Abstract
Although the neural systems supporting single word reading are well studied, there are limited direct comparisons between typical and dyslexic readers of the neural correlates of reading fluency. Reading fluency deficits are a persistent behavioral marker of dyslexia into adulthood. The current study identified the neural correlates of fluent reading in typical and dyslexic adult readers, using sentences presented in a word-by-word format in which single words were presented sequentially at fixed rates. Sentences were presented at slow, medium, and fast rates, and participants were asked to decide whether each sentence did or did not make sense semantically. As presentation rates increased, participants became less accurate and slower at making judgments, with comprehension accuracy decreasing disproportionately for dyslexic readers. In-scanner performance on the sentence task correlated significantly with standardized clinical measures of both reading fluency and phonological awareness. Both typical readers and readers with dyslexia exhibited widespread, bilateral increases in activation that corresponded to increases in presentation rate. Typical readers exhibited significantly larger gains in activation as a function of faster presentation rates than readers with dyslexia in several areas, including left prefrontal and left superior temporal regions associated with semantic retrieval and semantic and phonological representations. Group differences were more extensive when behavioral differences between conditions were equated across groups. These findings suggest a brain basis for impaired reading fluency in dyslexia, specifically a failure of brain regions involved in semantic retrieval and semantic and phonological representations to become fully engaged for comprehension at rapid reading rates.
Introduction
Reading fluency, the ability to read accurately and at a rate that enables comprehension [1], [2], is a cornerstone of skilled reading. Developmental dyslexia, defined as a specific learning disability with a neurological basis, manifests as difficulty in reading accurately or fluently at the single word level [3]. The ability to extract the meaning of text requires the coordination of multiple processing demands [4], and readers with dyslexia can struggle with reading comprehension due to impaired decoding and/or through a slow reading rate [5], [6]. Despite consensus that reading fluency is essential for efficient reading and that reading dysfluency is a severe problem encountered by adolescents and adults with a history of dyslexia, there is little direct evidence regarding the neural systems critical for reading fluency and disruptions of those neural systems in dyslexia. Our aim was to identify the neural systems associated with fluent reading in typical adult readers, and discover how those systems differed in adults with dyslexia.
Reading fluency deficits are persistent and widespread in both adolescents and adults with a history of dyslexia [7], [8]. In contrast to effective interventions focusing on phonological deficits [9]–[11], dysfluent reading is especially difficult to remediate [6], [12]–[14]. Particularly beyond elementary school, remediation attempts to boost reading fluency yield only minimal improvements for dyslexic readers [15]. Challenges with reading fluency are not restricted to readers of English, but rather play a prominent role across languages [8], [16]–[20].
The brain basis of single word reading has been the focus of many functional magnetic resonance imaging (fMRI) and magnetoencephalography (MEG) reading studies, but the brain basis of reading connected text has been investigated less often [21]–[24]. In typical readers, brain regions associated with sentence reading include greater left-hemisphere than right-hemisphere activation in the inferior frontal gyrus, posterior superior and middle temporal gyri, as well as left occipito-temporal cortex, bilateral occipital cortex, left cerebellar declive, and dorsolateral prefrontal cortex [25]. Studies using fMRI to examine the neural correlates of sentence reading indicate that semantic processing is associated with activation in the temporal lobes, greater on the left, and left inferior frontal gyrus (BA47) [25], [26], and that processing accelerated text presentation is associated with activation in left occipito-temporal cortex [27]. For typically developing readers, higher scores on single-word reading tests are associated with increased activation during sentence reading in left temporo-parietal and ventral occipito-temporal regions [28]. Studies comparing readers with dyslexia to typical readers on whole-sentence reading have found relative hypo-activation in bilateral parietal cortices [24] and left occipito-temporal gyrus [17], but hyper-activation in left inferior frontal gyrus [17]. A MEG study of silent passage reading with each word presented individually at a constant rate (700 ms) found that, compared to typical readers, dyslexic readers exhibited hypo-activation bilaterally in the temporo-parietal and occipital cortices [28].
To date, fMRI studies of reading fluency using sentence stimuli have not examined and compared directly fluency per se in typical and atypical reading development (i.e., by varying and comparing fluency demands). One indirect approach involved correlating out-of-scanner standardized scores on tests of reading fluency with brain activations measured during scanning on tasks that did not vary fluency demands (reading sentences versus noun strings) [29]. In typical readers, higher fluency scores were associated positively with activations in left occipitotemporal cortex (BA18) and negatively with activations in right superior temporal gyrus, left insula, and left cerebellum. In a study that varied presentation rate on a word-by-word sentence reading judgment task in Hebrew with slower and faster rates, activation differences based on rate comparisons were not reported. However, typical adult readers showed greater activation in left premotor, left anterior insula/inferior frontal gyrus, right anterior insula, left middle temporal gyrus, and bilateral extrastriate visual cortex for each rate compared to fixation [30]. Readers with dyslexia showed less activation than typical readers during the relatively fast rate condition (versus fixation) in the posterior right temporal regions. Although these imaging studies considered fluency and involved reading sentences, as opposed to isolated words, none of the studies directly examined brain systems underlying reading fluency in readers with and without dyslexia.
Impaired reading fluency could arise from several sources. First, dyslexia has often been associated with impaired phonological awareness, even before the onset of reading instruction, that is thought to slow single-word decoding and, in turn, connected text composed of single words [31]. Second, dyslexia has also been associated with impaired naming speed for lists of stimuli, even for nonverbal material [32], and such slowness in processing for a series of stimuli may slow the reading of a series of words that constitute a sentence. Third, dyslexia has been associated with other temporal processing impairments for both linguistic and non-linguistic stimuli, such as deficits in general auditory processing [33], speech-specific processing [34], rise-time discrimination [35], or auditory sampling at optimal frequencies for analyzing language sounds [36]. Slowed temporal processing could impede reading fluency.
The major impediment for direct identification of neural systems important for reading fluency is that the kinds of educational and clinical tests used to measure fluency are not easily translated for fMRI experimentation. Further, it is not obvious what task would serve as an informative baseline for fluent reading. Therefore, the current study investigated the neural correlates of reading fluency by parametrically varying the rate at which sentences were presented one word at a time that were read silently. Accuracy and speed of comprehension, as indicated by plausibility judgments, verified reading performance. There were three rates of word presentation that corresponded to typical silent reading rates in 3rd or 4th grades (150 words per minute (wpm) (slow rate)), in 8th or 9th grades (240 wpm) (medium rate), and surpassing typical college reader expectations (600 wpm) (fast rate).
We examined neural systems that may be important for fluency by identifying brain regions that changed activation in response to changes in presentation rate in typical young adult readers and readers with dyslexia. The use of three different reading rates allowed for a comparison of typical readers and readers with dyslexia with performance differences equated by examination of a faster rate in the typical readers compared to a slower rate in readers with dyslexia. Such performance-equated comparisons permit consideration of whether activation differences between typical readers and readers with dyslexia are simply a consequence of performance differences or are related more directly to the cause of reduced fluency in dyslexia [37]. We hypothesized that brain regions showing increasing activation with increasing rates of presentation would be important for reading fluency, and that activation patterns would differ in readers with and without dyslexia.
Methods
Ethics statement
Written informed consent for participation in the study, approved by the Massachusetts Institute of Technology (MIT) and Harvard University Institutional Review Boards, was obtained from all participants.
Participants
Participants recruited from online recruitment in the local community of urban and suburban areas met inclusion criteria of: between 18–35 years of age; native English speakers; completion of high school or higher levels of formal education; right handedness as indicated by responses to a questionnaire adapted from the Edinburgh Handedness Inventory [38]; no contraindications to MRI; and absence of neurological or psychiatric impairments or associated medications. Participants completed a behavioral testing session at MIT and an MR scanning session at the Athinoula A. Martinos Imaging Center, McGovern Institute for Brain Research at MIT.
