Abstract
Objective
Steroid-induced hyperglycemia is common in hospitalized patients with diabetes mellitus. Guidelines for glucose management in this setting are lacking.
Methods
We conducted a retrospective chart review of non-critically ill patients with diabetes receiving steroids, hospitalized from January 2009 to October 2012. Fifty-eight patients were identified from 247 consults. Multivariable linear regression was used to assess median daily insulin requirements of normoglycemic patients compared with hyperglycemic patients.
Results
Of the 58 total patients included in our study, 20 achieved normoglycemia during admission (patient-day weighted mean blood glucose [PDWMBG] level = 154 ± 16 mg/dL) and 38 remained hyperglycemic (PDWMBG level= 243 ± 39 mg/dL; P < 0.001). There were no differences between the 2 patient groups in age, sex, race, body weight, renal function, HbA1c level, glucose-altering medications, diabetes type, or disease duration. Following multivariable adjustment, compared with hyperglycemic patients, normoglycemic patients required similar units of basal insulin (median [interquartile range])(23.6 [17.9, 31.2] vs 20.1 [16.5, 24.4]; P = 0.35); higher units of nutritional insulin (45.5 [34.2, 60.4] vs 20.1 [16.4, 24.5]; P < 0.001]; and lower units of correctional insulin (5.8 [4.1, 8.1] vs 13.0 [10.2, 16.5]; P < 0.001]). Patients achieving normoglycemia required a significantly lower percentage of correction insulin (total daily dose [TDD]: 7.4% vs 23.4%; P < 0.001) and a higher percentage of nutritional insulin (TDD: 58.1% vs 36.2%; P < 0.001) than hyperglycemic patients. There was no difference in the TDD per kilogram, TDD per milligram hydrocortisone dose, or TDD per milligram hydrocortisone dose per kilogram weight between the 2 groups.
Conclusion
The data suggest that non-critically ill patients with hyperglycemia receiving steroids require a higher percentage of TDD insulin therapy as nutritional insulin to achieve normoglycemia.
Keywords: steroids, hyperglycemia, diabetes, in-patient management, insulin
Introduction
Diabetes is a highly prevalent condition in the United States1 and 1 of 4 patients admitted to the hospital have a history of diabetes.2 Many studies have suggested that hyperglycemia in hospitalized patients is associated with poor outcomes, increased mortality, increased incidence of infections, and prolonged length of stay.2-6 Glucocorticoids are commonly prescribed for the treatment of many inflammatory, allergic, immunologic, and malignant disorders. Hyperglycemia is frequently experienced by patients after steroid administration, with a prevalence of 20% to 50% among patients without a previous history of diabetes.7-9
The American Diabetes Association (ADA), the American Association of Clinical Endocrinologists, and the Endocrine Society have published guidelines about the appropriate management of patients who develop hyperglycemia secondary to steroid therapy; however, the recommendations are based on expert opinion rather than evidence-based data, which is lacking in this area.6,10 In the most recent statement by the ADA and the Endocrine Society,10 it is recommended that patients treated with glucocorticoids who develop hyperglycemia should be treated with a basal/bolus insulin regimen at a starting dose of 0.3 to 0.5 units/kg/day. Notably the Endocrine Society and the ADA, understanding the magnitude of the problem, recommended the use of continuous insulin infusion in patients who receive high-dose glucocorticoids and develop severe hyperglycemia,10 a treatment that can be easily implemented in the critical care/intensive care unit (ICU) setting but not in the non-critical care setting (eg, general wards). Most in-patient glucose management guidelines for subcutaneous insulin therapy recommend that 50% of the insulin TDD be administered as basal insulin and that 50% be administered as 3 equally divided doses of nutritional insulin.11,12 However, patients who receive steroids develop significant postprandial hyperglycemia due to decreased insulin-mediated glucose uptake into the peripheral tissues (ie, adipose tissue, skeletal muscle),13 leading diabetes experts to suggest that these patients may need to be treated with more nutritional than basal insulin.14
We therefore hypothesized that patients with diabetes treated with steroids who achieve normoglycemia in the hospital would have a higher nutritional and lower basal insulin requirement than those not achieving normoglycemia. To test our hypothesis, we performed a retrospective study of non-critically ill hospitalized patients with diabetes mellitus who received steroids to compare:1) total daily basal, nutritional, and correction insulin doses; 2) percentage each of basal, nutritional, and correction insulin to insulin TDD; 3) total daily insulin dose per kilogram body weight (units/kg); 4) ratio of insulin to hydrocortisone dose (units/mg); and 5) ratio of insulin to hydrocortisone dose per kilogram (units/mg/kg).
