 99.Zhang J., Dunk C. E., Lye S. J. 2013. Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum. Reprod. 28: 3026–3037. [DOI] [PubMed] [Google Scholar]
- 100.Murray P. J., Wynn T. A. 2011. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keul P., Lucke S., von Wnuck Lipinski K., Bode C., Gräler M., Heusch G., Levkau B. 2011. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circ. Res. 108: 314–323. [DOI] [PubMed] [Google Scholar]
- 102.Michaud J., Im D-S., Hla T. 2010. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J. Immunol. 184: 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Skoura A., Michaud J., Im D-S., Thangada S., Xiong Y., Smith J., Hla T. 2011. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31: 81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lewis N. D., Haxhinasto S. A., Anderson S. M., Stefanopoulos D. E., Fogal S. E., Adusumalli P., Desai S. N., Patnaude L. A., Lukas S. M., Ryan K. R., et al. 2013. Circulating monocytes are reduced by sphingosine-1-phosphate receptor modulators independently of S1P3. J. Immunol. 190: 3533–3540. [DOI] [PubMed] [Google Scholar]
- 105.Awojoodu A. O., Ogle M. E., Sefcik L. S., Bowers D. T., Martin K., Brayman K. L., Lynch K. R., Peirce-Cottler S. M., Botchwey E. 2013. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. USA. 110: 13785–13790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sola A., Weigert A., Jung M., Vinuesa E., Brecht K., Weis N., Brune B., Borregaard N., Hotter G. 2011. Sphingosine-1-phosphate signalling induces the production of Lcn-2 by macrophages to promote kidney regeneration. J. Pathol. 225: 597–608. [DOI] [PubMed] [Google Scholar]
- 107.Weichand B., Weis N., Weigert A., Grossmann N., Levkau B., Brune B. 2013. Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur. J. Immunol. 43: 3306–3313. [DOI] [PubMed] [Google Scholar]
- 108.McQuiston T., Luberto C., Del Poeta M. 2011. Role of sphingosine-1-phosphate (S1P) and S1P receptor 2 in the phagocytosis of Cryptococcus neoformans by alveolar macrophages. Microbiology. 157: 1416–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nathan C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6: 173–182. [DOI] [PubMed] [Google Scholar]
- 110.Rahaman M., Costello R. W., Belmonte K. E., Gendy S. S., Walsh M-T. 2006. Neutrophil sphingosine 1-phosphate and lysophosphatidic acid receptors in pneumonia. Am. J. Respir. Cell Mol. Biol. 34: 233–241. [DOI] [PubMed] [Google Scholar]
- 111.Allende M. L., Bektas M., Lee B. G., Bonifacino E., Kang J., Tuymetova G., Chen W., Saba J. D., Proia R. L. 2011. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 286: 7348–7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Finley A., Chen Z., Esposito E., Cuzzocrea S., Sabbadini R., Salvemini D. 2013. Sphingosine 1-phosphate mediates hyperalgesia via a neutrophil-dependent mechanism. PLoS ONE. 8: e55255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abraham S. N., John A. L. S. 2010. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10: 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sugita K., Kabashima K., Sakabe J-I., Yoshiki R., Tanizaki H., Tokura Y. 2010. FTY720 regulates bone marrow egress of eosinophils and modulates late-phase skin reaction in mice. Am. J. Pathol. 177: 1881–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kleinjan A., van Nimwegen M., Leman K., Hoogsteden H. C., Lambrecht B. N. 2013. Topical treatment targeting sphingosine-1-phosphate and sphingosine lyase abrogates experimental allergic rhinitis in a murine model. Allergy. 68: 204–212. [DOI] [PubMed] [Google Scholar]
- 116.Oskeritzian C. A., Price M. M., Hait N. C., Kapitonov D., Falanga Y. T., Morales J. K., Ryan J. J., Milstien S., Spiegel S. 2010. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J. Exp. Med. 207: 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. 2010. Development of monocytes, macrophages, and dendritic cells. Science. 327: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Idzko M., Panther E., Corinti S., Morelli A., Ferrari D., Herouy Y., Dichmann S., Mockenhaupt M., Gebicke-Haerter P., Di Virgilio F., et al. 2002. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 16: 625–627. [DOI] [PubMed] [Google Scholar]