Behavioral assessment
Standardized measures of cognitive, reading, and reading-related abilities were administered to participants by trained researchers at MIT. The testing battery included measures of cognitive ability, Wechsler Abbreviated Scale of Intelligence, 3rd Ed. (WASI) [39]; phonological processing, Comprehensive Test of Phonological Processing (CTOPP) [40]; and rapid naming, “Letters,” “Numbers,” and “2-set” from the Rapid Automatized Naming and Rapid Alternating Stimulus Tests (RAN/RAS) [41]. Untimed reading ability was indexed by accuracy for reading real words and pseudowords, “Word Identification” and “Word Attack” from the Woodcock Reading Mastery Test-Revised, NU (WRMT) [42]. Timed reading ability was indexed by accuracy for reading real words and pseudowords within time limits, “Sight Word Efficiency” and “Phonemic Decoding Efficiency” from the Test of Word Reading Efficiency (TOWRE) [43]. Untimed and timed measures of connected text reading were used to index reading comprehension ability by using cloze sentences, which are sentences in which a reader is asked to supply a word that has been removed from a passage in order to assess comprehension. These measures were “Passage Comprehension” from the WRMT, text passages from the Nelson-Denny Reading Test (NDRT) [44], and sentences that required a semantic plausibility judgment within a time limit, “Reading Fluency” from the Woodcock-Johnson III (WJ) [45]. Reading rate was recorded as the number of words in a text passage read silently at a typical pace within a time limit, “Reading Rate,” NDRT. Participants completed a background questionnaire regarding developmental history of language and literacy skills.
Participant groups
Two participant groups were included in this study. Typical readers (n = 12; 5 female) were between 18–28 years of age (M = 22.5; SD = 3.1) and earned a score at or above the 25th percentile on four measures of untimed or timed single word reading (TOWRE, WRMT; Table 1). Readers with dyslexia (n = 12; 8 female) were between 18–31 years of age (M = 23.3; SD = 4.1) and had both a history and a clinical diagnosis of reading disability, and were also currently scoring below the 25th percentile rank on at least two subtests of timed or untimed single word or pseudoword reading measures (TOWRE, WRMT; Table 1). All participants demonstrated cognitive performance at or above the expected mean range of 100±15 (WASI). The two groups did not differ significantly on age or nonverbal cognitive ability (Table 1). Among participants who reported ethnicity, 100% of adults in the control group reported having a Caucasian background. Among participants with dyslexia, 75% reported having a Caucasian background, one identified as Black, and 2 elected not to respond. There were no between group differences in education level attained.
Table 1. Participant Scores for Typical Readers and Readers with Dyslexia.
| Typical Reader | Dyslexic | p-values: Typical vs. | ||
| Group | Group | Dyslexic | ||
| N | 12 | 12 | – | |
| Age | 22.5±3.1 | 23.3±4.1 | .61 | |
| Construct | Behavioral Measure | |||
| Cognitive Abilities | WASI – Verbal | 121.17±13.04 | 109.00±7.31 | .010 |
| WASI – Performance | 114.67±8.2 | 110.58±6.05 | .181 | |
| Phonological Processing | CTOPP – Elision | 11.00±0.85 | 8.27±1.62 | .0005 |
| CTOPP – Blending Words | 11.00±1.41 | 8.83±2.76 | .024 | |
| CTOPP – Memory For Digits | 12.33±2.71 | 12.27±1.49 | .948 | |
| CTOPP – Nonword Repetition | 9.33±1.92 | 7.42±1.51 | .013 | |
| Sublexical Fluenc | RAN – Numbers | 112.83±5.28 | 105.00±6.84 | .005 |
| RAN – Letters | 112.00±3.24 | 102.00±7.39 | .0005 | |
| RAN – RAS | 112.33±5.85 | 101.67±11.20 | .008 | |
| Word Reading Accurac | WRMT – Word Identification | 108.83±10.07 | 90.25±8.97 | .0005 |
| WRMT – Word Attack | 111.00±12.23 | 93.92±6.99 | .0005 | |
| Word Reading Fluency | TOWRE – Sight Word Efficiency | 103.58±8.24 | 85.00±6.36 | .0005 |
| TOWRE – Phonemic Decoding Efficiency | 100.17±6.83 | 80.83±8.94 | .0005 | |
| Connected Text Reading Fluency | WJ – Reading Fluency | 118.55±8.65 | 93.92±10.03 | .0005 |
| Connected Text Reading Comprehension | WRMT – Passage Comprehension | 115.00±11.10 | 106.07±6.90 | .031 |
| NDRT – Reading Comprehension | 241.00±11.34 | 209.75±14.64 | .0005 | |
| Connected Text Reading Rate | NDRT – Reading Rate | 224.58±23.83 | 186.33±11.60 | .0005 |
| In-Scanner Task Performance | ||||
| Accuracy (% correct) | Fast | 81±10 | 64±11 | .001 |
| Medium | 94±6 | 83±9 | .002 | |
| Slow | 95±3 | 92±6 | .150 | |
| Reaction Time (ms) | Fast | 975.39±254.72 | 1230.13±312.70 | .040 |
| Medium | 629.88±187.12 | 954.92±259.49 | .002 | |
| Slow | 512.65±218.92 | 755.73±168.58 | .006 |
Mean ± SD; p values below .05 are statistically significant based on two-tailed t-tests. Note: Standard scores are based on a mean of 100 and a standard deviation of 15 (average range of 85-115) except for the CTOPP (based on mean of 10 and a standard deviation of 3; average range of 7-13) and NDRT (based on mean of 200 and a standard deviation of 25; average range of 175-225). SD = Standard deviation.
Task design and materials
The sentence reading paradigm consisted of five words presented sequentially, followed by a question mark, for each trial. Participants were asked to indicate via button press whether each sentence was semantically plausible (e.g., Bulls charge with great ferocity) or semantically nonplausible (e.g., Kangaroos type for their jobs). Participants practiced the paradigm with unique stimuli prior to the scanning session to ensure understanding of task directions and mastery of task demands.
Words in each sentence trial were presented at one of three speeds: Slow (400 milliseconds/word), Medium (250 milliseconds/word), or Fast (100 milliseconds/word). The slow sentence presentation rate, corresponding to 150 words per minute, was commensurate with a silent reading rate for typical readers in grade three or four [46], [47]. The medium sentence presentation rate, corresponding to 240 words per minute, was consistent with typical grade eight or nine silent reading rates [44], [46]. The fast sentence presentation rate, corresponding to a rate of 600 words per minute, was selected to be challenging for typical adult readers by surpassing the typical college-level silent reading rate of about 280 words per minute and efficient reading rates of about 500 words per minute [46], [47].
The nouns and verbs in sentence sets were matched for written frequency and number of syllables in three ways: between runs (run 1, run 2), between conditions (Slow, Medium, Fast), and between sentence types (plausible, nonplausible). One-way analysis of variance (ANOVA) was used to compare stimuli characteristics, which were compiled using the MRC Psycholinguistic Database (www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). First, sentences were balanced across run 1 and run 2 for written frequency [nouns: F(1, 295) = 1.08, p = .30; verbs: F(1, 150) = 1.30, p = .26] and for number of syllables [nouns: F(1, 318) = 0.02, p = .90; verbs: F(1, 154) = 1.65, p = .20]. Second, sentences were matched across the three conditions (Slow, Medium, Fast) for written frequency [nouns: F(2, 294) = 1.27, p = .28; verbs: F(2, 149) = 1.37, p = .26] and for number of syllables [nouns: F(2, 317) = 0.94, p = .39; verbs: F(2, 153) = 0.08, p = .93] to minimize effects of reading time that could be impacted by longer or less frequent words. Third, sentences were matched across the two types, plausible and nonplausible, for written frequency [nouns: F(1, 295) = 0.90, p = .34; verbs: F(1, 150) = 0.17, p = .68] and for number of syllables [nouns: F(1, 318) = 2.35, p = .13; verbs: F(1, 154) = 0.92, p = .34]. The sequence of sentence and rest trials was based on the output from a randomization program (OPTSEQ2) that generated three equivalent versions of the fluency task. Each version used the same sentence stimuli, but balanced the rate of presentation so that each sentence was presented in each condition across the three versions.
A jittered event-related design was used in which sentence conditions and a rest condition were randomly intermixed. The duration between trials, which constituted the rest condition, varied (i.e., was jittered). For the rest condition, participants were asked to stare at a fixation cross; this condition served as a low-level baseline. Rest trials were interspersed at random between sentence trials (i.e., jittered time periods), and the duration of the rest trials ranged from 200–2200 milliseconds at increments of 200 (e.g., 200 ms, 400 ms, 600 ms, … , 2200 ms). The total presentation time of each sentence condition was equal to the total duration of rest trials. Each sentence trial totaled four seconds and consisted of a sentence and a question mark. The question mark duration made up for the varying length of the sentence duration to total 4 seconds (i.e., 3500 ms for Fast, 2750 ms for Medium, and 2000 ms for Slow).
