Abstract
Sphingosine-1-phosphate (S1P) mediates several cytoprotective functions of HDL. apoM acts as a S1P binding protein in HDL. Erythrocytes are the major source of S1P in plasma. After glomerular filtration, apoM is endocytosed in the proximal renal tubules. Human or murine HDL elicited time- and dose-dependent S1P efflux from erythrocytes. Compared with HDL of wild-type (wt) mice, S1P efflux was enhanced in the presence of HDL from apoM transgenic mice, but not diminished in the presence of HDL from apoM knockout (Apom−/−) mice. Artificially reconstituted and apoM-free HDL also effectively induced S1P efflux from erythrocytes. S1P and apoM were not measurable in the urine of wt mice. Apom−/− mice excreted significant amounts of S1P. apoM was detected in the urine of mice with defective tubular endocytosis because of knockout of the LDL receptor-related protein, chloride-proton exchanger ClC-5 (Clcn5−/−), or the cysteine transporter cystinosin. Urinary levels of S1P were significantly elevated in Clcn5−/− mice. In contrast to Apom−/− mice, these mice showed normal plasma concentrations for apoM and S1P. In conclusion, HDL facilitates S1P efflux from erythrocytes by both apoM-dependent and apoM-independent mechanisms. Moreover, apoM facilitates tubular reabsorption of S1P from the urine, however, with no impact on S1P plasma concentrations.
Keywords: high density lipoprotein, kidney, megalin, chloride-proton exchanger ClC5, cystinosin
Sphingosine-1-phosphate (S1P) acts both as an intracellular signaling molecule and an extracellular agonist of at least five different G protein-coupled receptors. By its dual functions, S1P regulates the survival, proliferation, and migration as well as the functionality of many cells, eventually in opposite directions (1–4). Therefore the absolute and relative abundance of S1P in intracellular and extracellular compartments appears to be important for its biological functionality (4).
Most cells form S1P by the phosphorylation of sphingosine, a degradation product of ceramides, through sphingosine kinase and degrade S1P to phosphoethanolamine and fatty aldehyde through S1P-lyase (1–4). By contrast, not only erythrocytes and platelets, but also other cells which have low or no lyase activity, release S1P (4–6), probably by an as yet unidentified ABC transporter (4, 7, 8). Within the plasma compartment, the majority of S1P is transported by HDLs in which it exerts many cyto-protective and anti-inflammatory effects, for example, on endothelial cells (8–10). The enrichment of S1P in HDL has been explained by the presence of a specific S1P binding protein, namely apoM (10). Purified and recombinant apoM binds S1P with an IC50 of 0.9 μmol/l, which is in the range of physiological S1P plasma concentrations (11). X-ray crystallography of apoM identified an S1P binding domain which was confirmed by the recombinant generation of non-S1P binding apoM mutants (11). In agreement with these physicochemical data, S1P was copurified with apoM containing HDL, but not apoM-free HDL, from both human and murine plasma. Moreover, S1P concentrations were dramatically decreased in HDL of the apoM knockout (Apom−/−), but increased in HDL of mice transgenic for apoM (Apomtg). As an in vitro indication of functional relevance, the stimulatory effects of S1P on nitric oxide production by endothelial cells were mimicked by apoM-containing HDL, but not by apoM-free HDL. Finally, the physiological relevance of S1P binding by apoM was indicated by the reduced basal endothelial barrier function in lungs of Apom−/− mice (12). Despite this very strong in vitro and in vivo evidence for the limiting effect of apoM on the transport and function of S1P in plasma and HDL, concentrations of S1P and apoM in either plasma or HDL do not correlate with each other (12, 13). Moreover, stoichiometric calculations revealed that apoM is not saturated with S1P but present at an up to 8-f molar excess (12, 13). We therefore investigated the impact of apoM on two other potential pathways of S1P metabolism, namely efflux from erythrocytes and urinary excretion.
Plasma concentrations of S1P were recently shown to correlate with red blood cell counts (5, 6, 14), probably because the lack of the S1P-degrading lyase makes erythrocytes the main source of S1P in plasma (4, 5). Because HDL was previously found to induce S1P efflux from erythrocytes (5), we compared the S1P efflux capacity of HDL from wild-type (wt), Apom−/−, and Apomtg mice (15).
After glomerular filtration, apoM is reabsorbed from the primary urine into proximal tubular epithelial cells by binding to the endocytic receptor megalin [LDL receptor-related protein 2 (LRP2)]. Accordingly, mice with a conditional renal knockout of megalin excrete apoM in urine (16). Megalin and its coreceptor cubilin also mediate the tubular reabsorption of several small plasma proteins which carry small molecules and are filtrated through the glomeruli (17, 18). Not only megalin and cubilin, but also endosomal and lysosomal proteins such as chloride-proton exchanger ClC-5 (mutated in Dent’s disease) and the cystine transporter cystinosin (mutated in cystinosis), respectively, are key components of the machinery that rescues essential molecules such as vitamin B12 and vitamin D from inappropriate urinary loss (17, 19, 20). To test whether this is also of relevance for the metabolism of S1P, we compared the urinary excretion of S1P and apoM in wt and Apom−/− mice (15), as well as in mice with dysfunctional megalin (Lrp2−/−) (21), ClC-5 (Clcn5−/−) (22), or cystinosin (Ctns−/−) (23).
METHODS
Plasma and urine collection from mice
Mice with knockout of apoM (Apom−/−) or transgenic overexpression of human apoM (Apomtg) (15), as well as mice with defective expression of megalin (Lrp2−/−) (21), ClC-5 (Clcn5−/−) (22), or cystinosin (Ctns−/−) (23) were previously described. Blood samples were obtained by cardiac puncture immediately after euthanization. Plasma was prepared by 15 min of centrifugation of blood at 2,000 g. Urine samples were collected in metabolic cages for 8 h (Clcn5−/−, Ctns−/−, and their littermate controls), 16 h (Lrp2−/− and their littermate controls), or 24 h (Apom−/−, Apomtg, and their littermate controls) according to standard protocols. Each drop of urine was immediately cooled down to −20°C in the collector of the metabolic cages. All plasma and urine samples were kept frozen at −80°C before further use or analysis. The age of the mice, at which samples were obtained, is reported in the Results section. All animal procedures were approved by the appropriate National Research Council Guide for the Care and Use of Laboratory Animals/Animal Ethics Committee.
