Abstract
To determine dietary effects on circulating lipoprotein-associated phospholipase A2 (Lp-PLA2) activity and enzyme activity in peripheral blood mononuclear cells (PBMCs), 99 patients with impaired fasting glucose, impaired glucose tolerance, or newly-diagnosed T2D were randomly assigned to either a control group (usual diet with refined rice) or the whole grain and legume group. Substitution of whole grains and legumes for refined rice was associated with the replacement of 7% of energy from carbohydrates with energy from protein (about 4%) and fat. After 12 weeks, the whole grain and legume group showed a significant decrease in fasting glucose, insulin, homeostasis model assessment-insulin resistance, hemoglobin A1c, malondialdehyde, plasma Lp-PLA2 activity, and oxidized LDL (ox-LDL), and an increase in LDL particle size. The changes (Δs) in these variables in the whole grain and legume group were significantly different from those in controls after adjustment for the baseline levels. When all subjects were considered, Δ plasma Lp-PLA2 positively correlated with Δ glucose, Δ PBMC Lp-PLA2, Δ ox-LDL, and Δ urinary 8-epi-prostaglandin F2α after being adjusted for confounding factors. The Δ PBMC Lp-PLA2 correlated positively with Δ glucose and Δ ox-LDL, and negatively with Δ LDL particle size and baseline PBMC Lp-PLA2. The substitution of whole grains and legumes for refined rice resulted in a reduction in Lp-PLA2 activities in plasma and PBMCs partly through improved glycemic control, increased consumption of protein relative to carbohydrate, and reduced lipid peroxides.
Keywords: lipoprotein-associated phospholipase A2, peripheral blood mononuclear cell, type 2 diabetes
Lipoprotein-associated phospholipase A2 (Lp-PLA2) has been consistently higher among type 2 diabetics than nondiabetics (1–3), and independently predicts incident T2D (4). However, little is known about the dietary treatment that may alter circulating Lp-PLA2 activity levels. In a recent large cross-sectional study of men and women, the observation of an inverse association between protein intakes and circulating Lp-PLA2 activity suggests that nutritional factors may have the potential to influence Lp-PLA2 activity (5). Additionally, a small interventional study with low-calorie diet showed that an average 10 kg weight loss achieved over 4 months in healthy obese women was associated with a 10% decrease in Lp-PLA2 activity (6). These recent studies (5, 6) suggest that diet may represent a potentially modifiable pathway through which Lp-PLA2 activity can be altered. However, no study has examined the effects of dietary treatment on circulating Lp-PLA2 activity in patients with T2D.
According to the 2010 Korean National Health and Nutrition Survey (KNHANES V-1), carbohydrate-derived calories account for 65% of total caloric intake, and cooked refined rice is the major source of carbohydrates in middle-aged adults. Because of this high carbohydrate intake, the replacement of refined rice with whole grains and legumes has been suggested as a way to reduce the risk factors for T2D (7, 8). Although increased whole grain consumption in healthy subjects for a 16 week period did not affect any of the biomarkers of CVD risk, including insulin sensitivity (9), there was, however, a decreasing effect on blood glucose and insulin by whole grains and legumes reported in diabetic patients (7, 8). Therefore, we sought to determine the effects of dietary intervention (replacement of refined rice with whole grains and legumes and a high intake of vegetables) on circulating Lp-PLA2 activity and enzyme activity in peripheral blood mononuclear cells (PBMCs) in patients with impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or newly diagnosed T2D.
MATERIALS AND METHODS
Subjects and study design
Study subjects were selected from participants in a clinical nutrition study conducted by the National Leading Research Laboratory of Clinical Nutrigenetics/Nutrigenomics at Yonsei University. Study subjects were recruited from the Health Service Center during routine check-up at National Health Insurance Corporation Ilsan Hospital, Goyang, Korea (January 2012–December 2012). Based on the data screened from the Health Service Center, subjects who had IFG (100 ≤ fasting glucose < 126 mg/dl) or newly diagnosed T2D (fasting glucose ≥126 mg/dl) were referred to the Department of Family Medicine or Internal Medicine. Subjects were excluded if they had any diagnosis of vascular disease, cancer (clinically or by anamnesis), renal disease, liver disease, thyroid disease, chronic alcoholism, and acute or chronic inflammatory diseases; took antioxidants; were pregnant, breast feeding, or intending to become pregnant during the time of study; or were taking glucose-lowering, lipid-lowering, or antihypertensive medications. A total of 100 subjects were enrolled. The macronutrient composition of each subject’s usual diet was that of a typical diet with cooked refined rice, which is consumed by a substantial number of Koreans. All subjects provided written informed consent before participation in this study, which was approved by the Institutional Review Board of Yonsei University and performed in accordance with the Declaration of Helsinki.
Independently performed computer randomization was used to allocate numbers and divide the patients into two groups. The present study was carried out in two phases; a 2 week run-in phase consisting of the usual diet with refined rice and a 12 week intervention phase consisting of the usual diet of refined rice or consumption of whole grains and legumes as a carbohydrate source. At the beginning, a total 120 subjects were screened for the study. Twenty subjects were excluded; 13 subjects were taking medications or supplements which related to hypertension, and 7 subjects were taking blood glucose and lipid lowering drugs. During the run-in period, one subject, not having maintained energy intake, dropped out. The remaining 99 were randomly subdivided into two groups and were assigned to consume either their usual diet (refined rice) or the whole grains and legumes as a carbohydrate source for three meals a day during the 12 week intervention.
Dietary education and assessment of dietary intake/physical activity level
All subjects were given written and verbal instructions by a registered dietitian on completion of a 3 day (2 week days and 1 weekend day) dietary record every 2 weeks throughout the whole study. On the sheet, subjects were instructed to record the amount of foods before ingestion and any remaining after ingestion by weighing the foods. During the 2 week run-in period, all participants were advised to continue their usual diet of refined rice. Baseline measurements were also performed at the start of the run-in phase. After the run-in period, the subjects in the control group maintained their usual diet of refined rice, and the subjects in the whole grain group replaced refined rice intake with 1/3 of legumes (black soybeans), 1/3 of barley, and 1/3 of brown rice three times per day as a carbohydrate source and increased vegetable intake to at least 6 units (30–70 g/unit) per day for sufficient dietary fiber intake. To check participants’ compliance during the whole study period, the dietitian interviewed them during biweekly visits or by telephone. They were interviewed to discern whether they were following the program well, including dietary intake and weight changes. During the study period, all participants were also encouraged to maintain their usual lifestyle and were reminded not to make any changes to their usual dietary habits, except for the whole grain substitution in whole grain group.
Dietary energy values and nutrient content from the 3 day food records were calculated using a computer aided nutritional analysis program (CAN-pro 3.0; Korean Nutrition Society, Seoul, Korea). Total energy expenditure (kcal/day) was calculated from activity patterns including basal metabolic rate, physical activity for 24 h (10), and specific dynamic action of food. Basal metabolic rate for each subject was calculated with the Harris-Benedict equation (11).
