Abstract
The liver and the VLDL receptor (VLDLR) play major roles in TG and VLDL metabolism. However, the exact role of liver VLDLR is not well known because of the absence of or difficulty in detecting VLDLR in the liver. In this study, we demonstrate that fenofibrate, a PPARα agonist and widely used TG-lowering drug, markedly upregulated hepatic VLDLR, which is essential for lowering TG. This study also shows that the distinct regulatory roles of PPARα agonists on VLDLR in the liver and peripheral tissues including adipose tissues, heart, and skeletal muscles are due to the pattern of expression of PPARα. The in vivo portion of our study demonstrated that oral fenofibrate robustly increased liver VLDLR expression levels in hyperlipidemic and diabetic mice and significantly reduced the increase in serum TG observed in wt mice after feeding with high-fat diet (HFD) but not in Vldlr−/− mice or Pparα−/− mice. However, overexpression of mouse VLDLR in livers of Vldlr−/− mice significantly prevented the increase in serum TG induced by HFD. The in vitro portion of our study showed that fenofibrate upregulated VLDLR transcriptional activity through PPAR response element binding to the VLDLR promoter. The conclusions of our study provide a novel mechanism for the TG-lowering effects of fenofibrate in the treatment of dyslipidemia.
Keywords: dyslipidemia, very low density lipoprotein, peroxisome proliferator-activated receptor α, very low density lipoprotein receptor, liver
Dyslipidemia is considered one of the major risk factors for the pathogenic progression of many diseases, including CVD and non-insulin-dependent diabetes (1, 2). Dyslipidemia includes increased TG-related increases in VLDL and LDL particles and reductions in HDL particles in the plasma of patients. Among these measures, increases in TG-related VLDL are the most commonly observed in diabetic patients and are associated with the progression and increased severity of diabetic complications. The dysregulation of hepatic VLDL secretion is believed to be the mechanism underlying increases in TG-related VLDL and is responsible for the pathogenesis of hepatic insulin resistance in type 2 diabetes, representing a principal target for clinical intervention.
Fenofibrate, a fibrate-derived drug, was widely used in the treatment of dyslipidemia in diabetic patients for decades prior to the use of statins (3, 4). Fibrate drugs reduce plasma TG-related VLDLs, raise plasma HDLs (5), and have a modest effect on LDL cholesterol levels (6). Fenofibrate acts as a specific agonist of PPARα, a nuclear hormone receptor that functions as a transcription factor and regulates the expression of a number of genes involved in lipid metabolism and insulin resistance. Fenofibrate has been reported to reduce the body weight gain upon high-fat diet (HFD) stimulation (7). PPARα lowers serum TG by influencing many genes involved in VLDL production, lipid trafficking, and TG-rich lipoprotein clearance (8). For example, PPARα increases the expression of liver microsomal TG transfer protein (MTTP), a protein that mediates the lipidation of apoB100 to form a nascent VLDL (9); the expression of liver LPL, an enzyme that mediates the clearance of TG-rich VLDL and chylomicrons (10); and the synthesis of apoC-III (11), apoA-II (12), and apoA-V (13). However, the precise genes underlying the suppressive effect of PPARα agonists on hepatic VLDL metabolism currently are not well understood.
The VLDL receptor (VLDLR), a member of the LDL receptor (LDLR) family, is widely expressed in the heart, skeletal muscles, adipose tissues, and macrophages (14, 15). However, its levels are barely detectable in the liver under normal conditions. VLDLR mediates the uptake of VLDL by peripheral tissues through LPL-dependent lipolysis or receptor-mediated endocytosis (16–19), and thus plays an important role in VLDL metabolism. In addition, VLDLR has multiple protective roles against obesity, insulin resistance, premature heart disease, tumor growth, inflammation, and angiogenesis. In mammals, VLDLR is highly conserved in humans and rodents (14, 15). The deletion of VLDLR in mouse models usually does not directly lead to dyslipidemia; however, under stress conditions or after feeding with a HFD, these mice have significantly increased serum TG levels (20, 21). The reintroduction of VLDLR in VLDLR KO mice significantly increases atherosclerotic lesion development, indicating that the VLDLR expressed on macrophages is a pro-atherogenic factor (22). These characteristics make VLDLR KO mice a good model for the investigation of the pathological mechanisms underlying dyslipidemia. There are many potential mechanisms whereby PPARα agonists may reduce serum VLDL, including the decreased synthesis of VLDL and increased lipolysis of VLDL by lipase. However, the regulatory effect of fenofibrate on hepatic VLDLR, the key receptor for VLDL catabolism, has not been thoroughly elucidated.
In the present study, we investigated the lipid-regulatory role of fenofibrate in lipid metabolism and the tissue-specific relationships of VLDLR, PPARα, and LPL, thereby providing a novel mechanism by which fenofibrate inhibits the progression of metabolic diseases.
MATERIALS AND METHODS
Animals
Male Akita mice, Pparα−/− mice, Vldlr−/− mice, db/db mice, and their age-matched wild-type (wt) littermates (C57BL/6 mice) were purchased from Jackson Laboratory (Bar Harbor, MA). Brown Norway rats were purchased from Charles River (Wilmington, MA). Rodents were kept in a 12 h light-dark cycle with an ambient light intensity of 85 ± 18 lux. Care, use, and treatment of the animals were in strict agreement with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, and local ethics committee approval was obtained.
Differentiation of 3T3-L1
3T3-L1 cells were cultured in DMEM containing 10% FCS, 0.5 mM 3-isobutyl-1-methylxanthine (Sigma-Aldrich, St. Louis, MO), 0.25 μM dexamethasone (Spectrum Chemical Manufacturing Corp., San Diego, CA), 1 μg/ml insulin in the presence of 25 μM fenofibrate or 0.06% DMSO at 37°C in an environment containing 95% O2 and 5% CO2 for 48 h, and then maintained in DMEM with 10% FCS and 1 μg/ml insulin for 6 days.
Primary liver cell culture
Liver tissue (10 g) from Pparα −/− and age-matched wt mice was weighted, minced, and digested in Hank’s buffered salt solution with 0.25% trypsin (Sigma-Aldrich) at 37°C for 20 min. The digested material was filtered and centrifuged at 1,000 g for 10 min. The resulting pellets were washed and resuspended in MEM with 10% FCS and seeded in a 24-well plate.
Oral fenofibrate administration
Fenofibrate (Sigma-Aldrich) was given as a 0.06% admixture with rodent chow (5001; LabDiet/TestDiet, Ft. Worth, TX) or high-fat rodent chow containing 33.4% fat including total saturated FAs (17.77%), total monounsaturated FAs (7.74%), polyunsaturated FAs (3.70%), and with 60% kcal from fat, 18.8% from protein, and 21.7% from carbohydrates (5TA1; LabDiet/TestDiet). Diabetic animals and their respective controls were fed chow, with or without fenofibrate, for 8 weeks following diabetes onset.
Immunocytochemical analysis
The procedure for immunocytochemical analysis followed a protocol described previously (23).
Western blot analysis
The procedure for Western blot analysis followed a protocol described previously (23), using VLDLR (1:500 dilution; R&D Systems, Minneapolis, MN), PPARα (1:1,000 dilution; Santa Cruz Biotechnologies, Santa Cruz, CA), and β-actin (1:5,000 dilution; Sigma-Aldrich).
Quantitative real-time RT-PCR
The procedure for quantitative real-time RT-PCR was described previously (23). Primers were designed from the cDNA sequences spanning >1 kb introns using the Primer3 software.
Generation of adeno-associated virus-mediated mouse VLDLR
The mouse Vldlr gene was amplified from a cDNA clone by PCR. The resulting fragment was sequenced and inserted into pHelper AAV (pAAV)- ALBp. The constructs in the pAAV vectors were transfected into HEK293 cells and purified (Vector Biolabs, Eaglevilla, PA).
