Abstract
Psoriasis is characterized by an apoptosis-resistant and metabolic active epidermis, while a hallmark for allergic contact dermatitis (ACD) is T cell-induced keratinocyte apoptosis. Here, we induced ACD reactions in psoriasis patients sensitized to nickel (n = 14) to investigate underlying mechanisms of psoriasis and ACD simultaneously. All patients developed a clinically and histologically typical dermatitis upon nickel challenge even in close proximity to pre-existing psoriasis plaques. However, the ACD reaction was delayed as compared to non-psoriatic patients, with a maximum intensity after 7 days. Whole genome expression analysis revealed alterations in numerous pathways related to metabolism and proliferation in non-involved skin of psoriasis patients as compared to non-psoriatic individuals, indicating that even in clinically non-involved skin of psoriasis patients molecular events opposing contact dermatitis may occur. Immunohistochemical comparison of ACD reactions as well as in vitro secretion analysis of lesional T cells showed a higher Th17 and neutrophilic migration as well as epidermal proliferation in psoriasis, while ACD reactions were dominated by cytotoxic CD8+ T cells and a Th2 signature. Based on these findings, we hypothesized an ACD reaction directly on top of a pre-existing psoriasis plaque might influence the clinical course of psoriasis. We observed a strong clinical inflammation with a mixed psoriasis and eczema phenotype in histology. Surprisingly, the initial psoriasis plaque was unaltered after self-limitation of the ACD reaction. We conclude that sensitized psoriasis patients develop a typical, but delayed ACD reaction which might be relevant for patch test evaluation in clinical practice. Psoriasis and ACD are driven by distinct and independent immune mechanisms.
Introduction
Psoriasis and allergic contact dermatitis (ACD) are highly prevalent, complex inflammatory skin diseases characterized by a combination of epithelial alterations and deviated T cell immunity [1], [2].
In recent years, substantial progress was made in understanding the pathogenesis of both psoriasis and ACD. However, major scientific and clinical questions remain unresolved. In particular, the question whether psoriasis is primarily based on epithelial or immune alterations is still under debate. Valid arguments are found for both theories: while deletion of the epidermal proteins JunB/activator protein1, IKK2 or STAT3 overexpression are each sufficient to induce a psoriasis-like phenotype in mice [3]–[5], the description of patients with both psoriasis and eczema or ACD in parallel argues for specific local stimuli inducing either psoriasis or eczema [6].
In that context, a promising approach to investigate the pathogenesis of psoriasis and ACD or other dermatitis entities is to compare the molecular changes in lesional skin of both diseases. However, previous attempts to do so were limited by high inter-individual variability caused by different genetic background as well as environmental exposure, skin infections or trauma and eventual systemic or topical treatments [7]–[9]. These obstacles may largely be overcome by the possibility to investigate both diseases in the same patient at the same time. However, a prerequisite for general conclusions from such an analysis is the proof that sensitized psoriasis patients develop typical dermatitis either spontaneously [10] or to iatrogenic epicutaneous challenge with allergens or haptens (ACD), respectively.
Clinically, it should be easy to determine whether psoriasis patients may develop an ACD reaction. Psoriasis is characterized by squamous, well-demarcated red plaques of variable size [11]. In contrast, ACD is mainly characterized by diffuse dryness, erythema, papules, and vesicles followed by scaling [1]. The main histologic features of psoriasis plaques include epidermal hyperplasia with elongated rete ridges and altered keratinocyte cornification, neutrophil microabscesses and a T cellular infiltrate. Histological features of ACD are epidermotropism as well as dermal infiltration of T cells and epidermal spongiosis and apoptosis, reflecting a pathogenic hallmark of ACD: induction of keratinocyte apoptosis by T cells [12]–[15]. This key event opposes the pathogenesis of psoriasis. Here, the typical clinical picture is due to hyper-proliferation, apoptosis resistance and insufficient differentiation of keratinocytes. Thus, it is unclear to which extent psoriasis patients develop typical ACD reactions. This is in line with epidemiological studies reporting a decreased rate in type IV sensitizations in psoriasis patients [16], [17].
The aim of this study was an intra-individual comparison of psoriasis and ACD. We demonstrate that psoriasis patients can develop delayed, but otherwise typical contact dermatitis. Psoriasis and ACD differed in their immune cellular infiltrate and epithelial changes. Induction of ACD reactions directly on top of pre-existing psoriasis plaques did not alter the long-term clinical course of psoriasis, which indicates independent pathogenic mechanisms are the molecular basis of psoriasis and ACD.
Materials and Methods
Patients and material sampling
Patients with moderate-to-severe plaque type psoriasis and co-existing type IV sensitizations to nickel (allergic contact dermatitis, n = 14) were included into the study. A detailed clinical characterization of the patients is given in table 1. Exclusion criteria were treatment with immune-efficient medication prior to material sampling (wash-out phase 6 weeks for systemic, 2 weeks for local treatment). Diagnoses were made according to clinical as well as histological criteria as published previously [18]. Clinical evaluation of the patients was quantified using the PASI, and intensity of induced contact dermatitis reactions to nickel was documented according to guidelines as published previously [6]. For comparison of ACD kinetics, non-psoriatic patients admitted to our day care hospital with a positive epicutaneous patch test to nickel (n = 8) were included into the study. For analysis of the molecular changes of clinically non-involved skin, non-psoriatic individuals (patients with lichen planus, n = 3 as well as patients without any skin disease, n = 7) were included. The study was conducted according to the declaration of Helsinki. All patients gave their written consent to participate in the study, and the study was approved by the local ethical committee: Klinikum Rechts der Isar of the Technische Universität Munich (project number 5060/11).