Anthropometric parameters, blood pressure, and blood collection
Body weight and height were measured in the morning in unclothed subjects without shoes to calculate BMI (kg/m2). Waist circumference was measured at the umbilical level with the subjects standing after normal expiration and the hip girth was measured at the widest part of the hip, and the waist and hip ratio (WHR) was calculated. Blood pressure (BP) was measured in the left arm of seated patients with an automatic BP monitor (TM-2654; A and D, Tokyo, Japan) after a 20 min rest. Study subjects were interviewed for smoking and drinking behavior during their visit. After a 12 h fasting period, venous blood specimens were collected in EDTA-treated and plain tubes, centrifuged to produce plasma or serum, and stored at −70°C until analysis.
Oral glucose tolerance test, hemoglobin A1c, and homeostasis model assessment-insulin resistance
All subjects drank a 75 g glucose solution after a 12 h overnight fast. At 0 and 12 weeks, venous specimens were subsequently collected at, before, and 30, 60, and 120 min after loading to determine the serum levels and response areas of glucose, insulin, and C-peptide according to dietary intervention. Glucose was measured by a glucose oxidase method using the Beckman glucose analyzer (Beckman Instruments, Irvine, CA). Insulin and C-peptide were measured by radioimmunoassay with commercial kits from Immuno Nucleo Corp. (Stillwater, MN). Each response of glucose, insulin, C-peptide, and FFA was calculated with the area under each response curve using a trapezoidal method. Hemoglobin A1c (HbA1c) was measured by immunoturbidimetric analyzer using a turbidimeter. Insulin resistance was calculated from the homeostasis model assessment-insulin resistance (HOMA-IR) (12) using the following equation: HOMA-IR = [fasting insulin (μIU/ml) × fasting glucose (mmol/l)]/22.5.
Serum lipid profile, FFAs, and serum high-sensitivity C reactive protein
Fasting serum concentrations of total-cholesterol and triglycerides were measured using commercially available kits on a Hitachi 7150 autoanalyzer (Hitachi Ltd., Tokyo, Japan). After precipitation of apoB-containing lipoproteins with dextran sulfate-magnesium, HDL-cholesterol concentrations in the supernatants were measured enzymatically. LDL-cholesterol was indirectly estimated in participants with serum triglyceride concentrations less than 400 mg/dl using the Friedewald formula: LDL-cholesterol = total-cholesterol − [HDL-cholesterol + (triglycerides/5)]. FFAs were analyzed with a Hitachi 7150 autoanalyzer (Hitachi Ltd.). Serum high-sensitivity C reactive protein (hs-CRP) concentrations were measured with an Express PlusTM autoanalyzer (Chiron Diagnostics Co., Walpole, MA) using a commercially available high-sensitivity CRP-Latex(II) X2 kit (Seiken Laboratories Ltd., Tokyo, Japan).
Cytokine secretion in nonstimulated PBMCs
Whole blood was mixed with the same volume of RPMI 1640 (Gibco, Invitrogen, Carlsbad, CA). The sample was then centrifuged at 2,000 rpm for 20 min at 10°C. After the separation, a thin layer of PBMCs was isolated and washed twice with RPMI 1640. The pellet was resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (Gibco, Invitrogen) and seeded in 12-well plates (1 × 106 cells/ml; Nunc, Roskilde, Denmark) and incubated at 37°C with 5% CO2 for 24 h. After 24 h incubation, supernatants were collected and stored at 80°C until interleukin (IL)-1β, IL-6, TNF-α, and Lp-PLA2 activity levels were assayed (13, 14).
Cytokine assay for IL-1β, IL-6, and TNF-α levels in serum and PBMC supernatants
Levels of IL-1β, IL-6, and TNF-α in serum and PBMC supernatants were measured using the Bio-PlexTM reagent kit on the Bio-PlexTM (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instuctions.
Urinary 8-epi-prostaglandin F2α and plasma malondialdehyde
Urine was collected in polyethylene bottles containing 1% butylated hydroxytoluene after a 12 h fast. The bottles were immediately covered with aluminum foil and stored at −70°C until further analysis. The compound 8-epi-prostaglandin F2α (8-epi-PGF2α) was measured using an enzyme immunoassay (BIOXYTECH urinary 8-epi-PGF2α TM assay kit; OXIS International Inc., Portland, OR). Urinary creatinine levels were determined using the alkaline picrate (Jaffe) reaction. Plasma malondialdehyde (MDA) was measured from thiobarbituric acid-reactive substances (TBARS assay kit; Zepto-Metrix Co., Buffalo, NY).
Plasma oxidized LDL, LDL particle size, and Lp-PLA2 activity
Plasma oxidized LDL (ox-LDL) was measured using an enzyme immunoassay (Mercodia, Uppsala, Sweden). The resulting color reaction was read at 450 nm on a Wallac Victor2 multilabel counter (Perkin Elmer Life Sciences, Turku, Finland). Particle size distribution of LDL (1.019–1.063 g/ml) isolated by sequential flotation ultracentrifugation was examined by a pore gradient lipoprotein system (CBS Scientific, San Diego, CA) on commercially available nondenaturing polyacrylamide slab gels containing a linear gradient of 2–16% acrylamide (Alamo Gels Inc., San Antonio, TX). Standards of latex beads (34 nm), thyroglobulin (17 nm), apoferritin (12.2 nm), and catalase (10.4 nm) were used to estimate the relative migration rates of each band. Gels were scanned using a GS-800 calibrated imaging densitometer (Bio-Rad, Graz, Austria). The activity of Lp-PLA2 in plasma and PBMC supernatants was measured by using a modification of a previously described high-throughput radiometric activity assay (15).
Statistical analyses
Statistical analyses were performed using the SPSS version 12.0 for Windows (Statistical Package for the Social Sciences; SPSS Inc., Chicago, IL). A paired t-test was used to evaluate the effects of the dietary intervention. The primary endpoint was to assess changes of Lp-PLA2 activities in plasma and PBMC between groups. One-way ANOVA with the Bonferroni correction or independent t-test was used to test the inter-group comparison. Pearson and partial correlation coefficients were used to determine the relationship between changes in Lp-PLA2 activities in plasma and PBMC supernatants, and metabolic parameters. General linear model analysis was also used for the comparison with adjustment for confounding factors. Multiple linear regression analyses were performed to identify major factors associated with initial and changed variables, according to changes in Lp-PLA2 activities in plasma and PBMC supernatants. For descriptive purposes, mean values are presented using untransformed values. Results are expressed as the mean ± standard error. A two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
After the run-in period, 99 subjects who had maintained their energy intake participated in the intervention program and were randomly subdivided into the control group or the whole grain group. There were no significant differences between control and whole grain groups in baseline characteristics, such as age (55.4 ± 1.5 years vs. 56.3 ± 1.2 years; P = 0.618), male/female distribution (35/14 vs. 32/18; P = 0.429), education level, smoking, and drinking (data not shown).
