Abstract
Early life stress leads to several effects on neurological development, affecting health and well-being later in life. Instances of child abuse and neglect are associated with higher rates of depression, risk taking behavior, and an increased risk of drug abuse later in life. This study used repeated neonatal separation of rat pups as a model of early life stress. Rat pups were either handled and weighed as controls or separated for 180 minutes per day during postnatal days 2-8. In adulthood, male and female rats were tested for methamphetamine conditioned place preference reward and methamphetamine induced locomotor activity. Tissue samples were collected and mRNA was quantified for the norepinephrine transporter in the prefrontal cortex and the dopamine transporter in the nucleus accumbens. Results indicated rats given methamphetamine formed a conditioned place preference, but there was no effect of early separation or sex. Separated males showed heightened methamphetamine-induced locomotor activity, but there was no effect of early separation for females. Overall females were more active than males in response to both saline and methamphetamine. No differences in mRNA levels were observed across any conditions. These results suggest early neonatal separation affects methamphetamine-induced locomotor activity in a sex-dependent manner but has no effects on methamphetamine conditioned place preference.
Keywords: maternal separation, methamphetamine, conditioned place preference, locomotor activity
1. Introduction
Stress early in development, such as childhood abuse or neglect, negatively impacts physical and mental health later in life. Correlational research suggests chronic life stress increases the likelihood of developing psychological disorders, such as anxiety, depression, and substance abuse disorder. Although many studies have demonstrated this phenomenon, the underlying mechanisms remain difficult to study in the human population (Chapman et al., 2003; Dube et al., 2004). This increased vulnerability likely arises from multifaceted environmental interactions and biological changes which persist into adulthood. The time limitations, ethical constraints, and lack of satisfactory control groups considerably restrict human subjects research in this area.
Animal studies have helped elucidate many biological mechanisms of chronic early life stress. Maternal separation is a well-established model of early developmental stress. This paradigm takes advantage of a sensitive period during the development of the hypothalamic-pituitary-adrenal axis (HPA-axis). In rodents, during the first two weeks after birth, the dam regulates the HPA-axis activity of her pups. Removing pups from the dam activates the HPA-axis and her presence ceases further activation of this system, indicating absence of the dam results in persistent release of stress hormones during this sensitive period (Levine, 2001). Extended activation of the HPA-axis leads to long term changes which results in distinct biological and behavioral phenotypes in adulthood. However, the frequency and duration of HPA-axis activation required for long-term effects remains unclear, as animals can exhibit long-lasting alteration after a single, 24-hour separation, or daily one-hour separation for one week (Kuma et al., 2004; Zhang et al., 2002).
Repeated maternal separation is associated with many long-term behavioral adaptations, including behavioral responses to drugs of abuse. After an acute stressor, separated animals exhibit an increased locomotor response to low doses of cocaine and amphetamine relative to handled and non-handled controls (Brake et al., 2004; Meaney, 2002). A large body of evidence additionally supports the role of early life stress leading to increased sensitivity to the rewarding properties of drugs of abuse. Separated animals acquire cocaine self-administration earlier and escalate their intake more rapidly relative to control animals (Matthew et al., 1999; Moffet et al., 2007). Additionally, separated animals exhibit increased rewarding behavior to amphetamine in a conditioned place preference test (Der-Avakian & Markou, 2010). Although the reasons for these behavioral effects are not completely understood, several alterations in brain reward systems have been characterized.
The effects of early life stress on drug reward are likely a result of changes in multiple neural systems. Most predominantly, the mesolimbic dopamine system regulates aspects of drug reward and drug craving (Berridge, 2007). Maternally separated animals exhibit several alterations throughout the mesolimbic and mesostriatal dopamine systems. Separated animals show elevated dopamine levels in the nucleus accumbens in response to an acute stressor, suggesting a possible hypersensitivity in dopamine release after a stressor (Brake et al., 2004). Separated animals also exhibit decreased dopamine transporters in the nucleus accumbens core and striatum (Brake et al., 2004; Meaney et al., 2002). Decreased transporter availability may lead to a compromised ability to clear dopamine from the synapse leading to elevated dopamine levels. Interestingly, separated animals also exhibit increased dopamine release in the nucleus accumbens after an acute injection of cocaine or amphetamine, suggesting an increased sensitivity to the rewarding properties of these drugs may be due to changes in dopamine systems (Silvagni et al., 2008).
Although dopamine is an important neurotransmitter for regulating drug reward and sensitivity, effects of the dopamine system alone do not fully explain stimulant reward and locomotor activity. Noradrenergic systems have been implicated in stimulant reward and sensitivity. In particular, stimulation of α1 adrenergic receptors may facilitate dopamine release and associated psychostimulant locomotor activation and reward (Auclair et al., 2002; Drouin et al., 2002). Separated animals exhibit several alterations in norepinephrine systems including enhanced stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and reduced levels of the α2 adrenergic a utoreceptors, relative to handled controls (Liu et al., 2000). Thus, enhanced stress-induced noradrenergic activity in maternally separated rats may facilitate behavioral responses to psychostimulants.