Table 1. Clincial characterization of patients enrolled into the study.
| Patient | Age | Gender | Psoriasis onset [age] | Psoriasis arthritis | Family history | Grade of ACD reaction |
| 1 | 46 | female | 32 | no | pos | +++ |
| 2 | 31 | female | 28 | no | pos | ++ |
| 3 | 58 | female | 44 | yes | pos | +++ |
| 4 | 56 | female | 52 | no | neg | +++ |
| 5 | 70 | female | 51 | no | neg | +++ |
| 6 | 41 | female | 26 | no | neg | +++ |
| 7 | 46 | female | 12 | no | pos | +++ |
| 8 | 55 | female | 50 | no | neg | +++ |
| 9 | 57 | male | 43 | yes | pos | +++ |
| 10 | 61 | female | 40 | yes | pos | +++ |
| 11 | 52 | female | 45 | no | neg | ++ |
| 12 | 42 | female | 19 | no | neg | +++ |
| 13 | 38 | female | 13 | neg | neg | +++ |
| 14 | 48 | female | 37 | neg | neg | ++ |
Six-mm skin punch biopsies were obtained under local anaesthesia from one ACD lesion five days after hapten challenge, one active psoriasis plaque, one ACD reaction directly on top of a pre-existing psoriasis plaque, and clinically non-involved skin of all patients. Biopsies were divided into three parts for routine histological evaluation and isolation of total RNA and primary T cell lines, respectively.
Isolation of total RNA and cDNA transcription from skin biopsies
Skin tissue specimens were stored in PAXgene Tissue containers (Qiagen) at −20°C. For isolation of RNA, tissue samples were homogenized with a tissue lyzer (Qiagen) followed by the purification of total RNA with the PAXgene Tissue RNA Kit (Qiagen) according to the manufacturer’s protocol. The RNA yield and quality was determined with a Nanodrop ND1000 UV-vis Spectophotomer. cDNA was synthesized from 300 ng total RNA and transcribed using the High Capacity cDNA Reverse Transcript Amplification Kit (Applied Biosystems) according to the manufacturer’s instructions.
Primer design and Real-time PCR
Primers for real time PCR were designed using the publicly accessible Primer3 software (http://frodo.wi.mit.edu/primer3/). A list of used primers is shown in Table S1.
For amplification of genes of interest by real time PCR, cDNA was amplified using gene-specific primers and the Fast Start SYBR Green Master mix (Roche Applied Science). Reactions were performed in 384-well plates and fluorescence development monitored with a ViiA7 Real Time PCR machine (Applied Biosystems). The expression of transcripts was normalized to expression of 18S ribosomal RNA as housekeeper and fold change, relative to non-involved skin as calibrator, was determined using the ΔΔCt method.
Whole genome expression arrays
cRNA was synthesized from 25 ng total RNA and transcribed using the High Capacity cDNA Reverse Transcript Amplification Kit (Applied Biosystems) according to the manufacturer’s instructions. Samples were amplified and Cy3 labeled using the Agilent Low Input Quick Amp Labeling Kit (Agilent Technologies) according to the manufacturer’s instructions and hybridized to a SurePrint G3 Human GE 8X60K BeadChip (Agilent Technologies). After washing the hybridized arrays, fluorescence signals were detected by reading the arrays in the microarray scanner system iScan (Agilent Technology). The Agilent Feature Extraction software was used to read and process microarray image files.
Data analysis was performed using limma (Bioconductor, R) [19]. The “normexp” background corrected arrays have been normalized with the method “cyclicloess”. Only probes which are at least 10% brighter than the 95 percentile of the negative control probes were processed further. Control Probes and low expressed probes were filtered out. Differential gene expression was assessed using a two-sample, two-sided Welch’s t-test. To correct for multiple hypothesis testing the Benjamini and Hochberg approach (FDR) has been used. Fold-changes (FC) were computed using mean values and expressed as logarithm base 2. Significant differentially expressed genes were defined with a significance threshold of corrected p-values <0.05 and an absolute log FC cutoff of 1. The “weight01” algorithm of the topGO package was used to determine significantly enriched GO terms.
Immunohistochemistry
Skin samples were fixed in 10% formalin and embedded in paraffin. 5-µm sections were de-waxed and rehydrated. After quenching endogenous peroxidase, achieving antigen retrieval, and blocking nonspecific binding sites, sections were incubated with monoclonal antibodies against CD15 (BD-Pharmingen, Franklin Lakes, NJ, 1∶100 dilution), CD3 (BD-Pharmingen, 1∶20 dilution), BDCA-2 (Miltenyi Biotec, Bergisch Gladbach, Germany), Ki67 (Dako, Carpinteria, CA, mib1 clone, 1∶50 dilution), IL-17 (R&D Systems, Minneapolis, MN, 1∶40) and perforine (Leica Biosystems, Milan, Italy, 5B10 clone, 1∶20). Secondary biotinylated mAbs and staining kits were obtained from Vector Laboratories. Stainings were developed using 3-amino-9-ethylcarbazole (Vector Laboratories, Burlinagame, CA, USA). Slides were counterstained with hematoxylin. As a negative control, primary antibodies were omitted or replaced with an irrelevant isotype-matched monoclonal antibody. Figures depict one experiment that is representative of all the patients investigated (n = 10). Positive cells were counted in two visual fields per condition by two independent investigators in a blinded manner.
Blood flow analysis and clinical evaluation
To investigate whether ACD reactions might influence pre-existing psoriasis plaques, blood flow analysis of the resulting skin lesions was performed using laser Doppler imaging (Moor instruments) as previously published [20]. Additionally, psoriasis lesions were scored before and 21 days after nickel challenge according to local PASI criteria: erythema, squamation, and infiltration each ranging from 0 = none to 3 = severe. All values were added to calculate the total local PASI score.
Isolation of skin-infiltrating T cells
Skin biopsies were minced with a scalpel and placed in culture RPMI medium supplemented with 2 mM glutamine, 1 mM sodium pyruvate, 1% nonessential amino acids, 1% penicillin/streptomycin (all Invitrogen; RPMI complete), and 10% human serum (Sigma-Aldrich) 60 U/ml IL-2. After 2–5 d, T cells emigrated from tissue samples according to the IL-2 gradient and were further expanded by stimulation with aCD3/aCD28 antibodies (BD Biosciences).
Bioplex analysis
T cells isolated from skin biopsies were stimulated in RPMI complete and the presence or absence of aCD3/aCD28 antibodies for 48 hours. Cell-free supernatant was obtained and stored at −80°C until further analysis. Bioplex analysis was performed using the Bio-Plex Pro Human Cytokine 27-Plex Assay (Bio Rad) according to the manufacturer’s protocol. Quantification of protein content was determined with the Bio-Plex 200 System (Bio Rad).