Expression of mVLDLR in the liver in Vldlr−/− mice
Eight-week-old Vldlr−/− mice were injected in the tail vein with adeno-associated virus (AAV)-iALBp-mouse VLDLR (mVLDLR) or AAV-ALB-eGFP (8 × 1011/mouse). Four weeks after injection, the mice were fed a HFD or regular chow diet for 2 weeks.
TG measurement
The measurement of TG concentrations in plasma followed a manufacture’s procedure (Triglyceride Determination Kit; Sigma-Aldrich). Briefly, blood was collected and centrifuged at 700 g for 10 min at 4°C. The plasma in the supernatant was collected without disturbing the white buffy layer. The TG in the plasma was initiated enzymatically by adding lipase and incubated at 30°C for 10 min to convert TG to free FA and glycerol. The released glycerol is subsequently measured by a coupled enzymatic reaction system with a colorimetric readout at 540 nm. To determine the total TG level, the glycerol is continuously catalyzed at 37°C for 15 min by a reconstituted TG reagent including ATP, glycerol kinase, glycerol phosphate oxidase, and perioxidase; the released quninoneimine dye, directly proportional to TG concentration of the sample, was measured and recorded at 540 nm. The concentration of total TG and true TG in the sample was calculated as follows:
Electrophoretic mobility shift assay
The PPARα proteins extracted from Sf9 cells were mixed with purified retinoid X receptor. The mixture was preincubated with nonlabeled wt or mutant competitor oligonucleotides (20- or 100-fold) for 30 min and then incubated with biotinylated labeled PPAR response element (PPRE) oligonucleotides. The sequences of double-stranded oligonucleotides were as follows: mouse VLDLR PPRE, 5′-GATTTCAGTTTACAGGTCAGATGGCAGGCACAG-3′ (23); nonspecific competitor DNA, Poly (deoxyinosinic-deoxycytidylic). Double-stranded oligonucleotides were end-labeled with biotin using the Biotin 3′ End DNA labeling kit (Pierce Biotechnology, Rockford, IL). All of the electrophoretic mobility shift assay (EMSA) reactions were conducted according to the manufacturer’s instruction of LightShift® Chemiluminescent EMSA kit (Pierce Biotechnology, Rockford, IL). DNA-protein complexes were resolved by electrophoresis through 6% polyacrylamide gels.
VLDLR promoter luciferase reporter activity
HepG2 cells (1 × 104 cells/well of a 24-well dish) were cotransfected with 0.1 μg pGL-mouse VLDLR promotor Luc and 20 ng Renilla luciferase expression vector pGL4.75 (Promega, Madison, WI) as an internal control by lipofection. Twenty-four hours after achieving optimal efficiency, cells were exposed to a medium containing high glucose (30 mM) and/or VLDL (5 μM) in the presence or absence of fenofibrate (50 μM) and GW6471 (10 μM) for 24 h. Luciferase activities were assayed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI).
Protein-DNA binding assay
Nuclear protein was extracted from freshly prepared liver from mice (Millipore, Billerica, MA) and precipitated with rabbit polyclonal anti-PPARα antibody (H100, Santa Cruz Biotechnology) or normal rabbit IgG (Santa Cruz Biotechnology) and subjected to protein-DNA binding assay with double-stranded oligonucleotide probes according to the manufacturer’s instructions (Epigentek, Brooklyn, NY). The binding activity was measured at 520 nm on a Tecan Genios Pro microplate reader. The background absorbance with a blank was subtracted from the sample’s reading. The sequences of double-stranded oligonucleotides were as follows (only one strand is shown, and the half-site of the putative PPRE and the mutated PPRE are underlined): mouse VLDLR PPRE, 5′-TGATTTCAGTTTACAGGTCAGATGGCAGGCACAG-3′; mouse VLDLR mutant PPRE, 5′-TGATTTCGGTTTACATCGTTGATGGCTGGCACAG-3′.
Chromatin immunoprecipitation
Freshly prepared retina from mice treated with or without fenofibrate were chopped into small pieces. The DNA and protein were cross-linked with 1.5% formaldehyde for 15 min and stopped by 0.125 M glycine in a fume hood. Soluble chromatin was prepared using a chromatin immunoprecipitation (ChIP) assay kit (Upstate Biotechnology, Lake Placid, NY). After sonication, lysates from the retina were precipitated with rabbit polyclonal anti-PPARα antibody (H100) and normal rabbit IgG (Santa Cruz Biotechnology) according to the manufacturer’s instructions. Primers used for ChIP PCR were as follows: VLDLR-PPRE forward, 5′-TGAGGCCACAGATGATTTTG-3′; VLDLR-PPRE reverse, 5′-GGCTCTACACTCAACCTGGTG-3′; aP2-PPRE forward, 5′-ATGTCACAGGCATCTTATCCACC-3′; aP2-PPRE reverse, 5′-AACCCTGCCAAAGAGACAGAGG-3′; negative control primer forward, 5′-CTCCCCGATCACTGGAATAG-3′; and negative control primer reverse, 5′-ACCCTAGAGACACTGGTGGTG-3′. The negative control primers are located ∼2 kb upstream of the PPRE for VLDLR. PCR was performed for 45 cycles. PCR products were analyzed by 2% agarose gel electrophoresis.
Statistical analysis
The quantitative data of body weight in different diet groups of mice were analyzed by repeated measures ANOVA. All other quantitative data were analyzed and compared using the Mann-Whitney test. Statistical significance was set at P < 0.05.
RESULTS
Fenofibrate increased VLDLR expression in hepatic cells but not in adipose cells
LPL and VLDLR play crucial roles in the uptake and clearance of TG-rich VLDL. We investigated the effects of fenofibrate on VLDLR and LPL expression in liver and adipose cells.
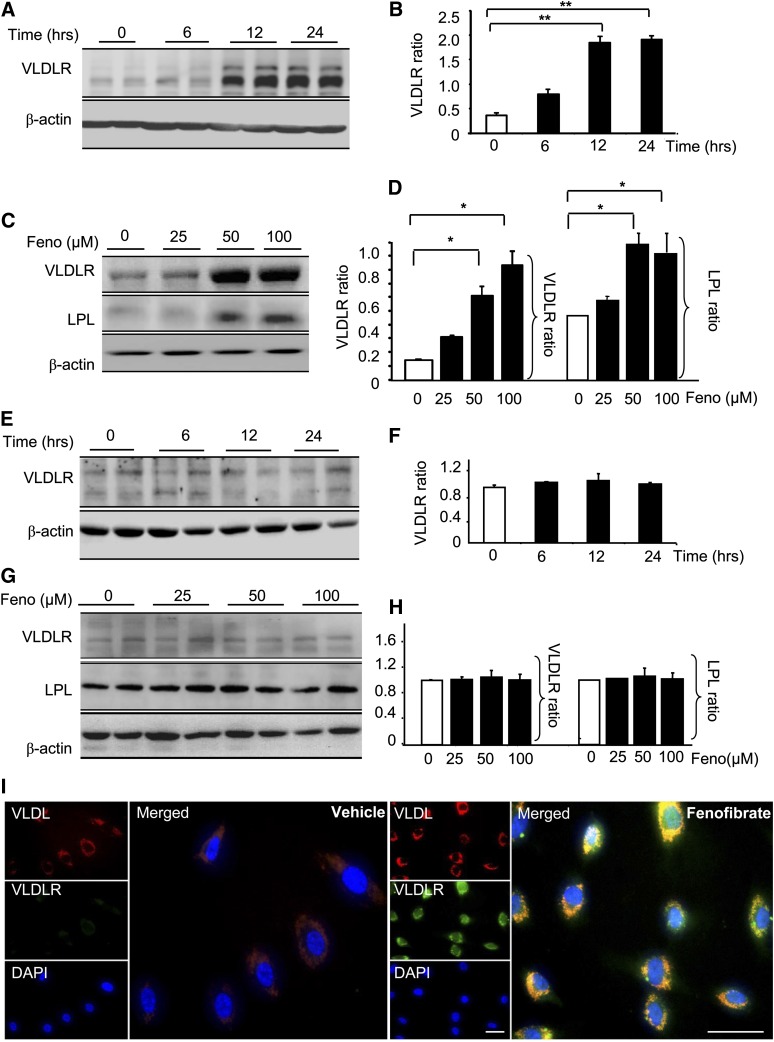
Confluent HepG2 cells and 3T3 cells were treated with different doses of fenofibrate for different durations, and the VLDLR levels were determined by Western blot analysis. At 24 h after treatment with fenofibrate, VLDLR had increased by 1.8-, 3.8-, and 5.5-fold over the basal level at concentrations of 25, 50, and 100 μM, respectively (n = 3, P < 0.05) (Fig. 1A, B). At a concentration of 50 μM, fenofibrate increased VLDLR by 1.1-, 3.6-, and 3.8-fold over the basal level at 6, 12, and 24 h, respectively (n = 3, P < 0.05) (Fig. 1C, D). Unlike the dose- and time-dependent increases in HepG2 cells, the VLDLR level was not significantly changed by fenofibrate in the 3T3 cells (Fig. 1E–H). Additional evidence for fenofibrate-regulated increases in VLDLR was obtained from immunohistochemistry staining of the HepG2 cells with the antibodies of VLDL and VLDLR. As shown in Fig. 1I, compared with the control, both VLDL and VLDLR signals were markedly increased in the hepatic cells treated with fenofibrate, consistent with the VLDL lowering effect of fenofibrate.