Though the effects of early life stress on cocaine sensitivity are generally consistent (Brake et al., 2004; Kikusui et al., 2005; Matthews et al., 1999), studies are mixed on the sensitivity of maternally separated animals to the locomotor activating effects of amphetamine (Hensleigh et al., 2011; Muhammad & Kolb, 2011; Weiss et al., 2001). Few studies have looked at the effects of early life stress on methamphetamine reward and locomotor activation (Dimatelis et al., 2012, Faure et al., 2009; Lewis et al., 2013). Previously, we found increased sensitivity to the locomotor activating effects of a low (1.0mg/kg) and medium (3.0mg/kg) dose of methamphetamine in separated animals (Pritchard, et al.,2012). Other studies found no difference or attenuation in adolescent separated animals relative to controls in methamphetamine reward (Dimatelis et al., 2012; Faure et al., 2009). However, these studies were done in adolescent rats and not adult animals and did not include female animals.
To further examine the effect of early life stress on drug reward and locomotor sensitivity, this study examined the immediate locomotor response to methamphetamine and the rewarding properties of methamphetamine using a conditioned place preference paradigm in adult animals. To better elucidate the underlying mechanisms associated with early life stress, locomotor activity, and methamphetamine reward, we quantified mRNA for dopamine and norepinephrine transporters. We hypothesized that maternal separation would increases sensitivity to the locomotor activating and rewarding effects of methamphetamine and decrease expression of catecholamine transporters in the mesocorticolimbic system.
2. Materials and Methods
2.1 Animals
Long-Evans rats (Charles River) were pair-housed in polypropylene cages and kept on 12:12 light/dark cycle with access to food and water ad libitum. Litters were derived from 12 male and female pairs bred in the facility. Pregnant dams were housed separately and remained untouched except for weekly cage changes. A total of 73 offspring, 34 males and 39 females, obtained from 10 litters, were used in the study.
2.2 Maternal Separation
The day of birth was designated as postnatal day (PND) 0. Litters were culled or fostered on PND 1 to obtain roughly equal litter sizes of no more than 12 pups. Litters were randomly assigned as control or separated. Control litters were transported to the testing room and weighed, resulting in separation from the dam for approximately 15 minutes every day between PND 2-8. Separated litters were transported to the testing room, weighed, and housed individually at nest temperature (30-33°C) by use of a heating pad for three hours every day during PND 2-8. Two to four pups from each litter were left with the dam to minimize stress reaction from the dam, and these animals were not tested in the subsequent conditioned place preference experiment. Litters were left undisturbed, except for cage changes, from PND 9-20 and were weaned into same-sex pair-housing on PND 21. All rats remained pair-housed and handled weekly during cage changes until testing began on PND 60. 2.3 Conditioned Place Preference
Methamphetamine conditioned place preference began between PND60-62. A three-compartment chamber (70cm × 30cm × 30cm, Kinder Scientific) was used to condition animals. Each larger side compartment had a distinct wall color (black or white) and a distinct floor texture (bars or grid), whereas the smaller middle chamber was gray with a smooth floor. Total activity and time in each compartment were recorded by a photobeam tracking system (Motor Monitor, Kinder Scientific). Animals were habituated to the testing room for 30 minutes before experimental manipulation each day. Testing occurred over ten days as follows: day1 – chamber habituation, days 2-9 – conditioning, day 10 – preference test. On day 1 of testing, animals were habituated to the testing apparatus for 30 minutes with free access to all chambers. After initial habituation, a single sample t-test that compared time spent in each chamber to 50% was run for each animal to determine if there was a baseline chamber preference. If animals spent significantly more than half of the habituation time in one chamber, the drug was paired with the non-preferred chamber. If animals did not display a preference, the drug-paired chamber was determined by random assignment. On days 2, 4, 6, and 8 animals were given saline injections and confined to the appropriate chamber for 30 minutes. On days 3, 5, 7, and 9, animals were given either a subcutaneous injection of saline (control) or 1.0mg/kg methamphetamine (Sigma, dissolved in a volume of 1.0ml/kg) and confined to the opposite chamber for 30 minutes. Drug dose was determined based on previous conditioning studies using the same dose (Pao-Pao et al., 2012) and preliminary data from our lab. On day 10, animals were not given an injection, placed in the center chamber and given free access to all chambers for 30 minutes. To control for individual variations in chamber preference, a ratio preference score was calculated for each animal. This ratio score was calculated by subtracting the total time spent in the drug-paired chamber on day 1 from the total time spent in the drug-paired chamber on day 10 and dividing by time spent in the drug-paired chamber on day 1 i.e. ((day 10-day1)/day1). This calculated the percent increase in time spent in the drug-paired chamber on day 10.
