Abstract
Nigeria is the most populous country in Africa, has a large proportion of the world's poor livestock keepers, and is a hotspot for neglected zoonoses. A review of the 127 accessible publications on brucellosis in Nigeria reveals only scant and fragmented evidence on its spatial and temporal distribution in different epidemiological contexts. The few bacteriological studies conducted demonstrate the existence of Brucella abortus in cattle and sheep, but evidence for B. melitensis in small ruminants is dated and unclear. The bulk of the evidence consists of seroprevalence studies, but test standardization and validation are not always adequately described, and misinterpretations exist with regard to sensitivity and/or specificity and ability to identify the infecting Brucella species. Despite this, early studies suggest that although brucellosis was endemic in extensive nomadic systems, seroprevalence was low, and brucellosis was not perceived as a real burden; recent studies, however, may reflect a changing trend. Concerning human brucellosis, no studies have identified the Brucella species and most reports provide only serological evidence of contact with Brucella in the classical risk groups; some suggest brucellosis misdiagnoses as malaria or other febrile conditions. The investigation of a severe outbreak that occurred in the late 1970s describes the emergence of animal and human disease caused by the settling of previously nomadic populations during the Sahelian drought. There appears to be an increasing risk of re-emergence of brucellosis in sub-Saharan Africa, as a result of the co-existence of pastoralist movements and the increase of intensive management resulting from growing urbanization and food demand. Highly contagious zoonoses like brucellosis pose a threat with far-reaching social and political consequences.
Introduction
Brucellosis is considered one of the most common global zoonoses [1]. Caused by the genus Brucella (the most common species being Brucella abortus, B. melitensis, and B. suis), the main clinical signs in animals are abortion and infertility. Brucellosis is highly contagious and is spread through contact with aborted foetuses, vaginal fluids, placentae, placental fluids, and milk, as well as congenitally and venereally. Animals are the only significant source of human brucellosis, and transmission is via direct contact (e.g., veterinarians, abattoir workers, and livestock keepers) and through consumption of unpasteurised dairy products. Human brucellosis is a grave and debilitating disease that may lead to permanent sequelae, requires prolonged and combined antibiotherapy, and is fatal in 1%–5% of untreated cases [2], [3]. Clinical signs are often ignored or incorrectly interpreted, and as a result, human brucellosis is severely underreported [1], [4], [5]. Eradicated in many developed countries after years of effort, brucellosis remains a major neglected zoonosis of low-income nations [1]. Low rates of transmission are typical of brucellosis in extensive systems, and intensification increases the risk of transmission because of higher stocking densities, increased animal contact, and higher birth index [1], [6]–[8]. Increasing co-location of pastoralist nomadism and transhumance with settled and commercial intensive farms may thus create conditions for brucellosis emergence. These circumstances occur in sub-Saharan Africa because of an exceptionally high rural–urban migration caused by the pull of expectation of a better life, and push of unfavourable environmental conditions on agriculture [9], [10].
There is a paucity of science-based evidence on brucellosis in sub-Saharan Africa [1], [4], [11]–[13], and an appraisal of historical and contemporary epidemiology (prevalence estimates, affected host species, potential reservoirs and Brucella species) is key to implementing measures for sustainable management of this disease. For a better understanding of these circumstances in the sub-Sahara, we present a review of reports on brucellosis in Nigeria.
Nigeria is the most populous country in Africa (over 170 million in 2012; http://esa.un.org/wpp/ASCII-Data/DISK_NAVIGATION_ASCII.htm) and has an estimated livestock population of 20.49 million cattle, 23.07 million sheep, 28.07 million goats, 6.54 million pigs (http://www.fao.org/ag/againfo/resources/en/glw/GLW_dens.html), 18,200–90,000 camels, and 210,000 horses (http://faostat.fao.org/site/573/default.aspx#ancor) [14]. Nigeria, India, Ethiopia, and Bangladesh account for 44% of poor livestock keepers globally, Nigeria ranking second [8]. Livestock production has always been important in Nigeria, and the rapidly emerging livestock sector now ranks second among the 20 poorest countries [8]. With a large pastoralist population, the livestock industry has been a major focus of government attention since the colonial era (Box 1). Approximately 70% of the population live in rural areas, but there is now considerable rural–urban drift. Increasing demand for animal products has resulted in expansion of animal trade, animal and human movements, and intensification of livestock production systems. The geographic, economic, and social conditions across Nigeria determine the ruminant livestock production systems (Box 2) [15].
Box 1. Some Events of Significance in the History of Brucellosis in Nigeria
Pre-colonial era (before 1900)
Livestock production (cattle and small ruminants) dominated by nomadic pastoralism (Fulani) in the savannah region of northern Nigeria. Agricultural land open to grazing post-harvest with mutual benefit of Fulani and farmers (fertilising effect of cow dung).
British colonial administration
1900–1930. Tsetse eradication, livestock breeding programmes, and mixed farming approaches. Establishment of Government Veterinary Field and Research Centres (Zaria, 1913; headquarters moved to Vom in 1924; expanded to include vaccine production).
1930s. Government sets up stock farms to improve local breeds (White Fulani, Gudali, and Shuwa). “Mixed Farming Policy” (use of grasslands and pasture by introducing fodder and selected browse plants) to promote agro-pastoralism and range management and livestock productivity.
1940s. Establishment of dairy herds and milk processing plants in Vom and Agege to meet expatriate population demand in Jos and Lagos.
Independence (1951) to Civil War (1967–1970)
-
1950s. Livestock Improvement and Breeding Centres established in Southwest to improve indigenous cattle (humpless dwarf Muturu and Keteku) by crossing with N'dama breed (from Guinea, Sierra Leone, and Congo). N'dama becomes the breed of choice in Southwest (white Fulani remain dominant in the North).
Western Nigeria Development Corporation established to promote importation of non-autochthonous breeds (South Devon cattle, Friesians, Holsteins, Brown Swiss, Jerseys) to upgrade local stock and increase milk production (most multiplication centres established in the Southwest, with some in the East and North).
Programmes to encourage settlement of nomadic pastoralists launched (supplementary feeding programme to secure year-round fodder [1962]; grazing reserves [1965 onwards] to protect grazing lands from expanding crop-farms and to resolve clashes over land-use).
Early 1960s. Smallholder steer fattening scheme (Food and Agriculture Organization project) using semi-intensive management systems introduced in the Southwest to ensure supply to local slaughterhouses.
Post-Civil War to present
Early 1970s. Nigerian Livestock and Meat Authority established to regulate all aspects of livestock industry and trade. Heavy investments in intensive feedlot fattening for beef.
1980s. Investment in direct livestock production reduces as the government focuses on livestock trade policy and oil industry. Dairy plants set up in Minna, Vom, Kaduna, but inadequate prices cause many to close down.
Post-1986. Government Structural Adjustment Programme Role (GSAPR) in livestock production initiated in 1986 to reform the Nigerian economy, including the livestock sector. The program dwindles, leading to a dominance of the private sector in livestock production. Research institutes (set up in the 1940s) no longer a priority for funding.
Box 2. Characteristics of Ruminant Livestock Production Systems in Nigeria
EXTENSIVE (SUBSISTENCE)
North—Pastoral systems (Nomadic or seminomadic)
Exclusive pastoralist
Livestock only (range, crop residues)
Large herds
Year-round movements, large range, no permanent homestead
Transhumant
Livestock more than crop (range)
Large herds
Seasonal migration (quality of grazing and tsetse flies)
Permanent homestead
Agro-pastoralists
Livestock more than crop (grazing near environs)
Medium-size herds
Semi-settled, low-range cattle movements
South and North—Traditional or village system (sedentary)
Seasonal tethering
Crop more than livestock (cut-and-carry)
Small herds
Fattening
Crop more than livestock (stall feeding)
Small herds
Scavenging
Crop more than livestock (scavenging of food scraps in village)
Small herds
Compound dairying
Crop more than livestock (stall-feeding or grazing close to homestead)
Small herds
INTENSIVE AND SEMI-INTENSIVE (COMMERCIAL)
All areas
Mixed farming
Crop equals livestock (integrated cropping with livestock rearing)
Variable size
South and North
Peri-urban and modern husbandry
Livestock only (crop residues, agricultural by-products, grazing)
Variable size
The climate varies from semi-arid in the North to tropical in the South. It is estimated that over a third of land that was cultivable 50 years ago is now desert across 11 of Nigeria's northern states and that over 15 million pastoralists are threatened by decreasing access to water and pasture [16]. About half of the semi-arid and sub-humid zones in northern Nigeria are livestock and mixed crop-livestock dominated. Dairy production is concentrated in the North and the beef industry, mostly in the South. Nomadic herdsmen manage about 90% of ruminants and practice seasonal transhumance or year-round nomadism [17], [18]. The Northeast has a hot, dry climate from January to June and rain from June to September. Transhumance is practiced to accommodate variations in available vegetation and agricultural practices and to avoid tsetse flies [19]. In the humid areas of the southern, western, and eastern states, mixed crop-livestock systems dominate, and sheep, goats, and pigs are more important. Pastoralism has been evolving in Nigeria, with farmers often combining cattle production with crop cultivation [20]. Herd sizes have been decreasing as pastoralists are becoming more settled, enabling them to pursue crop farming. Mohammed [21] mentions that a large population of agro-pastoralists settling in the hinterlands of the urban centres in Oyo State were cattle pastoralists displaced from their traditional territories in the North by a variety of agro-ecological and socioeconomic factors. This influx stimulated a new system of livestock production.
The majority (80%) of cattle, mainly Zebu, are concentrated in the savannah zone, with only 10% of the remaining 20% (mostly Bos taurus) in the South [15] in a range of management systems (Box 2). Cattle are usually extensively managed, either under nomadic or seminomadic pastoral systems or, to a lesser extent, under traditional village systems, often in contact with small ruminants belonging to the same household. There is more intimate contact between cattle and sheep as they are co-grazed, while goats are left to scavenge free-range. In nomadic systems, small ruminants are sold and exchanged, serving as a “current account,” whereas cattle are traded for status and serve as a “savings account” [22], [23]. Commercial, intensive farms are few and are located on the periphery of major towns in northern and western Nigeria. Cattle reared in extensive systems of the North and the Northeast are transported across Nigeria to the abattoirs of the Southwest to meet the high demand from the economically developed South [24], [25]. According to early reports, 20% of cattle are imported, mostly from Chad and Niger [13].
Methods
A database search (PubMed, GoogleScholar, Cabdirect, and African Journals Online) was undertaken using broad terms (Brucel* or zoonos* plus Nigeria or Africa) and screened for brucellosis and Nigeria. References in the identified articles were also screened, yielding a total of 164 publications, of which 37 were unobtainable (mostly local journals). Of the remaining 127 publications, 16 were excluded because they were duplicates or were not supported by diagnostic tests. The cattle and small ruminant studies rejected are presented in Tables S1 and S2, respectively.
We used this broad inclusion criterion because (i) only one study (limited to seroprevalence in cattle) met strict scientific criteria and (ii) a critical appraisal of grey literature allowed us to identify presence of the disease, limitations in the use of diagnostic tests, epidemiological aspects, and gaps from which lessons can be drawn. Both the first and corresponding author read all references.