Fig. 1.
Effects of fenofibrate on VLDLR expression in HepG2 and adipose cells. A, B: Representative blots (A) and relative expression of VLDLR (B) in HepG2 cells treated with 50 μM fenofibrate for the indicated time. C, D: Representative blots (C) and relative expression of VLDLR and LPL (D) in HepG2 cells treated with fenofibrate (Feno) for 24 h at the indicated dose. Western blots were performed from 60 μg of lysate proteins (mean ± SD, n = 6; *P < 0.05 vs. control). E–H: Representative blots (E) and relative expression of VLDLR (F) in 3T3-L1 cells treated with 50 μM fenofibrate for the indicated time. Representative blots (G) and relative expression of VLDLR and LPL (H) in 3T3-L1 cells treated with fenofibrate for 24 h at the indicated doses. Western blots were performed from 60 μg of lysate proteins (mean ± SD, n = 6; *P < 0.05, **P < 0.01). I: After 48 h lipoprotein deletion, primary cultured liver cells were cultured in a medium containing DiI-VLDL (5 μg/ml) with or without fenofibrate (100 μM) for 24 h and subjected to immunohistochemistry with an antibody against VLDLR. Green, VLDLR; red, VLDL. Scale bar, 20 μM.
We also investigated whether fenofibrate regulates LPL expression in liver and adipose cells. In accordance with the elevation of VLDLR in liver cells, fenofibrate significantly increased the cellular LPL levels in HepG2 cells to approximately 1.8-fold the basal levels at concentrations of 50 and 100 μM (n = 3, P < 0.05 vs. the group not treated with fenofibrate) (Fig. 1C, D). Fenofibrate did not significantly alter the cellular levels of LPL in the 3T3 cells at the same concentrations (Fig. 1E, F). These results suggest that fenofibrate regulates VLDLR and LPL differently in liver and adipose cells.
Fenofibrate upregulated hepatic VLDLR expression and the transcription-enhancing activity of the VLDLR gene in HFD-induced hyperlipidemic and diabetic animals
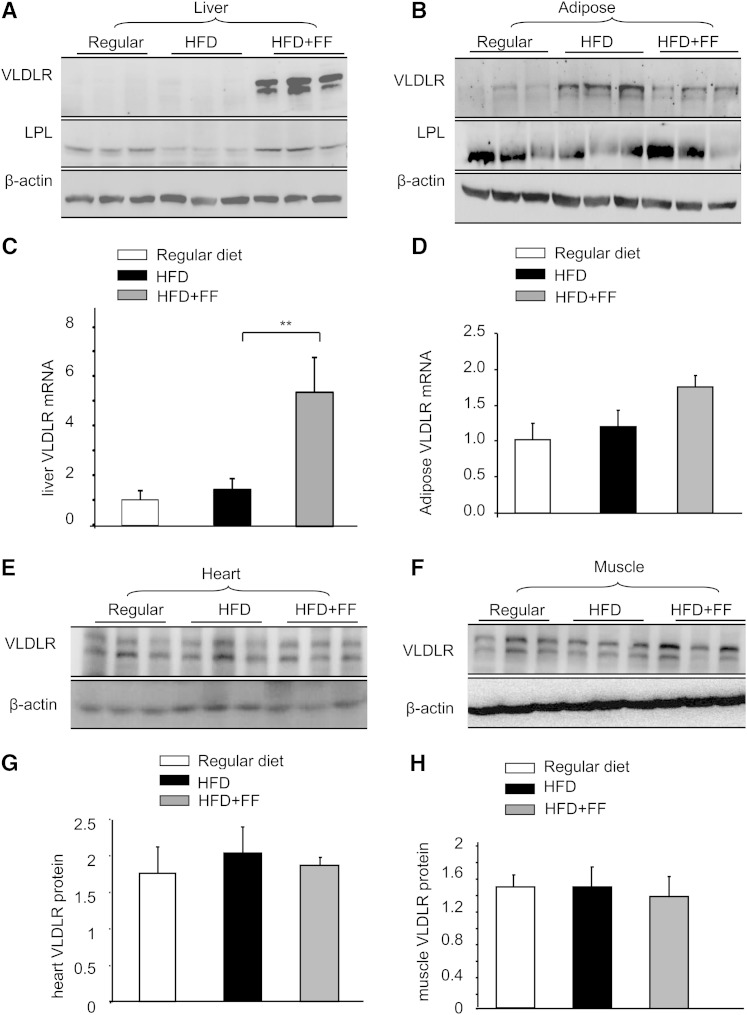
Our in vitro study established that fenofibrate regulates VLDLR differently in liver and adipose cells. We next examined how fenofibrate regulates VLDLR in vivo. Adult wt mice were fed a HFD either with or without fenofibrate (120 mg/kg/day) for 8 weeks; subsequently, the expression of VLDLR in the liver, adipose tissues, heart, and skeletal muscles was determined using Western blotting. Compared with the control mice, a HFD did not lead to a significant change in hepatic VLDLR levels, but feeding with fenofibrate resulted in a robust elevation in hepatic VLDLR levels by approximately 4-fold (n = 3, P < 0.05 vs. high-TG chow without fenofibrate) (Fig. 2A). However, in the adipose tissues, heart, or skeletal muscles, high-fat stimulation alone increased the VLDLR level, which was significantly decreased by fenofibrate administration (Fig. 2B).
Fig. 2.
Fenofibrate upregulated VLDLR in liver but not in adipose, cardiac muscles, and skeletal muscles in wt mice induced with a HFD. Eight-week-old wt and Vldlr−/− mice were fed with regular chow (Regular), HFD, and HFD containing with fenofibrate (HFD+FF) (120 mg/kg/day), respectively, for 8 weeks. A: Western blot analysis of VLDLR and LPL in the liver. Each lane represents one individual animal. B: Western blot analysis of VLDLR and LPL in the adipose tissues. Each lane represents one individual animal. C: Quantitative real-time PCR analysis of VLDLR mRNA in the liver. D: Quantitative real-time PCR analysis of VLDLR mRNA in the adipose tissues (mean ± SD, n = 5; *P < 0.05, **P < 0.01). E, F: Western blot analysis of VLDLR in the heart tissues and skeletal muscles. Each lane represents one individual animal. G: Semi-quantitative analysis of VLDLR protein in the cardiac muscles and in skeletal muscles. (mean ± SD, n = 5; *P < 0.05, **P < 0.01).