2.4 Tissue Collection
Twenty-four hours after the preference test (day 10), animals were euthanized by anesthetic overdose using Somnasol (390 mg pentobarbital and 50 mg phenytoin). The brain was chilled in ice-cold saline and dissected with the aid of a coronal brain matrix as described previously (Hondo et al., 1999). Prefrontal cortex and nucleus accumbens were collected for quantification of mRNA transcripts. Brain regions were snap frozen on dry ice and stored at −80°C until quantification with RT-PCR.
2.5 RT-PCR
Tissue samples were thawed on ice and homogenized by the manufacturer’s instructions using E.Z.N.A. Total RNA kit (Omega Bio-Tek). Briefly, tissue samples were homogenized in 1 ml of RNA Solv and 20 μl of 2-mercaptoethanol (30 mg of tissue) and incubated for 5 minutes at room temperature. After, 200 μl of chloroform was added and the homogenate was incubated at room temperature for 2 to 3 minutes, followed by centrifugation at 12,000 × g at 4°C for 15 minutes. The supernatant was transferred and combined with equal volume of 70% ethanol, loaded onto a spin column, and centrifuged for one minute at 10,000 × g at room temperature. Samples were further washed with 500 μl RNA Wash Buffer I, centrifuged at 12,000 × g at room temperature for one minute. The flow through was discarded and samples were washed twice more with 500 μl wash buffer II, and centrifuged at maximum speed for two minutes at room temperature. Final RNA was eluted into 55 μl of nuclease-free water by 2 minute centrifugation and stored at −80°C until real-time PCR.
Sample total concentration of RNA was determined by A260 measurement. Samples were diluted in nuclease-free water to a final concentration of 200 ng/μl. Transcripts of interest were amplified using a qScript One-Step SYBR Green qRT-PCR kit following manufacturer’s instructions (Quanta Biosciences). Samples were amplified in duplicate in a 96-well plate by use of an iCycler (BioRad) by using the following cycle parameters: 95°C for 10 minutes, 95°C for 30 seconds, 60°C for 1 minute with the final two steps repeated for 45 cycles. Melt curves were assessed for formation of primer dimers. Primers were designed using Primer-BLAST, and sequences are listed in table 1. Quantification of final mRNA products was achieved by normalizing the target mRNA to the house keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). A between-subject ANOVA revealed no differences in GAPDH among treatment groups, confirming that it was an appropriate normalizer. Values were calculated using the delta-delta CT method, and a standard curve was calculated using seven serial dilutions of total RNA (800ng-12.5ng). The target quantity in experimental samples was normalized to GAPDH and compared against the saline group. Final values were analyzed using between-subjects ANOVA.
Table 1.
| Target | Forward Primer | Reverse Primer |
|---|---|---|
| Rat GAPDH | 5′ - CTCAACTACATGGTCTACATGTTCCA - 3′ | 5′ - CTTCCCATTCTCAGCCTTGACT - 3′ |
| Rat DAT | 5′ - TGACGCAGGAGTCAGTCGAAGAAGAA - 3′ | 5′ - TTTAGCCGGGGCCACCACTGA - 3′ |
| Rat NET | 5′ - CAGCACCATCAACTGTGTTACC - 3′ | 5′ - AGGACCTGGAAGTCATCAGC - 3′ |
Primer sequences for (GAPDH), dopamine transporter (DAT), and norepinephrine transporter (NET).