Each participant completed two consecutive runs (10.4 minutes each) of the sentence-reading fMRI task. Each run consisted of 78 sentences, with 13 semantically plausible and 13 nonplausible sentences at each of the three rates. Thus, there were 156 sentences in total across both runs. Stimuli were presented on a rear projection screen in white on a black background via PsychToolBox software [48]. The screen size, zoom, and focus were calibrated for each participant to ensure that the entire visual field of the projected images was visible through the mirror mounted on the head coil.
Imaging data acquisition
Imaging was performed using a Siemens 3T MAGNETOM Trio, a Tim System, (Siemens Medical Solutions, Erlangen, Germany) and a commercial 12-Channel Matrix head coil (Siemens Medical Solutions, Erlangen, Germany). To minimize head movement, tetrahedron-shaped foam pads were placed between the head coil and either side of the participant's head. Sagittal localizer scans were aligned to a multi-subject atlas to derive automatic slice prescription for consistent head position across participants. At the beginning of each functional scan, five images (10 second duration) were discarded to allow for T1 equilibration. High-resolution structural whole-brain images were acquired using a T1-weighted anatomical scan (128 slices per slab; 256×256 matrix; 256 mm FOV; 1.33 mm slice thickness; 0.63 mm interslice gap; TR = 2530 ms; TI = 1100 ms; TE = 3.39 ms; flip angle = 7°).
Functional data were collected using a gradient echo T2*-weighted EPI sequence sensitive to the BOLD contrast. The gradient-echo EPI images were acquired with PACE [49], an online motion correction algorithm that minimizes movement-related artifacts by adjusting the system gradients and the acquisition field of view between one whole brain acquisition and another for participant movement. Thirty-two sagittal slices parallel to the anterior commissure-posterior commissure (AC-PC) line were imaged (voxel size of 3.1×3.1×4.0 mm, 64×64 mm matrix, 200 mm field-of-view, 4 mm slice thickness, 0.8 mm inter-slice gap). Other imaging acquisition parameters included: TR = 2000 ms, TE = 30 ms, flip angle = 90°, bandwidth = 2298 Hz/Px, echo spacing = 0.5 ms.
In-Scanner recording of performance
Accuracy and reaction time in judging semantic plausibility were recorded when participants responded via button press for each trial. The scanner paradigm was programmed to take one response from the time that the trial began (first word in the sentence) to the end of the trial (question mark).
fMRI data analysis
The neural correlates associated with increasing reading fluency demands were measured using a within-subjects design and a parametric modulation analysis, which creates a statistical parametric mapping of the significance of the correlation between cognitive parameters and physiology [50]. Preprocessing and statistical analysis were performed using statistical parametric mapping software (SPM8; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm). During preprocessing, data were realigned to the first functional volume and spatially normalized using the mean functional volume to the Montreal Neurological Institute (MNI) template. Normalized images were smoothed using a Gaussian filter (6-mm full width at half maximum) to decrease spatial noise.
Analysis included individual and group level statistics. For the individual level analysis, the stimuli (defined as the start of the first word and the end of the last word) were modeled as box-car functions aligned with the onset of each stimulus, the width of which corresponded to the duration of each stimulus. The expected BOLD responses to the stimuli were obtained by convolving a canonical hemodynamic response function with the modeled stimuli. A high-pass filter (cutoff = 128 s) was used on both the data and the model to reduce impact of physiological noise. The mean voxel value was used for the global calculation. Grand mean scaling was based on session specific parameters. Global normalization was not used.
Outlier image volumes in the BOLD time series were identified based on either the mean intensity of image volume greater than 3 standard deviations from the mean intensity of the time series or the largest voxel movement of the image volume greater than .5 mm, based on scan-to-scan movement. Image volumes were masked by a binary image created from the functional time series (using the same procedure as that used to create the SPM analysis mask). Outlier images were included as nuisance regressors in the first-level analysis per person. The typical reader group (M = 11.7, SD = 11.3) and the dyslexic group (M = 7.0, SD = 5.5) did not differ in the number of outlier images (t(22) = 1.27, p = .22).
A random effects model [51] was used to characterize group level effects (second-level analysis). Brain regions were identified using a threshold of p<.001 cluster-level FDR corrected for multiple comparisons and using a cluster extent threshold (ET) of 10 voxels or more. We used the Topological False Discovery Rate (FDR) calculations from SPM8. SPM8 computes FDR by assigning corrected p-values to the local maxima. Peak-wise FDR has fewer false positives than conventional voxel-wise FDR [52], [53].
The comparison between the typical reader group and the dyslexic group was based on differences between mean parameter estimates in a linear parametric modulation contrast (Fast > Medium > Slow) using a threshold of p<.01 cluster-level FDR corrected for multiple comparisons. An independent samples t-test was used to characterize clusters showing significant difference between groups. Within and between group comparisons were also completed for Medium > Slow to ensure that the parametric comparison was not driven by aberrant activations elicited by the fast rate, which exceeded expectations for even typical adult readers. For these analyses, all trials (correct and incorrect) were used to maximize power and not bias data-points in favor of the typical reader group, who answered more items correctly compared to readers with dyslexia. In addition, the Fast > Slow contrast for typical readers was compared to Medium > Slow for dyslexic readers because these contrasts yielded comparable behavioral performance between the groups. For this comparison, in-scanner accuracy changes in performance between presentation rates did not significantly differ between groups.
We also examined as an a priori region of interest the putative visual word form area (VWFA), which has been associated with rapid visual analysis of text for typical readers [54]. The ROI was defined as a 10 mm sphere with the location taken from the imaging literature (Standard Talairach Coordinates: x = −43, y = −54, z = −12; [55]).
Results
Behavioral measures
The typical reader group performed significantly better than the dyslexic group on standardized measures of verbal cognitive abilities, phonological processing (with the exception of Memory for Digits, an index of phonological memory), rapid naming (letters, numbers, 2-set), timed and untimed single word reading, timed and untimed text comprehension, and reading rate (Table 1; independent samples t-tests, two-tailed, all p<.05). The typical reader group and the dyslexic group did not differ significantly on non-verbal cognitive abilities (p = .18).
Scanner task performance
In-scanner performance (Table 1) for the typical reader group and the dyslexic group was analyzed with a 3×2 repeated measures ANOVA, with Condition (Fast, Medium, Slow) as a within-subjects factor and Group (typical readers vs. readers with dyslexia) as a between-subjects factor. The typical reader group was more accurate than the dyslexic group as indicated by a significant main effect for Group [F(1,22) = 14.16, p<.001]. Accuracy declined with greater rates of presentation as indicated by a significant main effect of Condition [F(2,21) = 61.34, p<.0005]. Accuracy differences between groups varied as a function of presentation rate as shown by a significant Group X Condition interaction [F(2,21) = 8.46, p<.002], with the dyslexic group performing significantly worse on Fast [t(22) = 4.05, p<.001] and Medium [t(22) = 3.59, p<.002] conditions, but not on the Slow condition [t(22) = 1.49, p = .15]. Groups did not differ on rates of response across conditions [t(22) = 1.77, p = .09], indicating that both groups of participants had sufficient time to respond to items.
The typical reader group was faster to respond than the dyslexic group as indicated by a significant main effect for Group [F(1,22) = 9.76, p<.005]. Responses were slower as presentation rates increased as shown by a significant main effect of Condition [F(2,21) = 49.06, p<.0001]. The Group X Condition interaction was not significant [F(2,21) = 2.10, p = .15].
In-scanner performances for the typical reader group and the dyslexic group were compared to determine if performance was comparable at the Fast rate for the typical reader group and at the Medium rate for the dyslexic group. Independent samples t-tests showed that performance for the typical reader group during the Fast-rate condition did not differ significantly from performance for the dyslexic group during the Medium-rate condition for accuracy [t(22) = 1.04, p = .31] or reaction time [t(22) = 0.49, p = .63]. Thus, we included these conditions as a performance-equated group comparison. Further analysis indicated that the difference between Slow and Fast conditions for the typical reader group and the difference between Slow and Medium conditions for the group with dyslexia was not statistically significant for accuracy [t(22) = 1.58, p = .13], but was for reaction time, with the typical reader group exhibiting a larger difference than dyslexic group [t(22) = 2.99, p<.05].