Materials and Methods
Study Population
We identified all hospitalized patients evaluated by our Inpatient Diabetes Management Service (IDMS) through our registry between January 2009 and October 2012, and determined which of the patients with a history of diabetes had received glucocorticoids (N = 247; Figure 1) from our electronic databases (ie, Physician Order Entry [POE] system, Electronic Patient Record [EPR]). To minimize potential non-glucocorticoid confounding factors that can alter glucose management (increasing or decreasing insulin needs), we excluded patients with infections that required treatment with systemic antibiotics (n = 32); individuals with a history of kidney failure (glomerular filtration rate [GFR] < 30 mL/min per 1.73m2) or liver failure (International Normalized Ratio [INR] > 1.7; n = 29); human immunodeficiency virus or lipodystrophy (n = 4); and patients receiving continuous tube feeds, parenteral nutrition, or > 1 L/day of 5% dextrose intravenous (IV) fluids (n = 21). We also excluded patients who were receiving glucocorticoids with hydrocortisone equivalent doses < 20 mg/day (n = 10), had missing data (n = 29), did not have a history of diabetes mellitus (n = 13), or had diabetes not managed by a basal-bolus insulin regimen (eg, treated with insulin pumps, premixed insulins, or oral medications; n = 34). We also excluded patients who had hypoglycemic events, as frequent hypoglycemia might lower overall average glucose despite significant glucose fluctuations (n = 13). Four duplicate patient records were also excluded. Our final patient sample (N = 58) included those patients who were eating, receiving a carbohydrate-controlled diet, and who were on a basal-bolus insulin regimen (glargine/aspart regimen) (n = 58). In our hospital, a carbohydrate-controlled diet consists of 60 grams of carbohydrates per tray. The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine.
Figure 1.
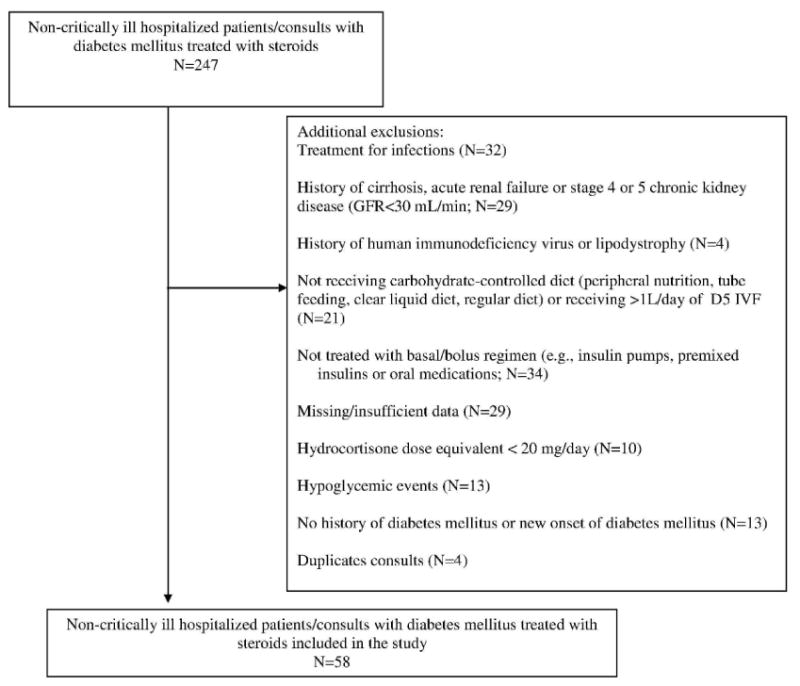
Flow diagram for selection of study population.
Abbreviations: CKD, chronic kidney disease; GFR, glomerular filtration rate; D5 IVF, 5% dextrose intravenous (IV) fluids.
Data Collection
Patient Data
From our POE and EPR systems, we collected demographic data (age, race, sex), diabetes duration and type (type 1 or type 2 diabetes mellitus, cystic fibrosis-related diabetes, post pancreatectomy diabetes), and recent (within last 3 months) glycated hemoglobin (HbA1c) level. Medical comorbidities were also collected and Charlson Comorbidity Index score was calculated.15 We also recorded any medications with hyperglycemic properties that patients were taking during their admission, including β-blockers, calcineurin inhibitors, clonidine, thiazide, and atypical antipsychotics.