Isolation and reconstitution of HDL
Human HDL was isolated from plasma of healthy blood donors (Kantosspital Schaffhausen, Switzerland) or mouse plasma by stepwise ultracentrifugation (d = 1.063–1.21 kg/l) at 360,000 g for 15 h at 15°C, as described previously (24), using solid potassium bromide (Sigma Aldrich, Buchs, Switzerland) for density adjustment. apoA-I was further purified from delipidated HDL as described previously (24). Discoidal reconstituted HDL (rHDL) particles were produced by the cholate dialysis method and contained apoA-I, POPC (Sigma), and sodium cholate (Sigma) in a molar ratio of 1/100/100 (24).
S1P efflux from erythrocytes
Erythrocytes were isolated from the blood of healthy adult volunteers. The blood was anticoagulated with sodium citrate and then centrifuged at 2,000 g for 5 min at 4°C. After removing the plasma, the sedimented erythrocytes were washed three times with sterile PBS and resuspended 1:1 in PBS (v/v) containing either BSA, human or murine HDL, rHDL, or lipid-free apoA-I at the concentrations indicated in the Results section and incubated at 37°C. Aliquots were removed at different time points (as indicated in the Results section) and immediately centrifuged at 2,000 g for 3 min at 4°C to sediment erythrocytes. The supernatants were carefully transferred into new tubes avoiding any contamination with erythrocytes. For S1P measurement, 25 μl aliquots of the supernatant were taken and processed as described below.
Quantification of S1P in plasma, HDL, and urine
S1P was quantified by LC-MS/MS after derivatization with acetic anhydride. The S1P concentrations in plasma or erythrocyte supernatants (25 μl), HDL (50 μg), and urine (500 μl) were analyzed after adding 10 pmol internal standard (D7S1P; Avanti Polar Lipids, Alabaster, AL). For calibration, S1P (Avanti Polar Lipids) was dissolved in DMSO/concentrated-HCl (100:2, v/v) at a concentration of 0.28 mmol/l stock solution. Each series of measurements was calibrated with 1, 2.5, 5, 10, 15, 20, and 25 pmol of S1P supplemented with 10 pmol of D7S1P as the internal standard (IS). Quality control samples with 7.5 and 22.5 pmol S1P were evaluated at the beginning and at the end of each sample series. Double blank and blank samples for carry-over control were prepared by adding methanol and internal standard, respectively, to 25 μl of water and processed as plasma samples.
Lipids were extracted with 1 ml of an organic solution consisting of ethyl acetate/2-propanol (6:1, v/v) and 50 μl of concentrated formic acid was added for phase separation (25). The upper organic phase was separated and evaporated to dryness under a stream of nitrogen. For the derivatization of the primary amino and secondary alcohol groups of S1P (26), the dried lipids were dissolved in 100 μl of pyridine and 50 μl of acetic anhydride and incubated at 40°C for 20 min. After evaporating the acetylation reagents, the reaction products were dissolved in 100 μl of methanol and transferred to glass vials prior to LC-MS/MS analysis.
Acetylated S1P [S1P(Ac)2] was analyzed on an LC-MS system consisting of an HTC PAL autosampler (CTC Analytics, Zwingen, Switzerland), a Rheos 2200 HPLC pump (Flux Instruments, Reinach, Switzerland), and a TSQ Quantum Access mass spectrometer (Thermo Fisher Scientific, Waltham, MA). Chromatographic conditions for reverse-phase separation of S1P(Ac)2 were modified from Berdyshev et al. (26). Separation of S1P(Ac)2 was done on a Nucleosil C18 HD column (125 × 2 mm, 100 Å, 5 μm) at 40°C. Mobile phase A consisted of water/methanol/formic acid (20:80:0.5, v/v) and mobile phase B consisted of methanol/acetonitrile/formic acid (59:40:0.5, v/v), both containing 5 mM ammonium formate. Elution started with 100% A for 1.0 min (0.25 ml/min) and increased to 100% B within 4.0 min and was then kept constant for 4 min. Finally the column was reequilibrated with 100% mobile phase A for 5.5 min. The injection volume was 10 μl. The injection system and syringe were washed twice with methanol/acetone/2-propanol (1:1:1, v/v), containing HCOOH 0.1% and acetone/methanol/water (2:2:1, v/v) solutions after every injection. S1P(Ac)2 and D7S1P(Ac)2 eluted at tr ∼6.3 min. Double blank and blank samples were analyzed before each set of calibrators and samples to exclude carryover.
For ionization, ESI was used and detection was performed in the positive mode, monitoring [M-H2O]+ ions using selective reaction monitoring for the transitions of m/z 446.2 → 264.2 (30 V) for S1P(Ac)2 and m/z 453.2 → 271.2 (27 V) for D7S1P(Ac)2 (spray voltage 5,000 V, skimmer offset 10 V, and ion transfer capillary temperature 300°C). Further ionization and detection parameters were optimized by tuning the system with S1P(Ac)2 standard. Data analysis was performed on XCalibur 2.0.6 (Thermo Scientific).
The standard curve was constructed by plotting the S1P/D7S1P peak area ratios against the concentrations of the S1P standards. The S1P concentration in samples was determined by linear regression obtained from the standard curve. The lower level of quantification was defined as the 10% functional assay sensitivity and amounted to 1 pmol extracted S1P corresponding to 40 nmol/l plasma or 2 nmol/l urine. At amounts of 7.5 and 22.5 pmol, the intra-day imprecision was 5.8 and 2.3%, respectively, and the inter-day imprecision was 4.3 and 8.1%, respectively.