3. Results
3.1 Conditioned Place Preference
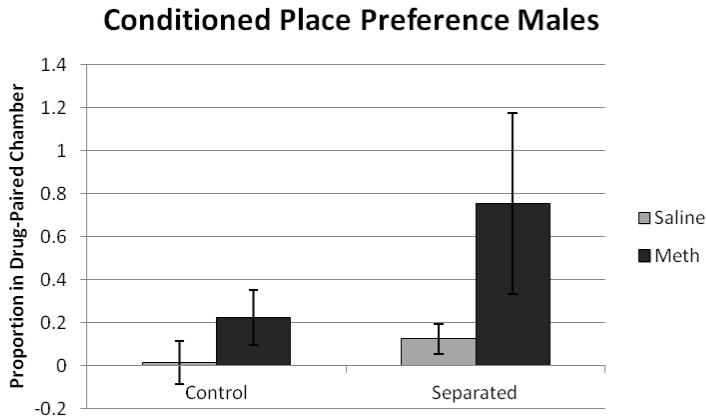
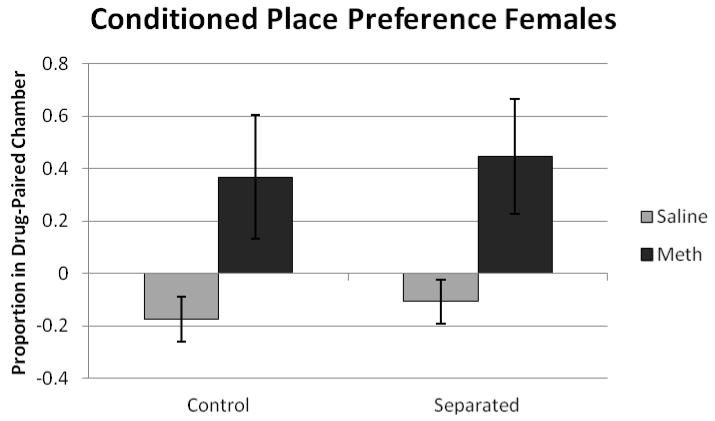
Results were analyzed using two ANOVAs, one for males and one for females with drug (saline or methamphetamine) by condition (separated or control) as between-subjects. Data were probed for outliers, and three animals had scores that fell at least two standard deviations from the group mean. When these data points were removed, the outcomes of all statistical analyses remained the same. For males, there was a significant effect of drug (F(1,33) = 5.92, p<0.05), with methamphetamine animals exhibiting a greater increase in time in the drug-paired chamber (figure 1). For females, there was a significant effect of drug (F(1,38) = 8.74, p<0.05), with methamphetamine increasing time spent in the drug-paired chamber. There was no significant effect of condition or significant drug × condition interaction (figure 2).
Figure 1.
The proportion of time spent in the drug paired chamber on preference day 10 for males. There was a significant effect of drug (F(1,33) = 5.92, p<0.05) with methamphetamine animals exhibiting a greater increase in time spent in the drug-paired chamber.
Figure 2.
The proportion of time spent in the drug paired chamber on preference day 10 for females. There was a significant effect of drug (F(1,38) = 8.74, p<0.05) with methamphetamine animals exhibiting a greater increase in time spent in the drug-paired chamber.
3.2 Locomotor Activity
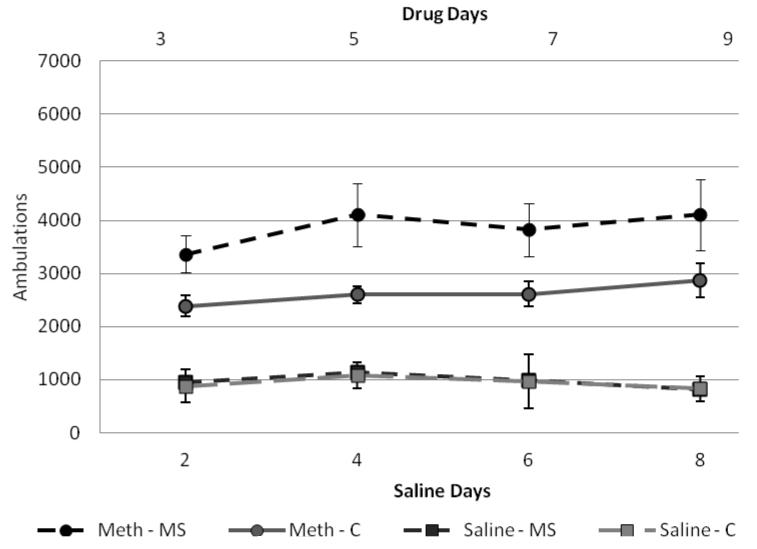
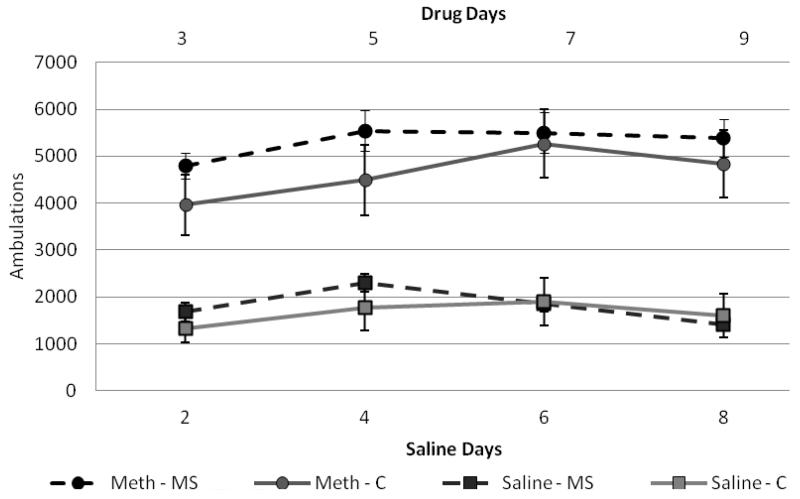
Two mixed-model ANOVAs were run to examine locomotor activity of the methamphetamine group for drug days (3, 5, 7, 9) and saline days (2, 4, 6, 8) for females and one for males. Between-subjects factors were condition (control × separated) and drug (saline × meth) and within-subjects factors were days (2, 4, 6, 8 for saline or 3, 5, 7, 9 for methamphetamine). For males, results for drug-paired days indicated no significant within-subjects effect (F(3,30) = 0.382, p>0.05) and no significant interactions between day and other factors. For between-subjects effects, there was a significant effect of condition (F(1,32) = 5.01, p<0.05) and drug (F(1,32) = 74.8, p<0.001) with separated males in the methamphetamine group exhibiting higher locomotor activity than control males in the methamphetamine group (figure 3). There were no significant effects on saline days. For females, results for drug-paired days indicated a significant within-subjects effect (F(3,26) = 7.51, p<0.05) and a significant day × drug interaction (F(3,26) = 39.84, p<0.05). There were no other significant within-subjects interactions. For between-subjects effects, condition was approaching significance (F(1,28) = 3.26, p=0.08) and there was a significant effect of drug (F(1,28) = 86.96, p<0.001) with females in the methamphetamine group exhibiting higher locomotor activity than those in the saline group (figure 4).