The studies were largely heterogeneous. To summarize their content, we first grouped data by host (cattle, sheep, goats, camels, pigs, horses and donkeys, chickens, dogs, and humans). The data extracted for cattle, small ruminants, and humans are summarised in Tables 1, 2, 3, and 4; Tables S3, S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15. Data for other species are discussed in the text (see “Brucellosis in other animals” below). When several hosts were included in the same study, we listed each in the corresponding Table (the common source can be identified in the references cited in the Tables). For cattle and small ruminants, studies were further separated out into farm studies, abattoir or meat market studies, and milk market studies. The farm studies were then further subdivided according to livestock production system (intensive, extensive, or not specified). Where multiple surveys (e.g., abattoir and farm) were reported in a single study, each survey was listed separately. Data were extracted from each reference on:
Table 1. Summary of brucellosis serology studies in cattle in Nigeria.
| Row label | Population/Production system | Tests, number studies, number individuals and number herds on which INDIVIDUAL PREVALENCE is based | Range of ind prev (%) | Tests, number studies and number. herds on which HERD PREVALENCE is based | Range of herd prev (%) | Refs. | ||||||
| Tests(number studies)1 | Number studies2 | Number individuals3 | Number studies4 | Number herds5 | Tests(number studies)6 | Number studies7 | Number herds8 | |||||
| Farm | ||||||||||||
| A | Intensive | SAT (6), RBT (4), RPT (2), MRT (1) | 13 | 4341 | 12 | >47 | 0–47 | SAT (5), RBT (4), MRT (1) | 10 | 37 | 0–100 | [25], [29], [32], [47], [53], [54], [97]–[103] |
| B | Extensive | RBT (2), MRT (2), MRT/RBT (1) | 5 | 4974 | 4 | >171 | 2–15 | MRT/RBT(1) | 1 | 8 | 13 | [27], [41], [104]–[106] |
| Int/Ext 9 | ||||||||||||
| C | Intensive | RBT (2), RPT (2), SAT/CFT (1), RBT/ELISA (1) | 6 | 3784 | 2 | >20 | 3–33 | SAT/CFT (1) | 1 | 9 | 100 | [13], [28], [39], [40], [42], [107] |
| D | Extensive | RBT (2), RPT (2), SAT/CFT (1), RBT/ELISA (1) | 6 | 6783 | 2 | >259 | 0–45 (41) 10 | SAT/CFT (1) | 1 | 4 | 0–100 | |
| E | Not specified | RBT (3), CT/MRT (1) | 4 | 5576 | 3.5 | >199 | 0–50 | RBT (2), CT/MRT (1) | 3 | 134 | 0–44 | [23], [38], [58], [108] |
| F | Abattoir | RBT (15), RPT (1), SAT (1) | 17 | 14265 | NA | NA | 0–22 | NA | NA | NA | NA | [13], [40], [58], [108]–[121] |
| G | Milk Market | MRT (2) | 2 | 410 | NA | NA | 7–12 | NA | NA | NA | NA | [41], [106] |
Range of diagnostic tests and respective number of studies for each test on which individual prevalence values in table have been based (see text).
Number of studies on which total number of individuals sampled and individual prevalence values have been based.
Sum of animal sample size for each study for which individual prevalence data is available.
Number of studies, out of total number of studies on which individual prevalence is based, which report number of herds sampled.
Minimum estimate of number of herds sampled for each production system category. Not all studies reported number of herds sampled, hence true value must be superior (>) to that in table.
Range of diagnostic tests and respective number of studies on which herd prevalence values in table have been based (see text).
Number of studies on which total number of herds sampled and herd prevalence values have been based.
Sum of number of herds sampled for each study for which herd prevalence data is available.
Studies sampling extensive and intensive flocks in parallel.
Value of 41% prevalence corresponds prevalence non-adjusted for sensitivity and specificity (apparent prevalence = [true prevalence (0.879+0.998−1)]+1−0.998]; 0.998 = specificity of RBT*ELISA in test series; 0.879 = sensitivity of test series, see Mai et al. 2012).
Table 2. Summary of brucellosis serology studies in sheep (S) and goats (G) in Nigeria.
| Row label | Population Production system | Tests, number studies and number individuals on which INDIVIDUAL PREVALENCE is based | Range of ind prev (%) | Tests, number studies and number flocks on which FLOCK PREVALENCE is based | Range of flock prev (%) | Refs. | ||||||||||||
| Test (number studies)1 | Number studies2 | Number Individuals3 | Test (number studies)4 | Number studies 5 | Number flocks6 | |||||||||||||
| Species | S | G | S | G | S | G | S | G | S | G | S | G | S | G | S | G | ||
| Farm | ||||||||||||||||||
| A | Intensive | RBT (4), RPT (1), SAT (1) | RBT (2), RPT (1) | 6 | 3 | 594 | 234 | 0-76 | 0-33 | RBT (4), SAT (1) | RBT (2) | 5 | 2 | 5 | 2 | 100 | 100 | [45], [47], [53], [101], [122], [123] |
| B | Extensive | RBT (1) | RBT (2) | 1 | 2 | 210 | 643 | 5 | 6-29 | NA8 | NA | 0 | 0 | NA | NA | NA | NA | [22], [124] |
| Int/Ext 7 | ||||||||||||||||||
| C | Intensive | RBT (2), SAT (1) | RBT (2) | 3 | 2 | 734 | 1053 | 0-21 | 5-21 | NA | NA | 0 | 0 | NA | NA | NA | NA | [54]–[56] |
| D | Extensive | RBT (2), SAT (1) | RBT (2) | 3 | 2 | 570 | 557 | 2-13 | 6-16 | NA | NA | 0 | 0 | NA | NA | NA | NA | |
| E | Not specified | RBT (1) | SAT (2), RBT (1) | 1 | 3 | 50 | 985 | 2 | 0-5 | NA | NA | 0 | 0 | NA | NA | NA | NA | [44], [54], [123] |
| F | Abattoir | RBT (6), SAT (1) | RBT (8), SAT (2) | 7 | 10 | 1376 | 6656 | 0-15 | 0-17 | NA | NA | NA | NA | NA | NA | NA | NA | [44], [50], [51], [55], [57], [58], [113], [117], [118], [123] |
Range of diagnostic tests and respective number of studies for each test on which individual prevalence values in table have been based (see text).
Number of studies on which total number of individuals sampled and individual prevalence values have been based.
Sum of animal sample size for each study for which individual prevalence data is available.
Range of diagnostic tests and respective number of studies on which flock prevalence values in table have been based (see text).
Number of studies on which total number of flocks sampled and herd prevalence values have been based.
Sum of number of herds sampled for each study for which flock prevalence data is available.
Studies sampling extensive and intensive flocks in parallel.
Not applicable.
Table 3. Summary of brucellosis RBT studies in cattle in Nigeria.
| Row label | Population/Production system | Number studies, number individuals and number herds on which INDIVIDUAL PREVALENCE is based | Range of ind prev (%) | Number studies and number herds on which HERD PREVALENCE is based | Range of herd prev (%) | Refs. | ||||
| Number studies1 | Number individuals2 | Number studies3 | Number herds4 | Number studies5 | Number herds6 | |||||
| Farm | ||||||||||
| A | Intensive | 4 | 333 | 4 | 12 | 0–33 | 4 | 12 | 0–100 | [32], [97], [98], [101] |
| B | Extensive | 2 | 3561 | 2 | 133 | 2–16 | 0 | NA8 | NA | [104], [105] |
| Int/Ext 7 | ||||||||||
| C | Intensive | 2 | 152 | 0 | NA | 3–8 | 0 | NA | NA | [39], [40], [42] |
| D | Extensive | 2 | 270 | 0 | NA | 5–12 | 0 | NA | NA | |
| E | Not specified | 3 | 3926 | 2.5 | >174 | 0–50 | 2.5 | 109 | 0–22 | [38], [58], [108] |
| F | Abattoir | 15 | 12079 | NA | NA | 0–22 | NA | NA | NA | [40], [58], [108]–[111], [113]–[116], [118]–[121] |
Number of studies using RBT on which individual prevalence values in table have been based (see text).
Sum of animal sample size for each study for which individual prevalence data is available.
Number of studies, out of total number of studies, on which individual prevalence is based, which report number of herds sampled.
Minimum estimate or true number of herds sampled for each production system category. Not all studies reported number of herds sampled, hence true value must be superior (>) to that in table.
Number of studies using RBT on which herd prevalence values in table have been based (see text).
Sum of number of herds sampled for each study for which herd prevalence data is available.
Studies sampling extensive and intensive flocks in parallel.
Not applicable.
Table 4. Summary of brucellosis RBT studies in sheep (S) and goats (G) in Nigeria.
| Row label | Population Production system | Number studies and number individuals on which INDIVIDUAL PREVALENCE is based | Range ind prev (%) | Number studies and number flocks on which FLOCK PREVALENCE is based | Range flock prev (%) | Refs. | ||||||||
| Number studies1 | Number individuals2 | Number studies3 | Number flocks4 | |||||||||||
| Species | S | G | S | G | S | G | S | G | S | G | S | G | ||
| Farm | ||||||||||||||
| A | Intensive | 4 | 2 | 179 | 124 | 14–76 | 21–33 | 4 | 2 | 4 | 2 | 100 | 100 | [47], [101], [122], [123] |
| B | Extensive | 1 | 2 | 210 | 643 | 5 | 6–29 | 0 | 0 | NA6 | NA | NA | NA | [22], [123], [124] |
| Int/Ext 5 | ||||||||||||||
| C | Intensive | 2 | 2 | 681 | 1053 | 0–21 | 5–21 | 0 | 0 | NA | NA | NA | NA | [54]–[56] |
| D | Extensive | 2 | 2 | 521 | 557 | 5–13 | 6–16 | 0 | 0 | NA | NA | NA | NA | |
| E | Not specified | 1 | 1 | 50 | 28 | 2 | 0 | 0 | 0 | NA | NA | NA | NA | [54] |
| F | Abattoir | 6 | 8 | 846 | 3890 | 0–15 | 0–17 | NA | NA | NA | NA | NA | NA | [50], [51], [55], [57], [58], [113], [118], [123] |
Number of studies using RBT on which individual prevalence values in table have been based (see text).
Sum of animal sample size for each study for which individual prevalence data is available.
Number of studies using RBT on which herd prevalence values in table have been based (see text).
Sum of number of herds sampled for each study for which herd prevalence data is available.
Studies sampling extensive and intensive flocks in parallel.
Not applicable.
population origin,
sampling method (probability or nonprobability sampling),
sampling approach (brucellosis investigation, random sampling, multistage sampling, systematic sampling, purposive selection, convenience sampling, etc.),
diagnostic test used and cut-off (see below),
bias and/or gaps in sampling method description,
location of study,
period of sampling,
sample size (total number of animals/humans sampled and total number of herds/flocks if information available),
seroprevalence (individual and herd/flock if available).
The intensive farm population (Rows A and C in Tables 1, 2, 3, and 4 and in Tables S3, S5, S10, S12) corresponds to commercial, government or research institutes, and the extensive farm population (Rows B and D in Tables 1, 2, 3, and 4 and in Tables S4, S5, S11, and S12) to Fulani or Indigene (one study only) herds/flocks exclusively. Based on personal field experience in Nigeria, we considered differences in livestock management (for example, nomadic and seminomadic Fulani) across herds of the same category to be of limited significance and merged the values. Studies where the population was not specified were categorised as such (Row E in Tables 1, 2, 3, and 4 and Tables S6 and S13). Some studies conducted surveys in extensively and intensively reared livestock in parallel, and the data for these have been considered separately under Row C and D of Tables 1, 2, 3, and 4 and in Tables S5 and S12. Data from abattoir or meat market studies are summarised in Row F of Tables 1, 2, 3, and 4 (and Tables S7 and S14) and milk market studies in Row G of Table 1 (and Table S8).