- 119.Maeda Y., Matsuyuki H., Shimano K., Kataoka H., Sugahara K., Chiba K. 2007. Migration of CD4 T cells and dendritic cells toward sphingosine 1-phosphate (S1P) is mediated by different receptor subtypes: S1P regulates the functions of murine mature dendritic cells via S1P receptor type 3. J. Immunol. 178: 3437–3446. [DOI] [PubMed] [Google Scholar]
- 120.Czeloth N., Bernhardt G., Hofmann F., Genth H., Förster R. 2005. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J. Immunol. 175: 2960–2967. [DOI] [PubMed] [Google Scholar]
- 121.Gollmann G., Neuwirt H., Tripp C. H., Mueller H., Konwalinka G., Heufler C., Romani N., Tiefenthaler M. 2008. Sphingosine-1-phosphate receptor type-1 agonism impairs blood dendritic cell chemotaxis and skin dendritic cell migration to lymph nodes under inflammatory conditions. Int. Immunol. 20: 911–923. [DOI] [PubMed] [Google Scholar]
- 122.Bajwa A., Huang L., Ye H., Dondeti K., Song S., Rosin D. L., Lynch K. R., Lobo P. I., Li L., Okusa M. D. 2012. Dendritic cell sphingosine 1-phosphate receptor-3 regulates Th1-Th2 polarity in kidney ischemia-reperfusion injury. J. Immunol. 189: 2584–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Niessen F., Schaffner F., Furlan-Freguia C., Pawlinski R., Bhattacharjee G., Chun J., Derian C. K., Andrade-Gordon P., Rosen H., Ruf W. 2008. Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature. 452: 654–658. [DOI] [PubMed] [Google Scholar]
- 124.Galicia-Rosas G., Pikor N., Schwartz J. A., Rojas O., Jian A., Summers-Deluca L., Ostrowski M., Nuesslein-Hildesheim B., Gommerman J. L. 2012. A sphingosine-1-phosphate receptor 1-directed agonist reduces central nervous system inflammation in a plasmacytoid dendritic cell-dependent manner. J. Immunol. 189: 3700–3706. [DOI] [PubMed] [Google Scholar]
- 125.Gräler M. H., Bernhardt G., Lipp M. 1998. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 53: 164–169. [DOI] [PubMed] [Google Scholar]
- 126.Harada J., Foley M., Moskowitz M. A., Waeber C. 2004. Sphingosine-1-phosphate induces proliferation and morphological changes of neural progenitor cells. J. Neurochem. 88: 1026–1039. [DOI] [PubMed] [Google Scholar]
- 127.Mizugishi K., Li C., Olivera A., Bielawski J., Bielawska A., Deng C-X., Proia R. L. 2007. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Invest. 117: 2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kimura A., Ohmori T., Kashiwakura Y., Ohkawa R., Madoiwa S., Mimuro J., Shimazaki K., Hoshino Y., Yatomi Y., Sakata Y. 2008. Antagonism of sphingosine 1-phosphate receptor-2 enhances migration of neural progenitor cells toward an area of brain. Stroke. 39: 3411–3417. [DOI] [PubMed] [Google Scholar]
- 129.Spohr T. C., Dezonne R. S., Nones J., dos Santos Souza C., Einicker-Lamas M., Gomes F. C., Rehen S. K. 2012. Sphingosine 1-phosphate-primed astrocytes enhance differentiation of neuronal progenitor cells. J. Neurosci. Res. 90: 1892–1902. [DOI] [PubMed] [Google Scholar]
- 130.Stessin A. M., Gursel D. B., Schwartz A., Parashar B., Kulidzhanov F. G., Sabbas A. M., Boockvar J., Nori D., Wernicke A. G. 2012. FTY720, sphingosine 1-phosphate receptor modulator, selectively radioprotects hippocampal neural stem cells. Neurosci. Lett. 516: 253–258. [DOI] [PubMed] [Google Scholar]
- 131.Kays J. S., Li C., Nicol G. D. 2012. Expression of sphingosine 1-phosphate receptors in the rat dorsal root ganglia and defined single isolated sensory neurons. Physiol. Genomics. 44: 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Camprubí-Robles M., Mair N., Andratsch M., Benetti C., Beroukas D., Rukwied R., Langeslag M., Proia R. L., Schmelz M., Ferrer Montiel A. V., et al. 2013. Sphingosine-1-phosphate-induced nociceptor excitation and ongoing pain behavior in mice and humans is largely mediated by S1P3 receptor. J. Neurosci. 33: 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mair N., Benetti C., Andratsch M., Leitner M. G., Constantin C. E., Camprubí-Robles M., Quarta S., Biasio W., Kuner R., Gibbins I. L. 2011. Genetic evidence for involvement of neuronally expressed S1P1 receptor in nociceptor sensitization and inflammatory pain. PLoS ONE. 6: e17268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Deogracias R., Yazdani M., Dekkers M. P., Guy J., Ionescu M. C. S., Vogt K. E., Barde Y-A. 2012. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA. 109: 14230–14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Herndon R. M. 2003. The pathology of multiple sclerosis and its variants. In Multiple Sclerosis: Immunology, Pathology, and Pathophysiology. R. M. Herndon, editor. Demos Medical Publishing, New York. 185–197. [Google Scholar]