Relation of in-scanner performance with standardized measures of reading fluency and phonological awareness
We examined the relationship between behavioral performance in the scanner and a standardized test of reading fluency on which the task was based (“Reading Fluency,” WJ) across participants in both groups using correlation analysis (uncorrected for multiple comparisons). There were significant negative correlations between age-standardized Reading Fluency scores and in-scanner reaction time at all three reading rates: Slow (standard scores: r = −.69, p<.01); Medium (standard scores: r = −.68, p<.01), and Fast (standard scores: r = −.54, p<.01). There were significant positive correlations between Reading Fluency scores and accuracy at the Medium and Fast rates, which did not show ceiling effects for accuracy: Medium (standard scores: r = .47, p<.05); Fast (standard scores: r = .63, p<.01). These correlations reflected group differences between the typical reader and dyslexic groups, because the correlations were not significant within either group alone.
We also examined the relationship between phonological processing and in-scanner performance. Standard scores from a measure of phonological awareness (“Elision,” CTOPP) showed significant correlations with in-scanner reaction time for Medium (r = −.49, p<.05) and Slow (r = −.43, p<.05) rates, and accuracy for Fast (r = .44, p<.05) and Medium (r = .47, p<.05) rates. Other measures of phonological awareness and phonological memory were not significantly correlated with in-scanner performance (p>.05). Once again, these correlations reflected group differences between the typical reader and dyslexic groups, because the correlations were not significant within either group alone.
fMRI Activation for Typical Readers
Fast > Medium > Slow
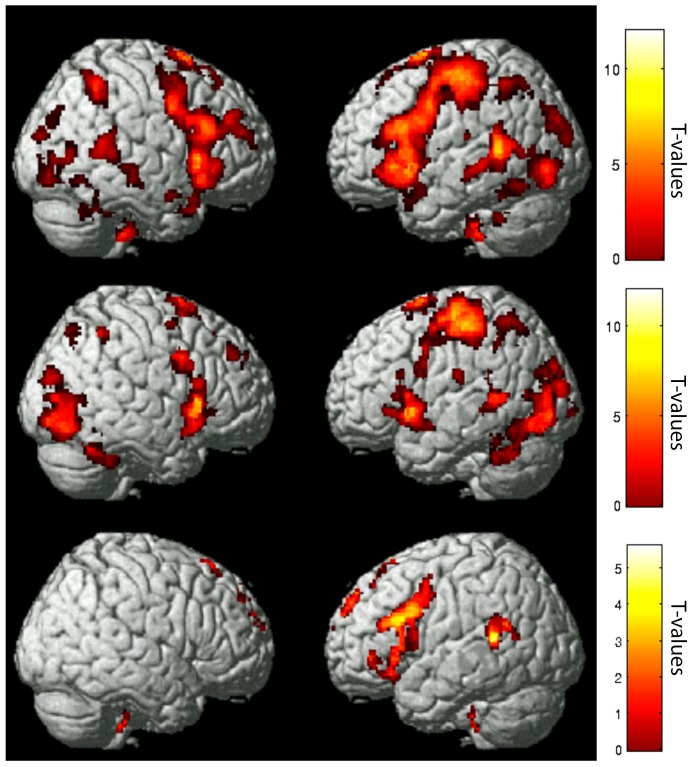
Typical readers showed greater activation for faster rates of word presentation in a distributed cortical network including peak activations in left superior frontal gyrus, left middle temporal gyrus, right superior temporal and insular regions, left inferior occipital gyrus, right middle occipital gyrus, and cerebellar and subcortical regions (Table 2 & Figure 1, top panel).
Table 2. Activations for Fast > Medium > Slow contrasts for Typical Readers.
| Region (peak activation) | BA | x | y | z | Cluster extent | p | Z score |
| Fast > Medium > Slow | |||||||
| Frontal Lobe | |||||||
| L. Superior frontal gyrus | 6 | −2 | 8 | 70 | 5008 | <.0001 | 6.61 |
| Temporal Lobe | |||||||
| R. Insula | 13 | 30 | 26 | 0 | 3841 | <.0001 | 6.11 |
| L. Middle temporal gyrus | 21 | −50 | −46 | 8 | 1270 | <.0001 | 5.53 |
| R. Superior temporal gyrus | 22 | 54 | −46 | 14 | 666 | <.0001 | 5.47 |
| Parietal Lobe | |||||||
| N/A | |||||||
| Occipital Lobe | |||||||
| L. Inferior occipital gyrus | 19 | −34 | −76 | −4 | 1082 | <.0001 | 5.32 |
| R. Middle occipital gyrus | 19 | 36 | −88 | 18 | 81 | .017 | 4.52 |
| R. Middle occipital gyrus | 19 | 48 | −78 | 4 | 327 | <.0001 | 4.04 |
| Cerebellum | |||||||
| L. Cerebellum | N/A | −38 | −46 | −38 | 199 | <.0001 | 4.42 |
| L. Cerebellum | N/A | −8 | −78 | −38 | 83 | .017 | 4.30 |
| Sub-lobar | |||||||
| Thalamus | N/A | −8 | −16 | 4 | 16152 | <.0001 | 6.22 |
| R. Caudate | N/A | 12 | 12 | 8 | 115 | .006 | 4.11 |
| Brainstem | N/A | 4 | −34 | −46 | 577 | <.0001 | 4.98 |
| Medium > Slow | |||||||
| Frontal Lobe | |||||||
| L. Superior frontal gyrus | 6 | −2 | 8 | 60 | 2605 | <.0001 | 6.42 |
| R. Middle frontal gyrus | 46 | 48 | 26 | 26 | 216 | .001 | 5.24 |
| R. Inferior frontal gyrus | 47 | 34 | 32 | −2 | 324 | <.0001 | 4.43 |
| R. Precentral gyrus | 6 | 38 | −4 | 38 | 90 | .028 | 4.42 |
| Temporal Lobe | |||||||
| R. Middle temporal gyrus | 22 | 50 | −40 | 6 | 104 | .021 | 4.39 |
| Parietal Lobe | |||||||
| L. Postcentral gyrus | 3 | −40 | −24 | 48 | 6078 | <.0001 | 5.98 |
| R. Inferior parietal lobule | 40 | 36 | −46 | 50 | 132 | .009 | 3.89 |
| R. Precuneus | 19 | 30 | −68 | 36 | 87 | .028 | 3.66 |
| Occipital Lobe | |||||||
| R. Lingual gyrus | 18 | 18 | −80 | −2 | 6923 | <.0001 | 5.46 |
p<.001, cluster level FDR corrected (T = 3.50); ET = 10. N/A = Not applicable. L. = Left hemisphere. R. = Right hemisphere. Coordinates reported in Talairach space.
Figure 1. Sentence presentation rate differentially impacts brain activation by group.
Fast > Medium > Slow parametric modulation (cluster level FDR corrected) for a) Typical Reader Group (p<.001) (top panel); b) Dyslexic Group (p<.001) (middle panel); c) Typical > Dyslexic Groups (p<.01) (bottom panel). Color bar indicates T-values.
Medium > Slow
In typical readers, comparisons excluding the fastest rate continued to demonstrate robust activations in networks including frontal systems with peaks in the superior frontal gyrus that was situated medially and extending to both hemispheres; right middle and inferior frontal gyrus; and right precentral gyrus. Activations also included clusters with peaks in the right middle temporal gyrus, left postcentral gyrus extending to inferior frontal gyrus, right inferior parietal lobule and precuneus, and a cluster with a peak in right lingual gyrus that included bilateral fusiform gyri and left cuneus (Table 2).
fMRI Activation for Dyslexic Readers
Fast > Medium > Slow
Dyslexic readers showed greater activation for faster rates of word presentation in a distributed cortical network including left superior frontal gyri, right middle frontal gyrus, bilateral insular and middle temporal regions, left postcentral gyrus, left superior parietal lobule, bilateral inferior parietal lobule, right precuneus, and cerebellar and subcortical regions (Table 3 and Figure 1, middle panel).