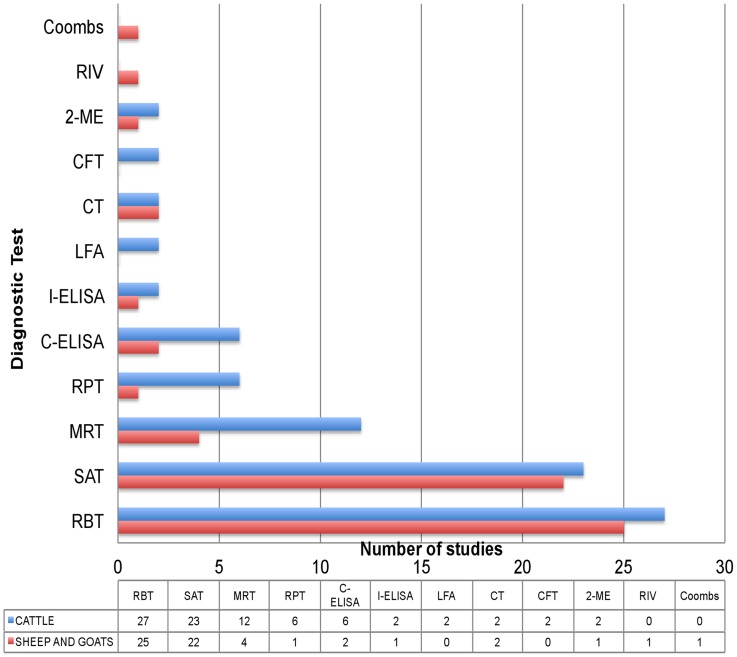
Most studies screened sera (blood or milk) with more than one serological assay and therefore report a seroprevalence value based on the results of each individual test. The number of cattle and small ruminant studies which have used classical tests such as the rose Bengal test (RBT), card test (CT), serum agglutination test (SAT), rapid plate test (RPT), 2-mercaptoethanol test (2-ME), rivanol test (RIV), coombs test, complement fixation test (CFT), milk ring test (MRT), and more recent diagnostic assays such as the competitive ELISA (C-ELISA), indirect ELISA (I-ELISA), and lateral flow assay (LFA) are summarised in Figure 1. To summarise and compare data we select one test seroprevalence value per study in this preferential order: RBT (or the equivalent Card Test), CFT, RPT, and SAT (all in blood serum). In studies where only milk was screened with MRT, these values are reported. The rationale for this preferential selection of tests is the superior sensitivity/specificity (in the absence of brucellosis vaccination) of the prioritized tests [26]. Four authors did not report individual test results: Esuruoso [13], who considered samples positive when they were positive for SAT confirmed by CFT for suspicious samples; Alausa [23], who considered samples positive when positive for the card test or MRT or both; Pullan [27], who used MRT screening at herd level and then RBT on individual animals of MRT positive herds; and Mai [28] who confirmed RBT positive or inconclusive samples with C-ELISA. In these cases, we used the positive/negative data provided.
Figure 1. Number of cattle and small ruminant studies which have used the rose Bengal test (RBT), card test (CT), serum agglutination test (SAT), rapid plate test (RPT), 2-mercaptoethanol test (2-ME), rivanol test (RIV), Coombs test, complement fixation test (CFT), milk ring test (MRT), and more recent diagnostic assays such as the competitive ELISA (C-ELISA), indirect ELISA (I-ELISA), and lateral flow assay (LFA) for serological screening.
The data table corresponds to total number of studies that have employed each test for each species. The overall number of studies is greater than the total number of papers retrieved because most papers screened sera with more than one serological assay.
The presentation of average prevalence values calculated from studies using different tests, in different populations, and using different sampling designs is not valid, and so we present only prevalence ranges. We did not average values across analogous livestock production systems using weighting approaches taking into account test performance or sample size because (i) the lack of standardization of tests (origin of antigens, positive and negative controls, cut-off criteria), (ii) the application of brucellosis vaccination in some of the herds tested in earlier studies, and (iii) nonprobability sampling across studies would have led to misleading estimates of average prevalence. These circumstances limit the interpretation of the range of prevalence values presented in Tables 1 and 2. In an attempt to overcome some of these limitations, we consider the RBT values only in Tables 3 and 4, which yield narrower ranges as they are based on fewer studies and a simpler, more robust test, but the overall pattern when comparing intensive and extensive populations is the same (see below).
Results
Period of sampling and spatial distribution
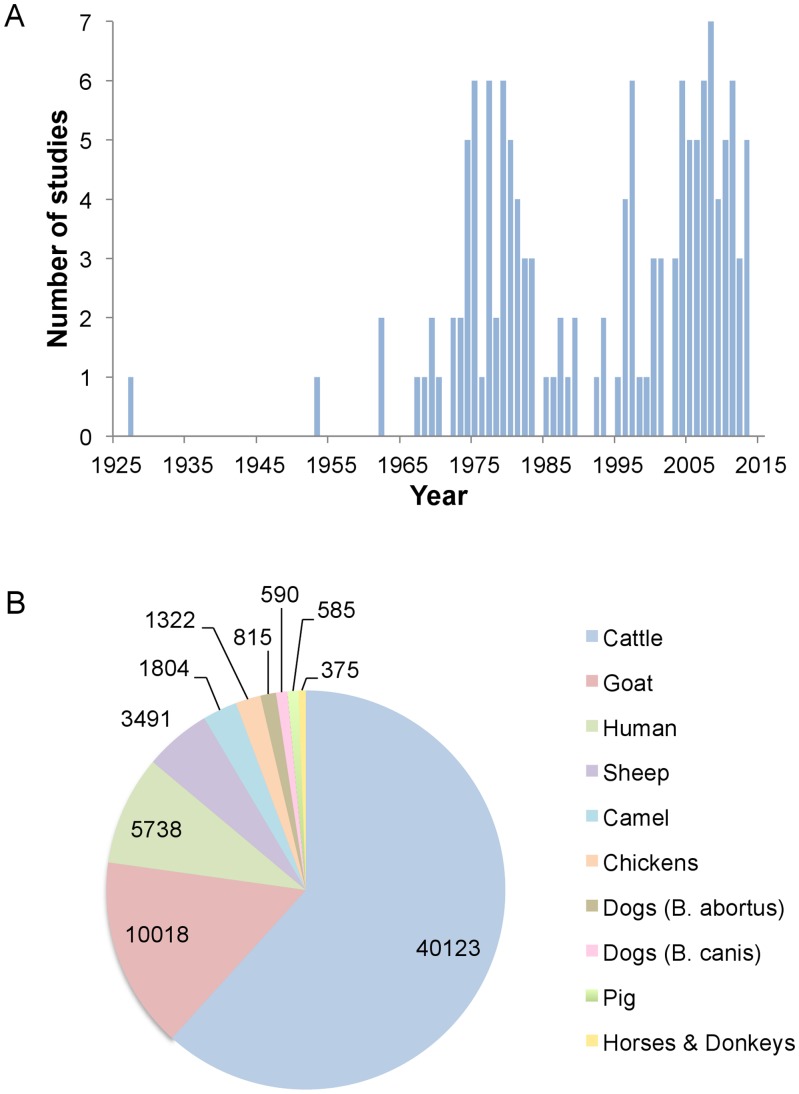
Historically, two peaks of brucellosis reporting are evident (Figure 2A): the first coincided with establishment of intensive government farms in the 1970s to promote meat production and reduce imports (Box 1); the second with the post-millennium development goals public health agenda, increased interest in neglected zoonotic diseases, and private sector growth. Significantly, the trough coincides with the oil boom of the 1970s (Box 1). Figure 2B shows studies by animal species and Figure 3, the spatial distribution of animal and human studies.
Figure 2. Distribution of studies on brucellosis in Nigeria according to (A) year of publication and (B) host investigated (numbers correspond to cumulative sample size across all studies for each host species).
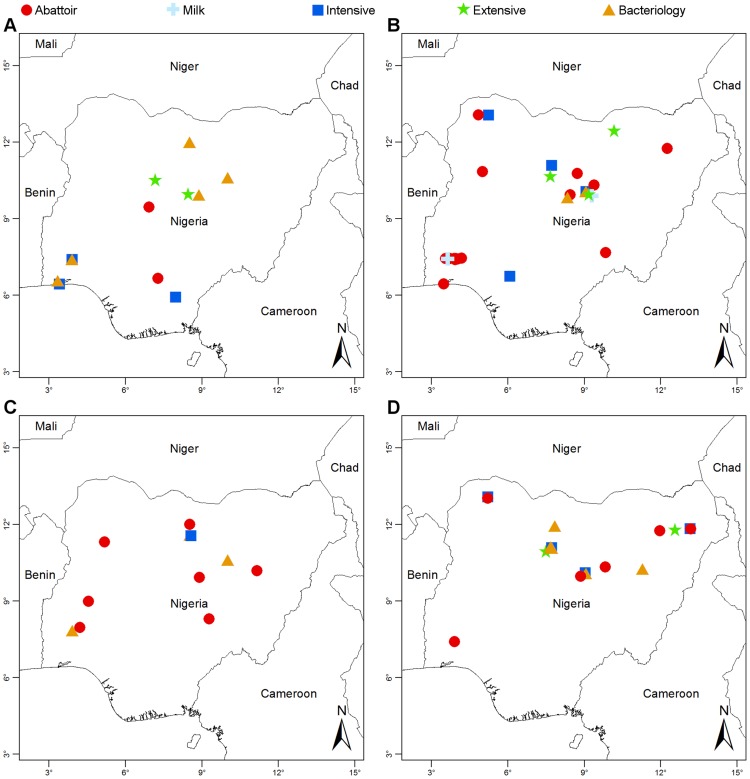
Figure 3. Location of brucellosis studies in Nigeria.
(A) cattle; (B) sheep and goats; (C) camels and pigs; and (D) humans.
Cattle brucellosis
To understand brucellosis epidemiology, it is necessary to determine the circulating Brucella species and biovars and, as antibodies are not species specific, bacterial isolation is essential. Since brucellosis was first reported in Nigeria in 1927 [29], only five studies have provided bacteriological data for cattle (Figure 3). In the West, studies in range cattle and in a University herd described the isolation of Brucella strains, probably B. abortus [30]. This species was properly identified in studies in government and private farms and in settled Fulani herds in the Centre and North [31]–[33]. In total, 58 isolates were classified as B. abortus biovar 1 (54 strains), biovar 2 (1 strain), biovar 3 (2 strains), and biovar 4 (1 strain) (see Table S9). However, re-examination of 20 of the biovar 1 isolates shows characteristics of biovar 3, the dominant biovar in countries proximal to Nigeria [34]. Moreover, VNTR genotyping [35] clusters these 20 strains with biovar 3a rather than 3b, the latter being typically reported in Europe (Ducrotoy, Bertu, Moriyón, and Ocholi, unpublished results). B. melitensis has not been reported in cattle, although there is close contact with small ruminants.
The bulk of the evidence is derived from serological studies (Figure 1), but limitations in the application of serological tests make data difficult to interpret. Early studies used RPT or SAT, two tests lacking sensitivity and specificity [26], [36], [37]. The RBT (or the equivalent Card Test) was applied shortly after its development and has been widely used (Tables 1 and 3; Figure 1). Despite the excellent specificity and sensitivity of RBT [26], [36], [37], the literature reviewed reflects the misconception that RBT is a test of low specificity which, in the absence of brucellosis vaccination or the false positive serological reaction phenomenon caused by crossreacting bacteria, needs to be confirmed. However, meta-analysis performed using strict criteria [26] shows that RBT specificity is in fact better than that of iELISA and cELISA, two tests used in some works to “confirm” the RBT results. Indeed, the OIE Manual (http://www.oie.int/en/international-standard-setting/terrestrial-manual/access-online/; Chapter 2.4.3. Bovine Brucellosis) clearly states that these other tests can also sometimes give a positive result because of S19 vaccination or of false-positive serological reactions.
