Abstract
Two groups derived neural and mesodermal cells from human fibroblasts by going through a partially reprogrammed intermediate.
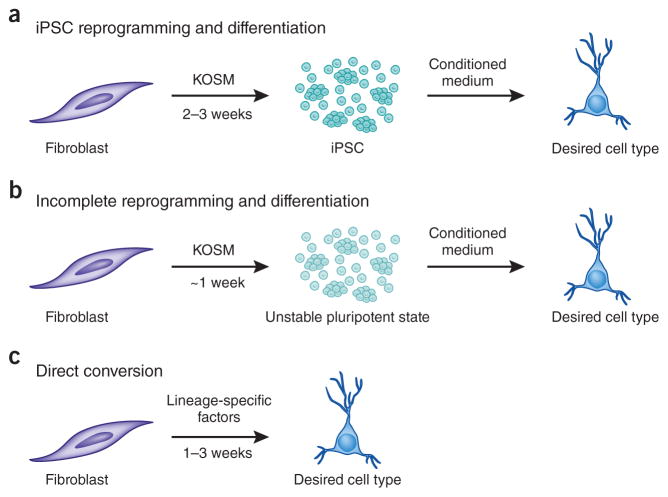
The ability to easily convert accessible human cells into disease-relevant cell types through cellular reprogramming has opened new doors for basic research and regenerative medicine1. Takahashi and Yamanaka ushered in contemporary reprogramming when they demonstrated that a combination of four transcription factors (Oct3/4, Sox2, Klf4 and c-Myc) could drive skin-derived fibroblasts to a pluripotent state that could be further differentiated into the desired cell type2 (Fig. 1a). But robust differentiation into specific lineages remains a stumbling block. Low efficiencies and week- to month-long protocols often give rise to mixed cultures requiring a second purification step. Purity matters, as remnant pluripotent cells can give rise to tumors after transplantation. Moreover, the yielded cells are typically immature (as in cardiomyocyte, hematopoietic or neuronal differentiation).
Figure 1.
Three current approaches to cellular reprogramming. (a–c) Reprogramming to a fully reprogrammed induced pluripotent cell (iPSC) with Yamanaka factors Klf4, Oct3/4, Sox2 and c-Myc (KOSM) and differentiation to the desired cell type (a); reprogramming to an unstable pluripotent state with KOSM and differentiation (b); and direct lineage conversion with lineage-specific transcription factors (c)
With these concerns in mind, we and other groups have sought to take a different approach where, instead of going through a pluripotent state, one somatic cell type can be directly converted to another with the correct combination of lineage-specific transcription factors3 (Fig. 1). Surprisingly, using this approach, cellular conversion is fast (2–3 weeks), does not require the derivation of pluripotent cells and is efficient.
Recently, several groups have taken yet another approach to cellular conversion by transiently expressing the Yamanaka factors to generate what appears to be a multipotent, partially reprogrammed intermediate that arises during reprogramming to the pluripotent state4 (Fig. 1b). These partially reprogrammed intermediates can be differentiated into multiple lineages and do not seem to give rise to pluripotent cells unless left in medium amenable to pluripotent cell derivation. Though not exactly the same as direct reprogramming, this approach has advantages: the time required to generate, expand and differentiate pluripotent cells is avoided, and the partially reprogrammed cells do not seem to give rise to teratomas. To date, the approach has been successful largely with mouse cells. In this issue of Nature Methods, work from two groups demonstrates the indirect approach on human cells, for mesodermal and neural lineages5,6.
Izpisua Belmonte and colleagues took this indirect approach by first identifying and optimizing mesodermal induction medium that could efficiently differentiate human embryonic stem cells into CD34+ angioblast-like cells after 8 days of treatment5. These angioblasts demonstrated bipotency: they could differentiate into endothelium and smooth muscle at high efficiencies. With this new medium, fibroblasts were converted to a partially reprogrammed state by transient 8-day expression of the Yamanaka factors via retroviral vectors and further differentiated to bipotent angioblasts by treatment with mesodermal induction medium for 8 days. Of note, all of the Yamanaka factors and growth conditions amenable for pluripotent cell generation were necessary for the first 8 days, suggesting that intermediate partially reprogrammed cells were necessary for conversion.
