Abstract
OBJECTIVE
We investigated the quality of care and factors associated with variations in care among a national cohort of Medicaid enrollees with incident lupus nephritis.
METHODS
Using Medicaid Analytic eXtract (MAX) files from 47 U.S. states and D.C. for 2000–2006, we identified a cohort of individuals with incident lupus nephritis. We assessed performance on three measures of health care quality: receipt of immunosuppressive, renal-protective anti-hypertensive, and anti-malarial medications. We examined performance on these measures over one year, and applied multivariable logistic regression models to understand whether sociodemographic, geographic or health care access factors were associated with higher performance on quality measures.
RESULTS
We identified 1711 Medicaid enrollees with incident lupus nephritis. Performance on quality measures was low at 90 days (21.9% for immunosuppressive therapy, 44.0% for renal protection and 36.4% for anti-malarials), but increased by one year (33.7%, 56.4%, and 45.8%, respectively). Younger individuals, Blacks and Hispanics were more likely to receive immunosuppressive therapy and hydroxychloroquine. Younger individuals were less likely to receive renal-protective anti-hypertensive medications. We found significant geographic variation in performance, with patients in the Northeast receiving higher quality of care compared to other regions. Poor access to health care, as assessed by having a greater number of treat-and-release emergency departments visits compared to ambulatory encounters, was associated with lower receipt of recommended treatment.
CONCLUSION
These nationwide data suggest low overall quality of care and potential delays in care for Medicaid enrollees with incident lupus nephritis. Significant regional differences also suggest room for quality improvement.
Lupus nephritis occurs in a substantial proportion of individuals with systemic lupus erythematosus (SLE), ranging from 25–75% in published studies [1–5]. Up to one-fifth of patients progress to end-stage renal disease (ESRD), and poor outcomes occur most frequently among racial/ethnic minorities and those with low socioeconomic status [6–9]. Although these health disparities are well-documented, their underlying mechanisms remain unclear. Differences in underlying disease severity, treatment resistance, or failure to receive timely or appropriate therapy may play a role. However, this latter aspect, which relates to the quality of care for lupus nephritis, has not been adequately studied.
Tools to measure health care quality in SLE are now available to address these gaps in our understanding of lupus care. The SLE Quality Indicators Project used a combination of scientific evidence reviews and expert consensus methodology to arrive at concepts representing high quality care for SLE [10]. Indicators related to lupus nephritis included use of appropriate immunosuppressive therapy within one month of lupus nephritis diagnosis and use of renal protective anti-hypertensive medications. Recent American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) Guidelines for lupus nephritis recommend use of anti-malarial medications in all patients with lupus nephritis [11, 12]. While no previous studies have directly applied these process measures, indirect evidence suggests uneven quality of care for lupus nephritis in the United States. For example, ESRD due to lupus nephritis is increased in areas with higher rates of hospitalizations for ambulatory-care sensitive conditions, a marker of poor access to ambulatory care [13]. In addition, studies have demonstrated that individuals with public insurance or no medical insurance develop ESRD at younger ages, a finding that suggests that limited access to care may shorten the time to ESRD [14].
Until recently, nationwide data were not available to more directly assess health care quality for lupus nephritis. In this study, we for the first time apply quality measures to a national sample of Medicaid enrollees with incident lupus nephritis. By applying explicit process measures, we sought to investigate quality of care for lupus nephritis in this vulnerable population and to understand factors associated with higher quality.
METHODS
Study Population and Data Sources
We assembled a national cohort of individuals with incident nephritis enrolled in the Medicaid program. Medicaid is the United States health insurance program for individuals with low income and resources (low income children, pregnant women, mothers and people with disabilities) and provides coverage for medical expenses and prescription drugs. Medicaid is jointly funded by the state and federal governments, but is managed by the states.
We used Medicaid Analytic eXtract (MAX) administrative data from the period 2000–2006. MAX files contain patient-level billing claims, pharmacy utilization and demographic information for Medicaid enrollees in 47 states and the District of Columbia (three states, Arizona, Tennessee and Maine, did not have data available). MAX data were linked to US Census Data from the year 2000 to assess the area-level socioeconomic status of the enrollees’ geographical location.