Western blotting of apoM in plasma and urine
Proteins were separated on 14% SDS polyacrylamide gels and transferred onto nitrocellulose membrane. The membranes were blocked with 5% milk in 0.1% TBS Tween (TBS-T) buffer for 1 h at room temperature. Thereafter, the membranes were incubated overnight at 4°C with a commercially available apoM antibody (LC-C51665; LifeSpam BioSciences). The 1:1,000 dilution of antibody was made in 5% milk TBS-T. The membranes were then washed three times with TBS-T and incubated with secondary antibody anti-rabbit coupled with HRP (1:10,000). After washing three times for 10 min, the membrane was developed with ECL Plus Western blotting detection reagent (Pierce) according to the manufacturer’s instructions.
Statistical analyses
Statistical analyses were performed by using Graph-Pad. Normality of the data was determined by using the Kolmogorov-Smirnov test. Normally distributed data were analyzed by the two-tailed unpaired Student’s t-test, and data that was not normally distributed by the Mann-Whitney U test. Differences in prevalences were analyzed by chi-square test.
RESULTS
HDL and apoM promote S1P efflux from erythrocytes
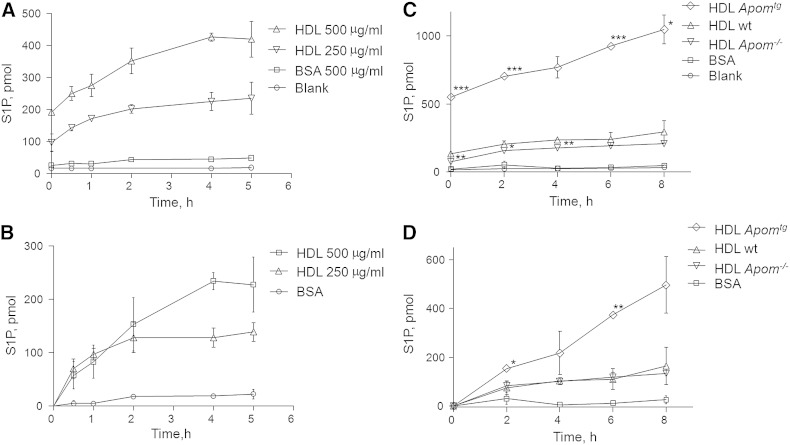
To confirm previous data that HDL induces S1P efflux from erythrocytes (5), 1 ml of washed human erythrocytes were incubated with two different concentrations of human HDL for increasing time. Figure 1A, B shows the time-dependent accumulation of S1P in human HDL without and with correction for the endogenous S1P content, respectively. Maximal efflux, which doubled the concentration of S1P preexisting in HDL, was reached after 4 h. Half-maximal efflux was reached after 1–2 h of incubation. Albumin (500 μg/ml), which is the second most important carrier of S1P in plasma (4, 10), elicited very little S1P efflux under the same conditions.
Fig. 1.
Time-dependent efflux of S1P from washed human erythrocytes in the presence of 500 μg/ml BSA (A–D), 250 or 500 μg/ml human HDL (A, B), or 300 μg/ml HDL from wt, Apom−/−, or Apomtg mice (C, D). A, C: Absolute S1P amounts in 1 ml of supernatant after incubation of HDL or albumin with erythrocytes for the indicated time intervals and subsequent removal of erythrocytes by centrifugation. B, D: Data on net S1P efflux which were obtained by subtracting the S1P concentrations of the various HDL preparations at baseline from the S1P concentrations at the indicated time points. Values are mean ± SD. Statistically significant differences between HDL of Apom−/− and wt mice, as well as between HDL of Apomtg and wt mice, are indicated by asterisks. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; Student’s t-test.
Next we monitored the time-dependent S1P efflux from erythrocytes in the presence of 300 μg/ml HDLs which were isolated from the plasma of wt, Apom−/−, or Apomtg mice in comparison with 300 μg/ml albumin. As reported previously, HDLs of these three mouse strains differ by endogenous S1P content (see also baseline data in Fig. 1C). Therefore, we present S1P efflux before and after correction for the endogenous S1P content of HDLs (Fig. 1C and Fig. 1D, respectively). During 8 h of incubation, S1P concentrations increased steadily in the HDL of all three mouse strains. S1P efflux capacity of HDL from wt mice resembled that of human HDL. The maximal net S1P efflux measured was significantly increased in the presence of HDL from Apomtg mice, but not decreased in the presence of HDL from Apom−/− mice (Fig. 1D). Also of note, S1P efflux in the presence of HDL from both wt and Apom−/− mice reached saturation after 2–4 h, whereas S1P efflux in the presence of HDL from Apomtg mice did not reach saturation within 8 h, the maximal time the experiment could be performed without hemolysis.
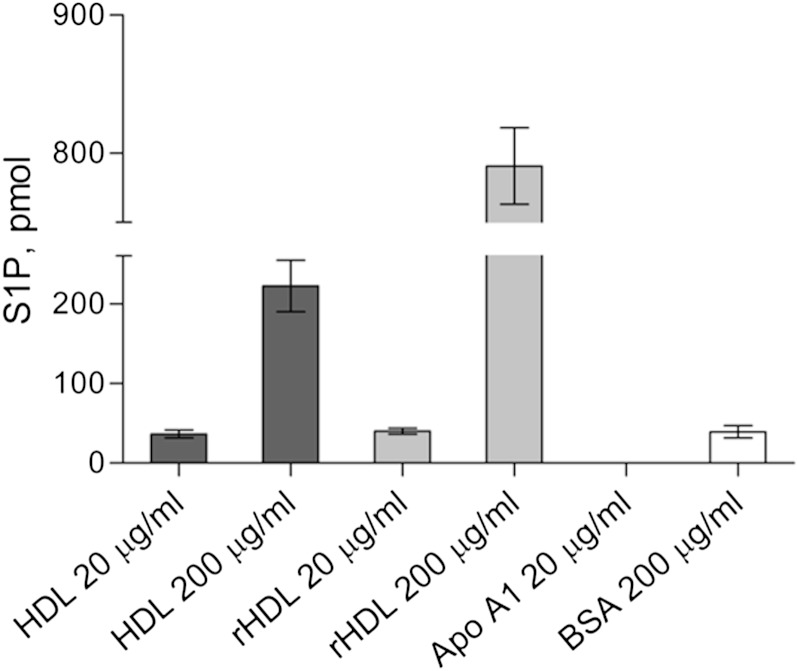
To provide further evidence that HDL can elicit S1P efflux independently of apoM, we compared the capacity of native HDL, rHDL, lipid-free apoA-I, and albumin to induce S1P efflux. Whereas lipid-free apoA-I was not capable of inducing S1P efflux, reconstituted apoM-free HDLs were even more active in stimulating S1P efflux than native HDLs at a concentration of 200 μg/ml (Fig. 2).