While RBT is a good choice, inadequate standardization results in considerable sensitivity (but not specificity) variation [37]. RBT standardization and origin was inadequately described in 15 out of 46 papers and six investigations used locally prepared antigens. Competitive or indirect ELISA kits were used according to manufacturer instructions but were never validated under local conditions (cut-offs established in brucellosis-free and good hygienic conditions cannot be extrapolated to endemic areas [38]).
Across Nigeria, 14,000, 11,000, and 8,000 cattle have been sampled in different studies from abattoirs (animals from both extensive and intensive systems), extensive, and intensive herds, respectively, but the data (Tables 1 and 3; Tables S3, S4, S5, S6, S7, S8, S9; Figures 2A and 3A) illustrate the limitations in time and space of the studies. A total of 1,800 cattle correspond to the North, half this number (1,000) to the West and only small numbers to the East and South. Abattoir studies cannot provide spatial information due to country-wide animal movements (see above). Only five out of the 46 prevalence studies applied probability based sampling methods [28], [39]–[42], and only one describes the method in sufficient detail [28], but even this study is biased, because herds were selected based on proximity to a reliable laboratory and farmer cooperation. Studies of intensive farms have focused mainly on infertility or abortion outbreaks, and few cattle were sampled (Table 1). Most intensive system studies were undertaken in the West before 1986 (Figures 2A and 3), a period of intense interest in the livestock sector (Box 1 and Table 1, Row A). Since 1986, more investigations have been reported in extensive cattle systems (Table 1, Row B) and from abattoirs (Table 1, Row F). Clearly there are few good-quality data on brucellosis in Nigeria, and discussion must bear in mind these limitations.
Extent to which the extensive and intensive cattle management systems are affected by brucellosis
In Nigeria, most cattle are reared extensively in the North and belong to nomadic, seminomadic or transhumant Fulani pastoralists. According to early official veterinary records, brucellosis was not regarded as a hazard in these herds [29], [43] and most studies conducted independently in the extensive and intensive systems suggest a lower prevalence in the former (Tables 1 and 3, Rows A and B; Table S4). This was the view of early investigators [13], [32]. Esuruoso wrote, “Cattle…in nomadic herds…on the move… are not likely to accumulate infection or spread it from one animal to the other as in settled herds. This factor, and the intense heat of the sun in fairly open country (Sudan Savannah zone) will provide some of the reasons for the low infection rate…in the northern herds… It would appear, therefore, that nomadic herding in Nigeria imposes a natural limit on the rate of brucellosis infection in cattle.” This observation is consistent with the low transmission deemed typical of pastoralist systems [7].
The inverse profile can be observed for studies that have looked at intensive and extensive system populations in parallel (Tables 1 and 3, Rows C and D; Table S5). A recent probability sampling study [28] (performed in Adamawa, Kaduna, and Kano, northern Nigeria), reports RBT seroprevalences of 45.1% (nomadic), 22.0% (seminomadic), 23.8% (commercial), and 15.9% (zero-grazing). Using a competitive ELISA kit as the reference, the authors assumed that 42.8% to 24.7% of these RBT results were false positives, but higher prevalence in the extensive than intensive system was also observed with the ELISA. Another recent, but more limited, work reported higher (but not statistically significant) numbers of RBT positives in extensively than in intensively managed herds (11.6% versus 3.1%, respectively) in Plateau State (North Central Nigeria) [42]. These results suggest that brucellosis prevalence has been on the increase in extensive systems over time [28]. However, in a recent cross-sectional survey using RBT standardised according to OIE criteria, seminomadic Fulani cattle (n = 2000) showed less than 1% individual seroprevalence in the Kachia Grazing Reserve (Kaduna) (ICONZ, 2013, www.iconzafrica.org). The reasons for the differences between this and earlier work are unclear. Although intensification provides opportunities for better control measures, their implementation cannot be taken for granted because this requires adequate infrastructure and training and, indeed, the risks of transmission are greatly increased [1], [6], [7]. None of these recent studies describe control measures in intensively managed herds that could account for the lower prevalence reported. On the other hand, at least in the Kachia Grazing Reserve, Fulani have intuitive disease-reducing management approaches (e.g., rapidly selling or slaughtering animals that abort and those with poor fertility or low milk yields), and low reproductive rates reduce transmission [7]. As discussed below, these aspects of brucellosis epidemiology are not trivial, and further studies are necessary to confirm whether there is an increase of brucellosis in extensively managed herds and its distribution across the country. Unfortunately, the gap in information between the early 1980s and late 1990s precludes any possibility of doing this with the data available (Figure 2A).
Extensive nomadic herds as reservoirs of disease
Brucellosis transmission is generally lower in pastoralist systems because of low reproductive rates, animal movements and environmental circumstances [7]. However, brucellosis transmission could increase as a result of the settling of migratory herds and emerge from increased contacts between these herds and unprotected intensive commercial or settled semi-intensive herds. This possibility has seldom been investigated in sub-Saharan Africa. One article provides evidence of this kind of transmission and of its dramatic impact on susceptible populations in the 1970s [23]. In a large brucellosis outbreak in Ibapara, out of ten governments, three private settled, and 12 Fulani herds tested, 11 herds were found to be positive using a combination of the MRT and Card Test. All 11 positive herds belonged to Fulani pastoralists, “nomadic herdsmen that move only within the district, and within few kilometres from previous settlements.” The outbreak coincided with the Sahelian drought that saw a general reduction in the cattle population of Nigeria and prompted an influx and settling of nomadic herds in Ibapara. The outcome was a widespread epidemic of bovine brucellosis with a severe increase in human cases. Fulani herdsmen complained of being unwell and unable to look after their cattle, and 51.5% of herdsmen, 23.5% of abattoir workers, and 3.1% of high school students were serologically positive with the Card Test. Calf losses were reported, resulting in a shortage of meat and protein undernutrition in the local populace.
Brucellosis in small ruminants
Small ruminants represent a major source of meat in Nigeria and are often reared alongside cattle. Their distribution is not known with certainty; Falade et al. [44] cite early sources, according to which 70% of goats were in the North, 20% in the East and 10% in the West, and about 60% of rural households in the northern, 50% in the eastern and 40% in the western states kept goats.15% of sheep and goats were reared under nomadic conditions at the end of the 20th century [22].
Bacteriological evidence for Brucella in small ruminants is scarce (Figure 3; Table S15). An early study claimed the isolation of B. abortus in sheep and goats, but the methodology used in species identification is unclear [45]. B. melitensis biovar 1 (22 strains) and B. abortus biovar 1 (8 strains) were isolated from goats in western Nigeria [46]. However, the reported biochemical characteristics of the B. melitensis strains are atypical. B. melitensis was recently described in sheep and goats in northern Nigeria but the ten strains were not definitively typed [24]. A study in Bauchi (central Nigeria) clearly demonstrated B. abortus but not B. melitensis in sheep [33]. Interestingly, seven B. abortus strains were isolated from sheep reared in contact with infected cattle [47]. Although B. abortus preferentially infects cattle, it is known to persist in sheep [48] and the significance of B. abortus infection in small ruminants in the mixed breeding systems of sub-Saharan Africa requires further investigation.
There are fewer and more limited serological studies in small ruminants than in cattle (Figure 2B; Tables 2 and 4; Tables S10, S11, S12, S13, S14). Significant misuse of tests were application of MRT (not useful in small ruminants [49]) in four studies and interpretation that animals were infected by B. melitensis based on a comparison of titres to B. abortus and B. melitensis antigens [50]–[52], a discrimination that is not possible by serology and indicates inadequate antigen standardization.
Studies in intensive or semi-intensive systems are not only scarce but also biased because most investigations focused on cattle abortions with simultaneous sampling of small ruminants (compare references in Tables 1 and 2 and Tables S3 and S10). In fact, contagion from cattle was often considered the origin of infection. Only one study was performed on intensively or semi-intensively raised small ruminants in the West [44]; the others for this region consisted of abattoir surveys (Tables 2 and 4). Studies in extensive systems were all undertaken in the North (Rows B and D in Tables 2 and 4; Table S11 and S12); hence, the epidemiology in sedentary and nomadic flocks in other regions is unknown. Although values broadly suggest that brucellosis prevalence is higher in intensive than extensive systems for small ruminants (Tables 2 and 4, Rows A, B, C, and D, Tables S10, S11, S12) these trends have to be interpreted with caution.
According to two studies performed in the 1960s, small ruminant brucellosis was not a problem on government farms, but most surveys were undertaken in the cattle-dominated North; hence, no information was available for other regions (Figure 3B) [53], [54]. Fifteen years later, one study in northern Nigeria later found significant rates of infection (13.8% and 15.1% averages for sheep and goats, respectively) [55]. This same study reported rates of infection in institutional (i.e., intensive) flocks about four times higher than in local (extensive) flocks for both sheep and goats (Table 2), and attributed the difference to an increased transmission caused by intensification [55]. A recent study [56] found overall prevalence values of 9.3% for sheep and 10.1% for goats, which are comparable to the values found 30 years previously [55], but husbandry-specific values were not obtained.
Ten studies have investigated sheep and goats for brucellosis in trade settings (Table 2, Row F; Table S14), and while values do not reflect the situation at farm level, they confirm the presence of brucellosis in small ruminants in the North. Two abattoirs studies in the West found low prevalence values (0.3%–0.9% and 0% for goat and sheep, respectively) [57], [58], but since animals come mostly from other parts of Nigeria, the situation in the West remains unknown.
Brucellosis in other animals
B. abortus has been isolated from horses [33], [59], and antibodies have been reported in donkeys [60], dogs [61]–[63], and fowl [64]–[67] in Nigeria (Figure 2B). However, the role of these nonruminant species in disease transmission has never been satisfactorily proven [68] and, as they are unable to act as reservoirs, once brucellosis is eradicated in domestic ruminants, they are considered as spillover hosts or sentinels.
Camels are distributed along the northern borders of Nigeria, and nomadism is common, often across borders. At the turn of the 20th century, estimated numbers of camels in Nigeria varied from 90,000 [14] to 25,000, substantially greater than an estimate of 18,000 in 1978 [69]. Both B. abortus and B. melitensis can infect camels, but Brucella has never been isolated from these animals in Nigeria [70]–[72]. Serological studies are particularly difficult to interpret because brucellosis tests have not been properly evaluated in these animals [73]. Abattoir studies in northern Nigeria reported 1.3%–14.8% seropositivity using SAT [14], [69], [74], [75] in camels from Nigeria and Chad, Niger, and Cameroon (Figure 3C). In Borno State, two MRT and RBT studies of range camels reported positive animals [70], [75]. However, the MRT has been proven useful only in cattle [49], and the RBT is dependent on the effect of acidic pH on ruminant IgG and IgM [76], [77]. Since camelids and ruminants differ markedly in immunoglobulin repertoire and structure [78], RBT results should be interpreted with caution. Camels are herded with sheep and goats and, to a lesser extent, cattle [69], and their role in the epidemiology of brucellosis in Nigeria is unclear.