We included all adults, aged 18 to 65 years, who met our case definition of incident lupus nephritis, which is based largely on a published algorithm validated for use in administrative data [15]. The algorithm combines ICD-9 and encounter codes to identify lupus nephritis with a moderate to high positive predictive value (80%, 95% CI 75–85%). Our definition algorithm, which built on this definition, requires the following: 1) ≥ 3 International Classification of Diseases, ninth revision (ICD-9) codes for SLE (710.0) from hospital discharge diagnoses or physician visit claims, each at least 60 days apart; 2) lupus nephritis as defined ≥ 2 ICD-9 hospital discharge diagnoses or physician billing claims for nephritis (ICD-9 codes 580–588), or proteinuria (ICD-9 code 791.0), on or after the SLE diagnosis, and at least 30 days apart, and 3) a minimum of 24 months of Medicaid enrollment without claims for the above codes for nephritis, proteinuria or renal failure and without ambulatory claims for hemodialysis, peritoneal dialysis or renal transplantation, to define the incident disease cohort. Individuals were therefore eligible for inclusion in the denominator once they had 24 months of Medicaid enrollment with no lupus nephritis-related claims.
We excluded individuals with ICD-9 codes indicating pregnancy during the measurement period (V22, 630–679, or 761.5; N=367), as well as the human immunodeficiency virus or acquired immunodeficiency syndrome (ICD9 042, V08, 795.71, N=33). These exclusions are consistent with the National Committee for Quality Assurance’s denominator exclusion for the use of immunosuppressive therapies in rheumatoid arthritis [16].
Quality Measures
We applied three quality measures for incident lupus nephritis to the denominator population with incident lupus nephritis. We examined performance on each measure at 3 time points, 30, 90 and 365 days after each patient met the case definition of incident lupus nephritis to assess whether performance improved with time. These time points were chosen for two reasons. First, the SLE QI project expert panels recommended institution of therapy for lupus nephritis within 30 days of diagnosis, and we therefore used the 30-day time period to be consistent with these recommendations [10]. However, previous analogous measures using administrative data to evaluate quality in rheumatic diseases (i.e. disease-modifying anti-rheumatic drug use in rheumatoid arthritis) have used a one-year period [17]. Our time points were chosen to reflect this range. Second, by choosing different time points, we hoped to evaluate whether there were delays in care for patients with incident disease.
Dispensation of at least one prescription for each drug was required in the preceding time period to pass each measure.
Measure 1
Percent of patients administered or dispensed an immunosuppressive agent (mycophenolate mofetil, mycophenolic acid, oral or intravenous cyclophosphamide, azathioprine, cyclosporine or tacrolimus). This measure was adapted from the SLE Quality Indicators Project [10]. Rituximab was not included because we found no patients in our denominator population that had received the drug.
Measure 2
Percent of patients dispensed an angiotensin-converting enzyme inhibitor (ACE) or angiotensin-receptor blocker (ARB). This measure was also adapted from the SLE Quality Indicators Project [10].
Measure 3
Percent of patients dispensed an anti-malarial drug (hydroxychloroquine or chloroquine). This measure is based on ACR and EULAR Guidelines for the treatment of lupus nephritis [11, 12].
Additional Variables
We extracted demographic data for all Medicaid enrollees including age, sex, and race/ethnicity (White, Black, African-American, Hispanic or Latino, Asian, Native American and Other). We determined location of residence by ZIP code and U.S. Census region (Northeast, Midwest, South or West). From the 2000 U.S. Census [18], we identified seven socioeconomic indicators at the ZIP code level: median household income, proportion with income below 200% of the federal poverty level, median home value, median monthly rent, mean education level, proportion of people age >25 who were college graduates, and proportion of employed persons with a professional occupation [19]. These were combined using a previously developed method into socioeconomic status quartiles [19].
Geographic location was categorized using the major census regions (Midwest, Northeast, South, West). In addition, to understand whether patients had adequate access to appropriate ambulatory care, we analyzed both acute care and ambulatory utilization claims. If ≥ 50% of these encounters were treat-and-release emergency department visits after the diagnosis of incident lupus nephritis and before the end of our study period in 2006, then the patient’s most common source of care was classified as the emergency department rather than the ambulatory setting.
Analysis
For all Medicaid enrollees with incident lupus nephritis during the study period, we calculated performance on each of the three quality measures, defined as the percentage of eligible enrollees who received the specified service. For each measure, multivariable logistic regression models were used to examine the factors associated with receipt of care. Models were adjusted for age, sex, race/ethnicity, geographic location, socioeconomic status quartiles, and most common source of care.
We calculated the adjusted group percentages and 95% confidence intervals from the predicted marginals derived from our model. We tested for interactions among variables of interest, including between geographic region and whether the most common source of care was the emergency department.