Statistical analysis
Statistical analysis was performed using the R software (http://www.r-project.org). A two-sample, two sided paired Welch’s t-test was used to compare released factors and cellular infiltrate in psoriasis and ACD reactions. We corrected for multiple hypothesis testing using the Benjamini and Hochberg method (FDR). A corrected p-value <0.05 was considered significant.
Results
Contact dermatitis is delayed in psoriasis patients
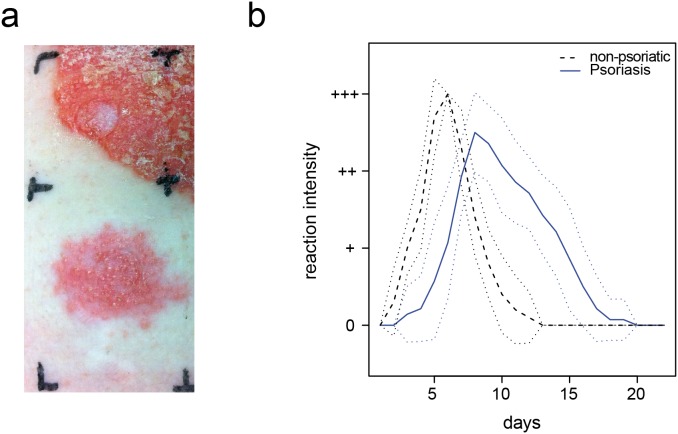
As a first step to investigate whether sensitized psoriasis patients develop a typical ACD reaction, we performed nickel patch tests in patients with plaque-type psoriasis and a known hypersensitivity reaction to nickel (n = 14). Epicutaneous application of nickel induced dermatitis in all patients, as characterized by diffuse erythema, papules, and vesicles followed by scaling even in close proximity to pre-existing psoriasis lesions with the typical clinical presentation of squamous, well-demarcated red plaques of variable size (Fig. 1A).
Figure 1. Induction of allergic contact dermatitis in psoriasis patients.
A Dermatitis 5 days after application of a nickel patch test in close proximity to a psoriasis plaque; B Comparison of reaction severity and time course of nickel patch test reactions to nickel in sensitized psoriasis patients (n = 14, mean is shown in blue line) and non-psoriatic individuals (n = 10, mean is shown in black dashed lines). Standard deviation is given by dotted lines.
As compared to non-psoriatic nickel sensitized individuals (n = 10), the psoriasis nickel-sensitized patients showed a delayed time course of the ACD reaction (Fig. 1B). While non-psoriatic patients usually developed a positive patch test reaction after 48–72 hours, patch tests were frequently still negative at that time point in the case of psoriasis patients. In line with that observation, the maximum intensity of the clinical reaction was reached after 3–5 days in the non-psoriatic group, but after 7 days in the psoriasis group. ACD reactions in the psoriasis patients showed also a slower resolution as compared to non-psoriatic patients (Fig. 1B).
Clinically non-involved skin of psoriasis patients shows altered metabolism and proliferation pathways
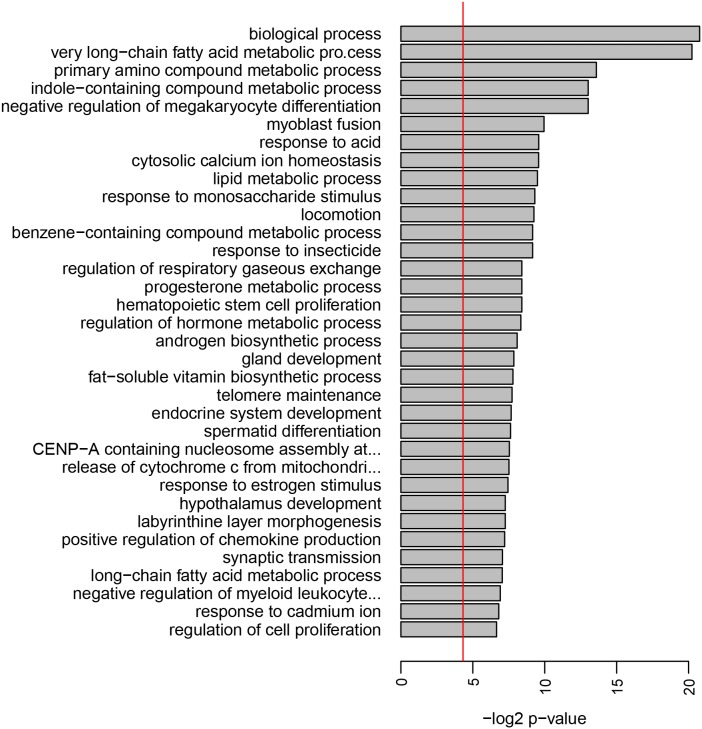
To understand the molecular basis of the delayed ACD time course in psoriasis patients, whole genome expression analysis of clinically non-involved skin of psoriasis patients as compared to non-psoriatic individuals was performed. Mean expression of 531 genes (1293 probes) was significantly different in the psoriasis group. A GO term pathway analysis showed that numerous pathways related to metabolism of lipids, amino acids, saccharides, and hormones as well as proliferation and differentiation pathways such as telomere maintenance, stem cell proliferation, and regulation of cell proliferation, were altered in psoriasis patients even in clinically non-involved skin (Fig. 2).
Figure 2. Clinically non-involved skin of psoriasis patients is altered regarding metabolism and proliferation.
Signaling pathway analysis of clinically non-involved skin of psoriasis patients (n = 8) versus healthy skin of non-psoriatic individuals (n = 10). Shown are the most significant hits for the Gene Ontology term “biological process”. The bar size indicates the level of significance for each pathway (negative log2 p-value), the vertical line shows the 0.05 significance level.