Fig. 2.
Net S1P efflux from washed human erythrocytes in the presence of native and rHDL, lipid-free apoA-I, or BSA. Presented are net amounts of S1P released by erythrocytes in 1ml of supernatant after 4 h incubation with 20 μg/ml or 200 μg/ml HDL isolated either from human plasma or artificially reconstituted by cholate dialysis of apoA-I and POPC (rHDL), 20 μg/ml lipid-free apoA-I, or 200 μg/ml BSA. Values are mean ± SD.
Urinary excretion of S1P is increased in Apom−/− mice, as well as in mice with dysfunctional tubular protein reabsorption
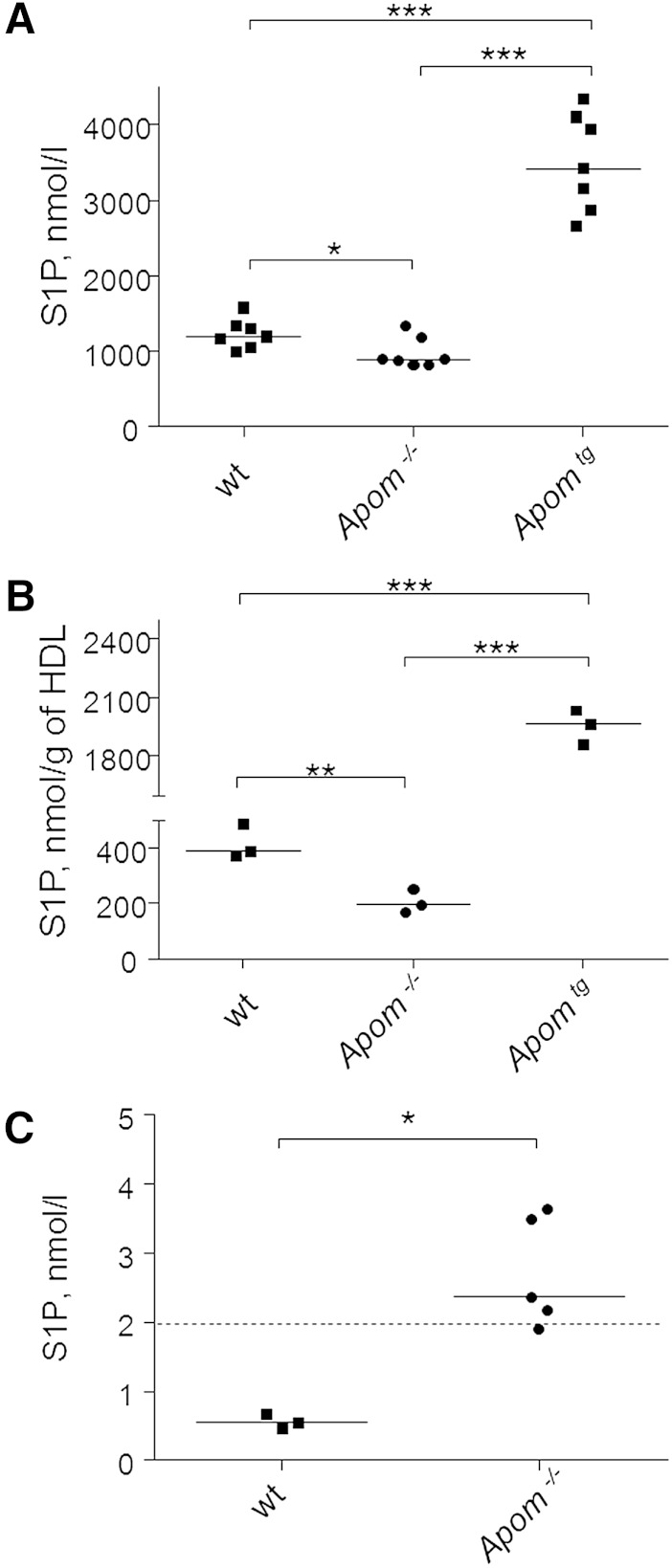
As reported previously (12, 13) and as compared with their wt littermates, S1P levels in plasma and HDL were significantly decreased by 20 and 50%, respectively, in 12–14-week-old Apom−/− mice, and were significantly increased by factors three and five, respectively, in plasma and HDL of age-matched Apomtg mice (Fig. 3A, B).
Fig. 3.
ApoM determines S1P concentration in plasma (A), HDL (B), and urine (C) of wt, Apom−/−, and Apomtg mice. A, B: S1P levels in plasma and HDL samples from 12–14-week-old wt, Apom−/−, and Apomtg mice. C: Urinary S1P excretion by 6- and 16-week-old Apom−/− mice and their wt littermates. Each point identifies data from individual mice. Solid lines indicate median values. The dashed line indicates the lower level of quantification of S1P by LC-MS in urine samples. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001; Mann-Whitney U-test.