Pigs represent approximately 4.5% of the meat market in Nigeria [79]. An early study claimed isolation of B. suis from animals positive in SAT [80] but a small-scale bacteriological study failed to isolate Brucella [33]. An investigation in government farms during a cattle abortion outbreak [53], a study in intensive and semi-intensive farms in the South [79], and an abattoir study in the West [58] found no or very few RBT positive animals. In contrast, a recent abattoir study in Central Nigeria reported 30% of 281 pigs RBT positive (Figure 3C) [81]. In the absence of bacteriological evidence or protein-based tests, these data have to be interpreted with caution, because pigs are prone to false positive serological reactions with RBT, CFT, and ELISA [82].
Control of animal brucellosis
Brucellosis control was initiated in colonial Nigeria in 1917; vaccination was applied to address widespread bovine abortions in government-owned farms and local production of a liquid S19 vaccine started at this time. A test and slaughter policy was also implemented [83], and its failure was attributed to a lack of rigor in implementation [28]. Production of lyophilised S19 started in 1950 [12], and by 1951, brucellosis eradication and control programmes succeeded in establishing brucellosis-free stock and reducing overall prevalence to less than 5% on government farms [28]. Efforts waned and vaccine production discontinued in 1954 [12] and today there is no government policy for brucellosis control in Nigeria. Nevertheless, local researchers estimated that brucellosis caused approximately 20% financial losses in traditional systems of cattle production in one Nigerian grazing reserve [84] and concluded that, as the nomads settle in these reserves, hygienic measures and brucellosis vaccination are profitable and should be implemented [85]. A recent study identified brucellosis and milk loss as the greatest components of the direct economic losses associated with reproductive disorders in settled herds in Zaria, Nigeria [86].
Human brucellosis
The first cases of human brucellosis confirmed by laboratory tests were reported in Nigeria in 1941 [87] and 1962 [88], and even during this period, underdetection was suspected [89]. A decade later, few laboratories could perform these tests and this, combined with low suspicion, was again thought to lead to underdetection [90]. This review shows that these circumstances have not changed.
Human seroprevalence data are summarized in Table 5, and Figure 3D shows the geographical location of studies. Although they strongly suggest the importance of the human disease, exact figures cannot be derived from most surveys. The studies based solely on RBT confirm exposure to Brucella of butchers, abattoir workers, and herdsmen. However, they do not necessarily represent the proportion of true disease, because a positive RBT result can be caused by contact or infection and needs to be interpreted according to the clinical picture [76]. Several studies complemented RBT with SAT and 2-mercaptoethanol tests, both of which detect only agglutinating antibodies; since these antibodies disappear in long-standing cases, the data only reflect recent infections. Moreover, SAT diagnostic titre varies from 50 to 200 international units (the diagnostic titre most often used in Nigeria was of 100 international units) depending on the origin (urban or rural and endemic or non-endemic areas) and exposure of the patient [76]. Complementary tests that detect non-agglutinating antibodies (competitive ELISA, Coombs, and CFT) were implemented in only two studies, one using competitive ELISA whose diagnostic cut-off for human brucellosis is unknown [76].
Table 5. Summary of brucellosis studies in humans in Nigeria.
| Region | Diagnostic test (cut-off) | Complementary tests | % Prevalence (n) | Refs. | |
| Occupationally exposed | |||||
| Abattoir workers | West | SAT (100 iu) | 2-ME | 39 (170) | [25] |
| West | RBT | 24 (51) | [23] | ||
| North | RBT | SAT | 0 (40) | [123] | |
| South | SAT (NS) | 27 (164) | [125] | ||
| Butchers & abattoir workers | West | RBT | 64 (11) | [58] | |
| Butchers | West | SAT (100 iu) | 2-ME | 21 (38) | [25] |
| West | SAT (100 iu) | 2-ME | 16 (51) | [25] | |
| North | RBT | SAT | 5 (101) | [113] | |
| Herdsmen | West | SAT (100 iu) | 2-ME | 74 (104) | [25] |
| West | SAT (100 iu) | 2-ME | 12 (99) | [25] | |
| West | SAT (100 iu) | 2-ME | 5 (44) | [25] | |
| North | SAT (100 iu) | 70 (71) | [126] | ||
| West | RBT | 2-ME | 51 (173) | [23] | |
| West | SAT (100 iu) | 2-ME | 7 (20) | [102] | |
| West | RBT | 0 (10) | [58] | ||
| North | RBT | SAT, c-ELISA | 7 (28) | [101] | |
| Veterinary workers | West | SAT (100 iu) | 2-ME | 5 (44) | [25] |
| South | SAT (NS) | 16 (86) | [125] | ||
| Cattle control post workers | West | SAT (100 iu) | 2-ME | 21 (18) | [25] |
| Agricultural college students | West | SAT (100 iu) | 2-ME | 12 (300) | [102] |
| Hospital studies | |||||
| Febrile individuals | |||||
| Students | North | RBT | SAT | 8 (122) | [127] |
| Civil servants | North | RBT | SAT | 4 (100) | [127] |
| Traders | North | RBT | SAT | 2 (53) | [127] |
| Housewives | North | RBT | SAT | 2 (62) | [127] |
| Crop farmers | North | RBT | SAT | 0 (6) | [127] |
| Health workers | North | RBT | SAT | 0 (10) | [127] |
| Children (1–15 years) | North | RBT | SAT | 10 (93) | [127] |
| Village farmers | North | RBT | SAT | 6 (114) | [91] |
| Traders and breeders | North | RBT | SAT | 34 (62) | [91] |
| Abattoir workers, butchers | North | RBT | SAT | 44 (32) | [91] |
| Civil servants | North | RBT | SAT | 4 (634) | [91] |
| Others | North | RBT | SAT | 6 (198) | [91] |
| Not specified | |||||
| Patients | West | SAT (50 iu) | RBT, Coombs, CFT | 6 (738) | [128] |
| Patients and personnel | West | SAT | 9 (176) | [129] | |
| Patients and personnel | North | RBT | SAT | 0 (64) | [123] |
| Personnel | North | RBT | SAT | 0 (90) | [123] |
| Blood donors, ante-natal women, male patients | West | SAT (100 iu) | 2-ME | 11 (1192) | [25], [130] |
| Blood donors | West | SAT (100 iu) | 2-ME | 21(178) | [25] |
| Blood donors | South | SAT (NS) | 12 (50) | [125] | |
| Others | |||||
| High school students | West | RBT | 3(65) | [23] |
There are no reports of Brucella isolation from human cases, and it is not known to what extent human brucellosis in Nigeria is caused by B. abortus or B. melitensis. Interpretation of human infection caused by B. melitensis or B. abortus on the basis of different titres with B. melitensis and B. abortus antigens is deceptive [91]. Misdiagnosis may be frequent; one abattoir study found that RBT positive individuals often complained of frequent treatments for malaria without showing improvement, while others complained of joint pain and general weakness [58].
Conclusion: Lessons from Nigeria
This review has identified major gaps in epidemiological data, diagnostics, and control, and misconceptions surrounding brucellosis. After 100 years, we know surprisingly little on the disease agent in Nigeria, and good-quality information—essential for evaluation of zoonotic potential and for establishment of control measures—is still lacking. Bacteriological studies are necessary to clarify the picture of both animal and human brucellosis. Preliminary evidence suggests that B. abortus biovar 3a is dominant or restricted to Africa, but little is known about its virulence and other biological properties. Also, the existence and distribution of B. melitensis and B. suis needs to be clarified. Likewise, a judicious choice of serological tests validated under local conditions and an understanding of their value in different contexts is key, as is implementation of clinical protocols and simple affordable tests for routine diagnosis in humans. Most sophisticated serodiagnostic tests were developed in high-income countries many years after brucellosis was eradicated, and these tests are better suited to epidemiological surveillance in well-equipped laboratories. Capacity building is a clear need, and the establishment of a reference laboratory for both human and animal brucellosis in sub-Saharan Africa would be a great asset.
The outbreak investigated by Alausa over 30 years ago [23] may be highly significant, because it shows the dramatic effect of the influx and settling of infected nomadic herds in areas where no control measures are implemented. This can happen in contemporary Nigeria where rural–urban migration, changing trends in livestock management and increased intensification could re-create the conditions for emergence of disease [6]. Climate change and desertification of the Sahel may also be an important driver for emergence, as it accounts in part for rural–urban migration [9] and is predicted to cause a reduction in the number of crop farmers in favour of livestock keepers [10]. Settling of nomadic Fulani in peri-urban areas and grazing reserves may be advantageous politically and economically, opening market chains for dairy products, offering formalised access to education and healthcare services, and avoiding disputes over land-use and clashes with crop farmers [92]. The emergence of brucellosis could, in these circumstances, have far-reaching social and political implications [84], [93], [94].
Prophylaxis and control of brucellosis requires contextual adaptation. Most evidence suggests differences in epidemiology between extensive livestock production systems and more intensive systems worldwide [1], [7]. This could apply to past situations in Nigeria, but we do not have a clear picture of the present status of the disease. An understanding of the dynamics of brucellosis in nomadic pastoralist systems and at the interface with settled populations is critical. Mass-vaccination approaches may be difficult to implement in extensively managed animals in Nigeria, but it is essential they be applied in the intensive and commercial systems. At a time when cost-effectiveness needs to be demonstrated, brucellosis control measures should be focused on settled populations that are at risk. This appeals to policy-makers, as settled populations are accessible and more amenable to mass-vaccination campaigns than nomadic pastoralist communities. Moreover, since differentiation of infected and vaccinated animals is not critical initially, the most effective vaccines (S19 in cattle and, if necessary, Rev1 in small ruminants [95]) should be used.
Nomadic pastoralism could offer a well-adapted management system for disease mitigation in Nigeria; if the disease exists at low levels, animals exhibit a low overall frequency of abortion and there are few opportunities for disease transmission. One Health and Eco Health approaches to disease reduction and prevention are particularly relevant in pastoralist communities, considering that pastoralism and transhumance is a desirable livelihood strategy in Nigeria [96].
Currently there is no coordinated policy for brucellosis in Nigeria. An assessment of the direct and indirect impact of brucellosis on these communities leading to culturally appropriate and locally adapted control options is overdue. There is a need to undertake a countrywide, evidence-based, and multidisciplinary study of brucellosis in the different livestock production systems of Nigeria to determine the extent, potential impact, and origin of brucellosis and to propose control template strategies of proven efficacy.
Key Learning Points
Despite imperfect evidence, an exhaustive review of studies in Nigeria suggests that brucellosis persists at low endemic levels in nomadic pastoralist systems. The settling of nomadic or transhumant pastoralist populations and intensification in livestock management, may favour disease transmission and conditions for brucellosis outbreaks.
There is an urgent need to study the dynamics of the disease at the interface between extensive pastoralist and intensive or settled livestock systems and to implement brucellosis control measures adapted to each of these situations.
The few attempts to implement a vaccination plus test and slaughter strategy in cattle show that this approach was not sustainable.
The role of small ruminants and camels in the epidemiology of brucellosis in Nigeria remains unknown because of insufficient bacteriological investigations and, for camels, properly validated serological tests.
The extent of the public health impact of brucellosis is largely unknown, and bacteriological studies to characterise the Brucella species infecting humans are lacking.
There is an imperfect understanding of the animal and human disease and of the value of the different diagnostic tests in different epidemiological contexts. One Health courses for veterinarians, medical doctors, and diagnostic laboratory personnel are necessary.
Top Five Papers in the Field
Ajogi I, Akinwumi JA, Esuruoso GO, Lamorde AG (1998) Settling the nomads in Wase-Zange grazing reserves in the Sudan Savannah zone of Nigeria III. Estimated financial losses due to bovine brucellosis. Nigerian Vet J 19: 86–94.
Alausa OK (1979) The investigation and control of a large-scale community outbreak of brucellosis in Nigeria. Public Health 93: 185–193.