Psoriasis plaques show a Th17 profile while ACD reactions are dominated by cytotoxic T cell responses in situ
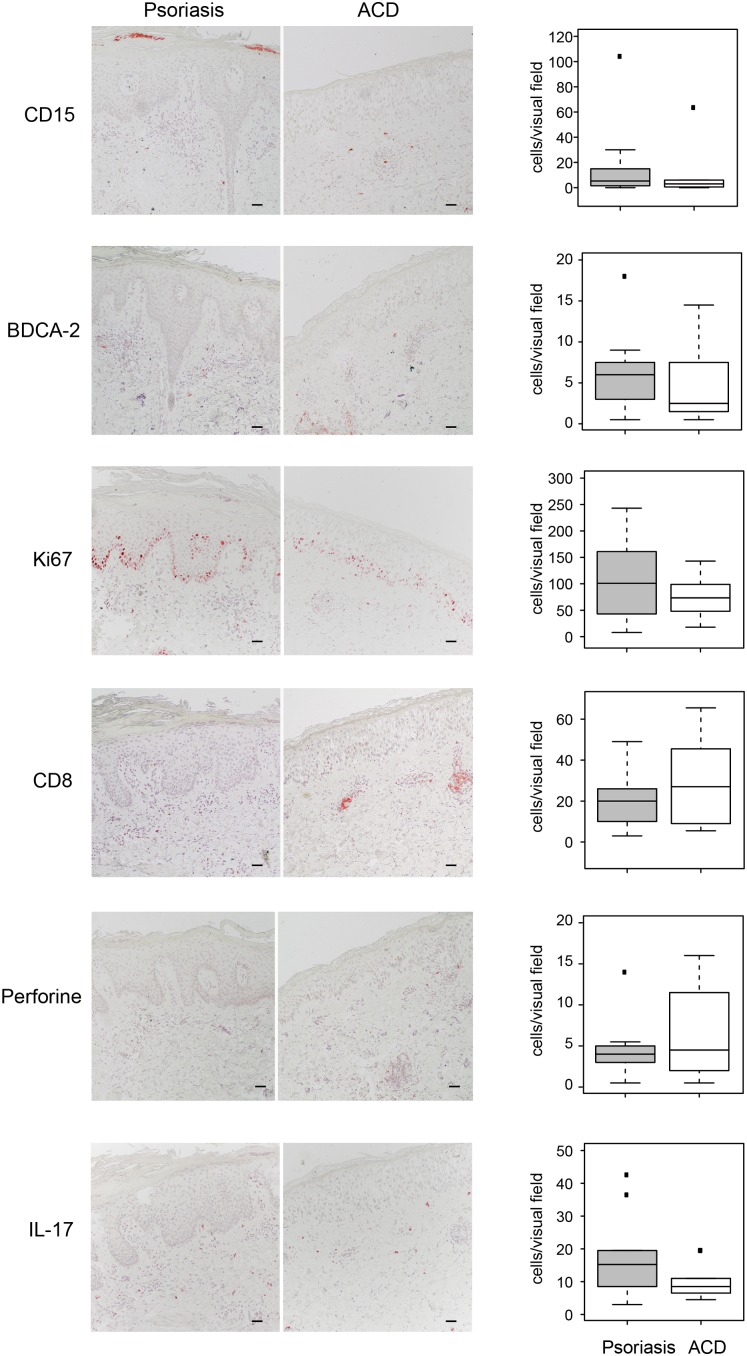
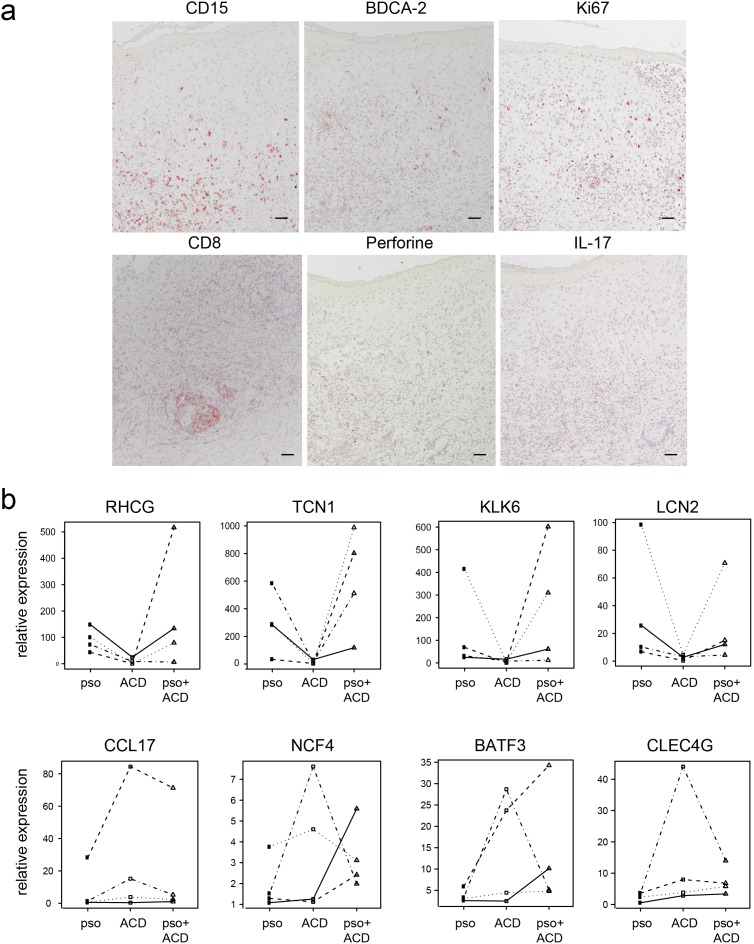
To further investigate the pathogenesis of psoriasis and ACD, we performed immunohistochemical studies on skin biopsies of acute psoriasis plaques and positive nickel patch test reactions. In psoriasis plaques, non-significant trends of a higher infiltration of CD15+ neutrophil granulocytes (17.1 versus 8.9 positive cells per visual field, p = 0.5), BDCA-2+ plasmacytoid dendritic cells (6.3 versus 4.5, p = 0.3), and IL-17+ T cells (17.8 versus 10.0, p = 0.3) as compared to ACD reactions was observed. Furthermore, the proliferation marker Ki67 was expressed higher in psoriasis plaques than in ACD lesions (108.6 versus 73.6, p = 0,3). Positivity for Ki67 was observed especially in basal layers of the epidermal compartment, but was also observed in higher epidermal layers in the case of psoriasis (Fig. 2). In contrast, a higher number of cytotoxic CD8+ T cells (29.2 versus 20.7, p = 0,46) and perforine positive cells (6.5 versus 4.6, p = 0,46) was observed in ACD reactions as compared to psoriasis plaques (Fig. 3).