Figure 3.
Male ambulations on conditioning days.
Total ambulations on representative drug days (3,5,7,9), represented as the top two lines with circle makers. And total ambulations for saline paired days (2,4,6,8), represented in the bottom two lines with square markers. Overall, males were more active on methamphetamine-paired days (F(1,30)=74.8, p<0.001) and separated males were most active on drug days (F(1,30)=5.01, p<0.05).
Figure 4.
Female ambulation on conditioning days
Total ambulations on representative drug days (3,5,7,9), represented as the top two lines with circle makers. And total ambulations for saline paired days (2,4,6,8), represented in the bottom two lines with square markers. Overall, females were more active on methamphetamine-paired days (F(1,35)=86.9, p<0.001) and separation was approaching significance (F(1,35)=3.26, p=0.08) with separated females exhibiting slightly higher ambulations during the first two drug-paired days.
3.3 RT-PCR
Final fold change values were run through two separate 2 × 2 ANOVAs (one for each gene) for each sex. For males and females, there were no significant effects of condition, drug or condition × drug interactions on NET in the prefrontal cortex or DAT in the nucleus accumbens (Table 2).
Table 2.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Meth-C | Meth-MS | Saline-C | Saline-MS | Meth -C | Meth-MS | Saline-C | Saline-MS | |
| PFC(NET) | 10.25 | 9.58 | 9.47 | 10.56 | 9.55 | 10.34 | 9.80 | 10.43 |
| SEM | 1.45 | 1.56 | 0.91 | 0.87 | 1.43 | 1.16 | 0.68 | 0.98 |
| N.Acc.(DAT) | 9.57 | 10.86 | 8.71 | 10.07 | 8.52 | 10.69 | 8.24 | 9.20 |
| SEM | 0.26 | 0.20 | 0.30 | 0.34 | 0.35 | 0.29 | 0.31 | 0.13 |
All genes were normalized to GAPDH and compared against the control group (control, saline). No significant differences were found for NET or DAT. Abbreviations: PFC (prefrontal cortex), N.Acc. (nucleus accumbens), C (control), MS (separated).
4. Discussion
Previous findings have elucidated the effects of early life stress on cocaine and d-amphetamine reward and locomotor sensitivity (Brake et al., Kikusui et al., 2005; Matthews et al., 1999; Meaney, 2002), though, few studies have examined the effects of early life stress on responses to methamphetamine (Faure et al., 2009; Lewis et al., 2013). Results from the present study indicate a main effect of drug and sex. However, maternal separation did not alter the rewarding properties of methamphetamine, but may increase sensitivity to the locomotor activating effects of the drug.
Females exhibited greater methamphetamine induced locomotor activity. These findings are similar to those of Milesi-Halle et al. (2007) and Schindler et al. (2002) who have shown female rats exhibit increased locomotor activity in response to methamphetamine. Our findings are also consistent with the extensive literature on increased sensitivity of females to psychostimulants in general, which is likely mediated by sex differences in drug metabolism (reviewed in Lynch et al., 2002). Clinical research also indicates an enhanced sensitivity to methamphetamine in females. More females abuse methamphetamine relative to their male counterparts. Females exhibit a greater likelihood for becoming dependent on methamphetamine and relapse to methamphetamine more frequently than males (Brecht et al., 2004; Rawson et al., 2005). Females also begin abusing methamphetamine at a younger age relative to males (Dluzen and Liu, 2008). These studies suggest females may have an increased sensitivity to methamphetamine leading to increased likelihood of addiction and relapse.