We performed several sensitivity analyses to test the robustness of our findings. Due to non-itemized hospital billing, the inpatient administration of medications, including intravenous cyclophosphamide, was not captured in our data. Thus, we calculated performance after excluding patients with a hospitalization with discharge diagnoses that included any code for renal disease in the period preceding the three time points examined. Additionally, we determined whether individuals not receiving immunosuppressive therapy consistent with quality measure 1 (immunosuppressive treatment) instead received therapy with glucocorticoids alone. This is important since patients receiving glucocorticoid monotherapy are likely receiving therapy for active disease, but with a regimen not recommended by ACR and European guidelines, which instead recommend treatment that includes immunosuppressive medications [11, 12, 20]. We included all oral and intravenous formulations of glucocorticoids, including prednisone, methylprednisolone, dexamethasone, hydrocortisone, prednisolone, and cortisone), and calculated the cumulative dose for those receiving any of these medications in the 30 days preceding each of three time points (30, 90, 365 days).
Finally, we explored the robustness of our findings to our definition of incident lupus nephritis. We altered our look-back period of 24 months with no claims for lupus nephritis to 12 months. In addition, we evaluated whether limiting the analysis to only those individuals with codes for acute renal disease, including glomerulonephritis, acute renal failure, proteinuria (represented by the subset of ICD-9 codes including 580, 580.9, 581, 581.9, 582, 582.9, 583, 584, 584.5, 791, 586) in the validated lupus nephritis algorithm would influence our findings.
All analyses were conducted in SAS, (Version 9.2, Cary, NC). Data were obtained from the Center for Medicare and Medicaid Services (CMS) through an approved data use agreement and are presented in accordance with their policies. The Institutional Review Board of Partners Healthcare waived human subjects approval for this study.
RESULTS
From 2000–2006, we found that 1711 Medicaid beneficiaries in the United States met our definition of incident lupus nephritis. Many (75%) of these individuals were younger than 50 years old, female (92%), Black (49%) or from other racial/ethnic minorities (25%), and resided in the U.S. South (36%). The emergency department was the most common source of care for 13% of patients (Table 1).
Table 1.
Characteristics of Medicaid recipients with incident lupus nephritis between 2000–2006 in the United States.
| N=1711 n (%) |
||
|---|---|---|
|
| ||
| Age at first encounter, y | ||
| 18–34 | 593 (34.7) | |
| 35–50 | 682 (39.9) | |
| 51–64 | 436 (25.5) | |
|
| ||
| Sex | ||
| Female | 1582 (92.5) | |
|
| ||
| Race/ethnicity | ||
| White | 456 (26.7) | |
| Black | 831 (48.6) | |
| Hispanic | 239 (14.0) | |
| Asian | 97 (5.7) | |
| Native | 21 (1.2) | |
| Other | 67 (3.9) | |
|
| ||
| Socioeconomic Status | ||
| Quartile 1, lowest | 90 (5.3) | |
| Quartile 2 | 152 (8.9) | |
| Quartile 3 | 312 (18.2) | |
| Quartile 4, highest | 1149 (67.2) | |
|
| ||
| Geographic Division | ||
| Midwest | 364 (21.3) | |
| Northeast | 336 (19.6) | |
| South | 613 (35.8) | |
| West | 398 (23.3) | |
|
| ||
| Most common source of care | ||
| Emergency department* | 221 (12.9) | |
| Ambulatory setting | 1490 (87.1) | |
Most common source of care was considered the emergency department if ≥ 50% of total visits over study period were treat-and-release emergency department visits.
Performance on quality measures was low at 30 days, but rose by 90 days, and again by 365 days (Table 2), with one-third of patients receiving immunosuppressive therapy by one year. Over half received therapy with ACE inhibition or ARBs, while 46% received therapy with anti-malarials by one year.
Table 2.
Performance on quality measures for Medicaid recipients with incident lupus nephritis between 2000–2006 in the United States.
| Quality measure* | Pass rate Day 30, (n=1734) | Pass rate Day 90 (n=1723) | Pass rate Day 365 (n=1710) |
|---|---|---|---|
| 1. Percent of patients receiving an immunosuppressant§ (i.e. mycophenolate mofetil, oral or intravenous cyclophosphamide, azathioprine, cyclosporine, tacrolimus) | 13.0% | 21.9% | 33.7% |
| 2. Percent of patients receiving an ACE inhibitor or ARB | 29.2% | 44.0% | 56.4% |
| 3. Percent of patients receiving anti-malarial therapy (hydroxychloroquine, chloroquine) | 24.7% | 36.4% | 45.8% |
ACE=angiotensin converting enzyme inhibitor, ARB=angiotensin receptor blocker.
Denominator Inclusions: Patients with at least three ICD-9 codes for systemic lupus erythematosus AND at least two encounter codes for renal disease. Denominator exclusions: Patients with pregnancy during the measurement year, and human immunodeficiency virus infection.