Glycemia Assessment
Glucose data, including glucose result, date, and time, were collected retrospectively. The data were collected from 2 sources: the point-of-care glucose database, which records all capillary glucose measurements; and the EPR, which records all serum results. Point-of-care capillary measurements were performed prior to meals and at bedtime (4 times daily) by nursing staff. Serum measurements are drawn generally in the early morning while the patient was in a fasting state and run in the central laboratory. If 2 blood glucose level results were recorded within 1 hour, the latter result was excluded in order to avoid bias from repeated testing for extreme low or high blood glucoses. For the purpose of our analysis, normoglycemia was defined as a patient-day-weighted mean blood glucose (PDWMBG) level of 70 to 179 mg/dL and hyperglycemia was defined as a PDWMBG level > 180 mg/dL.
Insulin and Medication Summary Variables
We recorded each patient's glucocorticoid type and dose, and daily insulin doses, including TDD and basal, nutritional, and correction insulin. For patients who achieved and did not achieve normoglycemia, we calculated the following variables: 1) total daily basal, nutritional, and correction insulin doses; 2) total daily insulin dose per kilogram body weight (units/kg); and 3) percentage each of basal, nutritional, and correction insulin to insulin TDD (basal/TDD, nutritional/TDD, correction/TDD). As patients received different types of glucocorticoids during the hospital stay, such as prednisone, methylprednisolone, hydrocortisone, and dexamethasone, and at different doses, we converted all the glucocorticoids to hydrocortisone equivalents to allow for comparability in glucocorticoid exposure between groups, then calculated the insulin TDD per mg of hydrocortisone dose for each group of patients. We also calculated the TDD/hydrocortisone dose/kg to potentially identify the required insulin dose/hydrocortisone/kg to safely achieve normoglycemia.
Statistical Analysis
Bivariate and multivariate analyses were performed. Both X2 and Fisher exact tests were used to compare categorical covariates, and t tests and Wilcoxon rank sum tests were used to compare means and medians of continuous covariates, respectively. Natural logarithmic transformation of the continuous insulin dose measures was necessary to normalize their distributions. Multivariable linear models were fit for each log-transformed outcome. Results were subsequently back transformed to the original scale, and reported as the geometric mean with 95% CI for those achieving and not achieving normoglycemia.
Two sets of multivariable linear models were specified: 1) minimally adjusted for age, sex, and race; and 2) fully adjusted for age, sex, race, diabetes type, diabetes duration, administration of medications with glucose-altering properties, GFR, chronic kidney disease (CKD) stage, and modified Charlson Index score. All covariates remained in the models regardless of statistical significance because they were considered to be potential confounders a priori. A 2-sided P value of < 0.05 was used as the cutoff for statistical significance. Statistical software SAS version 9.3 was used for analysis.
Results
Baseline Characteristics
Of the 58 patients included in our study, 20 patients achieved normoglycemia during admission (PDWMBG level 154 ± 16 mg/dL) and 38 patients remained hyperglycemic (PDWMBG level 243 ± 39 mg/dL; P < 0.001). There was no difference in age, sex, race, body weight, type or duration of diabetes, stage of CKD, HbA1c level, or use of medications with hyperglycemic properties between the 2 groups (Table 1). The 2 groups received similar median doses of glucocorticoids, 107 mg/day (80, 161 mg/day) and 120 mg/day (80, 391 mg/day) in those achieving and not achieving normoglycemia, respectively (P = 0.75). Notably however, the group that achieved normoglycemia had a higher Charlson Comorbidity Index score than the group that did not achieve normoglycemia (3.5 ± 2.7 vs 2.2 ± 2.2; Table 1).