- 136.Jaillard C., Harrison S., Stankoff B., Aigrot M. S., Calver A. R., Duddy G., Walsh F. S., Pangalos M. N., Arimura N., Kaibuchi K., et al. 2005. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J. Neurosci. 25: 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Novgorodov A. S., El-Alwani M., Bielawski J., Obeid L. M., Gudz T. I. 2007. Activation of sphingosine-1-phosphate receptor S1P5 inhibits oligodendrocyte progenitor migration. FASEB J. 21: 1503–1514. [DOI] [PubMed] [Google Scholar]
- 138.Sheridan G. K., Dev K. K. 2012. S1P1 receptor subtype inhibits demyelination and regulates chemokine release in cerebellar slice cultures. Glia. 60: 382–392. [DOI] [PubMed] [Google Scholar]
- 139.Miron V. E., Ludwin S. K., Darlington P. J., Jarjour A. A., Soliven B., Kennedy T. E., Antel J. P. 2010. Fingolimod (FTY720) enhances remyelination following demyelination of organotypic cerebellar slices. Am. J. Pathol. 176: 2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jackson S. J., Giovannoni G., Baker D. 2011. Fingolimod modulates microglial activation to augment markers of remyelination. J. Neuroinflammation. 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hu Y., Lee X., Ji B., Guckian K., Apicco D., Pepinsky R. B., Miller R. H., Mi S. 2011. Sphingosine 1-phosphate receptor modulator fingolimod (FTY720) does not promote remyelination in vivo. Mol. Cell. Neurosci. 48: 72–81. [DOI] [PubMed] [Google Scholar]
- 142.Noda H., Takeuchi H., Mizuno T., Suzumura A. 2013. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 256: 13–18. [DOI] [PubMed] [Google Scholar]
- 143.Trkov S., Stenovec M., Kreft M., Potokar M., Parpura V., Davletov B., Zorec R. 2012. Fingolimod–a sphingosine-like molecule inhibits vesicle mobility and secretion in astrocytes. Glia. 60: 1406–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seki N., Maeda Y., Kataoka H., Sugahara K., Chiba K. 2013. Role of Sphingosine 1-phosphate (S1P) receptor 1 in experimental autoimmune encephalomyelitis. Pharmacol. Pharm. 4: 628–637. [Google Scholar]
- 145.Choi J. W., Gardell S. E., Herr D. R., Rivera R., Lee C-W., Noguchi K., Teo S. T., Yung Y. C., Lu M., Kennedy G., et al. 2011. FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc. Natl. Acad. Sci. USA. 108: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Norimatsu Y., Ohmori T., Kimura A., Madoiwa S., Mimuro J., Seichi A., Yatomi Y., Hoshino Y., Sakata Y. 2012. FTY720 improves functional recovery after spinal cord injury by primarily nonimmunomodulatory mechanisms. Am. J. Pathol. 180: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 147.Fischer I., Alliod C., Martinier N., Newcombe J., Brana C., Pouly S. 2011. Sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 are functionally upregulated on astrocytes under pro-inflammatory conditions. PLoS ONE. 6: e23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Akahoshi N., Ishizaki Y., Yasuda H., Murashima Y. L., Shinba T., Goto K., Himi T., Chun J., Ishii I. 2011. Frequent spontaneous seizures followed by spatial working memory/anxiety deficits in mice lacking sphingosine 1-phosphate receptor 2. Epilepsy Behav. 22: 659–665. [DOI] [PubMed] [Google Scholar]
- 149.Barkhof F., Cohen J. A., Radue E., Kappos L., Calabresi P., Haring D., Sfikas N., Von Rosenstiel P., Francis G. 2013. Brain volume changes, on-study correlations and the link to disability in three fingolimod phase 3 studies (Abstract in 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Copenhagen, Denmark, October 2–5, 2013). [Google Scholar]
- 150.Kappos L., Cohen J. A., Barkhof F., Cappiello L., Zhang Y., Von Rosenstiel P. 2013. Relapse rates and disability remain consistently low with long-term fingolimod therapy: five year interim results of the LONGTERMS extension study (Abstract in 29th Congress of the European Committee for Treatment and Research in Multiple Sclerosis. Copenhagen, Denmark, October 2–5, 2013). [Google Scholar]