Separated male animals exhibited increased locomotor response after a methamphetamine injection relative to control males. This effect was significant in males but not in females. This finding is in contrast to our previous study, in which female, but not male MS rats exhibited increased locomotor responses to 1.0 mg/kg methamphetamine, relative to controls (Pritchard et al., 2012). It is possible that MS females in this study responded to 1.0 mg/kg methamphetamine with increased stereotyped behavior, which interfered with locomotor activity. However, we were unable to quantify stereotypy in this study due to the physical constratints of the CPP apparatus. Additionally, the smaller size and more complex configuration of the CPP chamber may have limited animals’ locomotor activity and prevented detection of the MS effect. These results, viewed in light of our previous findings, suggest early life stress likely affects the sensitivity to the motor activating effects of methamphetamine and that this effect differs by sex.
The current study demonstrated an effect of methamphetamine reward but not an effect of separation. Animals given methamphetamine formed a preference for the drug paired chamber, suggesting methamphetamine was rewarding. There was no effect of condition or sex for methamphetamine reward suggesting early life stress does not alter the rewarding properties of methamphetamine in adult males or females. These findings were in line with those of Faure, Stein, and Daniels (2009) who found no effect of maternal separation on methamphetamine conditioned place preference reward in adolescent males. An additional study by Dimatelis, et al. (2012) found maternal separation attenuated methamphetamine conditioned place preference reward, but this effect occurred in adolescent males and was not tested in adult males or females. Overall, our data are in line with previous studies indicating early life stress does not alter, or may even attenuate, methamphetamine reward in adolescent or adult male rats. Additionally, we indicated that separation did not alter female methamphetamine conditioned reward. Several procedural variations exist between this study and previous ones, such as duration of separation, age at CPP testing or inclusion of female animals. However, our results are likely not due to procedural differences as we demonstrated results similar to Faure, Stein, and Daniels in adult males as well as adult females. Furthermore, 1.0mg/kg methamphetamine caused a conditioning effect, which has been shown in other studies, suggesting that the lack of a difference in CPP scores between MS and control animals was not attributable to the dose used in this study. However, we cannot rule out an effect of MS on methamphetamine reward at higher or lower doses. Though high variability in CPP ratios may have limited our power to detect an effect of MS, the magnitude and variability of CPP observed in this study are consistent with other studies of methamphetamine CPP in the MS model (Faure et al., 2009). Lastly, little is known about female rats as most studies test males. As women are more likely to become addicted to methamphetamine relative to males, future studies need to further elucidate why.
Brain catecholamine systems have been implicated in the effects of early life experiences on drug reward. The current findings indicate no changes in mRNA for DAT and NET in the brain regions examined. Previous studies found that maternally separated animals exhibit decreased DAT levels in the nucleus accumbens using quantitative receptor autoradiography (Brake et al., 2004; Meaney et al., 2002). The decreased protein levels observed in Brake et al. (2004) may not be reflected in DAT mRNA levels in separated animals, as occurred in the current study. It is also possible that MS-induced changes in DAT expression or function occur only under specific conditions. For example, a recent study by Womersley et al. (2011) demonstrated that MS reduced DAT function in the striatum of spontaneously hypertensive, but not Wistar-Kyoto rats. Downregulation of DAT expression by maternal separation may only be evident in experimentally naïve animals. We examined DAT mRNA data for saline-treated animals separately and found no evidence of group differences in DAT expression. However, separated animals exhibit enhanced dopaminergic responses to stress (Brake et al., 2004). Thus, the repeated stress of saline injection in our study may have masked separation effects on DAT expression. Finally, changes in DAT expression in the MS model may be downstream adaptations to other changes in dopaminergic function, which were not examined in this study.
Maternal separation has been shown to alter norepinephrine systems but the current results indicated no significant changes norepinephrine transporter (NET) expression in the prefrontal cortex. Lui et al. (2000) found decreased α2 adrenergic receptor density in the locus coeruleus of separated animals relative to handled controls. Additionally, decreased norepinephrine levels were found in the cingulate cortex of separated animals relative to non handled controls (Arborelius & Eklund, 2007). These studies suggest early separation alters brain norepinephrine systems but likely through mechanisms other than prefrontal NET expression. Additionally, decreased norepinephrine levels in the frontal cortex were found 24 hours after eight days of escalating methamphetamine self administration but returned to normal after 24 hours of abstinence (Krasnova et al., 2010). Because of the short-lasting effects of acute methamphetamine on norepinephrine systems, alterations in NET would not likely occur without prolonged administration of methamphetamine. The moderate (1.0mg/kg), intermittent doses of methamphetamine administered in the current study were likely not sufficient to cause alterations in NET expression. The current results suggest early life stress and acute methamphetamine administration in adulthood do not impact prefrontal cortex NET expression long-term.