Table 1. Association of Demographics, Diabetes Characteristics, and Insulin Requirements in Patients Achieving and Not Achieving Normoglycemia.
| Parameter | Achieved Target (n = 20) | Did Not Achieve Target (n = 38) | P Value |
|---|---|---|---|
|
| |||
| Age, mean y ± SD | 60.0 ± 11.3 | 61.4 ± 15.0 | 0.73 |
|
| |||
| Sex, % | |||
| Women | 40.0 | 55.3 | 0.27 |
| Men | 60.0 | 44.7 | |
|
| |||
| Race, % | |||
| White | 55.0 | 50.0 | 0.80 |
| Black | 40.0 | 47.4 | |
| Other | 5.0 | 2.6 | |
|
| |||
| Diabetes type, % | |||
| Type 1 | 5.0 | 2.6 | 0.70 |
| Type 2 | 85.0 | 92.1 | |
| Othera | 10.0 | 5.3 | |
|
| |||
| Duration of diabetes, y (interquartile range) | 13 (8, 24) | 10 (4, 19) | 0.17 |
|
| |||
| Body weight, mean kg ± SD (n = 53) | 93 ± 19 | 94 ± 23 | 0.87 |
|
| |||
| GFR CKD classifications, % | |||
| Stages 1 & 2, > 60 | 35.0 | 60.5 | 0.18 |
| Stage 3A, 45–60 | 40.0 | 23.7 | |
| Stage 3B, 30–44 | 25.0 | 15.8 | |
|
| |||
| Charlson Index score, mean ± SD | 3.5 ± 2.7 | 2.2 ± 2.2 | 0.05 |
|
| |||
| HbA1c level, mean % ± SD (n = 55) | 7.9 ± 1.4 | 8.7 ± 2.7 | 0.15 |
|
| |||
| Taking medications with glucose-altering properties, % | 75.0 | 60.5 | 0.27 |
|
| |||
| Total PDWMB level ± SD | 154 ± 16 | 243 ± 39 | < 0.001 |
NOTE: X2 tests were used to compare proportional differences for categorical covariates; t tests and Wilcoxon rank sum tests were used to compare means and medians of continuous covariates by achieved target group.
Other types of diabetes (cystic fibrosis-related diabetes or post-pancreatectomy diabetes).
Abbreviations: CKD, chronic kidney disease; GFR, glomerular filtration rate; PDWMB, patient-day weighted mean blood; SD, standard deviation.
Insulin Requirements
The total daily basal insulin requirements were similar between the 2 groups (Table 2); however, there were significant differences in the proportion of nutritional and correction insulin required by the 2 groups. Compared with patients who did not achieve normoglycemia, those who achieved normoglycemia received significantly higher total daily nutritional insulin and lower correction insulin (Table 2). These associations persisted following minimal adjustment for age, race, and sex and multivariable adjustment for diabetes type and duration, length of stay, Chronic Kidney Disease (CKD) stage classification, Charlson Comorbidity Index score, and use of medications with glucose-altering properties. Similarly, patients achieving normoglycemia required a significantly lower percentage of basal insulin:TDD and correction insulin:TDD and a higher percentage of nutritional insulin:TDD than those who did not achieve normoglycemia (Table 2). The percentage of basal/total daily insulin was lower in the group that achieved normoglycemia compared with the hyperglycemic group, although the difference may not be clinically significant. Although not statistically significant, patients that achieved normoglycemia required a slightly higher TDD per kg compared to patients who that did not achieve normoglycemia (Table 2). There was no difference between the 2 groups on the ratio of insulin to hydrocortisone dose or the ratio of insulin to hydrocortisone per kg in univariate or multivariable analyses (Table 2); however, although not statistically significant, the ratios of total daily insulin to hydrocortisone dose and insulin to hydrocortisone dose per kg was higher in the normoglycemic group compared with the hyperglycemic group in the fully adjusted models.
Table 2. Insulin Requirements in Patients Receiving Steroids Who Did and Did Not Achieve Glycemic Target.