Table 3. Activations for Fast > Medium > Slow contrasts for Readers with Dyslexia.
| Region (peak activation) | BA | x | y | z | Cluster extent | p | Z score |
| Fast > Medium > Slow | |||||||
| Frontal Lobe | |||||||
| L. Superior frontal gyrus | 8 | 6 | 16 | 50 | 3902 | <.0001 | 6.06 |
| R. Middle frontal gyrus | 9 | 36 | 44 | 36 | 94 | .023 | 4.42 |
| R. Middle frontal gyrus | 6 | 28 | 4 | 46 | 84 | .029 | 4.16 |
| Temporal Lobe | |||||||
| L. Insula | 13 | −36 | 12 | 2 | 1432 | <.0001 | 5.93 |
| R. Insula | 13 | 30 | 24 | 2 | 1866 | <.000 | 6.15 |
| L. Middle temporal gyrus | 21 | −62 | −46 | 8 | 324 | <.0001 | 4.29 |
| R. Middle temporal gyrus | 21 | 48 | −34 | −4 | 69 | .049 | 3.41 |
| Parietal Lobe | |||||||
| L. Postcentral gyrus | 3 | −36 | −30 | 52 | 2626 | <.0001 | 5.77 |
| L. Superior parietal lobule | 7 | −30 | −52 | 52 | 817 | <.0001 | 4.65 |
| L. Inferior parietal lobule | 40 | −54 | −20 | 24 | 90 | .025 | 3.75 |
| R. Inferior parietal lobule | 40 | 46 | −50 | 52 | 96 | .023 | 3.71 |
| R. Precuneus | 7 | 26 | −68 | 50 | 223 | .001 | 3.81 |
| Occipital Lobe | |||||||
| N/A | |||||||
| Cerebellum | |||||||
| Anterior lobe | N/A | 0 | −54 | −30 | 216 | .001 | 3.96 |
| Sub-lobar | |||||||
| L. Thalamus | N/A | −10 | −20 | 0 | 11851 | <.0001 | 5.93 |
| Cingulate gyrus | 23 | 8 | −22 | 26 | 99 | .023 | 4.53 |
| Medium > Slow | |||||||
| Frontal Lobe | |||||||
| R. Superior frontal gyrus | 8 | 6 | 16 | 52 | 1744 | <.0001 | 6.15 |
| R. Middle frontal gyrus | 9 | 48 | 8 | 34 | 146 | .002 | 4.17 |
| R. Inferior frontal gyrus | 47 | 34 | 32 | 0 | 279 | <.0001 | 5.11 |
| L. Inferior frontal gyrus | 44 | −46 | 10 | 20 | 961 | <.0001 | 4.45 |
| Temporal Lobe | |||||||
| L. Middle temporal gyrus | 21 | −50 | −48 | 4 | 157 | .002 | 4.17 |
| L. Insula | 13 | −48 | −22 | 20 | 101 | .01 | 4.02 |
| Parietal Lobe | |||||||
| Postcentral gyrus | 3 | −38 | −22 | 52 | 2095 | <.0001 | 5.71 |
| Superior parietal lobule | 7 | −30 | −50 | 58 | 260 | <.0001 | 4.22 |
| Occipital Lobe | |||||||
| R. Cuneus | 17 | 8 | −78 | 10 | 4909 | <.0001 | 5.44 |
| L. Middle occipital gyrus | 18 | −22 | −92 | 16 | 572 | <.0001 | 4.83 |
| R. Middle occipital gyrus | 18 | 26 | −90 | 10 | 187 | .001 | 4.09 |
| Cerebellum | |||||||
| L. Anterior culmen | N/A | −8 | −28 | −10 | 476 | <.0001 | 4.68 |
p<.001, cluster level FDR corrected (T = 3.50); ET = 10. N/A = Not applicable. L. = Left hemisphere. R. = Right hemisphere. Coordinates reported in Talairach space.
Medium > Slow
Comparing the medium to slow rates of sentence presentation, readers with dyslexia showed activations with peaks in right superior, middle, and inferior frontal gyri and left inferior frontal gyrus; left middle temporal gyrus and insula, superior parietal lobule and postcentral gyrus; bilateral middle occipital gyrus and right cuneus; and cerebellar regions (Table 3).
Comparing Typical and Dyslexic Readers: Fast > Medium > Slow
Compared to readers with dyslexia on the parametric analysis (Fast > Medium > Slow), typical readers showed greater activation in a cluster with a peak in left middle frontal gyrus that extended into the inferior frontal gyrus, insula, and precentral gyrus; a cluster with a peak in left superior temporal gyrus that extended into the supramarginal gyrus, inferior parietal lobule, middle temporal gyrus, and cingulate cortex; and a cluster with a peak in the brainstem that extended into bilateral brainstem regions, medulla, and right cerebellum (Table 4; Figure 1, bottom panel). Readers with dyslexia did not show any activation greater than typical readers (Table 4).
Table 4. Activations for Typical Reader > Dyslexic Groups.
| Region (peak activation) | BA | x | y | z | Cluster extent | p | Z score |
| Typical(Fast > Medium > Slow) > Dyslexic(Fast > Medium > Slow) | |||||||
| L. Middle frontal gyrus | 9 | −54 | 22 | 26 | 1319 | <.0001 | 4.01 |
| L. Superior temporal gyrus | 22 | −60 | −46 | 16 | 324 | .048 | 4.29 |
| Cingulate gyrus | 32 | 14 | 20 | 44 | 772 | <.001 | 4.38 |
| Brainstem/Cerebellum | −2 | −32 | −38 | 380 | <.0001 | 4.01 | |
| Dyslexic(Fast > Medium > Slow) > Typical(Fast > Medium > Slow) | |||||||
| N/A | |||||||
| Typical(Fast > Slow) > Dyslexic(Medium > Slow) | |||||||
| L. Superior frontal gyrus | 6 | −4 | 10 | 66 | 7165 | <.0001 | 6.65 |
| L. Inferior frontal gyrus | 45 | −44 | 24 | 6 | 38925 | <.0001 | 6.39 |
| Dyslexic(Medium > Slow) > Typical(Fast > Slow) | |||||||
| L. Anterior cingulate gyrus | 24 | −6 | 24 | −4 | 2159 | <.0001 | 4.45 |
| Sub-lobar | 34 | −34 | −6 | 470 | .006 | 3.94 | |
| Sub-lobar | −22 | −8 | 26 | 699 | .001 | 3.88 | |
| Sub-lobar | 32 | −8 | −4 | 324 | .025 | 3.65 | |
p<.01, cluster level FDR corrected (T = 2.51); ET = 10. N/A = Not applicable. L. = Left hemisphere.
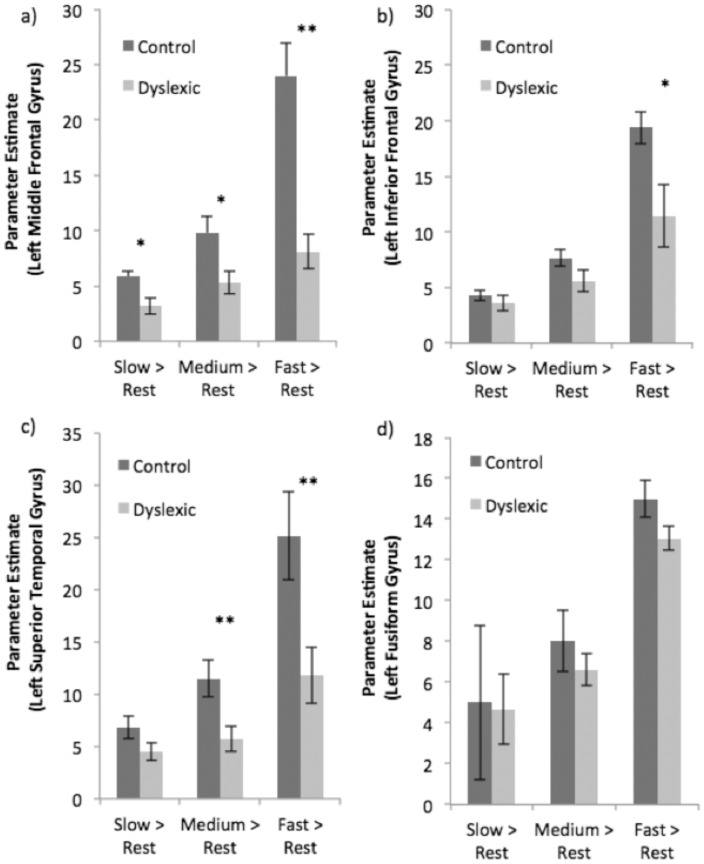
In order to characterize the nature of these group differences in activation, we extracted parameter estimate values for each reading rate, relative to the fixation baseline, from peaks of activation in regions showing greatest group differences (10 mm spheres around activation peaks in left middle (x = −54, y = 22, z = 26) and inferior (x = −44, y = 24, z = 6) frontal gyri and left superior temporal gyrus (x = −60, y = −46, z = 16)). In left middle frontal gyrus, typical readers showed significantly more activation than dyslexic readers at all rates (p = .01) (Figure 2a). In left inferior frontal (Figure 2b) and left superior temporal (Figure 2c) regions, there were no significant differences for the Slow condition (p>.05), but typical readers exhibited greater activation in the Medium [left middle frontal gyrus, t(22) = 2.47, p = .02; left superior temporal gyrus, t(22) = 2.73, p = .01] and Fast conditions [left middle frontal gyrus, t(22) = 4.78, p<.0005; left superior temporal gyrus, t(22) = 2.67, p = .02; left inferior frontal gyrus, t(22) = 2.67, p = .02]. In addition, the putative visual word form area (VWFA; left fusiform gyrus) region of interest exhibited greater activation as a function of rate (p<.0001), but there was neither an effect of group nor a group x rate interaction (p = .41) (Figure 2d).