Clinical characteristics, lipid profiles, inflammatory markers, and nutrient intake
Table 1 shows clinical characteristics, lipid profiles, inflammatory markers, and nutrient intakes before and after dietary intervention. Frequent interviews with the subjects did not suggest any important changes in physical activity patterns of the individual subjects during the study. As expected, BMI, WHR, total energy expenditure, and total energy intake were similar before and after treatment in both groups. However, IL-6 levels in serum and cultured nonstimulated PBMC supernatants significantly decreased from baseline in the whole grain and legume group. The changes (difference from baseline) in IL-6 levels in PBMC supernatants in the whole grain and legume group were significantly different from those in the refined rice group after adjusting for baseline levels. Posttreatment IL-6 levels in PBMC supernatants in the whole grain and legume group were significantly lower than those in the control group. With the substitution of whole grains and legumes for refined rice, energy percentages of protein (16.5 ± 0.1% vs. 20.4 ± 0.1%; P < 0.001) and fat (22.1 ± 0.1% vs. 24.9 ± 0.1%; P < 0.001) intake increased significantly, however, those of carbohydrate (61.8 ± 0.2% vs. 54.7 ± 0.1%; P < 0.001) significantly decreased in the whole grain and legume group. Compared with net differences of these macronutrient parameters, percent energy intakes of protein, fat, and carbohydrate were significantly different between the two groups (all P < 0.001), however, these significant differences disappeared after adjustment for baseline levels. There were significant increases from baseline in both fiber intake and the polyunsaturated-to-saturated FA ratio in the whole grain and legume group after dietary intervention. The whole grain and legume group showed lower posttreatment energy percentage of carbohydrate, higher posttreatment protein and fat calorie percent, and higher posttreatment fiber intake than the control group (Table 1).
TABLE 1.
Clinical characteristics, serum lipid profiles, inflammatory markers, and estimates of daily nutrient intake before and after 12 week dietary treatment
| Control (n = 49) | Whole Grain (n = 50) | |||||
| Before | After | Before | After | P | Pa | |
| BMI (kg/m2) | 24.1 ± 0.44 | 24.2 ± 0.44 | 24.0 ± 0.38 | 24.0 ± 0.38 | 0.451 | 0.449 |
| WHR | 0.90 ± 0.01 | 0.90 ± 0.01 | 0.90 ± 0.01 | 0.89 ± 0.01 | 0.172 | 0.174 |
| Systolic BP (mm Hg) | 126.3 ± 1.78 | 125.7 ± 1.65 | 124.6 ± 1.68 | 124.4 ± 1.51 | 0.826 | 0.859 |
| Diastolic BP (mm Hg) | 79.2 ± 1.21 | 79.6 ± 1.09 | 79.2 ± 1.26 | 79.9 ± 1.14 | 0.851 | 0.840 |
| Triglyceride (mg/dl)a | 130.7 ± 12.0 | 130.3 ± 10.6 | 139.4 ± 16.7 | 137.9 ± 15.6 | 0.942 | 0.922 |
| Total-cholesterol (mg/dl)a | 189.0 ± 5.42 | 193.7 ± 5.17 | 188.3 ± 4.56 | 186.7 ± 4.13 | 0.113 | 0.096 |
| HDL-cholesterol (mg/dl)a | 54.5 ± 1.66 | 55.4 ± 1.68 | 52.8 ± 1.53 | 53.0 ± 1.84 | 0.632 | 0.565 |
| LDL-cholesterol (mg/dl)a | 115.3 ± 5.47 | 116.9 ± 4.62 | 115.1 ± 4.33 | 113.3 ± 4.41 | 0.412 | 0.449 |
| hs-CRP (mg/dl)a | 1.49 ± 0.38 | 1.39 ± 0.32 | 1.42 ± 0.25 | 1.17 ± 0.19 | 0.711 | 0.610 |
| Serum TNF-α (pg/ml)a | 11.2 ± 0.86 | 11.2 ± 0.76 | 11.1 ± 0.74 | 9.96 ± 0.58 | 0.260 | 0.182 |
| Serum IL-6 (pg/ml)a | 4.12 ± 0.33 | 4.08 ± 0.41 | 4.27 ± 0.23 | 3.72 ± 0.23c | 0.154 | 0.271 |
| Serum IL-1β (pg/ml)a | 0.69 ± 0.07 | 0.66 ± 0.08 | 0.54 ± 0.03 | 0.53 ± 0.06 | 0.790 | 0.761 |
| Non-stimulated PBMC | ||||||
| TNF-α (pg/ml)a | 247.5 ± 36.6 | 273.8 ± 34.1 | 316.7 ± 47.7 | 284.6 ± 35.6 | 0.189 | 0.322 |
| IL-6 (pg/ml)a | 637.2 ± 139.3 | 971.4 ± 187.6 | 746.4 ± 139.3 | 438.0 ± 85.5ce | 0.003 | 0.002 |
| IL-1β (pg/ml)a | 7.13 ± 1.48 | 10.1 ± 1.70 | 11.1 ± 3.01 | 7.22 ± 1.80 | 0.041 | 0.099 |
| FFA (uEq/l)a | 512.5 ± 31.6 | 515.8 ± 32.9 | 496.1 ± 34.8 | 546.2 ± 40.4 | 0.374 | 0.433 |
| FFA response area (uEq/l/h)a | 669.0 ± 47.1 | 653.9 ± 50.5 | 582.7 ± 39.4 | 600.8 ± 63.0 | 0.672 | 0.872 |
| Total energy expenditure (kcal/day) | 2215 ± 38 | 2197 ± 35 | 2190 ± 29 | 2194 ± 30 | 0.136 | 0.164 |
| Estimates of daily nutrient intakes | ||||||
| Energy intake (kcal/day) | 2205 ± 37 | 2220 ± 39 | 2166 ± 32 | 2181 ± 32 | 0.997 | 0.924 |
| Carbohydrate (%) | 61.9 ± 0.13 | 61.9 ± 0.14 | 61.8 ± 0.16 | 54.7 ± 0.10de | <0.001 | 0.194 |
| Protein (%) | 16.1 ± 0.07 | 16.3 ± 0.10 | 16.5 ± 0.10 | 20.4 ± 0.09de | <0.001 | 0.452 |
| Fat (%) | 22.1 ± 0.14 | 22.1 ± 0.13 | 22.1 ± 0.14 | 24.9 ± 0.13de | <0.001 | 0.248 |
| Fiber (g)a | 24.2 ± 1.14 | 23.0 ± 1.01 | 24.1 ± 1.03 | 27.9 ± 1.01ce | 0.004 | 0.503 |
| PUFA/SFAa | 1.42 ± 0.16 | 1.43 ± 0.15 | 1.42 ± 0.13 | 1.90 ± 0.15b | 0.076 | 0.006 |
Data are expressed as mean ± standard error. SFA, saturated fatty acid.
Tested by logarithmic transformation.
P < 0.05.
P < 0.01.
P < 0.001 compared with initial value in each group tested by paired t-test.
†P < 0.01 compared between two groups at 12 week follow-up. P-value compared with net differences between 2 groups tested by independent t-test. Pa value compared with net differences between two groups after adjusting for baseline value.