Because the upregulation of VLDLR by fenofibrate could be caused by an increase in the transcriptional activation of the VLDLR gene or a decrease in VLDLR protein degradation, we examined the effect of fenofibrate on the hepatic VLDLR mRNA levels. As shown in Fig. 2C, consistent with the protein VLDLR levels, a high-TG diet did not result in any alterations in liver VLDLR mRNA, but fenofibrate administration led to significantly increased liver VLDLR mRNA levels (6-fold increase, P < 0.01 vs. HFD without fenofibrate). In contrast to the upregulation effect of fenofibrate on hepatic VLDLR, the adipose VLDLR mRNA levels were not altered by fenofibrate administration (Fig. 2D).
Fenofibrate upregulated hepatic VLDLR expression in type 1 and type 2 diabetic mice
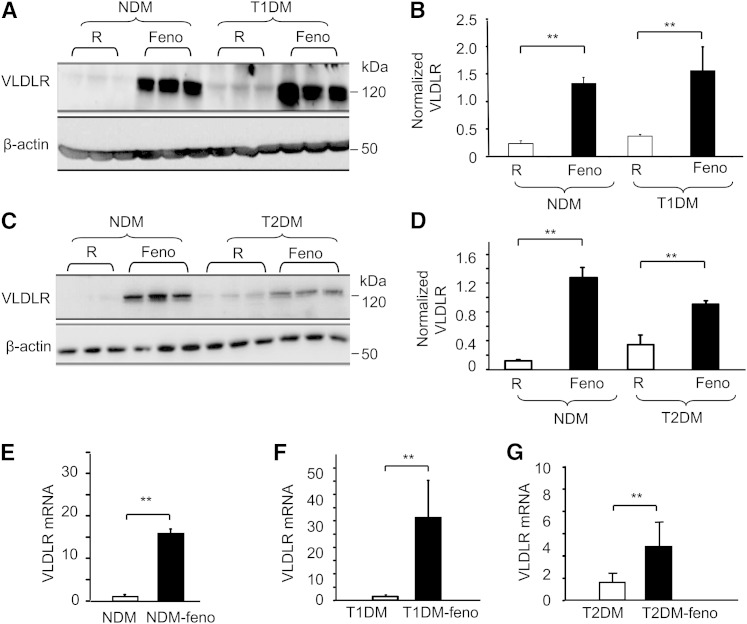
The liver VLDLR levels were also measured in both type 1 and type 2 diabetic mice. Compared with a regular diet, feeding with fenofibrate significantly increased the expression of liver VLDLR. The protein levels were increased 6.22-fold (n = 5, P < 0.01) in Akita mice (Fig. 3A, B) and 12-fold (n = 5, P < 0.01) in db/db mice (Fig. 3C, D); while the mRNA levels were increased 19-fold in the age-matched controls (n = 5, P < 0.01) (Fig. 3E), 30-fold in the Akita mice (n = 5, P < 0.01) (Fig. 3F), and 5-fold in the db/db mice (n = 5, P < 0.01) (Fig. 3G).
Fig. 3.
Fenofibrate upregulates hepatic VLDLR in both T1DM and T2DM mice. Eight-week-old Akita mice and db/db mice were fed with 8 weeks of fenofibrate (120 mg/kg/day). Western blot analysis of VLDLR (A) and relative protein expression (B) in liver tissue from C57 mice (non-diabetes mellitus, NDM model) and Akita mice (T1DM model) fed with normal chow (R) and chow with 0.06% fenofibrate (Feno), respectively. Representative blots (C) and relative protein expression (D) of VLDLR in liver tissue from C57 mice and db/db mice (T2DM model) fed with normal chow and chow with 0.06% fenofibrate, respectively. All Western blots were performed from 60 μg of lysate proteins (mean ± SD, n = 4; **P < 0.01). E–G: Total hepatic RNA was isolated from Akita mice, db/db mice, and their age-matched (16 weeks old) nondiabetic mice treated with or without fenofibrate chow (120 mg/kg/day, 8 weeks). The levels of VLDLR mRNA were determined by quantified real-time PCR (mean ± SD, n = 5; *P < 0.05, **P < 0.01).
Fenofibrate reduced TG levels induced by a HFD in wt mice but not in Vldlr−/− mice
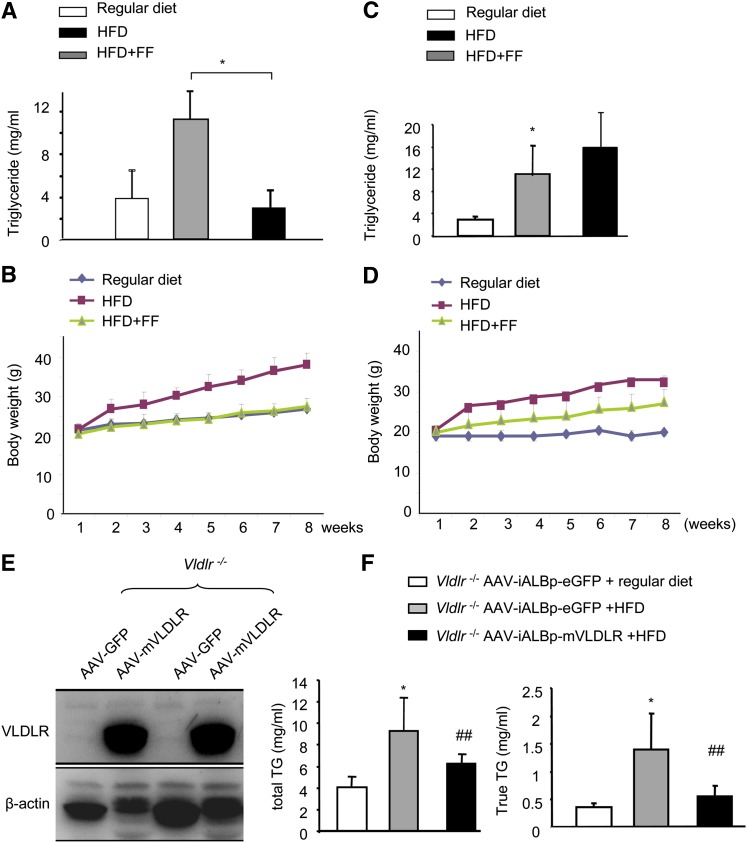
Nascent VLDL is assembled from TGs in the liver and redistributed into peripheral tissues via VLDLR. To determine whether the upregulation of VLDLR plays an essential role in decreasing TG levels, we compared the serum TG levels in wt mice and Vldlr−/− mice fed a HFD either with or without fenofibrate. The body weights were recorded weekly. Serum was collected at week 8 for the measurement of TG levels. In wt mice, a HFD induced a marked increase in serum TG levels and body weight, and these variables were significantly reduced by fenofibrate (P < 0.05) (Fig. 4A, B). However, fenofibrate did not significantly alter the serum TG levels in Vldlr−/− mice (Fig. 4C). Interestingly, although high-fat stimulation significantly increased body weight, this increase was only slightly reduced by fenofibrate (n = 5, P < 0.05) (Fig. 4D).
Fig. 4.
VLDLR is essential for the TG lowering effects of fenofibrate. Eight-week-old wt and Vldlr−/− mice were fed with regular diet, HFD, and HFD containing with fenofibrate (HFD+FF) (120 mg/kg/day), respectively, for 8 weeks. A, B: The serum total TG (A) and body weight (B) in wt mice. B: P < 0.01 by repeated measures ANOVA between wt mice fed with regular chow and HFD (mean ± SD, n = 5). C, D: The serum total TG (C) and body weight (D) in Vldlr−/− mice. D: P < 0.05 by repeated measures ANOVA between Vldlr−/− mice fed with regular diet and HFD (mean ± SD, n = 5); no significance (P > 0.05) by repeated measures ANOVA in body weight between mice fed with HFD and mice fed with HFD containing fenofibrate (mean ± SD, n = 5). E, F: Eight-week-old Vldlr−/− mice were injected with AAV-iALB-mVLDLR and AAV-ALB-eGFP, respectively. Four weeks after viral delivery, the mice were administered a HFD or a regular diet for 2 weeks. E: Liver expressing VLDLR 4 weeks after delivery of AAV-ALB-mVLDLRQ27. F: Measurement of plasma total TGs and true TGs. *P < 0.05 versus regular diet; ##P < 0.01 versus AAV-GFP (mean ± SD, n = 3).