- 151.Radue E. W., O’Connor P., Polman C. H., Hohlfeld R., Calabresi P., Selmaj K., Mueller-Lenke N., Agoropoulou C., Holdbrook F., de Vera A., et al. 2012. Impact of fingolimod therapy on magnetic resonance imaging outcomes in patients with multiple sclerosis. Arch. Neurol. 69: 1259–1269. [DOI] [PubMed] [Google Scholar]
- 152.Abbott N. J., Patabendige A. A. K., Dolman D. E. M., Yusof S. R., Begley D. J. 2010. Structure and function of the blood-brain barrier. Neurobiol. Dis. 37: 13–25. [DOI] [PubMed] [Google Scholar]
- 153.Neuwelt E. A., Bauer B., Fahlke C., Fricker G., Iadecola C., Janigro D., Leybaert L., Molnár Z., O’Donnell M. E., Povlishock J. T., et al. 2011. Engaging neuroscience to advance translational research in brain barrier biology. Nat. Rev. Neurosci. 12: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van Doorn R., Lopes Pinheiro M. A., Kooij G., Lakeman K., van het Hof B., van der Pol S. M. A., Geerts D., van Horssen J., van der Valk P., van der Kam E., et al. 2012. Sphingosine 1-phosphate receptor 5 mediates the immune quiescence of the human brain endothelial barrier. J. Neuroinflammation. 9: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wei Y., Yemisci M., Kim H-H., Yung L. M., Shin H. K., Hwang S-K., Guo S., Qin T., Alsharif N., Brinkmann V., et al. 2011. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 69: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kraft P., Göb E., Schuhmann M. K., Göbel K., Deppermann C., Thielmann I., Herrmann A. M., Lorenz K., Brede M., Stoll G., et al. 2013. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke. 44: 3202–3210. [DOI] [PubMed] [Google Scholar]
- 157.Liesz A., Sun L., Zhou W., Schwarting S., Mracskó É., Zorn M., Bauer H., Sommer C., Veltkamp R. 2011. FTY720 reduces post-ischemic brain lymphocyte influx but does not improve outcome in permanent murine cerebral ischemia. PLoS ONE. 6: e21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brunkhorst R., Kanaan N., Koch A., Ferreiros N. 2013. FTY720 treatment in the convalescence period improves functional recovery and reduces reactive astrogliosis in photothrombotic stroke. PLoS ONE. 8: e70124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pyne S., Pyne N. J. 2013. New perspectives on the role of sphingosine 1-phosphate in cancer. In Handbook of Experimental Pharmacology: Sphingolipids in Disease. E. Gulbins and I. Petrache, editors. Springer, Vienna. 55–71. [DOI] [PubMed] [Google Scholar]
- 160.Kunkel G. T., Maceyka M., Milstien S., Spiegel S. 2013. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 12: 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ogretmen B., Hannun Y. A. 2004. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat. Rev. Cancer. 4: 604–616. [DOI] [PubMed] [Google Scholar]
- 162.Hsu A., Zhang W., Lee J-F., An J., Ekambaram P., Liu J., Honn K. V., Klinge C. M., Lee M-J. 2012. Sphingosine-1-phosphate receptor-3 signaling up-regulates epidermal growth factor receptor and enhances epidermal growth factor receptor-mediated carcinogenic activities in cultured lung adenocarcinoma cells. Int. J. Oncol. 40: 1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rutherford C., Childs S., Ohotski J., McGlynn L., Riddick M., MacFarlane S., Tasker D., Pyne S., Pyne N. J., Edwards J., et al. 2013. Regulation of cell survival by sphingosine-1-phosphate receptor S1P1 via reciprocal ERK-dependent suppression of Bim and PI-3-kinase/protein kinase C-mediated upregulation of Mcl-1. Cell Death Dis. 4: e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sukocheva O., Wadham C., Xia P. 2013. Estrogen defines the dynamics and destination of transactivated EGF receptor in breast cancer cells: role of S1P3 receptor and Cdc42. Exp. Cell Res. 319: 455–465. [DOI] [PubMed] [Google Scholar]