Figure 2. Comparison between Typical Reader and Dyslexic Groups (with standard error bars) showing region-of-interest activations for a) left middle frontal gyrus; b) left inferior frontal gyrus; c) left superior temporal gyrus; and d) left fusiform gyrus (visual word form area, VWFA).
Note: *p<.05; **p<.01.
Comparing conditions matched for in-scanner performance
Typical readers (Fast > Slow) and readers with dyslexia (Medium > Slow) were compared based on matched in-scanner performance (Table 4). Typical readers showed significantly greater activation in almost all brain regions engaged by the task (Figure 3). Readers with dyslexia showed greater activations compared to typical readers in left anterior cingulate regions.
Figure 3. The Dyslexic Group showed reduced activation relative to the Typical Reader Group even when accuracy differences across conditions were equated between the groups.
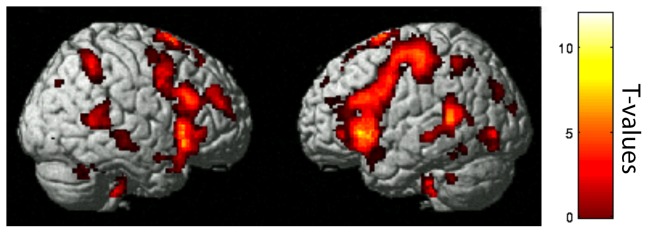
Greater activation for Fast > Slow contrast for Typical Readers versus Medium > Slow contrast for the Dyslexic Group (cluster level FDR corrected results displayed at p<.001). Color bar indicates T-values.
Discussion
We compared the neural correlates of reading fluency in adult readers with and without dyslexia using an fMRI sentence reading paradigm that parametrically varied fluency demands by increasing the rate at which sentences were presented for semantic plausibility judgments. Faster presentation rates resulted in slower responses and reduced judgment accuracy in both groups. Readers with dyslexia were slower and less accurate across rates than typical readers, and their accuracy declined disproportionately as rates increased. In-scanner behavioral performance correlated with standardized measures of reading fluency, indicating that the scanner task explored the same underlying fluency processes, and also standardized measures of phonological awareness. For both typical and dyslexic readers, a large bilateral network of cortical, subcortical, and cerebellar systems supported fluent sentence reading. Readers with dyslexia showed less of an increase in activation, as a function of reading rate, in left prefrontal and left superior temporal cortices, anterior cingulate, and brainstem/cerebellar regions. The relationship between performance levels did not account for the differences in activation, because equating performance at different rates between the typical and dyslexic readers enhanced, rather than reduced, group differences. These findings point to brain regions that are associated with reading fluency in typical reading and with reading dysfluency in dyslexia.
Defining and measuring fluent reading
The current study overcame several challenges in defining and measuring reading fluency directly using fMRI. Reading fluency was measured by using silent sentence reading in a word-by-word presentation format with the rate of word presentation manipulated on a sentence-by-sentence basis. Presentation rate was chosen as the independent variable because reading rate is a core aspect of reading fluency [56], and it can be manipulated in an fMRI task. Attention to the task was required as participants were asked to make semantic plausibility judgments following each sentence.
Silent reading of sentences was feasible for use in an fMRI study and similar to real world reading experiences. Implicit, or silent, word reading is effective in eliciting activations from brain areas associated with language processing [57]. Furthermore, for most adult readers, reading connected text silently is the most frequent interaction with written language, and predominantly involves decoding strings of words to extract meaning as opposed to reading isolated single words. Previous studies using word reading tasks have provided a basis for understanding the brain networks recruited for decoding and recognizing single words (i.e., isolated text), and studies using sentence reading tasks have identified brain regions recruited to process semantic or syntactic properties of sentences instantiated by relations among words. Sentence reading tasks can provide the additional advantage of localizing cognitive functions pertaining specifically to reading fluency - a dynamic aspect of reading behavior.
Limitations
We presented sentences one word at a time to control reading rate. A limitation of this approach is that some sentence-reading processes that are typically engaged during the reading of text, such as the voluntary allocation of different viewing times for different words, or looking back at words, were not invoked in this design. Several behavioral observations indicate, however, that this task probed reading fluency processes. First, faster rates reduced judgment accuracy and latency across all participants. Second, the dyslexic group exhibited the expected deficits in accuracy and latency, and accuracy decreased disproportionately as a function of increasing reading rate. Third, when reading was examined across participants from typical and dyslexic groups, in-scanner performance correlated with scores from a standardized reading fluency test that is widely used in educational and clinical testing. These behavioral findings support the validity of the reading rate manipulation as a test of reading fluency.
Brain regions associated with typical or impaired reading fluency
In typical readers, increased rates of word presentation likely influence many perceptual, phonological, semantic, syntactic, and pragmatic processes, and, correspondingly, resulted in increased activation in a large bilateral network of cortical, subcortical, and cerebellar regions. This activation pattern included brain regions implicated in processing visual (ventral occipital regions), phonological (inferior frontal gyrus, posterior superior temporal gyrus), and semantic (middle temporal gyrus, posterior superior temporal gyrus) information [58], [59].
The group with dyslexia showed many similarities to the typical group in regards to increased activation as a function of word presentation rate in a large bilateral network. The dyslexic group showed significantly less gain in activation relative to the control group in primarily left hemisphere regions, including left middle and inferior frontal gyri and left superior temporal gyrus. The left posterior superior temporal gyrus supports processing of semantic judgments, and shows greater activation as a function of greater semantic analysis [59]. The left inferior frontal gyrus has been implicated in semantic working memory [60], unrelated to general task difficulty [61], but related to competition between or selection among related semantic response options [62]. Although the current study did not directly investigate the distinct contributions of phonological and semantic processing, left inferior frontal gyrus activation in this study closely approximates the location identified in previous research (x = −37, y = 28, z = −9) showing greater activation for semantic versus phonological processing [63]. The left inferior frontal gyrus is also activated for extracting a coherent meaning from individual words in a sentence [64] and semantic processing of sentences [65]. At the same time, there is considerable overlap in brain regions associated with semantic and phonological analysis of language (e.g., the left posterior superior temporal gyrus) [58], [59]. To further characterize the relevant roles of the left inferior frontal gyrus and superior temporal gyrus in fluent reading, future analyses can compare activations for semantically appropriate and inappropriate sentences, or vary phonological demands.
Thus, some of the regions that showed significantly less activation in readers with dyslexia, which differed most at the fastest rate, are implicated in the control (left inferior frontal gyrus) or representation (left superior temporal gyrus) of semantic verbal knowledge. Weak responses in these regions associated with semantic processes during rapid or fluent reading could diminish comprehension during reading, as occurred for readers with dyslexia in the medium and fast conditions.