Figure 3. Immunohistochemical comparison of psoriasis and ACD reactions in the same patient.
Representative immunohistochemical stainings of psoriasis and ACD biopsy sections obtained from the same patient (right panel) and quantification of positive cells per visual field of all included patients (n = 10) (boxplots in left panel).
T cell secretion analysis reveals differences between psoriasis and ACD regarding the infiltrating T cell compartment
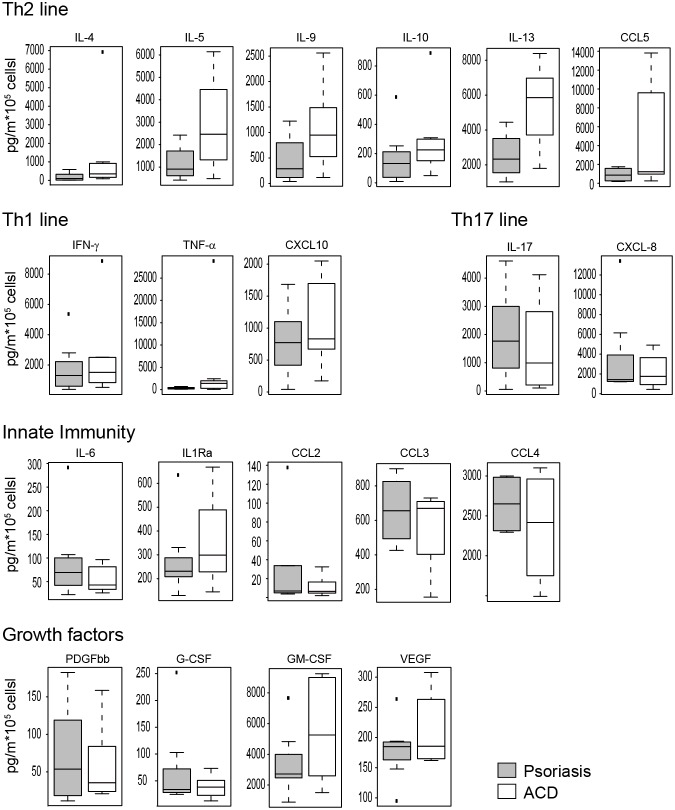
Lesional T cells were isolated from psoriasis plaques and positive ACD reactions simultaneously and analyzed in vitro (Fig. 4). Upon anti-CD3/anti-CD28 stimulation, psoriasis infiltrating T cells released more IL-17 (2.0 versus 1.5 ng/ml, p = 0.85) than ACD derived T cells. In contrast, the Th2 cytokines IL-4 (1.2 versus 0.2 ng/ml, p = 0.18), IL-5 (2.9 versus 1.2 ng/ml, p = 0.67), IL-9 (1.1 versus 0.5 ng/ml, p = 0.23), IL-10 (0.3 versus 0.2 ng/ml, p = 0.18), and IL-13 (5.3 versus 2.5 ng/ml, p = 0.96) showed a consistent trend of higher secretion in ACD as compared to psoriasis T cells. No marked difference was observed regarding the Th1 cytokine IFN-γ (2.4 versus 1.8 ng/ml, p = 0.38). IL-6 was secreted at low levels with slightly more in psoriasis plaque T cells (0.09 versus 0.05 ng/ml, p = 0.18), while the soluble IL-1 receptor α (0.36 in ACD versus 0.28 ng/ml in psoriasis, p = 0.18) and TNF-α (4.5 versus 0.3 ng/ml, p = 0.18) were secreted in higher amounts by ACD T cells. The Th17 chemokine CXCL8 was secreted to a higher amount by psoriasis-derived T cells (3.5 versus 2.3 ng/ml, p = 0.72), while the Th2-associated chemokine CCL-5 was released more by ACD T cells (4.5 versus 0.9 ng/ml, p = 0.53). No marked differences were observed regarding secretion of the Th1 chemokine CXCL10 as well as of CCL2, CCL3, and CCL4 (Fig. 4). Lesional T cells also released growth factors. PDGF-bb and G-CSF were expressed at very low levels, GM-CSF (5.5 versus 3.4 ng/ml, p = 0.67) and VEGF (0.21 versus 0.18 ng/ml, p = 0.18) were slightly higher released by ACD T cells.
Figure 4. Bioplex analysis of skin infiltrating T cells.
T cells infiltrating either the psoriatic plaque or the induced ACD lesion were stimulated with aCD3/aCD28 antibodies and cell-free supernatant was used for Bioplex analysis with 27 different analytes. Detected proteins were grouped according to association with the Th2 line (A), Th1 line (B), Th17 line (C), innate immunity (D) and growth factors (E). Shown are boxplots from five patients.
Induction of an ACD reaction on a pre-existing psoriasis plaque results in a mixed phenotype of both diseases
To investigate a possible direct interaction of psoriasis and ACD, we challenged nickel-sensitized psoriasis patients with nickel on top of a pre-existing, active psoriasis plaque in 11 patients. Resulting inflammatory reactions were analyzed histologically. Histologic hallmarks of both diseases were observed, namely psoriasiform acanthosis with neutrophilic micro-abscesses and dilated capillaries in the papillary dermis for psoriasis and marked spongiosis, epidermotropism and keratinocyte apoptosis defining dermatitis (Fig. 5A).
Figure 5. Characterization of ACD on top of psoriasis plaques.
ACD was induced by applying a nickel patch test on top of a pre-existing psoriasis plaque. A Immunohistochemical evaluation of hallmarks of ACD and psoriasis. B Real time PCR analysis of genes specific for psoriasis (upper panel) and ACD (lower panel). Non-involved skin served as control and data is shown as fold induction (2−ΔΔCT). Each line indicates samples coming from the same patient.
In addition, immunohistochemical investigation demonstrated that markers typical for both psoriasis and ACD, such as CD15, IL-17, and BDCA-2, and the cytotoxic marker CD8, respectively, were simultaneously present in the mixed psoriasis/ACD lesion (Fig. 5A). The expression of immune cells was spread in both dermis and epidermis and mostly distributed at the site of epidermal spongiosis and micro-vesiculation. Thus, the distribution of immune cells reflected the course of an acute inflammatory reaction (Fig. 5A).