Glucose, insulin, C-peptide, and oxidative stress
After dietary intervention, fasting glucose and HbA1c concentrations significantly increased in the control group, whereas fasting glucose, insulin, HOMA-IR index, response areas of glucose, insulin, and C-peptide during oral glucose tolerance test and HbA1c significantly decreased in the whole grain and legume group (Table 2). The change (Δ) in fasting glucose (P < 0.001), Δ insulin, Δ HOMA-IR index, Δ glucose response area, and Δ C-peptide response area during oral glucose tolerance test and Δ HbA1c in the whole grain and legume group were significantly different from those in the control group before and after adjustment for the baseline levels. Posttreatment fasting glucose, HOMA-IR index, and glucose response area in the whole grain and legume group were significantly lower than those in the control group. After 12 weeks of dietary intervention, plasma MDA concentrations of the control group significantly increased, whereas those of the whole grain and legume group significantly decreased. The Δ plasma MDA (P < 0.001) and Δ urinary 8-epi-PGF2α (P = 0.031) in the whole grain and legume group were significantly different from those in the control group after adjustment for the baseline levels. The whole grain and legume group showed lower levels of posttreatment plasma MDA (P = 0.015) and a trend toward a decrease in posttreatment urinary 8-epi-PGF2α (P = 0.053) than the control group (Table 2).
TABLE 2.
Effects of whole grain and legume consumption on glucose, insulin, HOMA-IR, and oxidative stress before and after 12 week dietary treatments
| Control (n = 49) | Whole Grain (n = 50) | P | a | |
| Glucose (mg/dl) | ||||
| Beforea | 123.8 ± 4.21 | 124.7 ± 4.35 | 0.865 | — |
| Aftera | 132.7 ± 4.62d | 113.9 ± 3.68d | <0.001 | — |
| Change | 8.88 ± 1.22 | −10.8 ± 1.17 | <0.001 | <0.001 |
| Insulin (uIU/ml) | ||||
| Beforea | 6.93 ± 0.39 | 7.08 ± 0.33 | 0.524 | — |
| Aftera | 6.85 ± 0.49 | 6.14 ± 0.26d | 0.517 | — |
| Change | −0.08 ± 0.32 | −0.95 ± 0.26 | 0.037 | 0.049 |
| HOMA-IRe | ||||
| Beforea | 2.12 ± 0.14 | 2.15 ± 0.11 | 0.503 | — |
| Aftera | 2.23 ± 0.16 | 1.71 ± 0.08d | 0.017 | — |
| Change | 0.11 ± 0.10 | −0.44 ± 0.08 | <0.001 | <0.001 |
| Glucose response area (mg/dl/h) | ||||
| Beforea | 413.0 ± 19.0 | 386.1 ± 16.7 | 0.294 | — |
| Aftera | 433.1 ± 22.5 | 352.1 ± 16.0d | 0.001 | — |
| Change | 20.1 ± 10.3 | −34.1 ± 7.72 | <0.001 | <0.001 |
| Insulin response area (uIU/ml/h) | ||||
| Beforea | 68.0 ± 10.1 | 77.8 ± 8.76 | 0.342 | — |
| Aftera | 74.4 ± 16.2 | 65.1 ± 6.64b | 0.944 | — |
| Change | 6.43 ± 7.81 | −11.5 ± 5.07 | 0.054 | 0.062 |
| C-peptide response area (ng/ml/h) | ||||
| Beforea | 14.0 ± 0.80 | 14.6 ± 0.86 | 0.556 | — |
| Aftera | 14.7 ± 1.12 | 13.6 ± 0.82c | 0.396 | — |
| Change | 0.73 ± 0.62 | −0.95 ± 0.38 | 0.022 | 0.025 |
| HbA1c (%) | ||||
| Beforea | 6.69 ± 0.16 | 6.82 ± 0.18 | 0.581 | — |
| Aftera | 6.82 ± 0.18b | 6.50 ± 0.09c | 0.111 | — |
| Change | 0.14 ± 0.06 | −0.33 ± 0.12 | 0.001 | <0.001 |
| MDA (nmol/ml) | ||||
| Beforea | 9.33 ± 0.42 | 9.36 ± 0.41 | 0.975 | — |
| Aftera | 10.0 ± 0.49b | 8.59 ± 0.34b | 0.015 | — |
| Change | 0.69 ± 0.26 | −0.78 ± 0.30 | <0.001 | <0.001 |
| 8-epi-PGF2α (pg/mg creatinine) | ||||
| Beforea | 2017.4 ± 148.7 | 1877.1 ± 129.1 | 0.622 | — |
| Aftera | 2,117.4 ± 150.1 | 1764.7 ± 95.4 | 0.053 | — |
| Change | 100.0 ± 95.4 | −112.5 ± 89.8 | 0.108 | 0.031 |
Data are expressed as means ± standard error.
Tested by logarithmic transformation.
P < 0.05.
P < 0.01.
P < 0.001 compared with initial value in each group tested by paired t-test. P values derived from independent t-test. Pa values derived from independent t-test after adjusted for baseline value.
HOMA-IR = [fasting insulin (μIU/ml) × fasting glucose (mmol/L)]/22.5.
Lp-PLA2 activity in plasma and nonstimulated PBMC, plasma ox-LDL, and LDL particle size
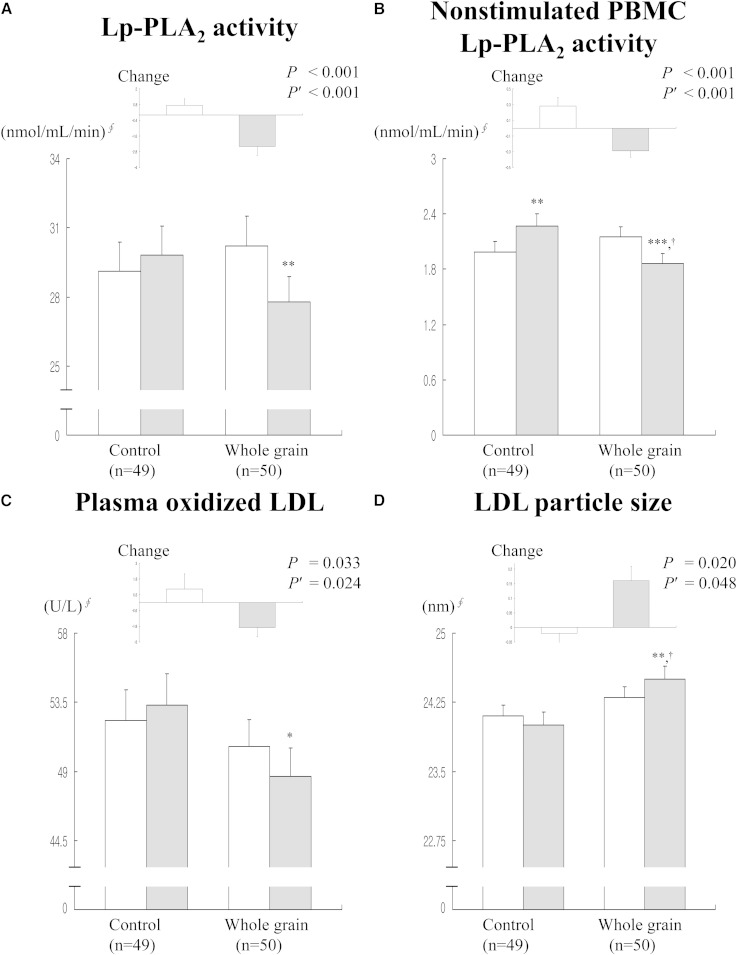
The 12 week intervention with the substitution of whole grains and legumes for refined rice resulted in a decrease in plasma Lp-PLA2 activity (30.2 ± 1.32 nmol/ml/min vs. 27.8 ± 1.08 nmol/ml/min; P = 0.002), Lp-PLA2 activity in cultured nonstimulated PBMC supernatants (2.15 ± 0.11 nmol/ml/min vs. 1.86 ± 0.11 nmol/ml/min; P < 0.001), and plasma ox-LDL (50.6 ± 1.78 U/l vs. 48.7 ± 1.82 U/l; P = 0.010), and an increase in LDL particle size (24.3 ± 0.12 nm vs. 24.5 ± 0.14 nm; P = 0.007) (Fig. 1). Lp-PLA2 activity in PBMC supernatants was increased in the control group (1.99 ± 0.11 nmol/ml/min vs. 2.27 ± 0.13 nmol/ml/min; P = 0.009). The Δ plasma Lp-PLA2 activity (0.75 ± 0.48 nmol/ml/min vs. −2.39 ± 0.69 nmol/ml/min; P < 0.001), Δ enzyme activity in PBMC supernatants (0.28 ± 0.11 nmol/ml/min vs. −0.29 ± 0.08 nmol/ml/min; P < 0.001), Δ plasma ox-LDL (1.04 ± 1.15 U/l vs. −1.90 ± 0.71 U/l; P = 0.024), and Δ LDL particle size (−0.02 ± 0.05 nm vs. 0.16 ± 0.05 nm; P = 0.048) in the whole grain and legume group were significantly different from those in the control group after adjustment for baseline levels. The whole grain and legume group showed lower posttreatment Lp-PLA2 activity in PBMC supernatants (2.27 ± 0.13 nmol/ml/min vs. 1.86 ± 0.11 nmol/ml/min; P = 0.014) and larger posttreatment LDL particle size (24.0 ± 0.14 nm vs. 24.5 ± 0.14 nm; P = 0.024) than the control group (Fig. 1).
