Abstract
Derivation of motor neurons from human pluripotent stem cells is inefficient and requires complex culture protocols. Recently in Nature Materials, Sun et al. (2014) report that differentiating human pluripotent stem cells on soft substrates increases the efficiency of mature motor neuron differentiation by altering cytoskeletal mechanotransduction through the Hippo/YAP/Smad pathway.
Efficient derivation of motor neurons (MNs) from human pluripotent stem cells (hPSCs) is limited by our current understanding of the mechanisms underlying MN differentiation as well as drawbacks arising from inefficient and lengthy existing protocols. Human MNs derived from in vitro cultures can be used for cellular mechanistic studies as well as in vivo studies testing cell replacement strategies and modelling human disease in animal models, with the ultimate goal of replacing dysfunctional MNs in degenerative disorders like amyotrophic lateral sclerosis. To enhance MN differentiation and surmount some of these existing difficulties, Jianping Fu and colleagues (Sun et al., 2014) developed a mechanical platform that enhances differentiation efficiency of hPSCs into MNs by >4-fold and significantly shortens the time period required for MN maturation.
This platform capitalizes on advances using micromoulded poly-(dimethylsiloxane) (PDMS) micropost arrays (PMAs) to culture cells (Fu et al., 2010). PDMS is widely used to construct microscale devices for cell culture and microfluidic applications (Fu et al., 2010). PDMS surfaces are continuous and thus cannot change rigidity without altering other characteristics of the material. In contrast, PMAs have a constant surface geometry whose rigidity is varied by adjusting heights of isolated microposts to cover the stiffness range exhibited by soft tissues, without altering the contact area available for cell-matrix interactions or the tethering of cell-surface integrins to the vitronectin-coated surface. The rigidity of soft tissues can range from 0.1 kPa to 100 kPa (Discher et al., 2009), in contrast to standard culture dishes with a rigidity of >104 kPa.
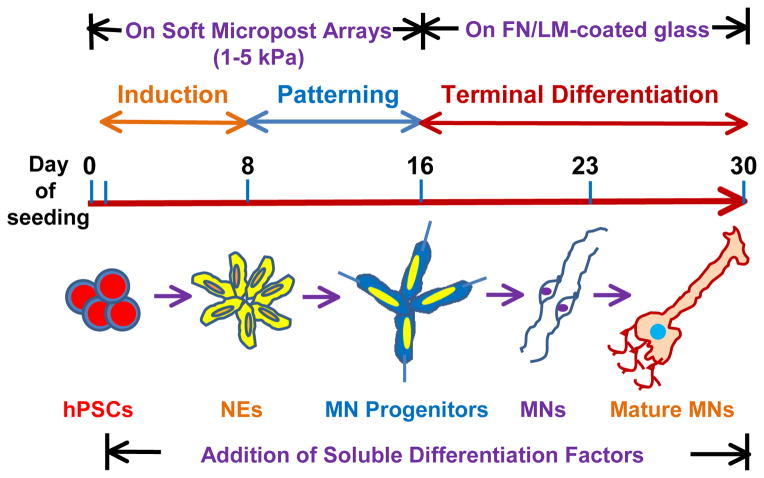
Sun et al. have harnessed the advantages for PMAs for directed differentiation of hPSCs into MNs (Fig. 1). hPSCs were seeded on vitronectin-coated PMAs of varying stiffness in neuroepithelial inducing culture and induction efficiency was assayed by expression of neuroectodermal marker PAX6. After 8 days, the percentage of PAX6+ neuroepithelial (NE) cells was 3-fold higher on soft PMAs (1–5 kPa) than on rigid PMAs (>14 kPa) or vitronectin-coated glass (>104 kPa). Cells were cultured for an additional eight days with retinoic acid (RA) and Purmorphamine to induce caudalization and ventralization patterning cues, and cells induced on soft PMAs showed an 8-fold enrichment for expression of the MN progenitor marker Olig2 as compared to cells maintained on stiffer PMAs. After transferring induced MN progenitors onto coverslips for 14 more days under conditions appropriate for MN maturation, cells from soft PMAs displayed a 13-fold enrichment in purity of mature MN subtypes over cells induced on stiff PMAs or glass. These MNs generated action potentials and expressed high levels of choline acetyltransferase, demonstrating derivation of mature MNs from hPSCs.