To further confirm that upregulating liver VLDLR plays an essential role in decreasing TG levels, we overexpressed liver VLDLR by delivery of AAV-mVLDLr into Vldlr−/− mice and then fed the mice with a HFD. As shown in Fig. 4E, F, feeding a HFD in Vldlr−/− mice injected with AAV-GFP induced a 2.3-fold increase in total TG and a 3.9-fold increase in true TG, whereas overexpression of mVLDLR decreased total TG by 34% and true TG by 60.7%. These results indicate that upregulating liver VLDLR plays an essential role in TG reduction.
Fenofibrate reduced TG levels and upregulated liver VLDLR levels in a PPARα dose-dependent manner
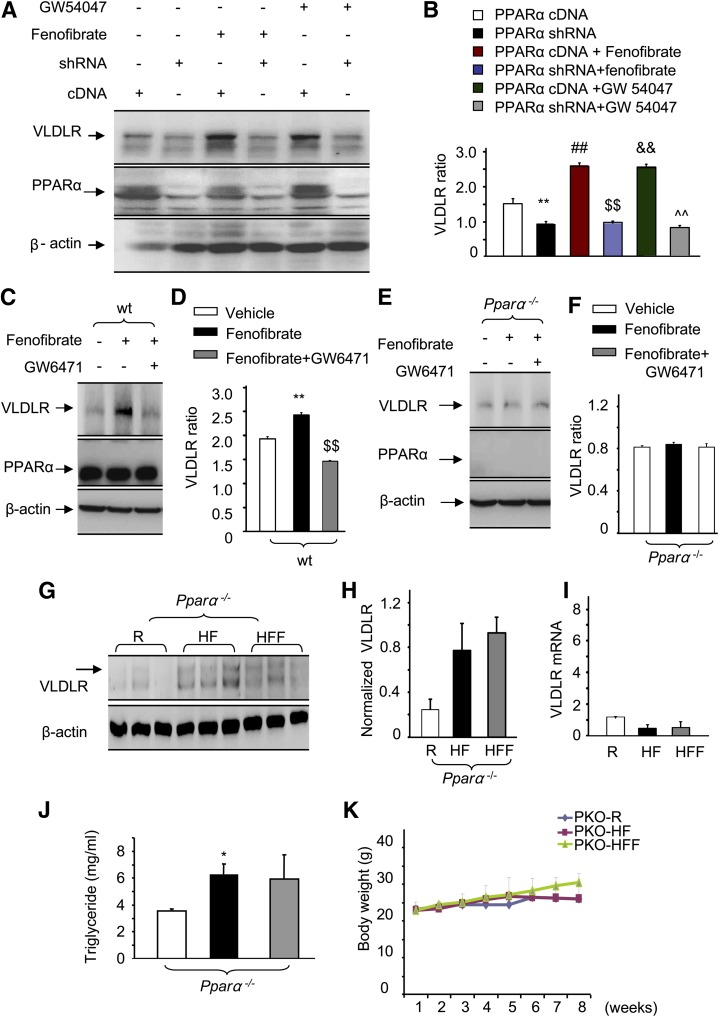
Fenofibrate is a PPARα-specific agonist. To determine whether fenofibrate upregulates VLDLR through PPARα activation, we tested the effects of different PPARα agonists on VLDLR expression in liver cells. Our data demonstrated that in the absence of activator, knockdown of PPARα alone reduced the VLDLR expression level. Overexpression of PPARα by transfecting the cells with cDNA upregulated VLDLR levels. Compared with the cells’ absence of PPARα agonists, the presence of fenofibrate or GW54047 remarkably increased VLDLR levels (Fig. 5A, B), indicating that fenofibrate upregulates hepatic VLDLR through PPARα activation.
Fig. 5.
PPARα is essential for fenofibrate upregulation of VLDLR levels. A, B: At confluence, HepG2 cells were transfected with vectors encoding PPARα shRNA or PPARα cDNA (scrambled shRNA as control). Forty-eight hours after achieving quiescence in a freshly prepared medium, the cells were exposed to a freshly prepared cultured medium in the presence of fenofibrate (50 μM) or GW54047 (10 nM) overnight. An equal amount of protein from whole cell lysis was used for Western blot analysis of VLDLR. C, D: Primary cultured liver cells from wt mice were cultured in a medium containing 50 μM fenofibrate in the presence or absence of 10 μM GW6471 for 24 h, the same amount of vehicle served as the control. A representative blot of VLDLR (C) and semi-quantification of VLDLR levels (D) in whole cell lysates (mean ± SD, n = 3; **P < 0.01 vs. vehicle, $$P < 0.01 vs. fenofibrate). E, F: Primary cultured liver cells from Pparα−/− mice were cultured in a medium containing 50 μM fenofibrate in the presence or absence of 10 μM GW6471 for 24 h, the same amount of vehicle served as the control. A representative blot of VLDLR (E) and semi-quantification of VLDLR levels (F) in whole cell lysates (mean ± SD, n = 3). G, H: Eight-week-old Pparα−/− mice were fed with regular diet (R), HFD, and HFD containing with fenofibrate (HFF) (120 mg/kg/day), respectively, for 8 weeks. Western blot analysis of VLDLR (G) and semi-quantification of VLDLR levels (H) in the livers from Pparα−/− mice fed with regular diet (R), HFD, and HFF. I: Real-time PCR quantification of VLDLR mRNA in the liver from wt mice and Pparα−/− mice (16 weeks old) treated with or without fenofibrate (120 mg/kg/day, 8 weeks). J, K: Serum total TG (J) and body weight (K) were determined. No significance (P > 0.05) by repeated measures ANOVA in body weight and serum TG by Mann-Whitney test between Pparα−/− mice fed with HFD and mice fed with HFD containing fenofibrate (mean ± SD, n = 5).
To further confirm that PPARα is required for fenofibrate upregulation of VLDLR expression, we examined the VLDLR expression in primary cultured mouse liver cells. As shown in Fig. 5C, the activation of PPARα with fenofibrate enhanced the expression of VLDLR in wt hepatic cells, whereas blocking PPARα activation by GW6471 returned the VLDLR expression to basal levels (Fig. 5C, D). However, in liver cells lacking PPARα, neither fenofibrate nor GW6471 altered the VLDLR levels (Fig. 5E, F), indicating the essential role of PPARα in fenofibrate-regulated liver VLDLR expression.
To further confirm the essential role of PPARα in the hepatic VLDLR activity in vivo, and thus to determine the possible function of fenofibrate for the treatment of dyslipidemia, we examined the effects of oral fenofibrate on hepatic VLDLR and serum TG levels in Pparα−/− mice. Neither a HFD nor fenofibrate affected the liver VLDLR levels in Pparα−/− mice (Fig. 5G, H). Additionally, we measured the liver VLDLR mRNA. Unlike the induction effect of fenofibrate on liver VLDLR mRNA levels, fenofibrate had no effect on the liver VLDLR mRNA levels (Fig. 5I).
Feeding a HFD resulted in a significant elevation of serum TG in Pparα−/− mice, but this increase was not altered by the administration of fenofibrate (Fig. 5J). Moreover, there were no significant changes in body weight among Pparα−/− mice fed a regular diet, a HFD, or a HFD with fenofibrate (Fig. 5K). These results confirm that the fenofibrate-regulated increase in hepatic VLDLR is a PPARα-dependent mechanism.