Fig. 1.
Effects of whole grain and legume consumption on circulating Lp-PLA2 activity (A), Lp-PLA2 activity in peripheral blood molecular cells (B), plasma ox-LDL (C), and LDL particle size before (white bars) and after (black bars) 12 weeks of dietary treatment (D). Data are expressed as mean ± standard error. §Tested by logarithmic transformation. *P < 0.05, **P < 0.01, ***P < 0.001 compared with baseline values in each group tested by paired t-test with the Wilcoxon signed rank test. P values derived from independent t-test with the Mann-Whitney U-test. P′, P value after adjusting for baseline value. †P < 0.01 compared between two groups at 12 week follow-up.
Relationship between changes in Lp-PLA2 activities in plasma and PBMC supernatants and metabolic parameters
When all subjects were considered, Δ plasma Lp-PLA2 activity positively correlated with Δ fasting glucose (r = 0.318, P = 0.002), Δ Lp-PLA2 activity in PBMC supernatants (r = 0.310, P = 0.002), Δ plasma ox-LDL (r = 0.314, P = 0.002), Δ serum IL-6 (r = 0.218, P = 0.034), and Δ urinary 8-epi-PGF2α (r = 0.346, P = 0.001) after adjustment for age, sex, BMI, smoking, and drinking. After adjusting for these confounding variables, Δ Lp-PLA2 activity in PBMC supernatants correlated positively with Δ fasting glucose (r = 0.386, P < 0.001), Δ plasma ox-LDL (r = 0.502, P < 0.001), Δ serum IL-6 (r = 0.233, P = 0.024), and Δ IL-6 in PBMC supernatants (r = 0.269, P = 0.009), and negatively with Δ LDL particle size (r = −0.356, P < 0.001) and baseline Lp-PLA2 activity in PBMC supernatants (r = −0.323, P = 0.002).
Fasting glucose change and baseline plasma Lp-PLA2 activity as independent predictors of Δ plasma Lp-PLA2 activity
Based on these results, we performed multiple regression analysis to determine independent predictors of Δ plasma Lp-PLA2 activity (Table 3). Age, baseline BMI, baseline fasting glucose, Δ fasting glucose, Δ HOMA-IR index, Δ plasma ox-LDL, Δ LDL particle size, baseline plasma Lp-PLA2 activity, baseline PBMC Lp-PLA2 activity, and Δ PBMC Lp-PLA2 activity were evaluated. The Δ fasting glucose was found to be an independent predictor of Δ plasma Lp-PLA2 activity (β = 0.113 ± 0.040; P = 0.006) together with baseline plasma Lp-PLA2 activity (β = −1.004 ± 0.494; P = 0.045).
TABLE 3.
Results of multiple linear regression analysis for changes in Lp-PLA2 activities in plasma and PBMC supernatant
| Δ Lp-PLA2 Activity in Plasma | Δ Lp-PLA2 Activity in Nonstimulated PBMC | |||||
| Variable | Regression Coefficients | 95% CI | P | Regression coefficients | 95% CI | P |
| Age | 0.011 | [−0.079; 0.101] | 0.809 | −0.006 | [−0.019; 0.007] | 0.351 |
| BMI | 0.185 | [−0.118; 0.488] | 0.228 | −0.033 | [−0.077; 0.011] | 0.140 |
| Serum glucosea | 3.533 | [−1.396; 8.463] | 0.158 | 0.112 | [−0.616; 0.840] | 0.760 |
| Δ Serum glucose | 0.113 | [0.033; 0.192] | 0.006 | 0.009 | [−0.003; 0.021] | 0.153 |
| Δ HOMA-IR | −1.023 | [−2.376; 0.331] | 0.137 | 0.016 | [−0.184; 0.217] | 0.872 |
| Δ Plasma ox-LDL | 0.134 | [−0.008; 0.277] | 0.064 | 0.033 | [0.013; 0.053] | 0.002 |
| Δ LDL particle size | 0.247 | [−2.171; 2.666] | 0.839 | −0.381 | [−0.725; −0.037] | 0.030 |
| Lp-PLA2 activity in plasmaa | −1.004 | [−1.985; −0.024] | 0.045 | 0.120 | [−0.025; 0.264] | 0.104 |
| Δ Lp-PLA2 activity in plasma | — | — | — | 0.019 | [-0.011; 0.050] | 0.216 |
| Lp-PLA2 activity in nonstimulated PBMCa | 1.518 | [−0.781; 3.817] | 0.193 | −0.423 | [−0.751; −0.096] | 0.012 |
| Δ Lp-PLA2 activity in nonstimulated PBMC | 0.9001 | [−0.536; 2.338] | 0.216 | — | — | — |
| R2 = 0.241, adjusted R2 = 0.154 | R2 = 0.407, adjusted R2 = 0.340 | |||||
| P = 0.005 | P < 0.001 | |||||
CI, confidence interval.
Tested by logarithmic transformation.