Esuruoso GO (1974) Bovine brucellosis in Nigeria. Vet Rec 95: 54–58.
Grace D, Mutua F, Ochungo P, Kruska R, Jones K (2012) Mapping of poverty and likely zoonoses hotspots. Zoonoses Project 4. Report to the UK Department for International Development. Nairobi, Kenya: ILRI. Available: http://cgspace.cgiar.org/handle/10568/21161. Accessed 27 June 2014.
Waters-Bayer AN, Bayer W (1994) Coming to Terms. Interactions between Immigrant Fulani Cattle-Keepers and Indigenous Farmers in Nigeria's Subhumid Zone. Cahiers d'Études Africaines 34: 213–229. doi:10.3406/cea.1994.2048
Supporting Information
Rejected brucellosis serology studies in cattle.
(DOCX)
Rejected brucellosis serology studies in sheep and goats.
(DOCX)
Brucellosis serology studies in cattle reared under intensive livestock systems.
(DOCX)
Brucellosis serology studies in cattle reared under extensive livestock systems.
(DOCX)
Brucellosis serology studies in cattle undertaken in extensive and intensive livestock systems in parallel.
(DOCX)
Brucellosis serology studies in cattle reared under non-specified livestock systems.
(DOCX)
Brucellosis abattoir serology studies in cattle.
(DOCX)
Brucellosis milk market milk serology studies in cattle.
(DOCX)
Brucellosis bacteriology studies in cattle.
(DOCX)
Brucellosis serology studies in sheep and goats reared under intensive livestock systems.
(DOCX)
Brucellosis serology studies in sheep and goats reared under extensive livestock systems.
(DOCX)
Brucellosis serology studies in sheep and goats undertaken in extensive and intensive livestock systems in parallel.
(DOCX)
Brucellosis serology studies in sheep and goats under non-specified livestock systems.
(DOCX)
Brucellosis abattoir serology studies in sheep and goats.
(DOCX)
Brucellosis bacteriology studies in sheep and goats.
(DOCX)
Acknowledgments
We are grateful to Professor Ian Maudlin for a critical reading of the manuscript. The constructive comments on data presentation made by an anonymous reviewer are also gratefully acknowledged.
Funding Statement
This research has received funding from the European Union's Seventh Framework Program (FP7/2007-2013) under grant agreement n° 221948, ICONZ (Integrated control of Neglected Zoonoses). Additional funding from the “Fundación para la Investigación Médica Aplicada (FIMA)” and from the University of Navarra is also gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McDermott J, Grace D, Zinsstag J (2013) Economics of brucellosis impact and control in low-income countries. Rev Sci Tech Off Int Epiz 32: 249–261. [DOI] [PubMed] [Google Scholar]
- 2.Dalrymple-Champneys W (1960) Prognosis. Brucella infection and undulant fever in man. Oxford & New York: Oxford University Press. pp. 151–155. [Google Scholar]
- 3.Zinsstag J, Schelling E, Solera J, Blasco JM, Moriyón I (2011) Brucellosis. In: Palmer SR, Soulsby L, Torgeson PR, Brown DG, editors. Handbook of Zoonoses. Oxford & New York: Oxford University Press. pp. 54–62. [Google Scholar]
- 4. Dean AS, Crump L, Greter H, Schelling E, Zinsstag J (2012) Global burden of human brucellosis: A systematic review of disease frequency. PLoS Negl Trop Dis 6: e1865 10.1371/journal.pntd.0001865.t004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seimenis A, Morelli D, Mantovani A (2006) Zoonoses in the Mediterranean region. Ann Ist Super Sanita 42: 437–445. [PubMed] [Google Scholar]
- 6. Jones BA, Grace D, Kock R, Alonso S, Rushton J, et al. (2013) Zoonosis emergence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA 110: 8399–8404 10.1073/pnas.1208059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Racloz V, Schelling E, Chitnis N, Roth F (2013) Persistence of brucellosis in pastoral systems. Rev Sci Tech Off Int Epiz 32: 61–70. [DOI] [PubMed] [Google Scholar]
- 8.ILRI (2012) Mapping of poverty and likely zoonoses hotspots. Nairobi, Kenya: ILRI. 1 p. Available: http://cgspace.cgiar.org/handle/10568/21161. Accessed 27 June 2014. [Google Scholar]
- 9. Barrios S, Bertinelli L, Strobl E (2006) Climatic change and rural–urban migration: The case of sub-Saharan Africa. J Urban Econ 60: 357–371 10.1016/j.jue.2006.04.005 [DOI] [Google Scholar]
- 10. Jones PG, Thornton PK (2009) Croppers to livestock keepers: livelihood transitions to 2050 in Africa due to climate change. Environ Sci Policy 12: 427–437 10.1016/j.envsci.2008.08.006 [DOI] [Google Scholar]
- 11. McDermott JJ, Arimi SM (2002) Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Vet Microbiol 90: 111–134. [DOI] [PubMed] [Google Scholar]
- 12. Ocholi RA, Kalejaiye JO, Okewole PA (1993) Brucellosis in Nigeria. A review. Trop Vet 11: 15–26. [Google Scholar]
- 13. Esuruoso GO (1974) Bovine brucellosis in Nigeria. Vet Rec 95: 54–58. [DOI] [PubMed] [Google Scholar]
- 14. Adamu NN, Ajogi I (1999) Serological investigations of camels (Camelus dromedarius) slaughtered at Kano municipal abattoir for evidence of brucellosis. Trop Vet 18: 45–48. [Google Scholar]
- 15.Aregheore EM (2009) Country Pasture/Forage Resource Profiles. Rome: FAO. Available: http://www.fao.org/ag/agp/AGPC/doc/Counprof/PDF%20files/Nigeria.pdf. Accessed 19 June 2014. [Google Scholar]
- 16. Stewart R (2010) Desertification in the Sahel. Environmental Science in the 21st century - An online textbook Available: http://oceanworld.tamu.edu/resources/environment-book/desertificationinsahel.html. Accessed 19 June 2014. [Google Scholar]
- 17. Rikin EU (1988) Brucellosis of cattle in Nigeria: proposals for a control program under intensive and extensive husbandry systems. Acta Vet Scand 84 (supplement) 94–97. [PubMed] [Google Scholar]
- 18.Suleiman H (1988) Policy issues in agropastoral development in Nigeria. Proceedings of the National Conference on Pastoralism in Nigeria. Nigeria: Ahmadu Bello University Zaria. [Google Scholar]
- 19. Majekodunmi AO, Fajinmi A, Dongkum C, Picozzi K, Thrusfield MV, et al. (2013) A longitudinal survey of African animal trypanosomiasis in domestic cattle on the Jos Plateau, Nigeria: prevalence, distribution and risk factors. Parasit Vectors 6: 239 10.1186/1756-3305-6-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iyayi EA, Okoruwa VO, Babayemi OJ, Busari AA, Peters OF (2003) Livestock production pattern of agropastoralists in peri-urban centres of south-west Nigeria. Nigerian J Anim Prod 30: 87–92. [Google Scholar]
- 21.Mohammed TA (1990) A study of peri-urban cattle agro-pastoralism in the derived savanna of Oyo State, southwest Nigeria. Ibadan, Nigeria: ILCA, Humid Zone Programme. 54pp. [Google Scholar]
- 22. Brisibe F, Nawathe DR, Bot CJ (1996) Sheep and goat brucellosis in Borno and Yobe states of arid northeastern Nigeria. Small Rumin Res 20: 83–88. [Google Scholar]
- 23. Alausa OK (1979) The investigation and control of a large-scale community outbreak of brucellosis in Nigeria. Public Health 93: 185–193. [DOI] [PubMed] [Google Scholar]
- 24. Bale JO, Nuru S, Addo PB, Adeyinka IA (2003) Bacteriological investigation of sheep and goat milk for brucellosis in government farms in Northern Nigeria. Nigerian J Anim Prod 30: 107–116 Available: http://www.ajol.info/index.php/njap/article/view/3321. Accessed 19 June 2014. [Google Scholar]
- 25. Alausa OK, Awoseyi A (1976) Brucellosis: the situation in Western Nigeria. Trop Geogr Med 28: 54–59. [PubMed] [Google Scholar]
- 26. Greiner M, Verloo D, De Massis F (2009) Meta-analytical equivalence studies on diagnostic tests for bovine brucellosis allowing assessment of a test against a group of comparative tests. Prev Vet Med 92: 373–381. [DOI] [PubMed] [Google Scholar]
- 27. Pullan NB (1980) Productivity of White Fulani cattle on the Jos Plateau, Nigeria. III. Disease and management factors. Trop Anim Health Prod 12: 77–84. [DOI] [PubMed] [Google Scholar]
- 28. Mai HM, Irons PC, Kabir J, Thompson PN (2012) A large seroprevalence survey of brucellosis in cattle herds under diverse production systems in northern Nigeria. BMC Vet Res 8: 144 Available: http://www.biomedcentral.com/1746-6148/8/144. Accessed 27 June 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banerjee AK, Bhatty MA (1970) A survey of bovine brucellosis in northern Nigeria (a preliminary communication). Bull Epizoot Dis Afr 18: 333–338. [PubMed] [Google Scholar]
- 30. Esuruoso GO (1974) Bovine brucellosis in two southern states of Nigeria; II. The incidence and implications of infection in range cattle. Bull Epizoot Dis Afr 22: 35–40. [PubMed] [Google Scholar]
- 31. Bale OO, Kumi-Diaka J (1981) Serological and bacteriological study of bovine brucellae from livestock investigation and breeding centres in Nigeria. Br Vet J 137: 256–261. [DOI] [PubMed] [Google Scholar]
- 32. Eze EN (1978) Isolation of Brucellae from the Nigerian livestock and the typing of such isolates. Bull Anim Hlth Prof Afr 26: 29–36. [PubMed] [Google Scholar]
- 33. Ocholi RA, Kwaga JK, Ajogi I, Bale JO (2004) Phenotypic characterization of Brucella strains isolated from livestock in Nigeria. Vet Microbiol 103: 47–53. [DOI] [PubMed] [Google Scholar]
- 34. Sanogo M, Abatih E, Thys E, Fretin D, Berkvens D, et al. (2013) Importance of identification and typing of Brucellae from West African cattle: A review. Vet Microbiol 164: 202–211 10.1016/j.vetmic.2013.02.009 [DOI] [PubMed] [Google Scholar]
- 35. Le Fleche P, Jacques I, Grayon M, Al-Dahouk S, Bouchon P, et al. (2006) Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol 6: 1–14 10.1186/1471-2180-6-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davies G (1971) The Rose Bengal test. Vet Rec 88: 447–449. [DOI] [PubMed] [Google Scholar]
- 37. Blasco JM, Garin-Bastuji B, Marín C, Gerbier G, Fanlo J, et al. (1994) Efficacy of different rose bengal and complement fixation antigens for the diagnosis of Brucella melitensis infection in sheep and goats. Vet Rec 134: 415–420. [DOI] [PubMed] [Google Scholar]
- 38. Greiner M, Gardner IA (2000) Epidemiologic issues in the validation of veterinary diagnostic tests. Prev Vet Med 45: 3–22. [DOI] [PubMed] [Google Scholar]