Fenofibrate upregulated transcription-enhancing activity of VLDLR by activating PPARα binding with PPRE in the VLDLR promoter region
Next, to further confirm that fenofibrate upregulates liver VLDLR through PPARα-regulated increases in VLDLR transcriptional activity, we measured mouse VLDLR promoter activity using a luciferase reporter assay. This assay was performed in the presence or absence of fenofibrate in HepG2 cells in which the PPARα was either activated or inactivated. As shown in Fig. 6A, activation of PPARα significantly increased the transcriptional activity of the VLDLR promoter, whereas inactivation of PPARα significantly lowered the transcriptional activity of the VLDLR promoter, indicating that fenofibrate increases mouse VLDLR promoter transcriptional activity through PPARα activation.
Fig. 6.
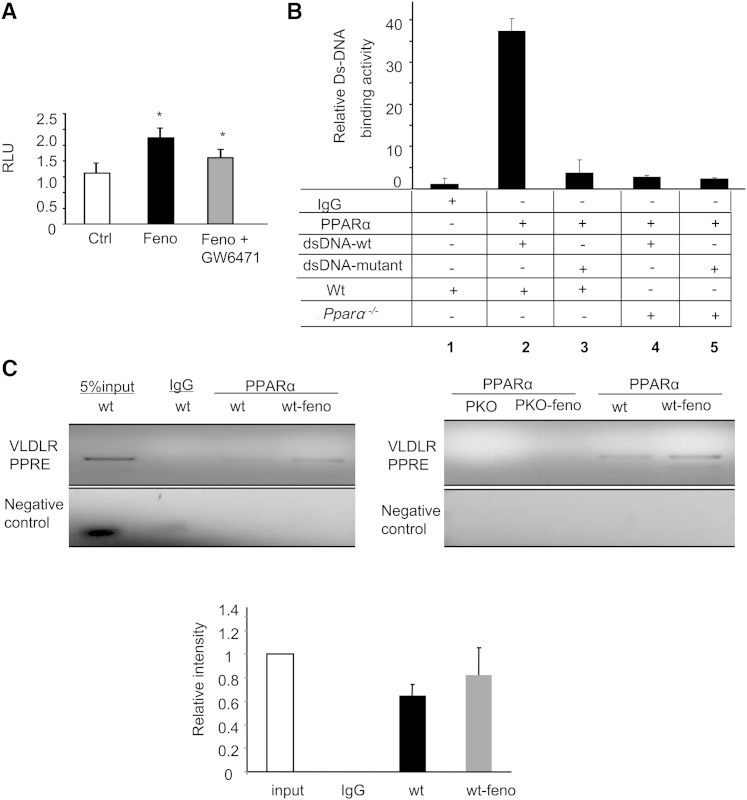
PPARα activation upregulation of VLDLR transcriptional activity through binding to PPRE. A: 293T cells were cotransfected with a vector containing VLDLR promoter and PRL-TK to normalize the transfection efficiency. Twenty-four hours later, after achieving quiescence in a freshly prepared medium, the transfected cells were then exposed to a cultured medium in the presence or absence of fenofibrate (Feno) (50 μM) with or without 10 μM GW6471 for 16 h; the same amount of vehicle served as control. The transcriptional activity of the VLDLR promoter was measured by luciferase assay and normalized by Renilla luciferase activity (mean ± SD, n = 3; *P < 0.05). B: Double-stranded oligonucleotides corresponding to the mouse VLDLR PPRE and mutant PPRE were incubated with the nuclear extract from the mouse tissues and precipitated with or without anti-mouse PPARα antibody. Lane 1, FITC-labeled VLDLR-PPRE oligonucleotides with nuclear extract from wt mice without being precipitated with PPARα antibody; lane 2, FITC-labeled VLDLR-PPRE oligonucleotides with PPARα antibody precipitated nuclear extract from wt mice; lane 3, FITC-labeled VLDLR-PPRE mutant oligonucleotides with PPARα antibody precipitated nuclear extract from wt mice; lanes 4 and 5, FITC-labeled oligonucleotides of VLDLR-PPRE (lane 4) and VLDLR-PPRE mutant (lane 5) with PPARα antibody precipitated nuclear extract from PPARα−/− mice. C: ChIP analysis of the association of PPARα with the VLDLR promoter in the wt mice and Pparα−/− mice treated with fenofibrate using primers located between ∼10 bp upstream and downstream of putative PPRE in the VLDLR promoter region, and control with primers located ∼2,000 bp upstream of the PPRE in the VLDLR promoter region.
The function of PPARα is activated by ligand-binding. Because fenofibrate was shown to increase VLDLR mRNA and VLDLR promoter activity through PPARα activation, we studied whether fenofibrate directly enhances VLDLR gene transcription via a putative PPRE in the VLDLR promoter region. A region at −2307 to −2288 bp PPRE motif in the mouse VLDLR promoter region was investigated to determine whether activated PPARα directly binds to this PPRE motif. The nuclear extracts from wt and Pparα−/− mice fed with fenofibrate were precipitated with control IgG or PPARα antibody prior to the addition of wt or mutated FITC-labeled PPRE motif probes. As depicted in Fig. 6B, the binding activity in wt with wt PPRE motif probes was 30-fold higher than in the control samples precipitated without the PPARα antibody, 8-fold higher than in the samples treated with a probe containing a mutant PPRE motif, and 8-fold higher than the activity in Pparα−/− mice. These results indicate that endogenous PPARα specifically binds to the PPRE motif in the mouse VLDLR promoter region and that the activation of PPARα by fenofibrate directly enhances VLDLR gene transcription via binding with the PPRE motif. To further investigate whether fenofibrate regulates the endogenous VLDLR gene transcription, we performed a ChIP assay using a primer set spanning the PPRE motif in the VLDLR promoter. As shown in Fig. 6C, treatment with fenofibrate in wt mice, but not in Pparα−/− mice, increased VLDLR association with PPRE.
DISCUSSION
Fenofibrate has been shown to lower TG-rich VLDL through the activation of LPL and to reduce the synthesis of TG-rich particles. However, the association between fenofibrate and liver VLDLR levels has not been reported previously. For the first time, our study demonstrated that PPARα activation substantially upregulates liver VLDLR, but not the VLDLR in adipose, heart, or skeletal muscle under various conditions, including in dyslipidemic and diabetic animal models. Our results also suggest that fenofibrate lowers TG at least partially through the upregulation of liver VLDLR. We also characterized the mechanism of the PPARα upregulation of VLDLR through binding with PPRE at the VLDLR promoter region. Our findings provide a novel mechanism of fenofibrate regulation of lipid metabolism in metabolic syndrome and diabetes.
VLDLR is believed to deliver VLDL-derived FAs to the peripheral tissues. FAs are primarily utilized by the muscles and heart, whereas adipose tissue acts as a reservoir for FA storage. Thus, VLDLR is most abundantly expressed in the heart, muscle, and adipose tissues, and VLDLR is located on the endothelial surface and the smooth muscle cells of vessels (24). Although the liver is one of the most important organs for lipid metabolism, the exact role of hepatic VLDLR remains largely unknown, possibly because of the absence or low levels of VLDLR in the liver. Fenofibrate effectively upregulated hepatic VLDLR levels in both diabetic animals and mice with HFD-induced hyperlipidemia; this conclusion is consistent with the observation that liver VLDLR is downregulated in PPARβ/δ−/− mice in the fed and fasted state (25). VLDLR upregulation was correlated with a reduction in serum TG. A lack of VLDLR significantly blunted the TG-lowering effect of fenofibrate in mice with HFD-induced hyperlipidemia, confirming that the upregulation of liver VLDLR is essential and necessary for VLDL-TG metabolism. This finding is in agreement with the results of a study by Tacken et al. (20), which showed that the overexpression of VLDLR was associated with decreased VLDL-rich TG levels in VLDLR transgenic mice, whereas the absence of VLDLR was associated with increased TG levels. Thus, VLDLR may be one of the major mediators for fenofibrate-regulated TG-rich VLDL clearance. As a result, the fenofibrate-regulated decrease in TG levels is most likely caused by an increase in TG clearance by VLDLR in the liver. However, increases in liver VLDLR may also potentially cause increased lipid uptake by the liver. Studies by Ferreira and Harano revealed that fenofibrate prevents hepatic steatosis in rats receiving orotic acid and in mice with spontaneous hepatic steatosis (26, 27). These studies support the theory that fenofibrate-regulated increases in the expression of liver VLDLR might not cause excess TG uptake but will improve TG clearance in the liver. Although some consequences of the upregulation of liver VLDLR are already well-described, further and more advanced studies investigating the mechanism of action of liver VLDLR are warranted.