| Parameter | Achieved Target (n = 20) | Did Not Achieve Target (n = 38) | P Value |
|---|---|---|---|
| Total daily basal insulin | |||
| Median (1st, 3rd IQR) | 22.8 (15.0, 45.5) | 20.0 (14.5, 30.0) | 0.35 |
| Minimally adjusteda | 23.5 (18.1, 30.6) | 20.4 (16.9, 24.6) | 0.39 |
| Fully adjustedb | 23.6 (17.9, 31.2) | 20.1 (16.5, 24.4) | 0.35 |
| Total Daily Nutritional Insulin Dose | |||
| Median (1st, 3rd IQR) | 43.5 (27.8, 75.0) | 21.5 (15.0, 27.5) | < 0.001 |
| Minimally adjusteda | 43.9 (33.5, 57.6) | 20.5 (16.9, 24.8) | < 0.001 |
| Fully adjustedb | 45.5 (34.2, 60.4) | 20.1 (16.4, 24.5) | < 0.001 |
| Total Daily Correctional Insulin | |||
| Median (1st, 3rd IQR) | 6.5 (3.5, 10.0) | 14.0 (9.5, 24.0) | < 0.001 |
| Minimally adjusteda | 5.5 (4.0, 7.6) | 13.6 (10.9, 17.1) | < 0.001 |
| Fully adjustedb | 5.8 (4.1, 8.1) | 13.0 (10.2, 16.5) | < 0.001 |
| Percentage of Basal/Total Daily Insulin | |||
| Median (1st, 3rd IQR) | 29.6 (26.9, 35.2) | 37.4 (31.0, 40.4) | 0.015 |
| Minimally adjusteda | 30.7 (27.5, 34.3) | 36.0 (33.3, 38.9) | 0.024 |
| Fully adjustedb | 30.2 (26.8, 34.0) | 36.2 (33.3, 39.4) | 0.02 |
| Percentage of Nutritional/Total Daily Insulin | |||
| Median (1st, 3rd IQR) | 59.2 (51.6, 65.0) | 37.6 (32.7, 42.0) | < 0.001 |
| Minimally adjusteda | 57.3 (50.4, 65.2) | 36.1 (33.0, 39.6) | < 0.001 |
| Fully adjustedb | 58.1 (50.9, 66.5) | 36.2 (32.9, 39.8) | < 0.001 |
| Percentage of Correction/Total Daily Insulin | |||
| Median (1st, 3rd IQR) | 9.4 (4.2, 12.8) | 24.3 (19.4, 34.2) | < 0.001 |
| Minimally adjusteda | 7.2 (5.3, 9.7) | 24.0 (19.4, 30.0) | < 0.001 |
| Fully adjustedb | 7.4 (5.3, 10.2) | 23.4 (18.6, 29.5) | < 0.001 |
| Total Daily Insulin Dose per kg Body Weight | |||
| Median (1st, 3rd IQR) | 0.82 (0.63, 1.38) | 0.64 (0.45, 0.93) | 0.06 |
| Minimally adjusteda | 0.81 (0.64, 1.03) | 0.63 (0.53, 0.75) | 0.10 |
| Fully adjustedb | 0.80 (0.62, 1.05) | 0.63 (0.52, 0.76) | 0.16 |
| Ratio of Insulin to Hydrocortisone Dose (units/mg) | |||
| Median (1st, 3rd IQR) | 0.66 (0.27, 1.21) | 0.60 (0.23, 1.01) | 0.40 |
| Minimally adjusteda | 0.56 (0.33, 0.96) | 0.44 (0.29, 0.65) | 0.46 |
| Fully adjustedb | 0.56 (0.31, 1.01) | 0.44 (0.28, 0.67) | 0.51 |
| Ratio of Insulin to Hydrocortisone Dose per kg (units/mg/kg) Multiplied by 100 | |||
| Median (1st, 3rd IQR) | 0.75 (0.31, 1.36) | 0.66 (0.18, 1.06) | 0.33 |
| Minimally adjusteda | 0.60 (0.35, 1.04) | 0.45 (0.30, 0.67) | 0.39 |
| Fully adjustedb | 0.57 (0.31, 1.04) | 0.46 (0.30, 0.72) | 0.59 |
NOTE: Natural logarithmic transformation of original outcomes was necessary to normalize distributions. Results of multivariate models were subsequently back transformed and reported as the geometric mean with 95% CI.
Adjusted for age, sex, and race
Adjusted for sex, race, age, diabetes type, duration of diabetes, GFR CKD stage classification, taking medications with glucose-altering properties, and the Charlson Comorbidity Index score.
Abbreviations: CKD, chronic kidney disease; GRF, glomerular filtration rate; IQR, interquartile.
Discussion
Our study showed that diabetic and/or hyperglycemic patients treated with glucocorticoids who achieved normoglycemia required more nutritional and less correction insulin relative to TDD compared with patients who did not achieve normoglycemia. There was also a trend toward a higher total daily insulin dose per kg in patients who achieved normoglycemia compared with those who did not, although this difference was not statistically significant in the multivariable models.