- 39. Junaidu AU, Oboegbulem SI, Salihu MD (2011) Serological survey of Brucella antibodies in breeding herds. J Microbiol Biotech Res 1: 60–65. [Google Scholar]
- 40. Cadmus SIB, Alabi PI, Adesokan HK, Dale EJ, Stack JA (2013) Serological investigation of bovine brucellosis in three cattle production systems in Yewa Division, south-western Nigeria. J S Afr Vet Assoc 84 10.4102/jsava.v84i1.217 [DOI] [PubMed] [Google Scholar]
- 41. Farouk UM, Ibrahim S, Ajogi I, Bale JO (2013) Brucella antibodies in milk and risk factors analysis in pastoralist herds in Jigawa State, Nigeria. Eur J Vet Med 2: 29–37. [Google Scholar]
- 42. Maurice NA, Wungak SY, Gana BA, Nanven MB, Ngbede EO, et al. (2013) Seroprevalence of bovine brucellosis in northern Plateau State, North Central Nigeria. Asian Pac J Trop Dis 3: 337–340 10.1016/S2222-1808(13)60081-X [DOI] [Google Scholar]
- 43.Anonymous (1958) Annual report on the Department of Veterinary Services of the Northern Region of Nigeria 1955–56. Kaduna: Government Printer. [Google Scholar]
- 44. Falade S, Ojo MO, Sellers KC (1974) A serological survey of caprine brucellosis in Nigeria. Bull Epizoot Dis Afr 22: 335–339. [PubMed] [Google Scholar]
- 45. Okoh AE (1980) Abortion in sheep near Kano, Nigeria. Trop Anim Health Prod 12: 11–14. [DOI] [PubMed] [Google Scholar]
- 46. Falade S (1981) Brucellae isolated from goats. Zentralbl Veterinarmed B 28: 205–209. [DOI] [PubMed] [Google Scholar]
- 47. Ocholi RA, Kwaga JK, Ajogi I, Bale JO (2005) Abortion due to Brucella abortus in sheep in Nigeria. Rev Sci Tech Off Int Epiz 24: 973–979. [PubMed] [Google Scholar]
- 48. Luchsinger DW, Anderson RK (1979) Longitudinal studies of naturally acquired Brucella abortus infection in sheep. Am J Vet Res 40: 1307–1312. [PubMed] [Google Scholar]
- 49.Alton GG, Jones LM, Angus RD, Verger JM (1988) Techniques for the brucellosis laboratory. Paris, France: INRA. [Google Scholar]
- 50. Junaidu AU, Daneji AI, Salihu MD, Magaji AA, Tambuwal FM, et al. (2010) Seroprevalence of brucellosis in goat in Sokoto, Nigeria. Curr Res J Biol Sci 2: 275–277. [Google Scholar]
- 51. Okewole PA, Eze EN, Okoh AE, Oyetunde IL, Odeyemi PS (1988) Small ruminant brucellosis in some parts of Northern Nigeria. Bull Anim Hlth Prof Afr 36: 251–254. [Google Scholar]
- 52. Onunkwo JI, Nwanta JA, Ezenduka EV, Oboegbulem SI, Onyenwe IW (2009) Seroepidemiological survey of Brucella infection in slaughterhouse goats in Enugu state of Southeastern Nigeria. Anim Sci Reporter 3: 123–127. [Google Scholar]
- 53. Adams JW, McKay J (1966) Brucella in government-owned livestock in Eastern Nigeria. Nature 212: 217–218. [DOI] [PubMed] [Google Scholar]
- 54. Kramer JW, Nduaka O, Uzoukwu M (1967) Serological survey of diseases of cattle, sheep and goats in the Eastern provinces of Nigeria. Bull Epizoot Dis Afr 15: 25–29. [PubMed] [Google Scholar]
- 55. Bale JO, Nuru S, Addo PB (1982) Serological study of sheep and goat brucellosis in Northern Nigeria. Bull Anim Hlth Prof Afr 30: 73–79. [PubMed] [Google Scholar]
- 56. Bertu WJ, Ajogi I, Bale JO, Kwaga JK, Ocholi RA (2010) Sero-epidemiology of brucellosis in small ruminants in Plateau State, Nigeria. Afr J Microbiol Res 4: 1935–1938. [Google Scholar]
- 57. Falade S (1980) Caprine Brucellosis: Serological studies and objectives for control in Nigeria. Bull Off Int Epizoot 92: 111–127. [Google Scholar]
- 58. Cadmus SIB, Ijagbone IF, Oputa HE, Adenosak HL, Stack JA (2006) Serological survey of brucellosis in livestock animals and workers in Ibadan, Nigeria. Afr J Biomed Res 9: 163–168. [Google Scholar]
- 59. Ocholi RA, Bertu WJ, Kwaga JK, Ajogi I, Bale JO, et al. (2004) Carpal bursitis associated with Brucella abortus in a horse in Nigeria. Vet Rec 155: 566–567. [DOI] [PubMed] [Google Scholar]
- 60. Sadiq MA, Tijjani AN, Auswal MS, Mustapha AR, Tijjani AO, et al. (2013) Prevalence of Brucella antibodies in donkeys (Equus asinus) in Borno and Yobe states, Nigeria. Sokoto J Vet Sci 11: 7–12. [Google Scholar]
- 61. Adesiyun AA, Abdullallahi SU, Adeyanju JB (1986) Prevalence of Brucella abortus and Brucella canis antibodies in dogs in Nigeria. J Small Anim Pract 27: 31–37. [Google Scholar]
- 62. Osinubi M, Ajogi I, Ehizibol OD (2005) Brucella abortus agglutinins in dogs in Zaria, Nigeria. Nigerian Vet J 25: 35–38 10.4314/nvj.v25i1.3464 [DOI] [Google Scholar]
- 63. Cadmus SIB, Adesokan HK, Ajala OO, Odetokun WO, Perrett LL, et al. (2011) Seroprevalence of Brucella abortus and B. canis in household dogs in southwestern Nigeria: a preliminary report. J S Afr Vet Assoc 82: 56–57. [DOI] [PubMed] [Google Scholar]
- 64. Bale JO, Nuru S (1982) Serological study of brucellosis in local fowls in Northern Nigeria. J Anim Prod Res 1: 53–55. [PubMed] [Google Scholar]
- 65. Junaidu AU, Salihu MO, Ahmed F, Ambursa MA, Gulumbe ML (2006) Brucellosis in local chickens in North Western Nigeria. International Journal of Poultry Science 5: 547–549. [Google Scholar]
- 66. Gugong VT, Maurice NA, Ngbede EO, Hambolu SE, Ajogi I (2012) Serological evidence of brucellosis in local chickens in Kaduna State, Nigeria. J Anim Vet Adv 11: 418–420. [Google Scholar]
- 67. Cadmus SIB, Adesokan HK, Oluwayelu DO, Idris AO, Stack JA (2011) Short Communication: Brucella abortus antibodies in the sera of indigenous and exotic avian species in Nigeria. Bull Anim Hlth Prof Afr 58 10.4314/bahpa.v58i4.64236 [DOI] [Google Scholar]
- 68.Rementsova MM (1985) Brucellosis in wild animals. New Delhi: Oxonian Pres Pvt. Ltd. [Google Scholar]
- 69. Kudi AC, Kalla D, Kudi MC, Kapio GI (1997) Brucellosis in camels. J Arid Environ 37: 413–417. [Google Scholar]
- 70. Adamu NB, Okoh AE, Azunku UJ (2007) Prevalence of brucellosis in nomadic herds of dromedaries in Borno State, Nigeria. J Camel Practice Res 14: 135–138. [Google Scholar]
- 71. Egbe-Nwiyi TN, Ameh JA, Zaria LT, Yita AB (1999) Sero-prevalence of camel (Camelus dromedarius) brucellosis in semi arid zones of North of North-Eastern Nigeria. Trop Vet 17: 119–123. [Google Scholar]
- 72. Zaria LT, Egwu GO, Nawathe DR, Ojeamiren ME (1990) A preliminary report on the sero-prevalence of brucellosis in camels (Camelus dromedarius) in Maiduguri. Zariya Vet 5: 64–65. [Google Scholar]
- 73. Sprague LD, Al-Dahouk S, Neubauer H (2012) A review on camel brucellosis: a zoonosis sustained by ignorance and indifference. Pathog Glob Health 106: 144–149 10.1179/2047773212Y.0000000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Okoh AE (1979) A survey of brucellosis in camels in Kano, Nigeria. Trop Anim Health Prod 11: 213–214. [DOI] [PubMed] [Google Scholar]
- 75. Sadiq MA, Ajogi I, Bale JO, Mosimabale FB, Tijjani AN, et al. (2010) Epidemiological investigations of brucellosis in one humped camels (Camel dromedarius) in lake Chad area of Borno Satate, Nigeria. J Camel Practice Res 17: 229–234. [Google Scholar]
- 76. Díaz R, Casanova A, Ariza J, Moriyón I (2011) The rose bengal test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLoS Negl Trop Dis 5: e950 10.1371/journal.pntd.0000950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Levieux D (1974) Immunoglobulines bovines et brucellose. II. Activité des IgG1, IgG2 et IgM du sérum dans las réactions d'agglutination, de Coombs, de fixation du complément et dans le test au rose bengale. Ann Rech Vet 5: 343–353. [Google Scholar]
- 78. Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, et al. (1993) Naturally occurring antibodies devoid of light chains. Nature 363: 446–448. [DOI] [PubMed] [Google Scholar]
- 79. Nwanta JA (2011) Production characteristics, disease prevalence, and herd-health management of pigs in Southeast Nigeria. J Swine Health Prod 19: 331–338. [Google Scholar]
- 80. Bale OOJ, Nuru S (1985) Swine brucellosis: bacteriological and serological investigation of naturally infected pigs from six piggeries in northern Nigeria. J Anim Prod Res 5: 193–199. [Google Scholar]
- 81. Ngbede EO, Momoh AH, Bala RS, Madaki BD, Maurice AN (2013) An abattoir-based study on serodiagnosis of swine brucellosis in Makurdi, Benue State, North-Central Nigeria. J Adv Vet Res 3: 57–59. [Google Scholar]
- 82. Dieste-Pérez L, Blasco JM, de Miguel MJ, Marín C, Barberán M, et al. (2013) Performance of skin tests with allergens from B. melitensis B115 and rough B. abortus mutants for diagnosing swine brucellosis. Vet Microbiol 1–8 10.1016/j.vetmic.2013.10.024 [DOI] [PubMed] [Google Scholar]
- 83. Falade S (1981) Studies on Brucella melitensis Rev. 1 vaccine in goats. Zentralbl Veterinarmed B 28: 749–758. [DOI] [PubMed] [Google Scholar]
- 84. Ajogi I, Akinwumi JA, Esuruoso GO, Lamorde AG (1998) Settling the nomads in Wase-Zange grazing reserves in the Sudan Savannah zone of Nigeria III. Estimated financial losses due to bovine brucellosis. Nigerian Vet J 19: 86–94. [Google Scholar]
- 85. Ajogi I (1998) Settling the nomads in Wase and Wawa-Zange grazing reserves in the Sudan savannah zone of Nigeria IV: strategies for the control of bovine brucellosis. Nigerian Vet J 19: 40–48. [Google Scholar]
- 86. Ate IU, Rekwot PI, Nok AJ, Tekdek LB (2007) Economic losses associated with reproductive disorders of cows in settled cattle herds in Zaria, Nigeria. J Anim Vet Adv 6: 867–871. [Google Scholar]
- 87. Elmes BGT (1941) Undulant fever in Nigeria. Ann Trop Med Parasit 35: 1. [Google Scholar]
- 88. Collard P (1962) Antibodies against brucellae in the sera of healthy persons in various parts of Nigeria. West Afr Med J 11: 172–174. [PubMed] [Google Scholar]
- 89. Collard P (1962) A sample survey to estimate the prevalence of certain communicable diseases in Katsina province, Nigeria. West Afr Med J 11: 3–31. [PubMed] [Google Scholar]
- 90. Alausa OK, Osoba AO (1975) Brucella seroreactivity in Western Nigeria: an epidemiological study. Trans R Soc Trop Med Hyg 69: 259–260. [DOI] [PubMed] [Google Scholar]
- 91.Ofukwu AR, Yohanna CA, Abuh H A (2007) Brucella infection among hospital patients in Makurdi, North Central Nigeria. Medicine on line. Available: http://www.priory.com/med/brucella.htm. Accessed 27 June 2014.