There was also greater activation in the group of typical readers in the cerebellum. Some studies have pointed to cerebellar anatomical differences in dyslexia [66], and it has been hypothesized that automaticity deficits in dyslexia may be associated with atypical cerebellar function [67]. The cerebellum, in addition to supplemental motor area (SMA) and primary motor cortex, shows increased recruitment for increasing rate (faster) and shorter duration when naming visually presented words [68], and for semantic and phonological processing [69]. The right cerebellar declive in particular has been implicated in automaticity in reading [70]. Functional connectivity between the cerebellum and inferior frontal and lateral temporal regions during reading suggests a coordinated cortico-cerebellar system that facilitates fluent reading [71]. The present findings are consistent with the impairment of the cerebellar component of this reading network in dyslexia.
Although activation was found in the purported visual word form area (VWFA) in the left temporo-occipital cortex and increased with reading rate, it did so similarly for both groups. This region has been associated with rapid processing of text for typical readers. Previously, this region has been found to show reduced activation in children with developmental dyslexia [72], [73]. Due to previous findings, we had expected to observe reduced activation in dyslexic adults as a function of reading rate. Perhaps the increasing intensity of visual processing across the faster rates of word presentation was such a strong manipulation that it dominated activation in the VWFA.
The most striking group differences occurred in left-hemisphere cortical regions implicated in semantic processing and required to perform the semantic analyses of the sentences. The present study, however, cannot determine what kinds of processing bottlenecks in the brain restricted the flow of information to brain regions involved in semantic analysis and judgment. Such bottlenecks may have been due to slow phonological decoding of single words, or impaired temporal processing that limited coordination of reading processes across words. The absence of a group difference in VWFA suggests the dyslexic group may not have been limited by the rate of orthographic processing per se, but only more targeted experiments can better elucidate the bases of the fluency impairment.
The activation differences between the groups could reveal the cause or the consequence of impaired fluency in dyslexia (or both). One approach towards this issue of interpretation is to compare typical and dyslexic groups under conditions where behavioral performance or comprehension is equated. This analytic approach was possible by comparing the difference between slow and medium rates in the dyslexic group to the difference between slow and fast rates in the typical reading group, because these comparisons did not show significant accuracy differences between the groups. When reading accuracies across conditions were equated, however, there remained large brain activation differences between the groups. Therefore, the activation differences between groups were not simply the consequence of worse performance by the group with dyslexia. Rather, weakened engagement of brain regions associated with semantic processing and automated reading may reflect the cause of the fluency deficits that make reading comprehension so challenging for many readers with dyslexia.
Acknowledgments
We thank the participants, A. A. Martinos Imaging Center at McGovern Institute for Brain Research (MIT) for making this research possible, as well as Tyler Perrachione, Bianca Levy, Alison Bloomfield, Rebecca Winter, Carlos Cardenas, Omar Hadzipasic, Sara Beach, for assistance with data collection. We thank Steve Shannon and Sheeba Arnold for data collection technical support.
Funding Statement
This work was supported by Ellison Medical Foundation; MIT Class of 1976 Funds for Dyslexia Research; Bard and Julie Richmond through the Martin Richmond Memorial Fund; Clinical Research Center (CRC) at the Massachusetts Institute of Technology, Grant Number UL1 RR025758- Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wolf M, Katzir-Cohen T (2001) Reading fluency and its intervention. Scientific Studies of Reading 5: 211–239. [Google Scholar]
- 2.National Institute of Child Heath and Human Development (2000) Report of the National Reading Panel. Teaching children to read: An evidence-based assessment of the scientific research literature on reading and its implications for reading instruction: Reports of the subgroups (NIH Publication No. 00–4754). Washington, DC: U.S. Government Printing Office. Available: www.nichd.nih.gov/publications/nrp/smallbook.cfm.
- 3. Lyon GR, Shaywitz SE, Shaywitz BA (2003) A definition of dyslexia. Annals of Dyslexia 53: 1–14. [Google Scholar]
- 4. Hudson RF, Pullen PC, Lane HB, Torgesen JK (2008) The complex nature of reading fluency: A multidimensional view. Reading & Writing Quarterly 25: 4–32. [Google Scholar]
- 5. Meyer MS, Felton RH (1999) Repeated reading to enhance fluency: Old approaches and new directions. Annals of Dyslexia 49: 283–306. [Google Scholar]
- 6. Torgesen JK, Alexander AW, Wagner RK, Rashotte CA, Voeller KK, et al. (2001) Intensive remedial instruction for children with severe reading disabilities: Immediate and long-term outcomes from two instructional approaches. Journal of Learning Disabilities 34: 33–58. [DOI] [PubMed] [Google Scholar]
- 7. Leinonen S, Müller K, Leppänen PH, Aro M, Ahonen T, et al. (2001) Heterogeneity in adult dyslexic readers: Relating processing skills to the speed and accuracy of oral text reading. Reading and Writing 14: 265–296. [Google Scholar]
- 8. Ziegler JC, Perry C, Ma-Wyatt A, Ladner D, Schulte-Körne G (2003) Developmental dyslexia in different languages: Language-specific or universal? Journal of Experimental Child Psychology 86: 169–193. [DOI] [PubMed] [Google Scholar]
- 10. Snowling MJ (1981) Phonemic deficits in developmental dyslexia. Psychological Research 43: 219–234. [DOI] [PubMed] [Google Scholar]
- 11. Gabrieli JDE (2009) Dyslexia: a new synergy between education and cognitive neuroscience. Science 325: 280–283. [DOI] [PubMed] [Google Scholar]
- 12. Roberts G, Torgesen JK, Boardman A, Scammacca N (2008) Evidence-based strategies for reading instruction of older students with learning disabilities. Learning Disabilities Research & Practice 23: 63–69. [Google Scholar]
- 13.Torgesen JK, Hudson RF (2006) Reading fluency: Critical issues for struggling readers. In S. J. Samuels & A. E. Farstrup (Eds.), What research has to say about fluency instruction (pp.130–158). Newark, DE: International Reading Assocation.
- 14. Lovett MW, Steinbach KA, Frijters JC (2000) Remediating the core deficits of developmental reading disability: A double-deficit perspective. Journal of Learning Disabilities 33: 334–358. [DOI] [PubMed] [Google Scholar]
- 15. Wexler J, Vaughn S, Edmonds M, Reutebuch CK (2008) A synthesis of fluency interventions for secondary struggling readers. Reading and Writing 21: 317–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katzir T, Shaul S, Breznitz Z, Wolf M (2004) The universal and the unique in dyslexia: A cross-linguistic investigation of reading and reading fluency in Hebrew-and English-speaking children with reading disorders. Reading and Writing 17: 739–768. [Google Scholar]
- 17. Kronbichler M, Hutzler F, Staffen W, Mair A, Ladurner G, et al. (2006) Evidence for a dysfunction of left posterior reading areas in German dyslexic readers. Neuropsychologia 44: 1822–1832. [DOI] [PubMed] [Google Scholar]
- 18. Landerl K, Wimmer H (2008) Development of word reading fluency and spelling in a consistent orthography: An 8-year follow-up. Journal of Educational Psychology 100: 150–161. [Google Scholar]
- 19. Thaler V, Ebner EM, Wimmer H, Landerl K (2004) Training reading fluency in dysfluent readers with high reading accuracy: Word specific effects but low transfer to untrained words. Annals of Dyslexia 54: 89–113. [DOI] [PubMed] [Google Scholar]
- 20. Wimmer H, Mayringer H (2002) Dysfluent reading in the absence of spelling difficulties: A specific disability in regular orthographies. Journal of Educational Psychology 94: 272. [Google Scholar]
- 21. Capek CM, Bavelier D, Corina D, Newman AJ, Jezzard P, et al. (2004) The cortical organization of audio-visual sentence comprehension: An fMRI study at 4 Tesla. Cognitive Brain Research 20: 111–119. [DOI] [PubMed] [Google Scholar]
- 22. Constable RT, Pugh KR, Berroya E, Mencl WE, Westerveld M, et al. (2004) Sentence complexity and input modality effects in sentence comprehension: An fMRI study. NeuroImage 22: 11–21. [DOI] [PubMed] [Google Scholar]