Hyperglycemia represents an important and frequent side effect in patients who receive glucocorticoids.10 Although glucocorticoids represent one of the most common medications prescribed in both outpatient and in-patient settings, there is very little data to support clinical practice recommendations for individuals with and without diabetes who develop significant hyperglycemia. Recently published guidelines recommend that patients being treated with glucocorticoids receive a starting total daily insulin dose of 0.3 to 0.5 units/kg/day.10 It is reasonable to initiate therapy with a more conservative insulin regimen, however, to our knowledge, no studies have been performed in order to identify the target TDD of insulin required to achieve normoglycemia in patients with diabetes receiving steroids. Our data indicate that patients achieving normglycemia required much higher insulin doses, close to 0.8 units/kg/day. Our finding of higher nutritional insulin requirements in patients receiving glucocorticoids who achieved glycemic targets is also consistent with expert opinion recommendations14; however, these recommendations were based on clinical observations rather than clinical research studies. Recently published guidelines did not specifically comment on the potential for increased nutritional insulin needs in patients receiving glucocorticoids but recommend IV insulin infusion, when the basal-bolus approach is not effective.10 Although IV insulin infusion treatment is likely the safest and most efficient approach to achieving normoglycemia in patients, the method may not be feasible in non-ICU, non-critical care settings (eg, general wards), necessitating development of alternative approaches to achieving glycemic targets using subcutaneous insulin.
In our study, glargine was the only basal insulin used. Insulin regimens using neutral protamine Hagedorn (NPH) have been suggested as alternative approaches intreating glucocorticoid- induced hyperglycemia in patients with or without history of diabetes mellitus, especially and in particular in patients who receive prednisone or prednisolone, as NPH and prednisone and prednisolone share similar pharmacodynamic properties.9 A review article9 also suggested an approach to adjusting the NPH dose in the setting of glucocorticoid taper. There are some acknowledged limitations to this approach. As the authors stated, the regimen should not be applied in patients who receive long-acting glucocorticoids, such as dexamethasone, when longer-acting insulins, such as glargine or detemir, may be more effective.9 A recent study showed that administration of NPH and glargine as basal insulin were equally effective in treating patients with prednisone-induce hyperglycemia.16 Seggelke et al performed a small study in patients with cystic fibrosis receiving methylprednisolone, assessing glucose outcomes in patients treated with glargine and lispro compared with glargine, lispro, and NPH.17 Results showed that both treatment groups had similar (normoglycemic) fasting blood glucose levels; however, the group that received NPH in addition to the basal-bolus regimen had better pre-lunch and pre-dinner glucose levels.17 The study was limited by the small number of patients (n = 20), short study duration (3 days), and the inclusion only of patients with cystic fibrosis, limiting generalizability of the findings.17 Additionally, the control group received similar amounts of glargine and lispro insulin, close to 50% each, so it is unknown if they would have achieved better postprandial glycemic control with higher doses of nutritional insulin. These early study results suggest combing NPH insulin with a multiple daily injection regimen of a once-daily basal insulin and rapid-acting analogs may be effective in treating glucocorticoid-induced hyperglycemia; however, although not reported, the use of 3 different insulin types could potentially lead to medication errors with deleterious effects for the patient.
An alternative approach is to increase the proportion of the total daily insulin dose administered as nutritional insulin to better treat postprandial hyperglycemia associated with glucocorticoid administration. Our data suggest that higher doses of nutritional insulin may be effective in achieving glycemic targets in patients with glucocorticoid-induced hyperglycemia when approximately 30% to 35% of the total daily insulin dose is administered as basal insulin and 65% to 70% is administered as nutritional insulin.