Results of the current study suggest early life stress does not affect methamphetamine conditioned place preference but may alter methamphetamine locomotor sensitivity, specifically in males. Future studies should characterize the effects of early life stress on methamphetamine sensitivity across a broad dose range and the possible mediating effects of sex. Overlapping, but different brain systems likely mediate the effects of MS on drug reward and drug-induced locomotor activity (Kalivas & Stewart, 1991; Koob & Le Moal, 1997). Conflicting findings among studies suggest the effects of early life stress on drug reward and locomotor sensitivity are variable (Kikusui, et al., 2005; Matthews et al., 2004; Vazquez et al., 2005). These inconsistent findings likely result from differences in drug class, variations among early life stress procedures, and paradigm differences used to measure drug reward and locomotor sensitivity. Based on the results of this study and others, it is suggested that early life stress alters methamphetamine induced locomotor sensitivity and does not influence methamphetamine conditioned place preference reward. Increased locomotor sensitivity to methamphetamine in male MS rats is likely not caused by changes in DAT or NET expression in the mesocorticolimbic system. Additionally, the effect of early life stress on methamphetamine sensitivity and reward appears more robust in males compared to females. However, females exhibit increased responses to the locomotor effects of methamphetamine which may have lead to a ceiling effect. Future research should focus on the sex related differences and the neural mechanisms underlying these variations. It will additionally be important to model methamphetamine abuse patterns observed in the human population and examine methamphetamine sensitivity and reward in relation to clinical early life histories and gender.
4.1 Conclusions
Overall, animals given methamphetamine formed a conditioned place preference for the drug. Separated animals did not show elevated methamphetamine induced reward. An effect of separation was approaching significance for increased methamphetamine induced locomotor activity, and this effect was more pronounced in males. There was no difference in DAT or NET mRNA levels for any conditions. These results suggest early life stress may sensitize animals to the motor effects of methamphetamine but may not alter methamphetamine reward.
Methamphetamine group developed a conditioned preference to the drug paired chamber
Separated animals did not exhibit increased meth conditioned place preference
Separated animals exhibited elevated activity in response to methamphetamine
Increased methamphetamine induced activity was more pronounced in males
No difference in mRNA levels was observed for DAT or NET
Acknowledgements
The project described was supported by grants from the National Center for Research Resources (5P20RR016464-11) and the National Institute of General Medical Sciences (8 P20 GM103440-11). The authors would also like to thank Matt Semmel, Sarah Lynch, Nicole Ridolfi, Meghan Pierce, and Ana Reyes for their help with the project.
Abbreviations
- HPA-axis
hypothalamic-pituitary-adrenal axis
- GR
glucocorticoid receptors
- PND
post natal day
- DAT
dopamine transporter
- NET
norepinephrine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Eklund MB. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience. 2007;145:738–750. doi: 10.1016/j.neuroscience.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Auclair A, Cotecchia S, Glowinski J, Tassin JP. D-amphetamine fails to increase extracellular dopamine levels in mice lacking alpha 1b-adrenergic receptors: Relationship between functional and non-functional dopamine release. J Neurosci. 2002;22:9150–9154. doi: 10.1523/JNEUROSCI.22-21-09150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ. Dopamine D-2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D-2/D-3 receptor agonists. Neuropharmacology. 1999;38:1389–1396. doi: 10.1016/s0028-3908(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Brecht ML, O’Brien A, von Mayhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82:217–225. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:307–316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170:1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimatelis JJ, Russell VA, Stein DJ, Daniels WM. Effects of maternal separation and methamphetamine exposure on protein expression in the nucleus accumbens shell and core. Metab Brain Dis. 2012;27:363–375. doi: 10.1007/s11011-012-9295-9. [DOI] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: A review. Gend Med. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: Influence of preexposure conditions and pharmacological characteristics. Synapse. 2002;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: The adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Faure J, Stein DJ, Daniels W. Maternal separation fails to render animals more susceptible to methamphetamine-induced conditioned place preference. Metab Brain Dis. 2009;24:541–559. doi: 10.1007/s11011-009-9158-1. [DOI] [PubMed] [Google Scholar]