- 165.Watson C., Long J. S., Orange C., Tannahill C. L., Mallon E., McGlynn L. M., Pyne S., Pyne N. J., Edwards J. 2010. High expression of sphingosine 1-phosphate receptors, S1P1 and S1P3, sphingosine kinase 1, and extracellular signal-regulated kinase-1/2 is associated with development of tamoxifen resistance in estrogen receptor-positive breast cancer patients. Am. J. Pathol. 177: 2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohotski J., Edwards J., Elsberger B., Watson C., Orange C., Mallon E., Pyne S., Pyne N. J. 2013. Identification of novel functional and spatial associations between sphingosine kinase 1, sphingosine 1-phosphate receptors and other signaling proteins that affect prognostic outcome in estrogen receptor-positive breast cancer. Int. J. Cancer. 132: 605–616. [DOI] [PubMed] [Google Scholar]
- 167.Ohotski J., Long J. S., Orange C., Elsberger B., Mallon E., Doughty J., Pyne S., Pyne N. J., Edwards J. 2012. Expression of sphingosine 1-phosphate receptor 4 and sphingosine kinase 1 is associated with outcome in oestrogen receptor-negative breast cancer. Br. J. Cancer. 106: 1453–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Emery S. M., Alotaibi M. R., Tao Q., Selley D. E., Lichtman A. H., Gewirtz D. A. 2014. Combined antiproliferative effects of the aminoalkylindole WIN55,212-2 and radiation in breast cancer cells. J. Pharmacol. Exp. Ther. 348: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pyne N. J., Pyne S. 2013. Sphingosine 1-phosphate is a missing link between chronic inflammation and colon cancer. Cancer Cell. 23: 5–7. [DOI] [PubMed] [Google Scholar]
- 170.Montrose D. C., Scherl E. J., Bosworth B. P., Zhou X. K., Jung B., Dannenberg A. J., Hla T. 2013. S1P1 localizes to the colonic vasculature in ulcerative colitis and maintains blood vessel integrity. J. Lipid Res. 54: 843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liang J., Nagahashi M., Kim E. Y., Harikumar K. B., Yamada A., Huang W-C., Hait N. C., Allegood J. C., Price M. M., Avni D., et al. 2013. Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer. Cancer Cell. 23: 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kawahara S., Otsuji Y., Nakamura M., Murakami M., Murate T., Matsunaga T., Kanoh H., Seishima M., Banno Y., Hara A. 2013. Sphingosine kinase 1 plays a role in the upregulation of CD44 expression through extracellular signal-regulated kinase signaling in human colon cancer cells. Anticancer Drugs. 24: 473–483. [DOI] [PubMed] [Google Scholar]
- 173.Murphy C. T., Hall L. J., Hurley G., Quinlan A., MacSharry J., Shanahan F., Nally K., Melgar S. 2012. The sphingosine-1-phosphate analogue FTY720 impairs mucosal immunity and clearance of the enteric pathogen Citrobacter rodentium. Infect. Immun. 80: 2712–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Beckham T. H., Cheng J. C., Lu P., Shao Y., Troyer D., Lance R., Marrison S. T., Norris J. S., Liu X. 2013. Acid ceramidase induces sphingosine kinase 1/S1P receptor 2-mediated activation of oncogenic Akt signaling. Oncogenesis. 2: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li M-H., Hla T., Ferrer F. 2013. FTY720 inhibits tumor growth and enhances the tumor-suppressive effect of topotecan in neuroblastoma by interfering with the sphingolipid signaling pathway. Pediatr. Blood Cancer. 60: 1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kluk M. J., Ryan K. P., Wang B., Zhang G., Rodig S. J., Sanchez T. 2013. Sphingosine-1-phosphate receptor 1 in classical Hodgkin lymphoma: assessment of expression and role in cell migration. Lab. Invest. 93: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Capitani N., Patrussi L., Trentin L., Lucherini O. M., Cannizzaro E., Migliaccio E., Frezzato F., Gattazzo C., Forconi F., Pelicci P., et al. 2012. S1P1 expression is controlled by the pro-oxidant activity of p66 Shc and is impaired in B-CLL patients with unfavorable prognosis. Blood. 120: 4391–4399. [DOI] [PubMed] [Google Scholar]
- 178.Liu Y., Deng J., Wang L., Lee H., Armstrong B., Scuto A., Kowolik C., Weiss L. M., Forman S., Yu H. 2012. S1PR1 is an effective target to block STAT3 signaling in activated B cell-like diffuse large B-cell lymphoma. Blood. 120: 1458–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Salas A., Ponnusamy S., Senkal C. E., Meyers-Needham M., Selvam S. P., Saddoughi S. A., Apohan E., Sentelle R. D., Smith C., Gault C. R., et al. 2011. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 117: 5941–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]