Excludes glucocorticoids alone during the measurement period.
Unadjusted analyses in which the outcome was performance on quality measures at one year yielded similar findings to the multivariable adjusted analysis, and we therefore present adjusted analyses in Table 3. We found that while younger patients were more likely to receive immunosuppressive and anti-malarial drugs (OR 5.8, 95% CI 4.2–7.9 for patients 18–34 years compared to those 51–64 years), they were less likely to receive ACE inhibitors or ARBs (OR 0.8, 95% CI 0.6–1.0 for those 35–50 years compared to 51–64 years). Blacks, Hispanics and Asians were more likely to receive immunosuppressive therapy compared to Whites. Blacks and Hispanics were more likely to receive ACE inhibitors or ARBs than Whites (OR 1.8, 95% CI 1.4–2.3 and OR 1.8, 95% CI 1.3–2.5, respectively). There were no consistent significant effects of either sex or socioeconomic status.
Table 3.
Adjusted performance and predictors of higher performance on three quality measures in the US Medicaid population with incident lupus nephritis at one year.
| Quality measure 1: Immunosuppressant OR (95% CI) | Quality measure 2: ACE/ARB OR (95% CI) | Quality measure 3: Hydroxychloroquine OR (95% CI) | |
|---|---|---|---|
|
| |||
| Age at first encounter | |||
| 18–34 years | 5.8 (4.2–7.9) | 0.9 (0.7–1.1) | 2.3 (1.7–2.9) |
| 35–50 years | 2.2 (1.6–3.0) | 0.8 (0.6–1.0) | 1.4 (1.1–1.8) |
| 51–64 years | -- | -- | -- |
|
| |||
| Sex | |||
| Male | 1.0 (0.7–1.5) | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) |
|
| |||
| Race/ethnicity | |||
| White | -- | -- | -- |
| Black | 1.4 (1.1–1.9) | 1.8 (1.4–2.3) | 1.4 (1.1–1.8) |
| Hispanic | 1.6 (1.1–2.3) | 1.8 (1.3–2.5) | 1.4 (1.0–2.0) |
| Asian | 2.2 (1.3–3.6) | 1.6 (1.0–2.6) | 0.9 (0.6–1.5) |
| Native | 1.0 (0.3–3.0) | 1.2 (0.5–3.0) | 0.5 (0.2–1.5) |
| Other | 1.5 (0.9–2.8) | 1.5 (0.9–2.6) | 1.4 (0.8–2.5) |
|
| |||
| Socioeconomic Status | |||
| Quartile 1, lowest | 1.4 (0.8–2.3) | 1.1 (0.7–1.8) | 1.1 (0.7–1.9) |
| Quartile 2 | 0.8 (0.5–1.3) | 0.8 (0.6–1.2) | 0.9 (0.6–1.4) |
| Quartile 3 | 0.9 (0.6–1.2) | 0.7 (0.5–0.9) | 1.3 (1.0–1.7) |
| Quartile 4, highest | -- | -- | -- |
|
| |||
| Geographic Division | |||
| Midwest | 0.5 (0.3–0.7) | 0.5 (0.3–0.7) | 0.4 (0.3–0.6) |
| Northeast | -- | -- | -- |
| South | 0.6 (0.4–0.8) | 1.0 (0.8–1.4) | 0.8 (0.6–1.1) |
| West | 0.7 (0.5–1.0) | 0.7 (0.5–0.9) | 0.9 (0.6–1.2) |
|
| |||
| Most common source of care | |||
| Emergency department* | 0.5 (0.3–0.7) | 0.8 (0.6–1.0) | 0.5 (0.3–0.6) |
| Ambulatory setting | -- | -- | -- |
Most common source of care was considered the emergency department if ≥ 50% of total visits were treat-and-release emergency department visits.
We found significant geographic variation in care, with those living in the U.S. South being less likely to receive immunosuppressive therapy (OR 0.6, 95% CI 0.4–0.8) compared to those living in the Northeast. Individuals living in the Midwest were less likely to receive all three of the quality measures examined (for immunosuppressive therapy OR 0.5, 95% CI 0.3–0.7, for ACE/ARB OR 0.5, 95% CI 0.3–0.6, for anti-malarials OR 0.4, 95% CI 0.3–0.6, compared to the Northeast). Those in the West were less likely to receive ACE/ARBs (OR 0.7, 95% CI 0.5–0.9 compared to the Northeast).