Changes in plasma ox-LDL, LDL particle size, and baseline PBMC Lp-PLA2 activity as independent predictors of Δ Lp-PLA2 activity in PBMCs
Through multiple regression analysis to determine independent predictors of Δ Lp-PLA2 activity in PBMC supernatants after dietary treatment, the Δ plasma ox-LDL was found to be an independent predictor of Δ Lp-PLA2 activity in PBMC supernatants (β = 0.033 ± 0.010; P = 0.002) together with baseline PBMC Lp-PLA2 activity (β = −0.423 ± 0.165; P = 0.012) and Δ LDL particle size (β = −0.381 ± 0.173; P = 0.030) (Table 3).
DISCUSSION
The 12 week intervention with the substitution of whole grains and legumes for refined rice was associated with substantial improvement in glucose and insulin metabolism as well as reduction in circulating and PBMC Lp-PLA2 activities. The positive and independent association between change in plasma Lp-PLA2 activity and change in fasting glucose suggests that improving glycemic control can reduce circulating Lp-PLA2 activity without significant changes in lipid profiles in patients with IFG, IGT, or newly-diagnosed T2D. Additionally, the finding of the positive correlation between changes in plasma and PBMC Lp-PLA2 activities suggests that a reduction in PBMC Lp-PLA2 activity could partly contribute to the reduction in circulating Lp-PLA2 activity. Indeed, Lp-PLA2 is known to be secreted from bone marrow-derived cells; mainly monocytes, macrophages, T lymphocytes, and mast cells (16, 17).
Improving glycemic control in the whole grain and legume group resulted in a decrease in plasma ox-LDL and an increase in LDL particle size as well as reducing plasma and PBMC Lp-PLA2 activities. Sanchez-Quesada et al. (18) reported that a 4 ± 2 month clinical trial with lifestyle counseling and pharmacologic hypoglycemic therapy in 42 patients with long-term T2D reduced Lp-PLA2 activity and significantly increased LDL particle size by 0.9%. The mechanism underlying reduced Lp-PLA2 activity has been suggested to be related to the increase in LDL particle size because the amount of Lp-PLA2 in small dense LDL is 5–10 times higher than in normal-sized LDL particles (19). Therefore, the extent of decrease in plasma Lp-PLA2 activity in the whole grain and legume group of this study may depend on not only PBMC synthesis of Lp-PLA2, but also LDL particle size carrying this enzyme in circulation.
PBMCs are constituted mostly by circulating T and B lymphocytes and monocytes, which are considered a useful cell source for molecular studies in humans (20). The positive and independent relationship between change in PBMC Lp-PLA2 and change in ox-LDL supports the previous finding of the direct effect of ox-LDL on the expression of Lp-PLA2 in monocytes (21). Enhanced Lp-PLA2 results in increasing levels of inflammatory lysophosphatidylcholine which induces the expression of several inflammatory cytokines. Inhibition of Lp-PLA2 has been, however, reported to abolish ox-LDL-induced expression of inflammatory genes, including IL-6 (22, 23). Thus the significant reductions in circulating and PBMC IL-6 concentrations might be related to the decrease in Lp-PLA2 activity in the whole grain and legume group.
Lp-PLA2 hydrolyzes oxidized phospholipids in LDL particles and generates bioactive oxidized FFAs and lysophosphatidylcholines (24). Stafforini et al. (25) showed that the secreted form of Lp-PLA2 released F2-isoprostanes, the end products of lipid oxidation, from the sn-2 position of phosphatidylcholines with high affinity. Kono et al. (26) have reported that intracellular type II Lp-PLA2, which shares homology with the plasma enzyme Lp-PLA2, is involved in the metabolism of esterified 8-iso-PGF2α. Similar to previous findings (3, 27), this study also showed a positive correlation between changes in circulating Lp-PLA2 activity and urinary 8-epi-PGF2α concentrations. Changes in urinary 8-epi-PGF2α concentrations were positively correlated with changes in fasting glucose, which were also strongly correlated with changes in plasma MDA, a marker of oxygen-derived free radicals. Similarly, recent studies of dietary treatment in patients with prediabetes or newly diagnosed T2D have shown that changes in MDA positively correlate with changes in fasting glucose and glucose response area during meal tolerance test (28).
The mechanisms that may link Lp-PLA2 activity with diet in this study may be through substantial improvement of glucose and insulin metabolism by replacing refined rice with whole grains and legumes. Recently, a positive association between Lp-PLA2 activity and insulin resistance (4), and a beneficial effect of improving glycemic control on Lp-PLA2 activity (18) have been reported. Alternatively, reduced Lp-PLA2 activity could be a marker of increased consumption of protein relative to carbohydrate intake in the whole grain and legume group. Substitution of whole grains and legumes for refined rice in this study was associated with the replacement of 7% of energy from carbohydrates with energy from protein (about 4%) and fat (about 3%). Hatoum et al. (5) found that the replacement of 5% of energy from carbohydrates with energy from protein was associated with 2.2 nmol·min−1·ml−1 lower levels of Lp-PLA2 activity, and this effect of protein intake on reduced Lp-PLA2 activity unrelated to the protein’s effect on lipid profiles. Additionally, whole grains, legumes, and vegetables contain many antioxidants, including vitamins, minerals, and phytochemicals (29, 30). Antioxidants can slow the rate of oxidation of oxidizable substrates (29, 31). In a randomized cross-design study, phytochemicals in soybeans were found to reduce lipid peroxidation in vivo and to increase the resistance of LDL to oxidation (32). Ox-LDL has been reported to induce Lp-PLA2 expression in monocytes and macrophages in vitro (33), supporting the positive and independent correlation between changes in PBMC Lp-PLA2 and plasma ox-LDL in this study.
There were limitations in this study. Several epidemiologic studies suggest that plasma Lp-PLA2 is an independent predictor of CVD events in primary and secondary prevention (34–37). A recent meta-analysis (36), which included 79,036 participants with or without coronary artery disease from 32 prospective studies, showed that Lp-PLA2 activity and mass each had a continuous association with the risk for coronary artery disease; as similarly, the present study showed positive correlation between Lp-PLA2 and CVD risk factors. On the other hand, Holmes et al. (38) suggested that reducing secretory phospholipase A2 (sPLA2)-IIA mass is unlikely to be a useful therapeutic goal for preventing cardiovascular events, which is associated with increased risk of incident and recurrence of major vascular events. PLA2G2A had large and specific effects on circulating sPLA2-IIA mass and a small-to-modest effect on sPLA2 enzyme activity (38). In addition, sPLA2 inhibitor, called varespladib, was not a useful strategy to reduce adverse cardiovascular outcomes after acute coronary syndrome (39). These inconsistencies of PLA2 families on CVD were emerged by causation and need to be further studied. An earlier study has shown that there were no significant differences in any markers of CVD risk between groups with BMI >25 kg/m2 (9). Also, Andersson et al. (40) did not find a significant effect of whole grain intake on insulin sensitivity, lipid peroxidation, inflammatory markers, or on any of the metabolic variables studied. However, a 6 week intervention might be too short to achieve significant effects on insulin sensitivity (40). We believe that the disparity in these findings from the previous observational studies and the present intervention study is caused by variations in subjects’ health characteristics, age, gender, and ethnicity, as well as the type of food, its structure, amount, and preparation. These are all important factors when interpreting metabolic responses, or lack of responses, to whole grain foods (41).