Disease-specific psoriasis and ACD genes are constant in the mixed phenotype reaction
In order to assess whether the psoriasis and ACD reactions could influence each other, previously identified transcripts specific for psoriasis or ACD [21] were quantified in biopsies from psoriasis, ACD, and psoriasis/ACD skin lesions of the same patient as compared to non-involved skin. Namely, RHCG, TCN1, KLK6, and LCN2 were chosen for psoriasis and CCL17, NCF4, BATF3, and CLEC4G as ACD-specific genes. Results revealed a strong up-regulation of the psoriasis-specific genes in psoriasis plaques, but not in ACD reactions. This was true for all markers investigated: RHCG (91 versus 9-fold up-regulation), TCN1 (285 versus 8-fold up-regulation), KLK6 50 versus 5-fold up-regulation), and LCN2 (18 versus 3-fold up-regulation) in psoriasis and CCL17 (1-fold versus 10-fold up-regulation), NCF4 (1.4-fold versus 3-fold up-regulation), BATF3 (3-fold versus 14-fold up-regulation), and CLEC4G (3-fold versus 6-fold up-regulation) in ACD. In the mixed psoriasis/ACD lesion, up-regulation of all markers was similar to the up-regulation in the corresponding single disease (Fig. 5B). Thus, psoriasis-specific genes are not markedly influenced by the simultaneous induction of an ACD reaction and vice versa.
The clinical course of psoriasis is not altered by ACD
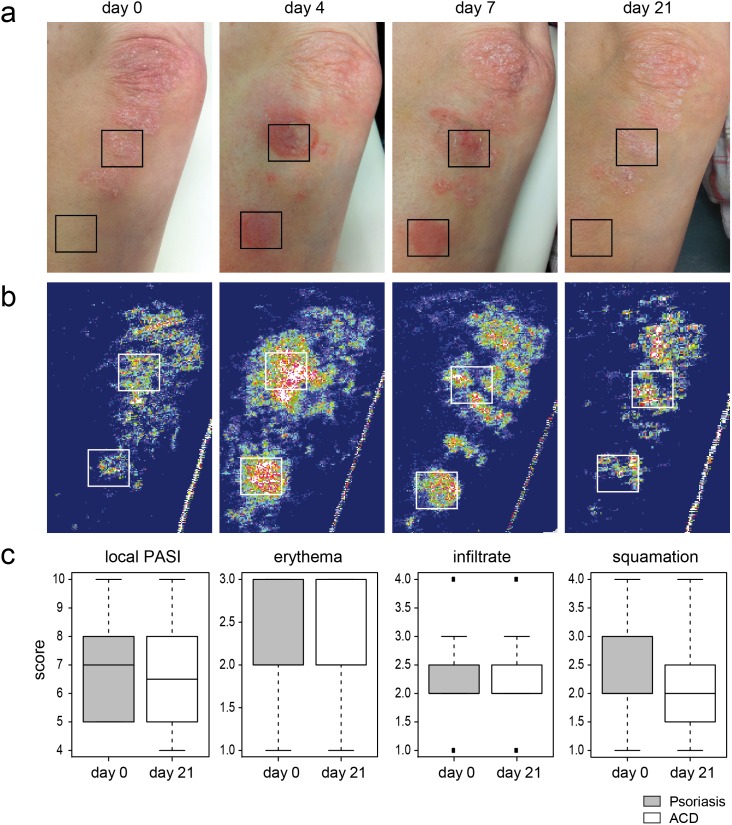
Since a specific and independent molecular signature was consistent for both psoriasis and ACD even upon ACD challenge on top of a psoriasis plaque, we sought to investigate whether the natural clinical course of these two conditions would be influenced by each other. Clinically, epicutaneous application of nickel on psoriasis plaques induced a strong local inflammatory reaction already after 48 hours in sensitized individuals. This inflammatory reaction was more severe than the nickel patch test reaction on previously non-involved skin, but it cleared following the natural course of an ACD reaction within two weeks. After 3–4 weeks, ACD reactions had resolved completely, but the initial psoriasis plaque remained largely unaltered (Fig. 6A). The subjective clinical investigation was confirmed by a laser blood flow analysis of the cutaneous reactions (Fig. 6B). This blood flow analysis confirmed that despite the strong temporary inflammation, the clinical course of psoriasis was not influenced by the local induction of an ACD reaction. This was confirmed by the degree of the local psoriasis area-and severity index (PASI), erythema, squamation and infiltration. All these parameter were similar, even if slightly reduced, in the psoriasis plaque after three weeks as compared to the plaque before nickel challenge (n = 11; Fig. 6C).
Figure 6. ACD and psoriasis are independent, stimulus-driven immune reactions.
Nickel patch tests were used to induce ACD lesions in close proximity to or on top of a psoriasis plaque (n = 11). A Representative clinical course of psoriasis, ACD, and psoriasis/ACD mixed reaction in one patient. B Subjective clinical evaluation was confirmed investigating the local blood flow as indicator for severity of inflammation via laser doppler imaging at day 0, day 4, day 7 and day 21 (B). C Changes in local PASI, erythema, infiltrate and squamation were determined for the nickel treated psoriasis plaque before and 21 days after nickel challenge (n = 11) and presented as boxplots.
Discussion
Patients that suffer from psoriasis and ACD simultaneously represent an interesting model to investigate common and distinct molecular mechanisms underlying those two disease patterns.
Dermatitis reactions can be elicited by epicutaneous challenge of common allergens or haptens in sensitized psoriasis patients (allergic contact dermatitis, ACD). This iatrogenic induction offers the possibility to control location and kinetics of the dermatitis response and thus standardizes the experimental setup. However, a prerequisite is that sensitized psoriasis patients develop an ACD reaction similar to non-psoriatic patients. This is doubtful, since hallmarks of psoriasis are antagonistic to ACD. Namely, keratinocytes of psoriasis patients show a higher proliferation and are less susceptible to apoptosis both in vivo and in vitro [22], [23]. In contrast, ACD is based on early loss of keratinocyte adherence and induction of apoptosis [14], [24]. In fact, when we compared ACD reactions to nickel of psoriatic and non-psoriatic individuals, we observed a delayed time course in psoriasis patients. This is likely due to the observed sub-clinical alterations in clinically non-involved skin of psoriasis patients, where numerous genes related to metabolism and proliferation are altered as compared to healthy skin of non-psoriatic individuals. Additionally, migration of antigen-presenting cells into the local lymph node is delayed in psoriasis patients [25]. These phenomena may also explain why some studies describe an inverse relationship of psoriasis and contact dermatitis reactions [16], [17]. The delayed time course observed in this study implies that epicutaneous patch tests should be additionally evaluated after seven days in psoriasis patients in daily clinical routine.