Our approach of treating postprandial hyperglycemia with higher doses of nutritional insulin in the setting of glucocorticoid administration has a physiologic rationale. Glucocorticoids can induce insulin resistance and therefore exacerbate postprandial hyperglycemia by different pathways, affecting either muscle, adipose tissue, liver, or bones.13 In rat L6 skeletal muscle cells, dexamethasone treatment decreased the expression of key elements in the insulin receptor pathway, such as insulin receptor substrate (IRS)-1, phospatidylinositol 3 kinase (PI3-K), and protein kinase B (PKB).18-20 Steroid administration in rats has also been shown to decrease the migration of glucose transporter 4 (GLUT-4) to the surface of the muscle.21 Glucocorticoids can also induce muscle skeletal atrophy by increasing protein degradation13 and the released amino acids have been shown to alter insulin signaling by inhibiting IRS-1 phosphorylation and PI3-K activation.22 In adipose tissue, glucocorticoids have been shown to down-regulate IRS-1 in 3T3L1 adipocytes,23 and also to alter the expression of several adipokines, such as adiponectin and resistin.13,24 Finally, in the liver, glucocorticoids can induce insulin resistance by different mechanisms. Administration of glucocorticoids induces hepatic insulin resistance by impairing the insulin suppressive effects in hepatic glucose production, especially in the postprandial state, and this was demonstrated in healthy subjects following short-term glucocorticoid exposure.13,25,26 Recently, it was reported that long-term treatment with glucocorticoids induces insulin resistance through suppression of the osteoblast-derived peptide osteocalcin.27
Our study had several limitations that should be kept in mind in interpreting our data. First, our sample size was small. Of the 247 patients consults considered where patients received steroids, only 58 patients did not have significant comorbidities that prevented them from being included. The rationale for this was to try to avoid any comorbidities unrelated to glucocorticoids that could significantly alter the patient's insulin requirements. Consequently, there may have been selection bias and our results are not generalizable to non-critical care settings or to patients with a greater severity of illness. Second, it is notable that the group of patients who achieved normoglycemia had a higher modified Charlson Comorbidity Index score compared with the patient group that did not achieve normoglycemia. Therefore, we cannot exclude the possibility that the provider caring for these patients may have perceived them to be at higher risk for hospital-related complications and may have treated them more aggressively, resulting in better glycemic control. Third, our study was retrospective, so we may not have had complete case ascertainment and we were unable to implement a specific insulin-management strategy and examine its effects prospectively. Fourth, our patients had pre-existing diabetes, so findings may differ in individuals without pre-existing diabetes who have isolated glucocorticoid-induced hyperglycemia. Fifth, in our analysis we included only patients who were ordered a 60-gm carbohydrate-controlled diet. Unfortunately we did not have data regarding their total caloric intake or the amount of carbohydrates actually consumed, which may be impacted by anorexia, nausea, vomiting, or diarrhea in the hospital setting. Finally, because of the retrospective nature of our study, there may have been residual confounding that may have influenced and biased our results.
Summary
In summary, our data suggest that non-critically ill patients with hyperglycemia receiving steroids require a higher percentage of their insulin TDD as nutritional insulin to achieve normoglycemia. Future clinical trials should examine the approach of using a basal-bolus insulin ratio of 35%:65% compared with the standard basal-bolus ratio of 50%:50%, or using insulin NPH to compare the effectiveness among treatments in reaching glycemic targets for patients. Such a study would fill an important research gap recently identified by the Planning Research in Inpatient Diabetes (PRIDE) Investigators in a recent overview of gaps in our current knowledge in delivering high-quality in-patient care to patients with diabetes and hyperglycemia.28
Acknowledgments
Elias K Spanakis, MD, was supported by an institutional training grant from the National Institute of Diabetes, Digestive, and Kidney Diseases (T32 DK062707). Nina Shah, MS, and Hsin-Chieh Yeh, PhD, were supported by the NIDDK Diabetes Research Center grant (P30DK079637).
Footnotes
Conflict of Interest Statement: Elias K Spanakis, MD, Nina Shah, MS, Keya Malhotra, MD, Terri Kemmerer, RN, MSc, CRNP, CDE, Hsin-Chieh Yeh, PhD, and Sherita Hill Golden, MD, MHS, disclose no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. 2011 National Diabetes Fact Sheet. Atlanta, GA: US Department of Health and Human Services; 2011. [Google Scholar]
- 2.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 3.Golden SH, Peart-Vigilance C, Kao WH, Brancati FL. Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes. Diabetes Care. 1999;22(9):1408–1414. doi: 10.2337/diacare.22.9.1408. [DOI] [PubMed] [Google Scholar]