In 21 urine samples of wt mice, we measured S1P at very low concentration below or close to the lower level of quantification of our method (10% functional assay sensitivity: 1 pmol S1P corresponding to 2 nmol S1P per liter of urine). In fact, only 5 of 21 samples of wt mice, but 4 of 5 samples Apom−/− mice, had S1P levels above this threshold (P = 0.018, chi-square test). In the direct comparison, urine concentrations of S1P were significantly higher in 6- and 16-week-old Apom−/− mice than in 6- and 16-week-old wt controls (P < 0.05, Fig. 3C).
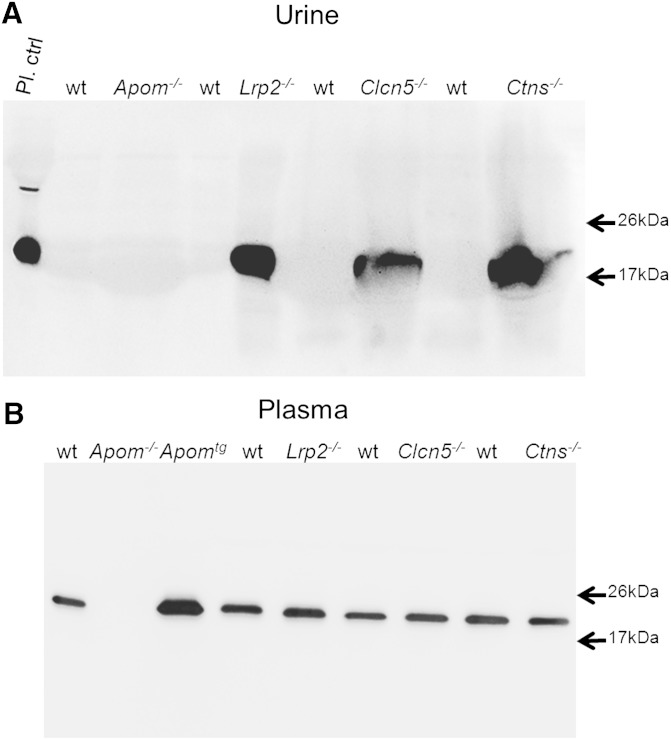
Next, we investigated whether disturbances of tubular apoM reabsorption are associated with increased urinary S1P excretion. By Western blotting, we found apoM present in the urine samples of mice with nonfunctional megalin (Lrp2−/−), ClC-5 (Clcn5−/−), or cystinosin (Ctns−/−) (Fig. 4A). ApoM was not detectable in the urine of wt littermates.
Fig. 4.
Western blotting of apoM in urine (A) and plasma (B) of Apom, Apomtg mice and mice with defective megalin (Lrp2−/−), ClC-5 (Clcn5−/−), and cystinosin (Ctns−/−) and their wt controls. Urine (20 μl) or 11.5 μl of 1/10 diluted plasma were separated by SDS-PAGE and immunoblotted as described in the Methods section. Note the absence of apoM from the urine samples of wt mice, but the presence in urine samples of mice with defective tubular transport (A). By contrast apoM plasma levels appear indistinguishable between wt mice and the different tubular proteinuria models.
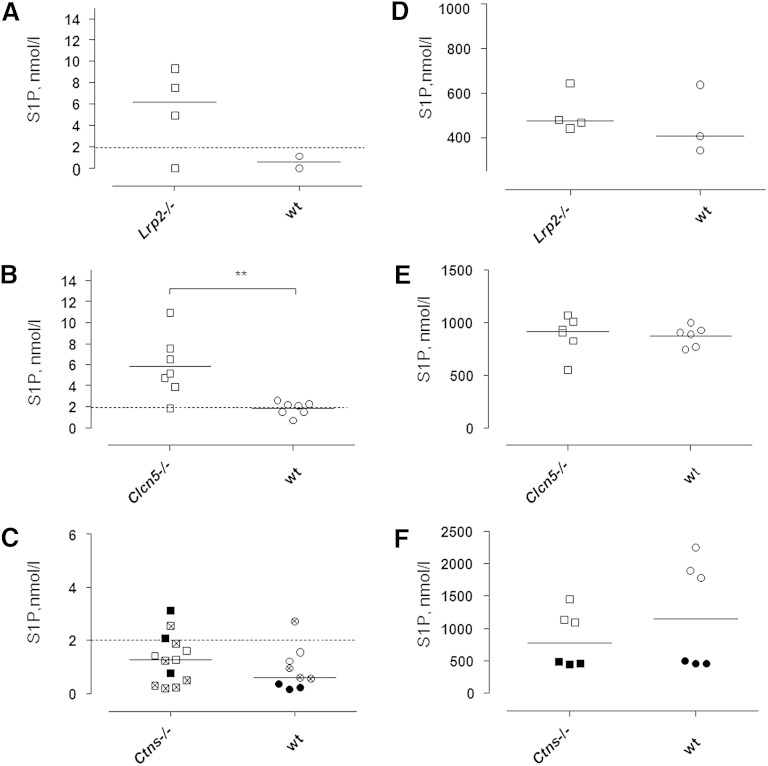
In the urine samples of 21 wt mice from three different laboratories, S1P was below or close to the level of quantification (2 nmol/l). By contrast, three of four urine samples from 11-week-old Lrp2−/− mice and six of seven samples from 12–16-week-old Clcn5−/− mice contained clearly quantifiable concentrations of S1P (Fig. 5A, B). Upon direct comparison of mutant mice and littermate controls, S1P excretion was significantly increased in Clcn5−/− mice (Fig. 5B), which are known to suffer from a severe dysfunction of the proximal tubule (27). At ages of 18 weeks, 20–24 weeks, or 30–38 weeks, neither male nor female Ctns−/− mice showed increased S1P excretion.
Fig. 5.