As viral integration of genes encoding the Yamanaka factors (which include the oncogene c-MYC (MYC)) may not be desirable for eventual clinical use, non-integrating episomal vectors carrying OCT4 (POU5F1), SOX2, KLF4, LMYC (MYCL1), LIN28 and a short hairpin RNAi against p53 were transiently expressed to induce the partially reprogrammed state. Bipotent CD34+ angioblast-like cells could be successfully derived with this approach, and no episomal vectors were detected in these cells. TRA 1–60 and TRA 1–81 pluripotent cells were also undetected in this culture, and cells injected into the mouse testis did not give rise to teratomas. Importantly, differentiated cells were shown to be functional, as smooth muscle cells could take up calcium and contract, and endothelial cells could form vessel-like structures in vitro and in vivo.
Taking a similar indirect approach, Pei and colleagues derived neural progenitor cells (NPCs) from cells obtained from adult human urine6. Fourteen days after transfection with episomal vectors carrying transgenes for Oct4, Sox2, SV40LT, Klf4 and miR302–miR367 and treatment with five small-molecule inhibitors, domed colonies formed that could be easily picked and replated. Upon replating, these colonies formed neural rosette–like cells, an early neural subpopulation that typically arises during neural differentiation of pluripotent cells. Separately, pluripotent stem cells could be derived if these colonies were replated in mTESR, a medium that supports pluripotent cell self-renewal, suggesting that the domed neural colonies were derived from intermediate partially reprogrammed cells. Further characterization showed that NPCs derived from these neural rosettes could differentiate into astrocytes and into mature, functional neurons, though only oligodendrocytes with immature morphologies were obtained (as is also seen with differentiation from pluripotent cells). Injection of the NPCs into the rat brain showed that the cells could give rise to neurons and astrocytes in vivo.
The ability to change the identity of one cell type into another has far-reaching implications for both basic and clinical research. The field now has three principal options for converting one cell type into another: (i) direct reprogramming from one somatic cell to another somatic cell, (ii) direct reprogramming to a stable pluripotent stem cell line followed by directed differentiation and (iii) indirect reprogramming by transient induction of a partially reprogrammed cell, followed by differentiation into the desired somatic cell type (Fig. 1). There are pros and cons for each approach. Direct reprogramming can be highly efficient, yielding more mature cells than pluripotent stem cell–derived cells. Somatic cells derived from direct and indirect reprogramming cannot be easily genetically manipulated, unlike stable iPS cell lines. Future studies will need to address whether cell types generated by indirect reprogramming are heterogeneous—a known complication for iPS cells—and the extent to which the cells generated by this method have epigenetic memory.
In summary, the reprogramming field continues to be exciting, and the spectrum of approaches to design the generation of a specific cell type of interest continues to increase.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Ernesto Lujan, Department of Genetics, Institute of Stem Cell Biology and Regenerative Medicine, Stanford University School of Medicine, Stanford, California, USA.
Marius Wernig, Email: wernig@stanford.edu, Department of Pathology, Institute of Stem Cell Biology and Regenerative Medicine, Stanford University School of Medicine, Stanford, California, USA.
References
- 1.Vierbuchen T, Wernig M. Nat Biotechnol. 2011;29:892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Yamanaka S. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Vierbuchen T, et al. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lujan E, Wernig M. Curr Opin Genet Dev. 2012;22:517–522. doi: 10.1016/j.gde.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kurian L, et al. Nat Methods. 2013;10:77–83. doi: 10.1038/nmeth.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, et al. Nat Methods. 2013;10:84–89. doi: 10.1038/nmeth.2283. [DOI] [PubMed] [Google Scholar]