Interestingly, unlike the fact that fenofibrate increases liver VLDLR, VLDLR upregulation does not occur in adipose tissues or adipocyte cells after fenofibrate treatment, suggesting that fenofibrate-regulated increases in liver VLDLR are tissue-specific responses. This difference is most likely due to the various expressional patterns of PPARα in the liver and the adipose tissues. The PPARα levels are extremely high in the liver but nearly absent in adipose tissues. Treatment with fenofibrate upregulates the liver PPARα level but has no effect on adipose PPARα, which is in accordance with the effect of fenofibrate on VLDLR levels. Functional differences may also be responsible for the differences between VLDLR expression in the liver and the peripheral tissues like adipose tissues. Immunostaining of VLDLR in naturally expressed sites, such as adipose tissue, heart, and muscle, revealed intense staining of VLDLR in the capillaries and arterioles but not in the tissues lacking endothelial cells. This finding indicates that the role of VLDLR is the transport of VLDL and the plasma constituent from the vascular compartment to adjacent tissues (28). A similar study conducted by Wyne et al. (28) showed that the expression of VLDLR on the endothelial lining of the venules in the liver is generally very low, which may explain the low liver VLDLR levels. In the naturally expressed peripheral tissues, the study by Tacken et al. (20) showed that VLDLR facilitates clearing TG and mainly affects the entry of VLDL TG into these tissues. Perman et al. (29) reported that heart VLDLR levels were upregulated in ischemic myocardial diseases and were associated with TG accumulation in cardiomyocytes. In the present study, we found that a HFD increased adipose VLDLR levels and body weight, whereas fenofibrate reduced adipose VLDLR levels and body weight. These results all indicate that the function of VLDLR may differ by tissue type. In tissues where VLDLR naturally occurs, such as adipose tissue, macrophage cells, and cardiac tissue, TG clearance is associated with TG uptake through VLDLR into peripheral tissues. Although our study found that activation of PPARα has no effect on the VLDLR in the peripheral tissue such as adipose tissues, several studies have reported that adipose VLDLR gene expression can be upregulated by PPARγ (30–32). Interestingly, unlike the low level of PPARγ in the liver and high level of PPARγ in the adipose tissues, PPARα is extremely abundant in the liver but very low in the adipose tissues. Whether the difference of PPARγ and PPARα in tissue distribution represents their different impacts on VLDLR functions requires more in-depth study.
We also investigated the relationships among PPARα, LPL, and VLDLR. LPL is a TG hydrolase and a ligand/bridging factor for receptor-mediated VLDL uptake (33, 34). Like VLDLR, LPL encoded by LPL is abundantly expressed in endothelial cells (34). A study done by Yagyu et al. (35) indicates that the presence of VLDLR is required for normal LPL regulation and that the disruption of VLDLR reduces LPL activity. However, Hu et al. (36) found that in the absence of VLDLR, LDLR, and apoE, three major apolipoprotein recognizing receptors, hepatic VLDL uptake is regulated by LPL. Our present study showed that a HFD reduced hepatic LPL levels, whereas treatment with fenofibrate increased LPL levels. In mice with deficient VLDLR levels, neither a HFD nor fenofibrate affected hepatic LPL levels (data not shown). Moreover, despite a certain amount of hepatic LPL activity in Vldlr−/− mice, fenofibrate lost its TG lowering effects in these animals. These findings are in accordance with the results that the presence of VLDLR is essential for LPL regulation. In contrast, this study did not find any evidence that fenofibrate had similar regulation effects on adipose LPL levels. Studies conducted by Schoonjans and colleagues demonstrated that PPARα agonists inhibit cardiac LPL activity (10) and that the response of LPL to a PPARα agonist is tissue-specific (11). It is unclear whether the variance of LPL levels in liver and adipose tissues is the same as the variance in VLDLR levels.
In conclusion, this study resulted in a novel finding that hepatic VLDLR upregulation plays an essential role in the TG lowering effect of fenofibrate via the activation of PPARα. The potential mechanisms linking VLDL metabolism and fenofibrate are multiple, overlapping, and highly correlated. Considerable interest should be raised regarding the tissue-specific effects of PPARα agonists on genes involved in VLDL metabolism and the precise mechanisms underlying the different functions of VLDLR in the liver and in other tissues.
Footnotes
Abbreviations:
- AAV
- adeno-associated virus
- ChIP
- chromatin immunoprecipitation
- EMSA
- electrophoretic mobility shift assay
- HFD
- high-fat diet
- LDLR
- LDL receptor
- PPRE
- PPAR response element
- VLDLR
- VLDL receptor
- wt
- wild-type
REFERENCES
- 1.Girach A., Manner D., Porta M. 2006. Diabetic microvascular complications: can patients at risk be identified? A review. Int. J. Clin. Pract. 60: 1471–1483. [DOI] [PubMed] [Google Scholar]
- 2.Blonde L. 2009. Current antihyperglycemic treatment strategies for patients with type 2 diabetes mellitus. Cleve. Clin. J. Med. 76(Suppl 5): S4–S11. [DOI] [PubMed] [Google Scholar]
- 3.Auwerx J., Schoonjans K., Fruchart J. C., Staels B. 1996. Regulation of triglyceride metabolism by PPARs: fibrates and thiazolidinediones have distinct effects. J. Atheroscler. Thromb. 3: 81–89. [DOI] [PubMed] [Google Scholar]
- 4.Schoonjans K., Staels B., Auwerx J. 1996. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim. Biophys. Acta. 1302: 93–109. [DOI] [PubMed] [Google Scholar]
- 5.Fruchart J. C., Duriez P. 2006. Mode of action of fibrates in the regulation of triglyceride and HDL-cholesterol metabolism. Drugs Today (Barc). 42: 39–64. [DOI] [PubMed] [Google Scholar]
- 6.McKeage K., Keating G. M. 2011. Fenofibrate: a review of its use in dyslipidaemia. Drugs. 71: 1917–1946. [DOI] [PubMed] [Google Scholar]
- 7.Guerre-Millo M., Gervois P., Raspe E., Madsen L., Poulain P., Derudas B., Herbert J. M., Winegar D. A., Willson T. M., Fruchart J. C., et al. 2000. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 275: 16638–16642. [DOI] [PubMed] [Google Scholar]
- 8.Cheng P. T., Mukherjee R. 2005. PPARs as targets for metabolic and cardiovascular diseases. Mini Rev. Med. Chem. 5: 741–753. [DOI] [PubMed] [Google Scholar]
- 9.Améen C., Edvardsson U., Ljungberg A., Asp L., Akerblad P., Tuneld A., Olofsson S. O., Lindén D., Oscarsson J. 2005. Activation of peroxisome proliferator-activated receptor alpha increases the expression and activity of microsomal triglyceride transfer protein in the liver. J. Biol. Chem. 280: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 10.Schoonjans K., Peinado-Onsurbe J., Lefebvre A. M., Heyman R. A., Briggs M., Deeb S., Staels B., Auwerx J. 1996. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 15: 5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 11.Auwerx J., Schoonjans K., Fruchart J. C., Staels B. 1996. Transcriptional control of triglyceride metabolism: fibrates and fatty acids change the expression of the LPL and apo C–III genes by activating the nuclear receptor PPAR. Atherosclerosis. 124(Suppl): S29–S37. [DOI] [PubMed] [Google Scholar]