Concentrations of S1P in urine (A–C) or plasma (D–F) of mice with defective megalin (Lrp2−/−) (A, D), ClC-5 (Clcn5−/−) (B, E), or cystinosin (Ctns−/−) (C, F) as compared with their wt controls. Each point represents data from an individual mouse sample. A, D: Urine and plasma levels of S1P in 11-week-old Lrp2−/− mice (open symbols). B, E: Urine and plasma levels of S1P in 12–16-week-old Clcn5−/− mice (open symbols). C, F: Urine and plasma S1P levels in 18-week-old Ctns−/− mice (open symbols), in 20–24-week-old Ctns−/− mice (closed symbols), and in 30–38-week-old Ctns−/− mice (crossed symbols). Dashed lines indicate the lower level of quantification of S1P by LC-MS in urine samples. **P ≤ 0.01; Mann-Whitney U-test. Note the higher medians of S1P excretion in mice with defective tubular protein reabsorption as compared with wt controls.
Western blotting did not provide any evidence for grossly altered concentrations of apoM in the plasma of Lrp2−/−, Clcn5−/−, or Ctns−/− mice (Fig. 4B). These three mouse models with tubular apoM proteinuria also did not show any statistically significant or consistent differences in plasma concentrations of S1P (Fig. 5D–F).
DISCUSSION
Previous work by the laboratories of Dahlbäck and colleagues and Nielssen and colleagues (11, 12) identified apoM as a physiologically relevant binding protein of S1P in HDLs of plasma. Our lab previously confirmed the limiting effect of apoM on S1P levels in plasma, but also found indications for more complex relationships between HDL, apoM, and S1P (13). Most notably, concentrations of apoM and S1P in total or apoB-depleted plasma did not correlate with each other (13), possibly due to the molar excess of apoM compared with its ligand S1P, which varies inter-individually between factors 1.2 and 8 and/or in the presence of alternative ligands, which compete with S1P for binding to apoM (10, 13). However, we also observed statistically significant correlations of S1P concentrations with concentrations of HDL-cholesterol, apoA-I, and other measures of HDLs (13). This raised the questions whether HDLs can handle S1P also independently of apoM and whether apoM may influence S1P plasma levels independently of its transport function in plasma.
To answer the first question, we investigated the capacity of HDLs to induce S1P efflux from erythrocytes. HDLs of both humans and wt mice were found to induce time-dependent and saturable S1P efflux leading to maximal doubling of the endogenous S1P concentration in normal human or murine HDLs (400 pmol/l S1P per mg HDL protein). Assuming that 80% of HDL protein mass corresponds to apoA-I, i.e., the predominant protein of HDLs which has a molecular mass of 28 kDa, the molar concentration of S1P per apoA-I amounts to 1/72. Assuming that the HDL particles contain an average of three molecules of apoA-I (28, 29), every 24th HDL particle in our experiments contained one molecule of S1P at baseline. The doubling of S1P content by efflux indicates the saturation of S1P efflux much below the HDL particle concentration. This can be explained by the presence of a specific S1P binding site in HDLs which is not saturated in HDLs isolated from plasma, such as apoM (10–12). At first sight and in agreement with this explanation, we found the net S1P efflux capacity of HDLs from Apomtg mice significantly increased without reaching saturation. By contrast, HDLs from both wt and Apom−/− mice elicited time-dependent and saturable S1P efflux from erythrocytes, which did not differ from each other and that was markedly greater than in the presence of albumin. Moreover, rHDL consisting only of apoA-I and POPC induced S1P efflux from erythrocytes. The apparent 2- to 3-fold higher efficacy of rHDL, as compared with native HDL, probably reflects the higher particle concentration at identical protein mass concentrations, because rHDL contains two molecules of apoA-I, whereas native HDL contains at least three molecules of apoA-I plus other proteins. Our finding of rHDL-induced S1P efflux does also explain the previous observation that, initially, S1P-free rHDLs exert cytoprotective effects on cardiomyocytes in a S1P receptor-dependent fashion (30).
Taken together, our findings indicate the presence of an additional apoM-independent mechanism by which HDLs can induce efflux and/or mediate the binding of S1P. Because of the saturation much below the concentration of HDL particles or phospholipids, it is unlikely that this apoM-independent fraction of HDL-induced S1P efflux is unspecific, for example, as the result of association with phospholipids. The nonsaturation of S1P efflux in the presence of HDLs from Apomtg mice indicates that apoM, rather than phosphatidylcholine, acts as the slow determinant of S1P binding capacity. The saturable S1P efflux in the presence of HDLs from Apom−/− mice points to the presence of an as yet unknown relatively fast inducer of S1P efflux. Because the rHDL only contains apoA-I in addition to phospholipids, apoA-I is the prime candidate for this activity. In this respect, it is noteworthy that ABCA1, ABCG1, and scavenger receptor B1, which are important cellular interaction partners for apoA-I- or HDL-induced efflux of cholesterol or phosphatidylcholine, have been suspected to promote or modulate cellular S1P efflux as well (4, 8). In fact, glyburide, a pharmacological inhibitor of ABC transporters including ABCA1, was previously found to inhibit S1P efflux from red blood cells. However, although present in erythrocytes, functional experiments excluded ABCA1 and ABCA7 as the mediators of S1P efflux from these cells (7). In agreement with the lack of involvement of ABCA1, we did not find any S1P efflux induced by lipid-free apoA-I, which, as the primary interaction partner of ABCA1, stimulates efflux of cholesterol and phosphatidylcholine from many cells. The roles of ABCG1 and scavenger receptor BI, which typically interact with both native and artificially lipidated HDL (29) for S1P efflux, are as yet unknown.