Beyond the delayed onset, ACD reactions in psoriasis patients were similar to non-psoriatic individuals regarding all clinical and histological parameters even in close proximity to pre-existing psoriasis plaques. This strengthens the concept that not only ACD, but also psoriasis is driven primarily by immune responses to specific stimuli [6]. Recent evidence argue for epidermal antigens such as desmoglein 3 as potential trigger of a psoriatic T cell response [26]. While in line with the literature, in nickel-induced ACD a Th2 and a cytotoxic immune response mediated by CD8+ T cells was more frequent than in psoriasis [18], psoriasis lesions were infiltrated by a higher number of IL-17+ immune cells [27]. A trigger of psoriasis may be a complex of antimicrobial peptides and self-DNA that is released after cellular damage and consecutively activates plasmacytoid dendritic cells to drive a Th17 dominated immune response [28]. This observation fits with the fact that psoriasis shares several aspects of a wound healing reaction [29], [30]. Interestingly, numbers of plasmacytoid dendritic cells were comparable in psoriasis and ACD in our patients, but none developed a psoriatic lesion at the site of hapten challenge. This fact highlights that the specific process of epidermal apoptosis observed in ACD is not sufficient to initiate the pathologic cascade described in psoriasis plaques.
The model described here can further be used for interventional studies. Since the common psoriasis therapy dithranol clears psoriasis lesions by an inflammatory reaction of unknown basis [31], we thought whether induction of an ACD reaction right on top of a pre-existing psoriasis plaque might influence the clinical course of the psoriasis plaque. Our hypothesis was further strengthened by our observation that the ACD reaction was associated with an influx of Th2 cells, since IL-4 was shown to be an efficient psoriasis therapy [32]. As expected, nickel induced a strong inflammatory reaction on top of the psoriasis plaque. In contrast to ACD reactions on non-involved skin, kinetics of ACD on top of psoriasis was not delayed, which indicates common innate immune mechanisms of ACD and psoriasis. Histological analysis of the resulting lesions revealed a mixed phenotype of psoriasis and ACD, with acanthosis and neutrophilic micro-abscesses as well as spongiosis and epidermal apoptosis. Surprisingly, within three weeks the self-limited ACD reaction cleared and left a psoriasis plaque closely resembling the one prior to ACD induction. This means the specific inflammatory reaction induced by nickel acting via Toll like receptor 4, activation of the inflammasome, and direct induction of keratinocyte apoptosis [33]–[35] results in epidermal damage, but not in elimination of the psoriasis trigger. It further means that dithranol acts beyond induction of an unspecific inflammation, maybe via development of free radicals [36] or induction of keratinocyte apoptosis via mitochondrial action [37]. Finally, this observation implies that the factors leading to limitation of the ACD such as hapten-specific regulatory T cell responses are either ACD-specific or not strong enough to limit a psoriasis plaque formation.
In summary, we demonstrate that nickel sensitized psoriasis patients develop a delayed, but otherwise typical ACD reaction to nickel even in close proximity to pre-existing psoriasis plaques. Intra-individual comparison of the pathogenesis of psoriasis and ACD reveals a dominated Th17 response in psoriasis and a stronger Th2 and cytotoxic immune response in ACD. Psoriasis and ACD are distinct immune-driven reactions that do not influence each other.
Supporting Information
Sequences of the primers used for real-time PCR analysis.
(DOCX)
Acknowledgments
The authors thank Jana Sänger for excellent technical support.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data except array raw data are included in the paper; array data were uploaded to the GEO system accession number GSE58121.
Funding Statement
This work was supported by the German Research Foundation (EY97/3-1, TR22, SPP1395/InKoMBio Busch 900/6-1), the Helmholtz Association (“Impuls- und Vernetzungsfonds”), the Bavarian Academy of Sciences (Young scholar program), CK-CARE (Christine Kühne Center for Allergy Research and Education), and FONDATION ACTERIA. This study was performed with samples of the biobank Biederstein of the Technische Universität Munich. F.J.T. is supported by the European Union within the European Research Council Grant LatentCauses/ ERC grant agreement no. [259294]11. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nestle FO, Kaplan DH, Barker J (2009) Psoriasis. N Engl J Med 361: 496–509. [DOI] [PubMed] [Google Scholar]
- 2. Martin SF (2012) Allergic contact dermatitis: xenoinflammation of the skin. Curr Opin Immunol 24: 720–729. [DOI] [PubMed] [Google Scholar]
- 3. Zenz R, Eferl R, Kenner L, Florin L, Hummerich L, et al. (2005) Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature 437: 369–375. [DOI] [PubMed] [Google Scholar]
- 4. Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, et al. (2005) Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med 11: 43–49. [DOI] [PubMed] [Google Scholar]
- 5. Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, et al. (2002) TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature 417: 861–866. [DOI] [PubMed] [Google Scholar]
- 6. Eyerich S, Onken AT, Weidinger S, Franke A, Nasorri F, et al. Mutual antagonism of T cells causing psoriasis and atopic eczema. N Engl J Med 365: 231–238. [DOI] [PubMed] [Google Scholar]
- 7. Wenzel J, Peters B, Zahn S, Birth M, Hofmann K, et al. (2008) Gene expression profiling of lichen planus reflects CXCL9+-mediated inflammation and distinguishes this disease from atopic dermatitis and psoriasis. J Invest Dermatol 128: 67–78. [DOI] [PubMed] [Google Scholar]
- 8. Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, et al. (2003) Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: a gene microarray analysis. J Allergy Clin Immunol 112: 1195–1202. [DOI] [PubMed] [Google Scholar]