- 12.Vu-Dac N., Schoonjans K., Kosykh V., Dallongeville J., Fruchart J. C., Staels B., Auwerx J. 1995. Fibrates increase human apolipoprotein A-II expression through activation of the peroxisome proliferator-activated receptor. J. Clin. Invest. 96: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schultze A. E., Alborn W. E., Newton R. K., Konrad R. J. 2005. Administration of a PPARalpha agonist increases serum apolipoprotein A-V levels and the apolipoprotein A-V/apolipoprotein C–III ratio. J. Lipid Res. 46: 1591–1595. [DOI] [PubMed] [Google Scholar]
- 14.Webb J. C., Patel D. D., Jones M. D., Knight B. L., Soutar A. K. 1994. Characterization and tissue-specific expression of the human ‘very low density lipoprotein (VLDL) receptor’ mRNA. Hum. Mol. Genet. 3: 531–537. [DOI] [PubMed] [Google Scholar]
- 15.Oka K., Ishimura-Oka K., Chu M. J., Sullivan M., Krushkal J., Li W. H., Chan L. 1994. Mouse very-low-density-lipoprotein receptor (VLDLR) cDNA cloning, tissue-specific expression and evolutionary relationship with the low-density-lipoprotein receptor. Eur. J. Biochem. 224: 975–982. [DOI] [PubMed] [Google Scholar]
- 16.Tacken P. J., Beer F. D., Vark L. C., Havekes L. M., Hofker M. H., Willems Van Dijk K. 2000. Very-low-density lipoprotein binding to the apolipoprotein E receptor 2 is enhanced by lipoprotein lipase, and does not require apolipoprotein E. Biochem. J. 347: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi S., Suzuki J., Kohno M., Oida K., Tamai T., Miyabo S., Yamamoto T., Nakai T. 1995. Enhancement of the binding of triglyceride-rich lipoproteins to the very low density lipoprotein receptor by apolipoprotein E and lipoprotein lipase. J. Biol. Chem. 270: 15747–15754. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S., Sakai J., Fujino T., Hattori H., Zenimaru Y., Suzuki J., Miyamori I., Yamamoto T. T. 2004. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J. Atheroscler. Thromb. 11: 200–208. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S., Sakai J., Fujino T., Miyamori I., Yamamoto T. T. 2003. The very low density lipoprotein (VLDL) receptor–a peripheral lipoprotein receptor for remnant lipoproteins into fatty acid active tissues. Mol. Cell. Biochem. 248: 121–127. [DOI] [PubMed] [Google Scholar]
- 20.Tacken P. J., Teusink B., Jong M. C., Harats D., Havekes L. M., van Dijk K. W., Hofker M. H. 2000. LDL receptor deficiency unmasks altered VLDL triglyceride metabolism in VLDL receptor transgenic and knockout mice. J. Lipid Res. 41: 2055–2062. [PubMed] [Google Scholar]
- 21.Goudriaan J. R., Tacken P. J., Dahlmans V. E., Gijbels M. J., van Dijk K. W., Havekes L. M., Jong M. C. 2001. Protection from obesity in mice lacking the VLDL receptor. Arterioscler. Thromb. Vasc. Biol. 21: 1488–1493. [DOI] [PubMed] [Google Scholar]
- 22.Eck M. V., Oost J., Goudriaan J. R., Hoekstra M., Hildebrand R. B., Bos I. S., van Dijk K. W., Van Berkel T. J. 2005. Role of the macrophage very-low-density lipoprotein receptor in atherosclerotic lesion development. Atherosclerosis. 183: 230–237. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y., Hu Y., Lu K., Flannery J. G., Ma J. X. 2007. Very low density lipoprotein receptor, a negative regulator of the wnt signaling pathway and choroidal neovascularization. J. Biol. Chem. 282: 34420–34428. [DOI] [PubMed] [Google Scholar]
- 24.Multhaupt H. A., Gafvels M. E., Kariko K., Jin H., Arenas-Elliot C., Goldman B. I., Strauss J. F., 3rd, Angelin B., Warhol M. J., McCrae K. R. 1996. Expression of very low density lipoprotein receptor in the vascular wall. Analysis of human tissues by in situ hybridization and immunohistochemistry. Am. J. Pathol. 148: 1985–1997. [PMC free article] [PubMed] [Google Scholar]
- 25.Sanderson L. M., Boekschoten M. V., Desvergne B., Muller M., Kersten S. 2010. Transcriptional profiling reveals divergent roles of PPARalpha and PPARbeta/delta in regulation of gene expression in mouse liver. Physiol. Genomics. 41: 42–52. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira A. V., Parreira G. G., Porto L. C., Mario E. G., Delpuerto H. L., Martins A. S., Botion L. M. 2008. Fenofibrate prevents orotic acid–induced hepatic steatosis in rats. Life Sci. 82: 876–883. [DOI] [PubMed] [Google Scholar]
- 27.Harano Y., Yasui K., Toyama T., Nakajima T., Mitsuyoshi H., Mimani M., Hirasawa T., Itoh Y., Okanoue T. 2006. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, reduces hepatic steatosis and lipid peroxidation in fatty liver Shionogi mice with hereditary fatty liver. Liver Int. 26: 613–620. [DOI] [PubMed] [Google Scholar]
- 28.Wyne K. L., Pathak K., Seabra M. C., Hobbs H. H. 1996. Expression of the VLDL receptor in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 16: 407–415. [DOI] [PubMed] [Google Scholar]
- 29.Perman J. C., Bostrom P., Lindbom M., Lidberg U., StAhlman M., Hagg D., Lindskog H., Scharin Tang M., Omerovic E., Mattsson Hulten L., et al. 2011. The VLDL receptor promotes lipotoxicity and increases mortality in mice following an acute myocardial infarction. J. Clin. Invest. 121: 2625–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takazawa T., Yamauchi T., Tsuchida A., Takata M., Hada Y., Iwabu M., Okada-Iwabu M., Ueki K., Kadowaki T. 2009. Peroxisome proliferator-activated receptor gamma agonist rosiglitazone increases expression of very low density lipoprotein receptor gene in adipocytes. J. Biol. Chem. 284: 30049–30057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tao H., Aakula S., Abumrad N. N., Hajri T. 2010. Peroxisome proliferator-activated receptor-gamma regulates the expression and function of very-low-density lipoprotein receptor. Am. J. Physiol. Endocrinol. Metab. 298: E68–E79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao H., Hajri T. 2011. Very low density lipoprotein receptor promotes adipocyte differentiation and mediates the proadipogenic effect of peroxisome proliferator-activated receptor gamma agonists. Biochem. Pharmacol. 82: 1950–1962. [DOI] [PubMed] [Google Scholar]
- 33.Tan M. H. 1978. The lipoprotein lipase system: new understandings. Can. Med. Assoc. J. 118: 675–680. [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H., Eckel R. H. 2009. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 297: E271–E288. [DOI] [PubMed] [Google Scholar]
- 35.Yagyu H., Lutz E. P., Kako Y., Marks S., Hu Y., Choi S. Y., Bensadoun A., Goldberg I. J. 2002. Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity. Possible causes of hypertriglyceridemia and reduced body mass with VLDL receptor deficiency. J. Biol. Chem. 277: 10037–10043. [DOI] [PubMed] [Google Scholar]
- 36.Hu L., van der Hoogt C. C., Espirito Santo S. M., Out R., Kypreos K. E., van Vlijmen B. J., Van Berkel T. J., Romijn J. A., Havekes L. M., van Dijk K. W., et al. 2008. The hepatic uptake of VLDL in lrp-ldlr−/−vldlr−/− mice is regulated by LPL activity and involves proteoglycans and SR-BI. J. Lipid Res. 49: 1553–1561. [DOI] [PubMed] [Google Scholar]