Using the emergency department as the most common source of care was associated with lower receipt of recommended treatment (OR 0.5 (95% CI 0.3–0.7) for immunosuppressive therapy, OR 0.8 (95% CI 0.6–1.0) for ACE/ARB use, and OR 0.5 (95% CI 0.3–0.6) for anti-malarial use.
We did not find a statistically significant interaction between geographic region and using the ED as the most common source of care. In Figure 1, we depict the adjusted percentage of patients receiving immunosuppressive therapy by 365 days, by geographic region and whether the emergency department was the most common source of care.
Figure 1.
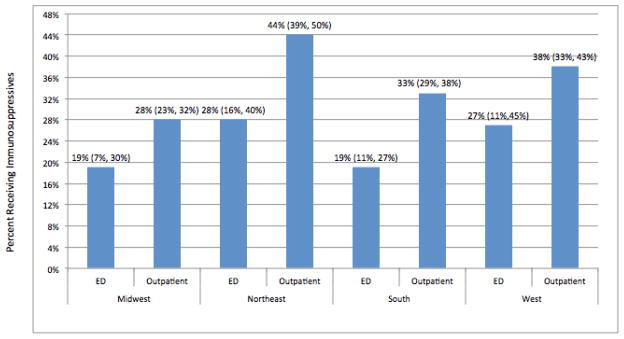
Adjusted percentage of lupus nephritis patients receiving immunosuppressive therapy at 365 days, by region and whether emergency department was the most common source of care.
Immunosuppressive therapy users were identified as those patients with one of the following drugs administered or dispensed by 365 days after onset of lupus nephritis: mycophenolate mofetil, oral or intravenous cyclophosphamide, azathioprine, cyclosporine, or tacrolimus. Results reflect the adjusted group percentages and 95% confidence intervals from the predicted marginals derived from our regression model, which adjusted for age, sex, race/ethnicity, geographic region and most common source of care.
In sensitivity analyses, excluding individuals with hospitalizations preceding the time points examined did not impact our results for quality measure 1; performance remained low (13.6% at 30 days for N=1159; 20.7% at 90 days for N=1086; 30.8% at 365 days for N=947. When examining the robustness of our findings to our definition of incident lupus nephritis, altering the look-back period from 24 months to 12 months of no claims for lupus nephritis did not change our results. For example, at 365 days, we found virtually identical pass rates among the 2723 patients in the revised denominator (34.4% for immunosuppressive use, 56% for ACE/ARB use, and 46% for anti-malarial use). Similarly, excluding individuals with chronic renal disease ICD-9 codes but no acute glomerulonephritis codes (n=54) did not change our findings.
Finally, we found that among individuals with incident lupus nephritis who did not receive immunosuppressive therapy, over one-third received glucocorticoids at relatively high cumulative 30-day doses (Table 4). Table 4 also illustrates that the median and interquartile range cumulative glucocorticoid dose over the preceding thirty days was slightly higher in the group of patients receiving immunosuppressive therapy at all three time points compared to the group receiving glucocorticoid monotherapy.
Table 4.
Median glucocorticoid dose at 30, 60 and 365 days for Medicaid recipients with incident lupus nephritis between 2000–2006, by receipt of immunosuppressive therapy.
| Cumulative median prednisone dose (intra-quartile range) in previous 30 day period | |||
|---|---|---|---|
| Day 30 | Day 90 | Day 365 | |
| Glucocorticoid monotherapy* users | 330 mg (135–859 mg) N=548 |
350 mg (150–780 mg) N=561 |
280 mg (140–550 mg) N=302 |
| Immunosuppressant therapy users§ | 400 mg (145–1040 mg) N=151 |
410 mg (207–866 mg) n=263 |
300 mg (155–560 mg) n=269 |
Patients who had no dispensed immunosuppressant (i.e. mycophenolate mofetil, oral or intravenous cyclophosphamide, azathioprine, cyclosporine, tacrolimus) during the measurement year.
Patients with at least one dispensed immunosuppressant (i.e. mycophenolate mofetil, oral or intravenous cyclophosphamide, azathioprine, cyclosporine, tacrolimus).
Discussion
In this population-based study of Medicaid enrollees with incident lupus nephritis between 2000–2006, we found low performance on three measures of health care quality. Our data suggest delays in care for a significant proportion of patients, since performance on quality measures was low at 30 days but rose substantially by one year. Moreover, we found geographic variation in care, with the Northeast outperforming other regions on the measures examined. Our data also suggest that over 1 in 8 individuals with incident lupus nephritis used the emergency department as the most common source of care, and quality of care was lower for this group of patients.