- 92. Ajogi I, Esuruoso GO (1996) Settling the nomads in Wase Wase-Zange grazing reserves in the Sudan Savannah zone of Nigeria. I. Livestock, grazing and water resources. Nigerian Vet J 1 (special edition) 73–79. [Google Scholar]
- 93. Ajogi I, Akinwumi JA (2001) Cash-flow model of the cost of brucellosis in traditionally managed cattle herds in Nigeria. Bull Anim Hlth Prof Afr 49: 169–173. [Google Scholar]
- 94. Ehizibolo DO, Ehizibolo PO, Ehizibolo EE, Sugun MY, Idachaba SE (2011) The control of neglected zoonotic diseases in Nigeria through animal intervention. Afr J Biomed Res 14: 81–88. [Google Scholar]
- 95. Godfroid J, Scholz HC, Barbier T, Nicolas C, Wattiau P, et al. (2011) Brucellosis at the animal/ecosystem/human interface at the beginning of the 21st century. Prev Vet Med 102: 118–131. [DOI] [PubMed] [Google Scholar]
- 96. Waters-Bayer A, Bayer W (1994) Coming to terms. Interactions between immigrant Fulani cattle-keepers and indigenous farmers in Nigeria's subhumid zone. Cahiers d'études africaines 34: 213–229 10.3406/cea.1994.2048 [DOI] [Google Scholar]
- 97. Anene BM, Chime AB, Anika SM (1991) The production performance of imported Friesian cattle under heavy Trypanosoma challenge in a rain forest zone of Nigeria. Br Vet J 147: 275–282. [DOI] [PubMed] [Google Scholar]
- 98. Ate IU, Andrew PIR, Nok J, Tekdek LB (2007) Seroprevalence of brucellosis in puerperal cows and its public health implications in Zaria. Northern Nigeria. J Anim Vet Adv 6: 863–866. [Google Scholar]
- 99. Esuruoso GO, Hill DH (1971) A serological survey of bovine brucellosis in dairy herds in the Western State of Nigeria. J Agriculture 8: 147–154. [Google Scholar]
- 100. Esuruoso GO, van Blake HE (1972) Bovine brucellosis in two southern states of Nigeria. I. An investigation of selected herds. Bull Epizoot Dis Afr 20: 269–274. [PubMed] [Google Scholar]
- 101. Junaidu AU, Oboegbulem SI, Salihu MD (2008) Seroprevalence of brucellosis in prison farm in Sokoto, Nigeria. Asian J Epidemiol 1: 24–28. [Google Scholar]
- 102. Alausa OK (1980) Incidence and seasonal prevalence among an occupationally-exposed population to brucellosis. Trop Geogr Med 32: 12–15. [PubMed] [Google Scholar]
- 103. Esuruoso GO, Ayanwale FO (1980) Bovine brucellosis in Lagos State of Nigeria. Bull Anim Hlth Prof Afr 28: 11–15. [PubMed] [Google Scholar]
- 104. Mbuk EU, Ajogi I, Bale JO, Umoh JU (2011) Prevalence of Brucella antibodies in migratory Fulani cattle herds in Kaduna State, Nigeria. Nigerian Vet J 1: 26–29. [Google Scholar]
- 105. Ocholi RA, Ezeokoli CD, Akerejola OO, Saror DI (1996) Use of the enzyme-linked immunosorbent assay for screening cattle for Brucella antibodies in Nigeria. Vet Q 18: 22–24. [DOI] [PubMed] [Google Scholar]
- 106. Bertu WJ, Dapar M, Gusi AM, Ngulukun SS, Leo S, et al. (2010) Prevalence of antibodies in marketed milk in Jos and environs. Afr J Food Sci 4: 62–64. [Google Scholar]
- 107. Nuru S, Dennis SM (1975) Bovine brucellosis in Northern Nigeria: A serological survey. J Nigerian Vet Med Asoc 4: 3–8. [Google Scholar]
- 108. Bertu WJ, Gusi AM, Hassan M, Mwankon E, Ocholi RA, et al. (2012) Serological evidence for brucellosis in Bos indicus in Nigeria. Trop Anim Health Prod 44: 253–258 10.1007/s11250-011-0011-2 [DOI] [PubMed] [Google Scholar]
- 109. Ajogi I (1997) Seroprevalence of brucellosis in slaughtered cattle in four Northern states of Nigeria. Trop Vet 15: 21–24. [Google Scholar]
- 110. Cadmus SIB, Adesokan HK, Adedokun BO, Stack JA (2010) Seroprevalence of bovine brucellosis in trade cattle slaughtered in Ibadan, Nigeria, from 2004–2006. J S Afr Vet Assoc 81: 50–53. [DOI] [PubMed] [Google Scholar]
- 111. Cadmus SIB, Adesokan HK, Stack JA (2008) Co-infection of brucellosis and tuberculosis in slaughtered cattle in Ibadan, Nigeria: a case report. Vet Ital 44: 557–558. [PubMed] [Google Scholar]
- 112. Cadmus SIB, Adesokan HK, Stack JA (2008) The use of the milk ring test and rose bengal test in brucellosis control and eradication in Nigeria. J S Afr Vet Assoc 79: 113–115. [DOI] [PubMed] [Google Scholar]
- 113. Gusi AM, Bertu WJ, Mwankwon ES, Hassan M, Ocholi RA, et al. (2010) Prevalence of Brucella antibodies in animals and butchers at Jos abattoir, Nigeria. Vom J Vet Sci 7: 30–34. [Google Scholar]
- 114. Ishola, Ogundipe GAT (2000) Seroprevalence of brucellosis in trade cattle slaughtered in Thadan, Nigeria. Bull Anim Hlth Prof Afr 48: 53–55. [Google Scholar]
- 115. Junaidu AU, Garba HS (2006) Application of competitive ELISA (Compelisa) rose bengal plate test (RBPT) and serum agglutination test (SAT) for detection of antibodies to Brucella infection in slaughter cattle in Sokoto, Nigeria. Sahel J Vet Sci 5: 9–12 Available: http://www.ajol.info/index.php/sjvs/article/view/41460. Accessed 19 June 2014. [Google Scholar]
- 116. Ogundipe GAT, Oyeyemi MO, Ijagbone IF (1994) Sero-prevalence of Brucella abortus agglutinins in slaughtered cattle in Ibadan. Trop Vet 12: 158–161. [Google Scholar]
- 117. Shehu LM, Yusuf H, Kudi AC, Kalla DU (1999) Seroprevalence of brucellosis in ruminants in Bauchi and environs. Nigerian Vet J 20: 64–74. [Google Scholar]
- 118. Tijjani AO, Musa HI, Ousoumanou O, Akintola OO (2009) Prevalence of brucellosis in food animals slaughtered at Damaturu abattoir, Yobe State, Nigeria. Sahel J Vet Sci 8: 55–60 Available: http://www.ajol.info/index.php/sjvs/article/view/44475. Accessed 19 June 2014. [Google Scholar]
- 119. Cadmus SIB, Osikoya IE, Adesokan HK (2009) Brucellosis in trade cattle in Lagos state: An investigation of two abattoirs. Nigerian Vet J 29: 43–46. [Google Scholar]
- 120. Oyejide A, Adu FD, Makinde AA, Ezeh EN (1987) The prevalence of antibodies of Brucella abortus, Dermatophilus congolensis and bovine leukaemia virus in Nigerian slaughter cattle. Vet Q 9: 83–85. [DOI] [PubMed] [Google Scholar]
- 121. Chukwu CC (1987) Studies on sero-prevalence of bovine brucellosis in Enugu and Nsukka, Nigeria. Zariya Vet 2: 383–387. [Google Scholar]
- 122. Onoja II, Ajani AJ, Mshelia WP, Andrew A, Ogunkoya AB, et al. (2011) Brucellosis outbreak in a flock of seventeen sheep in Zaria. Bull Anim Hlth Prof Afr 7 10.4314/sokjvs.v7i2 [DOI] [Google Scholar]
- 123. Brisibe F, Nawathe DR, Bot CJ (1993) Serological prevalence of brucellosis in sheep goats and human beings in Maiduguri Metropolis. Trop Vet 11: 27–33. [Google Scholar]
- 124. Kaltungo BY, Saidu SNA, Sackey AKB, Kazeem HM (2013) Serological evidence of brucellosis in goats in Kaduna North senatorial district of Kaduna State, Nigeria. ISRN Vet Sci 2013: 1–6 10.1111/j.1865-1682.2010.01171.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Useh MF, Udo SM, Oghomu CJ (1996) Sero-epidemiology and perception of human brucellosis in Calabar, Nigeria. Cent Afr J Med 42: 184–185. [PubMed] [Google Scholar]
- 126. Alausa OK, Osoba AO (1977) Subclinical human Brucella infection in Ibadan, Nigeria. Ghana Med J 16: 251–254. [Google Scholar]
- 127. Baba MM, Sarkindared SE, Brisibe F (2001) Serological evidence of brucellosis among predisposed patients with pyrexia of unknown origin in the North Eastern Nigeria. Cent Eur J Public Health 9: 158–161. [PubMed] [Google Scholar]
- 128. Falade S (1974) Brucella agglutinating antibodies in the sera of persons dwelling in Ibadan and the surrounding districts. J Nigerian Vet Med Asoc 3: 21–23. [Google Scholar]
- 129. Sixl W, Rosegger H, Schneeweiss H, Withalm H, Schuhmann G (1987) Serological investigations in Nigeria for anthropozoonoses in human sera: brucellosis, echinococcosis, toxoplasmosis, chlamydial diseases, listeriosis, rickettsiosis (Coxiella burneti and Rickettsia conori). J Hyg Epidemiol Microbiol Immunol 31: 493–495. [PubMed] [Google Scholar]
- 130. Alausa OK (1977) Brucellosis: epidemiology and practical problems of control in Nigeria. Public Health 91: 141–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rejected brucellosis serology studies in cattle.
(DOCX)
Rejected brucellosis serology studies in sheep and goats.
(DOCX)
Brucellosis serology studies in cattle reared under intensive livestock systems.
(DOCX)
Brucellosis serology studies in cattle reared under extensive livestock systems.
(DOCX)
Brucellosis serology studies in cattle undertaken in extensive and intensive livestock systems in parallel.
(DOCX)
Brucellosis serology studies in cattle reared under non-specified livestock systems.
(DOCX)
Brucellosis abattoir serology studies in cattle.
(DOCX)
Brucellosis milk market milk serology studies in cattle.
(DOCX)
Brucellosis bacteriology studies in cattle.
(DOCX)
Brucellosis serology studies in sheep and goats reared under intensive livestock systems.
(DOCX)
Brucellosis serology studies in sheep and goats reared under extensive livestock systems.
(DOCX)
Brucellosis serology studies in sheep and goats undertaken in extensive and intensive livestock systems in parallel.
(DOCX)
Brucellosis serology studies in sheep and goats under non-specified livestock systems.
(DOCX)
Brucellosis abattoir serology studies in sheep and goats.
(DOCX)
Brucellosis bacteriology studies in sheep and goats.
(DOCX)