In summary, the present study showed significant beneficial effects from the substitution of whole grains and legumes for refined rice on circulating Lp-PLA2 activity, enzyme activity in PBMCs, plasma ox-LDL, and LDL particle size in patients with IFG, IGT, or newly-diagnosed T2D. These effects are likely to substantially reduce the risk factors of CVD in patients with IFG, IGT, or newly-diagnosed T2D. The biological mechanism whereby whole grains and legumes may exert their reducing effects on circulating and PBMC Lp-PLA2 is not clear in the present study, but it is likely to be due to multiple mechanisms, such as substantial improvement of glucose and insulin metabolism, increased consumption of protein relative to carbohydrates, and antioxidant-reduced lipid peroxides including ox-LDL. Therefore, grains should be consumed in a minimally refined form, and frequent consumption of vegetables and legumes should be recommended to reduce cardiovascular risk factors in patients with IFG, IGT, or newly-diagnosed T2D.
Acknowledgments
The authors thank the research volunteers who participated in the studies described in this article.
Footnotes
Abbreviations:
- BP
- blood pressure
- Δ
- change
- 8-epi-PGF2α
- 8-epi-prostaglandin F2α
- HbA1c
- hemoglobin A1c
- HOMA-IR
- homeostasis model assessment-insulin resistance
- hs-CRP
- high-sensitivity C reactive protein
- IFG
- impaired fasting glucose
- IGT
- impaired glucose tolerance
- IL
- interleukin
- Lp-PLA2
- lipoprotein-associated phospholipase A2
- MDA
- malondialdehyde
- ox-LDL
- oxidized LDL
- PBMC
- peripheral blood mononuclear cell
- sPLA2
- secretory phospholipase A2
- WHR
- waist and hip ratio
This research was supported by the Bio and Medical Technology Development Program (2006-2005306 and 2012M3A9C4048762) and Mid-Career Researcher Program (2010-0015017) of the National Research Foundation (NRF) funded by the Ministry of Science, ICT, and Future Planning, Republic of Korea). The authors declare no financial conflicts of interest.
REFERENCES
- 1.Nelson T. L., Kamineni A., Psaty B., Cushman M., Jenny N. S., Hokanson J., Furberg C., Mukamal K. J. 2011. Lipoprotein-associated phospholipase A2 and future risk of subclinical disease and cardiovascular events in individuals with type 2 diabetes: the Cardiovascular Health Study. Diabetologia. 54: 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serban M., Tanaseanu C., Kosaka T., Vidulescu C., Stoian I., Marta D. S., Tanaseanu S., Moldoveanu E. 2002. Significance of platelet-activating factor acetylhydrolase in patients with non-insulin-dependent (type2) diabetes mellitus. J. Cell. Mol. Med. 6: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ha C. Y., Kim J. Y., Paik J. K., Kim O. Y., Lee E. J., Lee J. H. 2012. The association of specific metabolites of lipid metabolism with markers of oxidative stress, inflammation and arterial stiffness in men with newly diagnosed type 2 diabetes. Clin. Endocrinol. (Oxf.). 76: 674–682. [DOI] [PubMed] [Google Scholar]
- 4.Nelson T. L., Biggs M. L., Kizer J. R., Cushman M., Hokanson J. E., Furberq C. D., Mukamal K. J. 2012. Lipoprotein-associated phospholipase A2 (Lp-PLA2) and future risk of type 2 diabetes: results from the cardiovascular health study. J. Clin. Endocrinol. Metab. 97: 1695–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatoum I. J., Nelson J. J., Cook N. R., Hu F. B., Rimm E. B. 2010. Dietary, lifestyle, and clinical predictors of lipoprotein-associated phospholipase A2 activity in individuals without coronary artery disease. Am. J. Clin. Nutr. 91: 786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzotzas T., Filippatos T. D., Triantos A., Bruckert E., Tselepis A. D., Kiortsis D. N. 2008. Effects of a low-calorie diet associated with weight loss on lipoprotein-associated phospholipase A2 (Lp-PLA2) activity in healthy obese women. Nutr. Metab. Cardiovasc. Dis. 18: 477–482. [DOI] [PubMed] [Google Scholar]
- 7.Jang Y., Lee J. H., Kim O. Y., Park H. Y., Lee S. Y. 2001. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arterioscler. Thromb. Vasc. Biol. 21: 2065–2071. [DOI] [PubMed] [Google Scholar]
- 8.Chung H. K., Chae J. S., Hyun Y. J., Paik J. K., Kim J. Y., Jang Y., Kwon H. M., Song Y. D., Lee H. C., Lee J. H. 2009. Influence of adiponectin gene polymorphisms on adiponectin level and insulin resistance index in response to dietary intervention in overweight-obese patients with impaired fasting glucose or newly diagnosed type 2 diabetes. Diabetes Care. 32: 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brownlee I. A., Moore C., Chatfield M., Richardson D. P., Ashby P., Kuznesof S. A., Jebb S. A., Seal C. J. 2010. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br. J. Nutr. 104: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christian J. L., Greger J. L. 1994. Energy sources and uses. In Nutrition for Living. 4th edition. Benjamin/Cummings Publishing Co., Redwood City, CA. 242–266. [Google Scholar]
- 11.Butte N. F., Caballero B. 2006. Energy needs: assessment and requirements. In Modern Nutrition in Health and Disease. 10th edition. M. E. Shils, M. Shike, A. C. Ross, et al., editors. Lippincott Williams & Wilkins, Philadelphia, PA. 136–148. [Google Scholar]
- 12.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 13.Rachoń D., Mysliwśka J., Suchecka-Rachoń K., Wieckiewicz J., Myśliwski A. 2002. Effects of oestrogen deprivation on interleukin-6 production by peripheral blood mononuclear cells of postmenopausal women. J. Endocrinol. 172: 387–395. [DOI] [PubMed] [Google Scholar]
- 14.von Haehling S., Genth-Zotz S., Sharma R., Bolger A. P., Doehner W., Barnes P. J., Coats A. J., Anker S. D. 2003. The relationship between age and production of tumor necrosis factor-alpha in healthy volunteers and patients with chronic heart failure. Int. J. Cardiol. 90: 197–204. [DOI] [PubMed] [Google Scholar]
- 15.Kim J. Y., Hyun Y. J., Jang Y., Lee B. K., Chae J. S., Kim S. E., Yeo H. Y., Jeong T. S., Jeon D. W., Lee J. H. 2008. Lipoprotein-associated phospholipase A2 activity is associated with coronary artery disease and markers of oxidative stress: a case-control study. Am. J. Clin. Nutr. 88: 630–637. [DOI] [PubMed] [Google Scholar]
- 16.Zalewski A., Macphee C. 2005. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler. Thromb. Vasc. Biol. 25: 923–931. [DOI] [PubMed] [Google Scholar]