This is the first study to apply explicit process measures to understand quality of care in a national sample of patients with SLE. The study examined a racial/ethnically diverse group of low-income individuals, a population that bears a high burden of lupus nephritis and is more likely to develop ESRD [2, 21]. We found that younger individuals and Black and Hispanic patients were more likely to receive care consistent with the quality measures, which is reassuring given that these groups have more severe forms of lupus nephritis [6, 22, 23]. Similarly, use of antihypertensive agents was higher among Blacks and older patients compared to younger patients, likely reflecting the higher prevalence of hypertension in these groups [23, 24]. However, it is important to note that the quality measures examined in this study apply to all patients with lupus nephritis. Therefore, our results suggest that younger individuals may be under-treated with anti-proteinuric drugs, and older individuals may be under-treated with immunosuppressant drugs.
We did not find significant variation in care according to the patients’ area-level socioeconomic status, implying that area-level socioeconomic status has no independent effect on receipt of quality measures above personal poverty, a requirement for Medicaid eligibility. Previous studies have documented disparities in health outcomes for low-income populations with lupus nephritis [2, 14, 25], but the mechanisms of these disparities remain unclear. By demonstrating delays in appropriate therapy as well as substantial variation in care, our data suggest that poor quality of care may be one important mechanism for these disparities and warrants further investigation and attention.
We found that a substantial proportion of Medicaid recipients with SLE had a greater number of treat-and-release emergency room visits than ambulatory visits, suggesting inadequate access to outpatient care or poor care transitions between health care settings. This finding is consistent with previous studies reporting that Medicaid beneficiaries with SLE may face problems with health care access. For example, Medicaid beneficiaries travel significantly longer distances to see a physician for SLE, suggesting geographic barriers to care [26]. Similarly, the incidence of ESRD due to SLE is higher in areas with higher proportions of residents who have public insurance and higher rates of avoidable hospitalizations, suggesting that limited access to care may contribute to poor renal outcomes in SLE [14]. Among patients with lupus nephritis who develop ESRD, those with private medical insurance are also older when they begin ESRD treatment than those with Medicaid or no insurance. The fact that progression to ESRD varies with medical insurance status may also signal differences in quality or access to care [19]. Taken together, these data suggest that greater attention to coordinating care and increasing access to appropriate ambulatory specialty care for individuals with this complex disease is needed.
The quality measures examined in our study are supported by professional consensus as well as clinical evidence. Over the last several decades, landmark clinical trials and subsequent meta-analyses in lupus nephritis have demonstrated that induction therapy with immunosuppressive medications improves patient outcomes, including reducing renal damage and increasing renal survival [27]. Moreover, anti-malarial drugs are recommended as background therapy in all patients with lupus nephritis. This is based on a randomized controlled trial showing that flare rates are lower for patients continuing hydroxychloroquine compared to those who switched to placebo, as well as accumulating observational studies showing lower damage accrual, lower mortality, and reduced risk of thrombosis [28–32]. Finally, treatment with either angiotensin-converting enzyme (ACE inhibitors or angiotensin receptor blockers (ARBs) is recommended in all patients with significant proteinuria [11]. This recommendation is based on the fact these drugs reduce proteinuria by approximately one-third and delay doubling of serum creatinine and progression to end-stage renal disease in patients with non-diabetic chronic renal disease [33]. The quality measures in this study therefore represent processes of care that current scientific evidence suggests can significantly influence clinical outcomes, including preventing or delaying ESRD. Unwarranted variations in performance and delays in care therefore signal room for quality improvement.
Although no previous studies have specifically applied quality measures to a population with incident lupus nephritis, our data on rates of hydroxychloroquine and anti-proteinuric therapy use among Medicaid enrollees are similar to previously reported rates in clinical cohorts. For example, in a longitudinal cohort study of 881 individuals with clinically confirmed cases of SLE, the prevalence of hydroxychloroquine use was 55 per 100 person-years and remained constant throughout the multi-year observation period [34]. Similarly, in the Lupus in Minorities, Nature versus Nurture (LUMINA) cohort, 56% of patients were treated with anti-malarial drugs at the time of study enrollment [29]. We found that 46% of Medicaid enrollees with lupus nephritis were dispensed a prescription for anti-malarials after one year of observation, a result that is slightly lower than that for patients enrolled in clinical studies who largely have access to rheumatology subspecialty care. In addition, one previous study found that 49% of individuals with prevalent lupus nephritis received anti-proteinuric therapy, a result that is very similar to the performance on the quality measure in this study [35]. Taken together, these studies suggest that there is underutilization of anti-malarial and anti-proteinuric therapy in SLE.