- 9. de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, et al. (2005) High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol 125: 1163–1173. [DOI] [PubMed] [Google Scholar]
- 10. Henseler T, Christophers E (1995) Disease concomitance in psoriasis. J Am Acad Dermatol 32: 982–986. [DOI] [PubMed] [Google Scholar]
- 11. Bieber T (2008) Atopic dermatitis. N Engl J Med 358: 1483–1494. [DOI] [PubMed] [Google Scholar]
- 12. Eyerich K, Novak N (2013) Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy 68: 974–982. [DOI] [PubMed] [Google Scholar]
- 13. Traidl C, Sebastiani S, Albanesi C, Merk HF, Puddu P, et al. (2000) Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J Immunol 165: 3058–3064. [DOI] [PubMed] [Google Scholar]
- 14. Pennino D, Eyerich K, Scarponi C, Carbone T, Eyerich S, et al. (2010) IL-17 amplifies human contact hypersensitivity by licensing hapten nonspecific Th1 cells to kill autologous keratinocytes. J Immunol 184: 4880–4888. [DOI] [PubMed] [Google Scholar]
- 15. Trautmann A, Akdis M, Kleemann D, Altznauer F, Simon HU, et al. (2000) T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J Clin Invest 106: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jovanovic M, Boza P, Karadaglic D, Brkic S, Petrovic A, et al. (2009) Contact sensitivity in patients with psoriasis in Vojvodina. Int Arch Allergy Immunol 148: 311–320. [DOI] [PubMed] [Google Scholar]
- 17. Bangsgaard N, Engkilde K, Thyssen JP, Linneberg A, Nielsen NH, et al. (2009) Inverse relationship between contact allergy and psoriasis: results from a patient- and a population-based study. Br J Dermatol 161: 1119–1123. [DOI] [PubMed] [Google Scholar]
- 18.Eyerich K, Bockelmann R, Pommer AJ, Foerster S, Hofmeister H, et al.. (2009) Comparative in situ topoproteome analysis reveals differences in patch test-induced eczema: cytotoxicity-dominated nickel versus pleiotrope pollen reaction. Exp Dermatol. [DOI] [PubMed]
- 19. Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3. [DOI] [PubMed] [Google Scholar]
- 20. Huss-Marp J, Eberlein-Konig B, Breuer K, Mair S, Ansel A, et al. (2006) Influence of short-term exposure to airborne Der p 1 and volatile organic compounds on skin barrier function and dermal blood flow in patients with atopic eczema and healthy individuals. Clin Exp Allergy 36: 338–345. [DOI] [PubMed] [Google Scholar]
- 21.Quaranta M, Knapp B, Garzorz N, Mattii M, Pullabhatla V, et al.. (2014) Intra-individual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci Transl Med. 9th July, epub ahead of print. [DOI] [PubMed]
- 22. Sestito R, Madonna S, Scarponi C, Cianfarani F, Failla CM, et al. (2011) STAT3-dependent effects of IL-22 in human keratinocytes are counterregulated by sirtuin 1 through a direct inhibition of STAT3 acetylation. FASEB J 25: 916–927. [DOI] [PubMed] [Google Scholar]
- 23. Madonna S, Scarponi C, Pallotta S, Cavani A, Albanesi C (2012) Anti-apoptotic effects of suppressor of cytokine signaling 3 and 1 in psoriasis. Cell Death Dis 3: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cavani A, De Luca A (2010) Allergic contact dermatitis: novel mechanisms and therapeutic perspectives. Curr Drug Metab 11: 228–233. [DOI] [PubMed] [Google Scholar]
- 25. Cumberbatch M, Singh M, Dearman RJ, Young HS, Kimber I, et al. (2006) Impaired Langerhans cell migration in psoriasis. J Exp Med 203: 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishimoto S, Kotani H, Tsuruta S, Shimizu N, Ito M, et al. (2013) Th17 cells carrying TCR recognizing epidermal autoantigen induce psoriasis-like skin inflammation. J Immunol 191: 3065–3072. [DOI] [PubMed] [Google Scholar]
- 27. Fischer-Stabauer M, Boehner A, Eyerich S, Carbone T, Traidl-Hoffmann C, et al. (2012) Differential in situ expression of IL-17 in skin diseases. Eur J Dermatol 22: 781–784. [DOI] [PubMed] [Google Scholar]
- 28. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, et al. (2007) Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449: 564–569. [DOI] [PubMed] [Google Scholar]
- 29. Lai Y, Di Nardo A, Nakatsuji T, Leichtle A, Yang Y, et al. (2009) Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med 15: 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, et al. (2009) Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119: 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mason A, Mason J, Cork M, Hancock H, Dooley G (2013) Topical treatments for chronic plaque psoriasis: An abridged Cochrane Systematic Review. J Am Acad Dermatol 69: 799–807. [DOI] [PubMed] [Google Scholar]
- 32. Ghoreschi K, Thomas P, Breit S, Dugas M, Mailhammer R, et al. (2003) Interleukin-4 therapy of psoriasis induces Th2 responses and improves human autoimmune disease. Nat Med 9: 40–46. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt M, Raghavan B, Muller V, Vogl T, Fejer G, et al. (2010) Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat Immunol 11: 814–819. [DOI] [PubMed] [Google Scholar]
- 34. Martin SF, Esser PR, Weber FC, Jakob T, Freudenberg MA, et al. (2011) Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy 66: 1152–1163. [DOI] [PubMed] [Google Scholar]
- 35. Schmidt M, Hupe M, Endres N, Raghavan B, Kavuri S, et al. (2010) The contact allergen nickel sensitizes primary human endothelial cells and keratinocytes to TRAIL-mediated apoptosis. J Cell Mol Med 14: 1760–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Muller K (1997) Antipsoriatic and proinflammatory action of anthralin. Implications for the role of oxygen radicals. Biochem Pharmacol 53: 1215–1221. [DOI] [PubMed] [Google Scholar]
- 37. McGill A, Frank A, Emmett N, Turnbull DM, Birch-Machin MA, et al. (2005) The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J 19: 1012–1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences of the primers used for real-time PCR analysis.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data except array raw data are included in the paper; array data were uploaded to the GEO system accession number GSE58121.