- 23. Gernsbacher MA, Kaschak MP (2003) Neuroimaging studies of language production and comprehension. Annual Review of Psychology 54: 91–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyler A, Keller TA, Cherkassky VL, Gabrieli JDE, Just MA (2008) Modifying the brain activation of poor readers during sentence comprehension with extended remedial instruction: A longitudinal study of neuroplasticity. Neuropsychologia 46: 2580–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cutting L, Clements A, Courtney S, Rimrodt S, Schafer J, et al. (2006) Differential components of sentence comprehension: Beyond single word reading and memory. NeuroImage 29: 429–438. [DOI] [PubMed] [Google Scholar]
- 26. Glaser YG, Martin RC, Van Dyke JA, Hamilton AC, Tan Y (2013) Neural basis of semantic and syntactic interference in sentence comprehension. Brain and Language 126: 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benjamin CF, Gaab N (2012) What's the story? The tale of reading fluency told at speed. Human Brain Mapping 33: 2572–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simos PG, Rezaie R, Fletcher JM, Juranek J, Papanicolaou AC (2011) Neural correlates of sentence reading in children with reading difficulties. NeuroReport 22: 674–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rimrodt S, Clements-Stephens A, Pugh K, Courtney S, Gaur P, et al. (2009) Functional MRI of sentence comprehension in children with dyslexia: Beyond word recognition. Cerebral Cortex 19: 402–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karni A, Morocz I, Bitan T, Shaul S, Kushnir T, et al. (2005) An fMRI study of the differential effects of word presentation rates (reading acceleration) on dyslexic readers' brain activity patterns. Journal of Neurolinguistics 18: 197–219. [Google Scholar]
- 31. Wagner RK, Torgesen JK (1987) The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin 101: 192–212. [Google Scholar]
- 32. Wolf M, Bowers PG (1999) The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology 91: 415–438. [Google Scholar]
- 33. Tallal P (1980) Auditory temporal perception, phonics, and reading disabilities in children. Brain and Language 9: 182–198. [DOI] [PubMed] [Google Scholar]
- 34. Mody M, Studdert-Kennedy M, Brady S (1997) Speech perception deficits in poor readers: Auditory processing or phonological coding. Journal of Experimental Child Psychology 64: 199–231. [DOI] [PubMed] [Google Scholar]
- 35. Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, et al. (2002) Amplitude envelope onsets and developmental dyslexia: A new hypothesis. Proceedings of the National Academy of Sciences 99: 10911–10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lehongre K, Ramus F, Villiermet N, Schwartz D, Giraud A-L (2011) Altered low-gamma sampling in auditory cortex accounts for the three main facets of dyslexia. Neuron 72: 1080–1090. [DOI] [PubMed] [Google Scholar]
- 37. Hoeft F, Hernandez A, McMillon G, Taylor-Hill H, Martindale JL, et al. (2006) Neural basis of dyslexia: A comparison between dyslexic and nondyslexic children equated for reading ability. The Journal of Neuroscience 26: 10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oldfield RC (1971) The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 39.Wechsler D (1999) Manual for the Wechsler Abbreviated Intelligence Scale. San Antonio, TX: The Psychological Corporation.
- 40.Wagner R, Torgesen J, Rashotte C (1999) Comprehensive Test of Phonological Processing. Austin, TX: Pro-Ed.
- 41.Wolf M, Denckla MB (2005) RAN/RAS: Rapid Automatized Naming and Rapid Alternating Stimulus Tests. Austin, TX: Pro-Ed.
- 42.Woodcock RW (1998) Woodcock Reading Mastery Tests, Revised Normative Update. Circle Pines, MN: American Guidance Service.
- 43.Torgesen J, Wagner R, Rashotte C (1999) Test of Word Reading Efficiency. Austin, Texas: Pro-Ed. Inc.
- 44.Brown JI, Fishco VV, Hanna G (1993) Nelson-Denny Reading Test. Itasca, IL: Riverside Publishing Company.
- 45.Woodcock RW, McGrew KS, Schrank FA, Mather N (2007) Woodcock-Johnson III Normative Update. Rolling Meadows, IL: Riverside Publishing.
- 46.Cioffi G (2010) Fluency. University of New Hampshire, Durham, NH.
- 47.Taylor SE, Frackenpohl H, Pettee JL (1960) Grade level norms for the components of the fundamental reading skill. NY: Educational Developmental Laboratories.
- 48. Brainard DH (1997) The psychophysics toolbox. Spatial Vision 10: 433–436. [PubMed] [Google Scholar]
- 49. Thesen S, Heid O, Mueller E, Schad LR (2000) Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine 44: 457–465. [DOI] [PubMed] [Google Scholar]
- 50. Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, et al. (1994) Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping 2: 189–210. [Google Scholar]
- 51. Friston KJ, Holmes AP, Price C, Büchel C, Worsley K (1999) Multisubject fMRI studies and conjunction analyses. NeuroImage 10: 385–396. [DOI] [PubMed] [Google Scholar]
- 52. Chumbley J, Worsley K, Flandin G, Friston K (2010) Topological FDR for neuroimaging. NeuroImage 49: 3057–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chumbley JR, Friston KJ (2009) False discovery rate revisited: FDR and topological inference using Gaussian random fields. NeuroImage 44: 62–70. [DOI] [PubMed] [Google Scholar]
- 54. Mechelli A, Friston KJ, Price CJ (2000) The effects of presentation rate during word and pseudoword reading: A comparison of PET and fMRI. Journal of Cognitive Neuroscience 12: 145–156. [DOI] [PubMed] [Google Scholar]
- 55. McCandliss BD, Cohen L, Dehaene S (2003) The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences 7: 293–299. [DOI] [PubMed] [Google Scholar]
- 56.Breznitz Z (2001) The determinants of reading fluency: A comparison of dyslexic and average readers. In M.Wolf (Ed.), Dyslexia, Fluency and the Brain (pp.245–276) Cambridge, MA: York Press.
- 57. Price CJ, Wise R, Frackowiak R (1996) Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex 6: 62–70. [DOI] [PubMed] [Google Scholar]
- 58. Jobard G, Crivello F, Tzourio-Mazoyer N (2003) Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. NeuroImage 20: 693–712. [DOI] [PubMed] [Google Scholar]
- 59. Ruff I, Blumstein SE, Myers EB, Hutchison E (2008) Recruitment of anterior and posterior structures in lexical–semantic processing: An fMRI study comparing implicit and explicit tasks. Brain and Language 105: 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gabrieli JD, Poldrack RA, Desmond JE (1998) The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences 95: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, et al. (1995) Semantic encoding and retrieval in the left inferior prefrontal cortex: A functional MRI study of task difficulty and process specificity. Journal of Neuroscience 15: 5870–5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thompson-Schill SL, D'Esposito M, Kan IP (1999) Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron 23: 513–522. [DOI] [PubMed] [Google Scholar]
- 63. Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, et al. (1999) Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- 64. Zhu Z, Zhang JX, Wang S, Xiao Z, Huang J, et al. (2009) Involvement of left inferior frontal gyrus in sentence-level semantic integration. NeuroImage 47: 756–763. [DOI] [PubMed] [Google Scholar]
- 65. Dapretto M, Bookheimer SY (1999) Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron 24: 427–432. [DOI] [PubMed] [Google Scholar]
- 66. Pernet C, Poline J, Demonet J, Rousselet G (2009) Brain classification reveals the right cerebellum as the best biomarker of dyslexia. BMC Neuroscience 10: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nicolson RI, Fawcett AJ (2011) Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex 47: 117–127. [DOI] [PubMed] [Google Scholar]
- 68. Price C, Moore C, Frackowiak R (1996) The effect of varying stimulus rate and duration on brain activity during reading. NeuroImage 3: 40–52. [DOI] [PubMed] [Google Scholar]
- 69. Fulbright RK, Jenner AR, Mencl WE, Pugh KR, Shaywitz BA, et al. (1999) The cerebellum's role in reading: A functional MR imaging study. American Journal of Neuroradiology 20: 1925–1930. [PMC free article] [PubMed] [Google Scholar]
- 70. Pernet C, Andersson J, Paulesu E, Demonet JF (2009) When all hypotheses are right: A multifocal account of dyslexia. Human Brain Mapping 30: 2278–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Booth JR, Wood L, Lu D, Houk JC, Bitan T (2007) The role of the basal ganglia and cerebellum in language processing. Brain Research 1133: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, et al. (2007) Age-related changes in reading systems of dyslexic children. Annals of Neurology 61: 363–370. [DOI] [PubMed] [Google Scholar]
- 73. Van der Mark S, Bucher K, Maurer U, Schulz E, Brem S, et al. (2009) Children with dyslexia lack multiple specializations along the visual word-form (VWF) system. NeuroImage 47: 1940–1949. [DOI] [PubMed] [Google Scholar]