Although administrative data provide an otherwise unattainable picture of health care quality at the population level, it is important to recognize their limitations and to interpret our data with these in mind. First, although our study strongly suggests that care is uneven for lupus nephritis across the United States and that delays in care are common in the Medicaid population, the estimates of performance on the quality measures examined are likely imprecise. Clinically detailed information, including factors such as disease activity, the etiology of renal disease (or class of lupus nephritis), or other factors such as drug contraindications cannot be obtained from these data. It is therefore likely that some individuals in our denominator population did not have lupus nephritis, or had mild forms of disease (e.g. mesangial disease or Class V nephritis with low proteinuria) that did not require therapy. To partly address this issue, we examined glucocorticoid exposure among individuals who did not receive immunosuppressive medications for their disease. The fact that many individuals received substantial doses of glucocorticoid monotherapy during the study period suggests that this group likely had active SLE.
Additionally, while our algorithms did capture intravenous cyclophosphamide given in infusion units, the MAX dataset does not capture inpatient medications and thus receipt of medications dispensed to patients while they were hospitalized may have been missed. It is possible that inpatient administration of cyclophosphamide may have been relatively frequent among vulnerable patients with low socioeconomic status during the study period, and quality of care for the measure examining immunosuppressant use is likely therefore an underestimate. However, excluding patients with hospitalizations preceding the three time points examined did not improve performance, suggesting that failure to capture inpatient administration of cyclophosphamide does not entirely explain low performance. An additional limitation is that the data used in our study pre-dated both the SLE Quality Indicators Project and the ACR Lupus Nephritis Guidelines [10, 11], both of which served to consolidate and disseminate information from clinical trials in the preceding decades. It is also important to note that landmark clinical trials supporting the use of currently used induction regimens were also occurring during the study interval and later likely served to change clinical practice. Although it is possible that care has improved over time, our initial findings suggest that more contemporary, clinically-rich data streams are urgently needed to assess and improve care for this vulnerable population of patients.
Moving forward, administrative data can continue to serve as one source of information for studies of quality in SLE, particularly as newer coding schemes (ICD-10) potentially add specificity to diagnoses, and as linkage between laboratory, pharmacy and billing data is more readily available. However, as noted above, administrative data will continue to have significant limitations and attention should therefore also be focused on developing related, but newly specified quality measures for quality improvement. Developing measures for large-scale application to clinically-rich data streams, such as electronic-health records, will likely help physicians, regional specialists, health systems, and also potentially geographic regions, better organize care for patients with SLE.
In conclusion, our study suggests important gaps in quality of care for Medicaid enrollees with lupus nephritis from 2000–2006. Many patients experienced substantial delays in care, and almost 1 in 8 patients used the emergency department as the most common source of care, implying inadequate access to ambulatory care. We also found geographic variation in quality, with those in the Northeast receiving consistently higher quality of care. Although these administrative data provide an otherwise unattainable population-based view of variations in care for SLE, moving forward, there is a need to build on these findings to develop and apply measures to clinically rich data streams as they become available. Collecting and reporting such measures can serve as an important tool for monitoring and aiming to improve care for SLE and for attempting to reduce the striking disparities in the condition. The severity of SLE, and its contribution to system-wide resource utilization, justify further research in this area.
Significant findings.
This is the first study to apply newly developed quality measures to a national sample of patients with SLE.
Our data suggest important gaps in quality of care for Medicaid enrollees with lupus nephritis from 2000–2006. Many patients experienced substantial delays in care, and almost 1 in 8 patients used the emergency department as the most common source of care, implying inadequate access to ambulatory care.
We also found geographic variation in quality, with those in the Northeast receiving consistently higher quality of care.
Acknowledgments
This study was funded by NIH K23 AR060259 (Yazdany), NIH R01 AR057327 (Costenbader) and the Rosalind Russell Medical Research Center for Arthritis, and in part by the Intramural Research Program, National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health (Ward).
Footnotes
Disclosures: No financial or other commercial relationships.