To address the second question, whether apoM may regulate S1P plasma concentrations beyond transport in plasma, we compared the urinary excretion of S1P of wt and Apom−/− mice. Not unexpectedly, the urine of wt mice did not contain much S1P. However, quantifiable amounts of S1P were excreted with the urine by Apom−/− mice. These data indicated that apoM plays some role for preventing urinary S1P excretion, but does not indicate whether the excreted S1P is of plasmatic or renal origin. On the one hand and next to hepatocytes, the epithelial cells lining the proximal tubules of the kidney are the only cells which express apoM (31). Hence, kidney-derived apoM may play an important role in handling S1P, for example for the interaction with S1P receptors. In fact, S1P and S1P receptors were reported to convey protection of the proximal tubule epithelium against oxidative stress induced by ischemia/reperfusion injury (32). On the other hand, the plasma-derived 22 kDa large murine apoM or the 26 kDa large human apoM is ultrafiltrated through glomeruli and reabsorbed from the primary urine by the endocytic receptor megalin into the proximal tubule epithelial cells (16), so that the lack of apoM could interfere with the tubular recovery of S1P. To discriminate between these two explanations, we investigated the urinary excretion of apoM and S1P by mice which lack megalin, the chloride-proton exchanger ClC-5, or the lysosomal cystine transporter cystinosin, which all play important roles for the tubular reabsorption of carrier proteins and their cargo from the primary urine (17, 19, 20). We confirmed the previously observed urinary apoM excretion by megalin knockout mice (16). In addition, mice without CLC-5 or cystinosin showed a urinary loss of apoM. Like Apom−/− mice, Lrp2−/− and Clcn5−/− mice showed clearly increased urinary S1P excretion. This difference in urinary S1P excretion was much less prominent, if not absent, in Ctns−/− mice. This may reflect the fact that the cystinosis model develops tubular dysfunction later in life (33). In our hands, even at the age of 30–38 weeks when tubular dysfunction is manifested, we did not see any increase in S1P excretion, although apoM was excreted with the urine. Nevertheless, the unusual urinary excretion of S1P in the urine of Apom−/−, Lrp2−/−, and Clcn5−/− mice suggests that tubular reabsorption of apoM interferes with the urinary loss of S1P. However, in Lrp2−/− and Clcn5−/− mice, the urinary loss of apoM and S1P appears to be too low to substantially decrease the plasma concentrations of apoM and S1P. Therefore, it is also unlikely that the urinary loss contributes to the lower plasma and HDL-S1P levels observed in Apom−/− mice (12, 13). However, cubilin deficiency was previously reported to increase the turnover and decrease plasma albumin and apoA-I concentrations slightly, but significantly (18). Quantitative studies in larger numbers of mice may hence unravel subtle differences.
In conclusion, our studies of mice differing by the expression of apoM or the activity of proteins involved in the tubular endocytosis of apoM indicate that binding of S1P by apoM plays at least two further roles in S1P metabolism beyond mediating S1P transport in HDL. First, HDL facilitates S1P efflux from erythrocytes by both apoM-dependent and apoM-independent mechanisms. Second, apoM facilitates the reabsorption of S1P from the primary urine into tubular epithelial cells, however, with no impact on S1P plasma concentration. By the two newly identified activities, apoM may modulate the paracrine and autocrine interactions of S1P with vascular endothelial and renal tubular epithelial cells, respectively. In addition we provide evidence for an additional HDL-associated factor which contributes to S1P efflux and transport by HDLs.
Acknowledgments
The authors gratefully acknowledge the support by Dr. Thomas Willnow (Max Delbrück Centrum, Berlin-Buch, Germany), who provided plasma and urine samples of Lrp2−/− mice. Furthermore, the authors wish to thank Yvette Cnops for providing them with urine samples of Ctns−/− mice.
Footnotes
Abbreviations:
- Apom−/−
- apoM knockout mice
- Apomtg
- mice transgenic for apoM
- IS
- internal standard
- LRP2
- LDL receptor-related protein 2
- rHDL
- reconstituted HDL
- S1P
- sphingosine-1-phosphate
- S1P(Ac)2
- acetylated S1P
- TBS-T
- TBS Tween
- wt
- wild-type
This work was supported by grants from the Zurich Center of Integrated Human Physiology, University of Zurich (ZIHP) (to A.v.E, which paid the salary of I.S; and to T.H. and O.D. for research on sphingolipids and diseases of the proximal tubules, respectively). A.v.E. was supported by grants from the Swiss National Science Foundation and the 7th Framework Program of the European Commission (“RESOLVE” project number 305707 and TransCard project number 603091). O.D. received funding from the Belgian agencies Fonds de la Recherche Scientifique (Belgium) and Fonds de la Recherche Scientifique Médicale (Belgium) as well as from the National Centre of Competence in Research (NCCR) Kidney CH and the Swiss National Science Foundation (project grant 310030_143929), the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant number 305608 (EURenOmics). A.v.E., O.D., and T.H. are members of the Clinical Research Priority “Rare Diseases Initiative Zurich (RaDiZ)” of the University of Zurich. A.v.E. is a member of the European Cooperation in Science and Technology (COST) Action “HDLnet” (BM904).