- 17.Caslake M. J., Packard C. J. 2003. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr. Opin. Lipidol. 14: 347–352. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-Quesada J. L., Vinagre I., de Juan-Franco E., Sánchez-Hernández J., Blanco-Vaca F., Ordóñez-Llanos J., Pérez A. 2012. Effect of improving glycemic control in patients with type 2 diabetes mellitus on low-density lipoprotein size, electronegative low-density lipoprotein and lipoprotein-associated phospholipase A2 distribution. Am. J. Cardiol. 110: 67–71. [DOI] [PubMed] [Google Scholar]
- 19.Gazi I., Lourida E. S., Filippatos T., Tsimihodimos V., Elisaf M., Tselepis A. D. 2005. Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. Clin. Chem. 51: 2264–2273. [DOI] [PubMed] [Google Scholar]
- 20.Tomé-Carneiro J., Larrosa M., Yáñez-Gascón M. J., Dávalos A., Gil-Zamorano J., Gonzálvez M., Garcia-Almagro F. J., Ruiz Ros J. A., Tomás-Barberán F. A., Espin J. C., et al. 2013. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 72: 69–82. [DOI] [PubMed] [Google Scholar]
- 21.Wang W. Y., Li J., Yang D., Xu W., Zha R. P., Wang Y. P. 2010. OxLDL stimulated lipoprotein-associated phospholipase A2 expression in THP-1 monocytes via PI3K and p38 MAPK pathways. Cardiovasc. Res. 85: 845–852. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y., Zhang P., Zhang L., Osman H., Mohler E. R., III, Macphee C., Zalewski A., Postle A., Wilensky R. L. 2007. Role of lipoprotein-associated phospholipase A2 in leukocyte activation and inflammatory response. Atherosclerosis. 191: 54–62. [DOI] [PubMed] [Google Scholar]
- 23.Carpenter K. L., Dennis I. F., Challis I. R., Osborn D. P., Macphee C. H., Leake D. S., Arends M. J., Mitchinson M. J. 2001. Inhibition of lipoprotein-associated phospholipase A2 diminishes the death inducing effects of oxidized LDL on human monocyte-macrophages. FEBS Lett. 505: 357–363. [DOI] [PubMed] [Google Scholar]
- 24.Steinbrecher U. P., Pritchard P. H. 1989. Hydrolysis of phosphatidylcholine during LDL oxidation is mediated by platelet-activating factor acetylhydrolase. J. Lipid Res. 30: 305–315. [PubMed] [Google Scholar]
- 25.Stafforini D. M., Sheller J. R., Blackwell T. S., Sapirstein A., Yull F. E., Mclntyre T. M., Bonventre J. V., Prescott S. M., Roberts L. J., II 2006. Release of free F2-isoprostanes from esterified phospholipids is catalyzed by intracellular and plasma platelet-activating factor acetylhydrolases. J. Biol. Chem. 281: 4616–4623. [DOI] [PubMed] [Google Scholar]
- 26.Kono N., Inoue T., Yoshida Y., Sato H., Matsusue T., Itabe H., Niki E., Aoki J., Arai H. 2008. Protection against oxidative stress-induced hepatic injury by intracellular type II platelet-activating factor acetylhydrolase by metabolism of oxidized phospholipids in vivo. J. Biol. Chem. 283: 1628–1636. [DOI] [PubMed] [Google Scholar]
- 27.Kim J. Y., Park J. Y., Kim O. Y., Ham B. M., Kim H. J., Kwon D. Y., Jang Y., Lee J. H. 2010. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS). J. Proteome Res. 9: 4368–4375. [DOI] [PubMed] [Google Scholar]
- 28.Kwak J. H., Paik J. K., Kim H. I., Kim O. Y., Shin D. Y., Kim H. J., Lee H. J., Lee J. H. 2012. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis. 224: 457–464. [DOI] [PubMed] [Google Scholar]
- 29.Belobrajdic D. P., Bird A. R. 2013. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr. J. 12: 62–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S., Manson J. E., Lee I. M., Cole S. R., Henneckens C. H., Willett W. C., Buring J. E. 2000. Fruit and vegetable intake and risk of cardiovascular disease: the Women`s Health Study. Am. J. Clin. Nutr. 72: 922–928. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs D. R., Jr, Andersen L. F., Blomhoff R. 2007. Whole-grain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory disease in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 85: 1606–1614. [DOI] [PubMed] [Google Scholar]
- 32.Crujeiras A. B., Parra D., Abete I., Martinez J. A. 2007. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic. Res. 41: 498–506. [DOI] [PubMed] [Google Scholar]
- 33.De Keyzer D., Karabina S. A., Wei W., Geeraert B., Stengel D., Marsillach J., Camps J., Holvoet P., Ninio E. 2009. Increased PAFAH and oxidized lipids are associated with inflammation and atherosclerosis in hypercholesterolemic pigs. Arterioscler. Thromb. Vasc. Biol. 29: 2041–2046. [DOI] [PubMed] [Google Scholar]
- 34.Anderson J. L. 2008. Lipoprotein-associated phospholipase A2: an independent predictor of coronary artery disease events in primary and secondary prevention. Am. J. Cardiol. 101: 23F–33F. [DOI] [PubMed] [Google Scholar]
- 35.Tsimikas S., Willeit J., Knoflach M., Mayr M., Egger G., Notdurfter M., Witztum J. L., Wiedermann C. J., Xu Q., Kiechl S. 2009. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur. Heart J. 30: 107–115. [DOI] [PubMed] [Google Scholar]
- 36.Lp-PLA(2) Studies Collaboration, Thompson A., Gao P., Orfei L., Watson S., Di E., Kaptoge S., Ballantyne C., Cannon C. P., Criqui M., Cushman M., et al. 2010. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 375: 1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sabatine M. S., Morrow D. A., O’Donoghue M., Jablonksi K. A., Rice M. M., Solomon S., Rosenberg Y., Domanski M. J., Hsia J.; PEACE Investigators. 2007. Prognostic utility of lipoprotein-associated phospholipase A2 for cardiovascular outcomes in patients with stable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 27: 2463–2469. [DOI] [PubMed] [Google Scholar]
- 38.Holmes M. V., Simon T., Exeter H. J., Folkersen L., Asselbergs F. W., Guardiola M., Cooper J.A., Palmen J., Hubacek J.A., Carruthers K.F., et al. 2013. Secretory phospholipase A(2)-IIA and cardiovascular disease: a mendelian randomization study. J. Am. Coll. Cardiol. 62: 1966–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicholls S. J., Kastelein J. J., Schwartz G. G., Bash D., Rosenson R. S., Cavender M. A., Brennan D. M., Koenig W., Jukema J. W., Nambi V., et al. , VISTA-16 Investigators. 2014. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 311: 252–262. [DOI] [PubMed] [Google Scholar]
- 40.Andersson A., Tengblad S., Karlström B., Kamal-Eldin A., Landberg R., Basu S., Aman P., Vessby B. 2007. Whole-grain foods do not affect insulin sensitivity or markers of lipid peroxidation and inflammation in healthy, moderately overweight subjects. J. Nutr. 137: 1401–1407. [DOI] [PubMed] [Google Scholar]
- 41.Hallfrisch J., Facn, Behall K. M. 2000. Mechanisms of the effects of grains on insulin and glucose responses. J. Am. Coll. Nutr. 19: 320S–325S. [DOI] [PubMed] [Google Scholar]