References
- 1.Cervera R, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82(5):299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 2.Feldman CH, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 65(3):753–63. doi: 10.1002/art.37795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alarcon GS, et al. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11 (2):95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, et al. Systemic lupus erythematosus in Malaysia: a study of 539 patients and comparison of prevalence and disease expression in different racial and gender groups. Lupus. 1997;6(3):248–53. doi: 10.1177/096120339700600306. [DOI] [PubMed] [Google Scholar]
- 5.Seligman VA, et al. Demographic differences in the development of lupus nephritis: a retrospective analysis. Am J Med. 2002;112(9):726–9. doi: 10.1016/s0002-9343(02)01118-x. [DOI] [PubMed] [Google Scholar]
- 6.Costenbader KH, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 63(6):1681–8. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alarcon GS, et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med. 2006;3(10):e396. doi: 10.1371/journal.pmed.0030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States, 1996–2004. J Rheumatol. 2009;36(1):63–7. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korbet SM, et al. Severe lupus nephritis: racial differences in presentation and outcome. J Am Soc Nephrol. 2007;18(1):244–54. doi: 10.1681/ASN.2006090992. [DOI] [PubMed] [Google Scholar]
- 10.Yazdany J, et al. A quality indicator set for systemic lupus erythematosus. Arthritis Rheum. 2009;61 (3):370–7. doi: 10.1002/art.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn BH, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken) 64(6):797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertsias GK, et al. Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis. 71(11):1771–82. doi: 10.1136/annrheumdis-2012-201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 37(6):1158–63. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward MM. Access to care and the incidence of endstage renal disease due to systemic lupus erythematosus. J Rheumatol. 2010;37(6):1158–63. doi: 10.3899/jrheum.091199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chibnik LB, Massarotti EM, Costenbader KH. Identification and validation of lupus nephritis cases using administrative data. Lupus. 2010;19(6):741–3. doi: 10.1177/0961203309356289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.2010 National Committee for Quality Assurance’s Quality Compass®. [Accessed September 10, 2011]; Available at: http://www.ncqa.org/tabid/60/Default.aspx.
- 17.Schmajuk G, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. Jama. 305(5):480–6. doi: 10.1001/jama.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bureau USC. [Accessed March 2013];United States Census 2000. http://www.census.gov/main/www/cen2000.html.
- 19.Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol. 2007;34(10):2024–7. [PubMed] [Google Scholar]
- 20.Bertsias G, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics. Ann Rheum Dis. 2008;67(2):195–205. doi: 10.1136/ard.2007.070367. [DOI] [PubMed] [Google Scholar]
- 21.Ward MM. Socioeconomic status and the incidence of ESRD. Am J Kidney Dis. 2008;51(4):563–72. doi: 10.1053/j.ajkd.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Frankovich JD, Hsu JJ, Sandborg CI. European ancestry decreases the risk of early onset, severe lupus nephritis in a single center, multiethnic pediatric lupus inception cohort. Lupus. 21(4):421–9. doi: 10.1177/0961203312437805. [DOI] [PubMed] [Google Scholar]
- 23.Hiraki LT, et al. Prevalence, incidence, and demographics of systemic lupus erythematosus and lupus nephritis from 2000 to 2004 among children in the US Medicaid beneficiary population. Arthritis Rheum. 64(8):2669–76. doi: 10.1002/art.34472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon SS, et al. Hypertension among adults in the United States, 2009–2010. NCHS Data Brief. (107):1–8. [PubMed] [Google Scholar]
- 25.Alarcon GS, et al. Systemic lupus erythematosus in three ethnic groups. VII [correction of VIII]. Predictors of early mortality in the LUMINA cohort. LUMINA Study Group. Arthritis Rheum. 2001;45(2):191–202. doi: 10.1002/1529-0131(200104)45:2<191::AID-ANR173>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Gillis JZ, et al. Medicaid and access to care among persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57(4):601–7. doi: 10.1002/art.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson L, et al. Treatment for lupus nephritis. Cochrane Database Syst Rev. 12:CD002922. doi: 10.1002/14651858.CD002922.pub3. [DOI] [PubMed] [Google Scholar]
- 28.The Canadian Hydroxychloroquine Study Group. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med. 1991;324(3):150–4. doi: 10.1056/NEJM199101173240303. [DOI] [PubMed] [Google Scholar]
- 29.Alarcon GS, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L) Ann Rheum Dis. 2007;66(9):1168–72. doi: 10.1136/ard.2006.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis. 2009;68(2):238–41. doi: 10.1136/ard.2008.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52(5):1473–80. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 32.Jung H, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 62(3):863–8. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 33.Gansevoort RT, et al. Antiproteinuric effect of blood-pressure-lowering agents: a meta-analysis of comparative trials. Nephrol Dial Transplant. 1995;10(11):1963–74. [PubMed] [Google Scholar]
- 34.Schmajuk G, et al. Hydroxychloroquine treatment in a community-based cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 62(3):386–92. doi: 10.1002/acr.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yazdany J, et al. Quality of care in systemic lupus erythematosus: application of quality measures to understand gaps in care. J Gen Intern Med. 27(10):1326–33. doi: 10.1007/s11606-012-2071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