REFERENCES
- 1.Fyrst H., Saba J. D. 2010. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat. Chem. Biol. 6: 489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hla T., Dannenberg A. J. 2012. Sphingolipid signaling in metabolic disorders. Cell Metab. 16: 420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornemann T., Worgall T. S. 2013. Sphingolipids and atherosclerosis. Atherosclerosis. 226: 16–28. [DOI] [PubMed] [Google Scholar]
- 4.Nishi T., Kobayashi N., Hisano Y., Kawahara A., Yamaguchi A. 2014. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta. 1841: 759–765. [DOI] [PubMed] [Google Scholar]
- 5.Bode C., Sensken S. C., Peest U., Beutel G., Thol F., Levkau B., Li Z., Bittman R., Huang T., Tolle M., et al. 2010. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J. Cell. Biochem. 109: 1232–1243. [DOI] [PubMed] [Google Scholar]
- 6.Ono Y., Kurano M., Ohkawa R., Yokota H., Igarashi K., Aoki J., Tozuka M., Yatomi Y. 2013. Sphingosine 1-phosphate release from platelets during clot formation: close correlation between platelet count and serum sphingosine 1-phosphate concentration. Lipids Health Dis. 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi N., Kobayashi N., Yamaguchi A., Nishi T. 2009. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J. Biol. Chem. 284: 21192–21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Xiong S. L., Yi G. H. 2012. ABCA1, ABCG1, and SR-BI: transit of HDL-associated sphingosine-1-phosphate. Clin. Chim. Acta. 413: 384–390. [DOI] [PubMed] [Google Scholar]
- 9.Sattler K., Levkau B. 2009. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovasc. Res. 82: 201–211. [DOI] [PubMed] [Google Scholar]
- 10.Christoffersen C., Nielsen L. B. 2013. Apolipoprotein M: bridging HDL and endothelial function. Curr. Opin. Lipidol. 24: 295–300. [DOI] [PubMed] [Google Scholar]
- 11.Sevvana M., Ahnstrom J., Egerer-Sieber C., Lange H. A., Dahlback B., Muller Y. A. 2009. Serendipitous fatty acid binding reveals the structural determinants for ligand recognition in apolipoprotein M. J. Mol. Biol. 393: 920–936. [DOI] [PubMed] [Google Scholar]
- 12.Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnstrom J., Sevvana M., Egerer-Sieber C., Muller Y. A., Hla T., Nielsen L. B., et al. 2011. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. USA. 108: 9613–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karuna R., Park R., Othman A., Holleboom A. G., Motazacker M. M., Sutter I., Kuivenhoven J. A., Rohrer L., Matile H., Hornemann T., et al. 2011. Plasma levels of sphingosine-1-phosphate and apolipoprotein M in patients with monogenic disorders of HDL metabolism. Atherosclerosis. 219: 855–863. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa R., Nakamura K., Okubo S., Hosogaya S., Ozaki Y., Tozuka M., Osima N., Yokota H., Ikeda H., Yatomi Y. 2008. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann. Clin. Biochem. 45: 356–363. [DOI] [PubMed] [Google Scholar]
- 15.Wolfrum C., Poy M. N., Stoffel M. 2005. Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat. Med. 11: 418–422. [DOI] [PubMed] [Google Scholar]
- 16.Faber K., Hvidberg V., Moestrup S. K., Dahlback B., Nielsen L. B. 2006. Megalin is a receptor for apolipoprotein M, and kidney-specific megalin-deficiency confers urinary excretion of apolipoprotein M. Mol. Endocrinol. 20: 212–218. [DOI] [PubMed] [Google Scholar]
- 17.Christensen E. I., Birn H., Storm T., Weyer K., Nielsen R. 2012. Endocytic receptors in the renal proximal tubule. Physiology (Bethesda). 27: 223–236. [DOI] [PubMed] [Google Scholar]
- 18.Aseem O., Smith B. T., Cooley M. A., Wilkerson B. A., Argraves K. M., Remaley A. T., Argraves W. S. 2014. Cubilin maintains blood levels of HDL and albumin. J. Am. Soc. Nephrol. 25: 1028–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilmer M. J., Emma F., Levtchenko E. N. 2010. The pathogenesis of cystinosis: mechanisms beyond cystine accumulation. Am. J. Physiol. Renal Physiol. 299: F905–F916. [DOI] [PubMed] [Google Scholar]
- 20.Devuyst O., Thakker R. V. 2010. Dent’s disease. Orphanet J. Rare Dis. 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nykjaer A., Dragun D., Walther D., Vorum H., Jacobsen C., Herz J., Melsen F., Christensen E. I., Willnow T. E. 1999. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 96: 507–515. [DOI] [PubMed] [Google Scholar]
- 22.Christensen E. I., Devuyst O., Dom G., Nielsen R., Van der Smissen P., Verroust P., Leruth M., Guggino W. B., Courtoy P. J. 2003. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc. Natl. Acad. Sci. USA. 100: 8472–8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nevo N., Chol M., Bailleux A., Kalatzis V., Morisset L., Devuyst O., Gubler M. C., Antignac C. 2010. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol. Dial. Transplant. 25: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 24.Ohnsorg P. M., Rohrer L., Perisa D., Kateifides A., Chroni A., Kardassis D., Zannis V. I., von Eckardstein A. 2011. Carboxyl terminus of apolipoprotein A-I (ApoA-I) is necessary for the transport of lipid-free ApoA-I but not prelipidated ApoA-I particles through aortic endothelial cells. J. Biol. Chem. 286: 7744–7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. 2009. Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 579: 443–467. [DOI] [PubMed] [Google Scholar]
- 26.Berdyshev E. V., Gorshkova I. A., Garcia J. G., Natarajan V., Hubbard W. C. 2005. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal. Biochem. 339: 129–136. [DOI] [PubMed] [Google Scholar]
- 27.Wang S. S., Devuyst O., Courtoy P. J., Wang X. T., Wang H., Wang Y., Thakker R. V., Guggino S., Guggino W. B. 2000. Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum. Mol. Genet. 9: 2937–2945. [DOI] [PubMed] [Google Scholar]
- 28.Segrest J. P., Cheung M. C., Jones M. K. 2013. Volumetric determination of apolipoprotein stoichiometry of circulating HDL subspecies. J. Lipid Res. 54: 2733–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annema W., von Eckardstein A. 2013. High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ. J. 77: 2432–2448. [DOI] [PubMed] [Google Scholar]
- 30.Frias M. A., Lang U., Gerber-Wicht C., James R. W. 2010. Native and reconstituted HDL protect cardiomyocytes from doxorubicin-induced apoptosis. Cardiovasc. Res. 85: 118–126. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X. Y., Dong X., Zheng L., Luo G. H., Liu Y. H., Ekstrom U., Nilsson-Ehle P., Ye Q., Xu N. 2003. Specific tissue expression and cellular localization of human apolipoprotein M as determined by in situ hybridization. Acta Histochem. 105: 67–72. [DOI] [PubMed] [Google Scholar]
- 32.Koch A., Pfeilschifter J., Huwiler A. 2013. Sphingosine 1-phosphate in renal diseases. Cell. Physiol. Biochem. 31: 745–760. [DOI] [PubMed] [Google Scholar]
- 33.Raggi C., Luciani A., Nevo N., Antignac C., Terryn S., Devuyst O. 2014. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum. Mol. Genet. 23: 2266–2278. [DOI] [PubMed] [Google Scholar]