- Goodwin JS, Larson GA, Swant J, Sen N, Javitch JA, Zahniser NR, et al. Amphetamine and methamphetamine differently affect dopamine transporters in vitro and in vivo. J Biol Chem. 2009;284:2978–2989. doi: 10.1074/jbc.M805298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensleigh E, Smedley L, Pritchard LM. Sex, but not repeated maternal separation during the first postnatal week, influences novel object exploration and amphetamine sensitivity. Dev Psychol. 2011;53:132–140. doi: 10.1002/dev.20499. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Faccidomo S, Miczek KA. Repeated maternal separation in cocaine-induced behavioral sensitization in adult male and female mice. Psychopharmacology (Berl) 2005;178:202–210. doi: 10.1007/s00213-004-1989-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, LeMoal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PloS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuma H, Miki T, Matsumoto Y, Gu H, Li H, Kusaka T, et al. Early maternal deprivation induces alterations in brain-derived neurotrophic factor expression in the developing rat hippocampus. Neuroscience Letters. 2004;372(1-2):68–73. doi: 10.1016/j.neulet.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic-pituitary-adrenal axis in the rat. Physiol Behav. 2001;73:255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Lewis CR, Staudinger K, Schech L, Olive MF. The effects of maternal separation on adult methamphetamine self-administration, extinction, reinstatement, and MeCP2 immunoreactivity in the nucleus accumbens. Front Psychiatry. 2013;4(55) doi: 10.3389/fpsyt.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci. 2007;25:3091–3098. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Caroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl) 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology (Berl) 1999;141:123–134. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: A neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Milesi-Halle A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female sprague-dawley rats. Pharmacol Biochem Behav. 2007;86:140–149. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Vicentic A, Kozel M, Plotsky P, Francis DD, Kuhar MJ. Maternal separation alters drug intake patterns in adulthood in rats. Biochem Pharmacol. 2007;73:321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res. 2011;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Pao-Pao Y, Huang EYK, Fu YY, Ho TS, Tao PT. Post-treatment of dextromethorphan on methamphetamine-induced drug seeking and behavioral sensitization in rats. Synapse. 2012;66:858–869. doi: 10.1002/syn.21576. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pritchard LM, Hensleigh E, Lynch S. Altered locomotor and stereotyped responses to acute methamphetamine in adolescent, maternally separated rats. Psychopharmacology (Berl) 2012;223:27–35. doi: 10.1007/s00213-012-2679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Obert JL, McCann MJ, Brethen P. Methamphetamine use among treatment-seeking adolescents in Southern California: Participant characteristics and treatment response. J Subst Abuse Treat. 2005;29:67–74. doi: 10.1016/j.jsat.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB. Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol. 2002;442:231–235. doi: 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Silvagni A, Barros VG, Mura C, Antonelli MC, Carboni E. Prenatal restraint stress differentially modifies basal and stimulated dopamine and noradrenaline release in the nucleus accumbens shell: an ‘in vivo’ microdialusis study in adolescent and young adult rats. Eur J Neurosci. 2008;28:744–758. doi: 10.1111/j.1460-9568.2008.06364.x. [DOI] [PubMed] [Google Scholar]
- Tilley MR, O’Neill B, Han DD, Gu HH. Cocaine does not produce reward in absence of dopamine transporter inhibition. Neuroreport. 2009;20:9–12. doi: 10.1097/WNR.0b013e32831b9ce4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention Youth Risk Behavior Surveillance — United States, 2011. Surveillance Summaries. 2012;61(4) [Google Scholar]
- Vazquez V, Farely S, Giros B, Dauge V. Maternal deprivation increases behavioral reactivity to stressful situations in adulthood: suppression by the CCK2 antagonist L365,260. Psychopharmacology (Berl) 2005;181:706–713. doi: 10.1007/s00213-005-0029-0. [DOI] [PubMed] [Google Scholar]
- Weaver SA, Diorio J, Meaney MJ. Maternal separation leads to persistent reductions in pain sensitivity in female rats. J Pain. 2007;8:962–963. doi: 10.1016/j.jpain.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Domeney AM, Heidbreder CA, Moreau JL, Feldon J. Early social isolation, but not maternal separation, affects behavioral sensitization to amphetamine in male and female rats. Pharmacol Biochem Behav. 2001;70:397–409. doi: 10.1016/s0091-3057(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Womersley JS, Hsieh JH, Kellaway LA, Gerhardt GA, Russell VA. Maternal separation affects dopamine transporter function in the spontaneously hypertensive rat: an in vivo electrochemical study. Behavioral and Brain Functions. 2011;7:49. doi: 10.1186/1744-9081-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Huang E, Fu Y, Ho T, Tao P. Post-treatment of dextromethorphan on methamphetamine-induced drug-seeking and behavioral sensitization in rats. Synapse. 2012;66(10):858–869. doi: 10.1002/syn.21576. [DOI] [PubMed] [Google Scholar]
- Zhang LX, Levine S, Dent G, Zhan Y, Xang G, Okimoto D, et al. Maternal deprivation increases cell death in the infant rat brain. Brain Research: Developmental Brain Research. 2002;133(1):1–11. doi: 10.1016/s0926-6410(01)00118-5. [DOI] [PubMed] [Google Scholar]