- 4.McAlister FA, Majumdar SR, Blitz S, Rowe BH, Romney J, Marrie TJ. The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community-acquired pneumonia. Diabetes Care. 2005;28(4):810–815. doi: 10.2337/diacare.28.4.810. [DOI] [PubMed] [Google Scholar]
- 5.Baker EH, Janaway CH, Philips BJ, et al. Hyperglycaemia is associated with poor outcomes in patients admitted to hospital with acute exacerbations of chronic obstructive pulmonary disease. Thorax. 2006;61(4):284–289. doi: 10.1136/thx.2005.051029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Daibetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353–369. doi: 10.4158/EP09102.RA. [DOI] [PubMed] [Google Scholar]
- 7.Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Prac. 2006;12(4):358–362. doi: 10.4158/EP.12.4.358. [DOI] [PubMed] [Google Scholar]
- 8.Xiao JZ, Ma L, Gao J, et al. Glucocorticoid-induced diabetes in severe acute respiratory syndrome: the impact of high dosage and duration of methylprednisolone therapy. Zhonghua Nei Ke Za Zhi. 2004;43(3):179–182. [PubMed] [Google Scholar]
- 9.Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15(5):469–474. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- 10.Umpierrez GE, Hellman R, Korytkowski MT, et al. Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
- 11.McDonnell ME, Umpierrez GE. Insulin therapy for the management of hyperglycemia in hospitalized patients. Endocrin Metab Clin North Am. 2012;41(1):175–201. doi: 10.1016/j.ecl.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. New Engl J Med. 2006;355(18):1903–1911. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 13.van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? European J Clin investigation Feb. 2009;39(2):81–93. doi: 10.1111/j.1365-2362.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- 14.Clement S, Braithwaite SS, Magee MF, et al. American Diabetes Asspcoatopm Diabetes in Hospitals Writing Committee. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553–591. doi: 10.2337/diacare.27.2.553. [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 16.Dhital SM, Shenker Y, Meredith M, Davis DB. A retrospective study comparing neutral protamine hagedorn insulin with glargine as basal therapy in prednisone-associated diabetes mellitus in hospitalized patients. Endocr Pract. 2012;18(5):712–719. doi: 10.4158/EP11371.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seggelke SA, Gibbs J, Draznin B. Pilot study of using neutral protamine Hagedorn insulin to counteract the effect of methylprednisolone in hospitalized patients with diabetes. J Hosp Med. 2011;6(3):175–176. doi: 10.1002/jhm.874. [DOI] [PubMed] [Google Scholar]
- 18.Ewart HS, Somwar R, Klip A. Dexamethasone stimulates the expression of GLUT1 and GLUT4 proteins via different signalling pathways in L6 skeletal muscle cells. FEBS Lett. 1998;425(1):179–183. [PubMed] [Google Scholar]
- 19.Long W, Barrett EJ, Wei L, Liu Z. Adrenalectomy enhances the insulin sensitivity of muscle protein synthesis. Am J Physiol Endocrinol Metab. 2003;284(1):E102–E109. doi: 10.1152/ajpendo.00028.2002. [DOI] [PubMed] [Google Scholar]
- 20.Saad MJ, Folli F, Kahn JA, Kahn CR. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. J Clin Invest. 1993;92(4):2065–2072. doi: 10.1172/JCI116803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weinstein SP, Wilson CM, Pritsker A, Cushman SW. Dexamethasone inhibits insulin-stimulated recruitment of GLUT4 to the cell surface in rat skeletal muscle. Metabolism. 1998;47(1):3–6. doi: 10.1016/s0026-0495(98)90184-6. [DOI] [PubMed] [Google Scholar]
- 22.Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. J Clin Invest. 1998;101(7):1519–1529. doi: 10.1172/JCI1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turnbow MA, Keller SR, Rice KM, Garner CW. Dexamethasone down-regulation of insulin receptor substrate-1 in 3T3-L1 adipocytes. J Biol Chem. 1994;269(4):2516–2520. [PubMed] [Google Scholar]
- 24.Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46(12):1594–1603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen MF, Caumo A, Chandramouli V, et al. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercortisolemia in healthy subjects. Am J Physiol Endocrinol Metab. 2004;286(1):E102–E110. doi: 10.1152/ajpendo.00566.2002. [DOI] [PubMed] [Google Scholar]
- 26.Rooney DP, Neely RD, Cullen C, et al. The effect of cortisol on glucose/glucose-6-phosphate cycle activity and insulin action. J Clin Endocrinol Metab. 1993;77(5):1180–1183. doi: 10.1210/jcem.77.5.8077310. [DOI] [PubMed] [Google Scholar]
- 27.Brennan-Speranza TC, Henneicke H, Gasparini SJ, et al. Osteoblasts mediate the adverse effects of glucocorticoids on fuel metabolism. J Clin Invest. 2012;122(11):4172–4189. doi: 10.1172/JCI63377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draznin B, Gilden J, Golden SH, Inzucchi SE PRIDE Investigators. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care. 2013;36(7):1807–1814. doi: 10.2337/dc12-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]