Figure 1. Schematic for generating motor neurons (MNs) from human pluripotent stem cells (hPSCs) on PDMS-based micropost arrays (PMAs).
hPSCs were seeded and maintained on vitronectin-coated soft micropost arrays before transfer onto FN/LM-coated glass to facilitate induction and maturation of MNs. Different combinations of soluble signalling factors were added at the beginning of each stage (induction, patterning, or terminal maturation) of hPSC differentiation. NEs=neuroepithelial cells; FN=fibronectin; LM=laminin.
The authors next examined whether varying substrate rigidity influenced mechanotransduction during hPSC differentiation to enhance MN generation. Human mesenchymal stem cells (MSCs) differentiate towards different lineages in response to culturing on matrices of differing rigidity (Engler et al., 2006), and mouse ESCs differentiate in response to applied shear forces even in the presence of factors which maintain the pluripotent state, such as leukemia inhibitory factor (LIF) (Chowdhury et al., 2010). The YAP/TAZ pathway is required for MSC differentiation by transducing mechanical signals arising from matrix rigidity to the nucleus to coordinate transcriptional responses (Dupont et al., 2011). When Sun et al. compared the effects of culturing cells on soft PMAs versus rigid PMAs, they found that phosphorylation of YAP was higher in the cytoplasm of the cells cultured on soft PMAs than those on rigid PMAs, with a corresponding decrease in nuclear YAP localization suggesting an inability of p-YAP to translocate into the nucleus. When in the nucleus, YAP regulates the activity of Smad transcription factors, and the authors found that YAP and p-Smad were co-localized only in the nuclei of cell cultured on rigid PMAs. Consistently, Smad phosphorylation was decreased in cells cultured on soft PMAs. The authors further demonstrated that the cytoskeleton regulates YAP localization in this system, as nuclear localization of YAP responded to altering the actin cytoskeleton. Nuclear YAP localization was dramatically decreased by inhibiting ROCK and could be enhanced by stimulating RhoA, two enzymes which promote actin remodelling and stress fiber formation in response to mechanic cues, suggesting that tension-dependent mechanotransduction is important for regulating YAP translocation and signalling.
The findings of Fu and colleagues that MN differentiation is enhanced by YAP-dependent reductions in p-Smad levels is consistent with findings showing that YAP/TAZ-mediated p-Smad accumulation in the nucleus is necessary for maintaining hPSC pluripotency (Valeras et al., 2008). The mechanisms which underlie these effects, however, remain unresolved. For example, it is not clear how culturing cells on soft PMAs leads to downregulated phosphorylation of Smad proteins. Cells grown on soft PMAs exhibited few stress fibers, suggesting that altered actin dynamics may release Lats1, a kinase that phosphorylates YAP and prevents it from translocating into the nucleus. This is supported by the finding that stimulating RhoA activity using lysophosphatidic acid (LPA) completely blocked induction of Pax6+ neuroepithelial cells on soft PMAs, and siRNA-induced silencing of Lats1 expression decreased the percentage of Pax6+ cells on soft PMAs to the same level as that observed when culturing cells on rigid PMAs (Sun et al., 2014). Interestingly, Sun et al. find that culture on soft PMAs elevated expression of neural posterior markers HOXB1, HOXB4, and HOXB8, even in the absence of caudalizing RA, suggesting that local matrix compliance alone could alter the fate of anterior/posterior patterning. Furthermore, soft PMAs alone promoted much higher expression of HOXB8 than RA, implying that that local matrix rigidity can be more potent than soluble factors in determining anterior/posterior patterning, consistent with recent findings that insoluble matrix softness is important in organization of germ layers in an in vitro mouse ESC culture (Poh et al., 2014).
The findings of Sun et al. (2014) demonstrating an improved protocol via substrate rigidity manipulation and the finding of crosstalks between YAP/TAZ and soft-matrix mediated mechanotransduction represent a significant step toward better understanding of MN differentiation from hPSCs. Another recent report describes high efficiency of MN differentiation from hPSCs with shortened maturation duration by utilizing small molecules in combination with seeding cells on a combination of four matrix proteins (Qu et al., 2014), and it will be interesting to determine whether YAP and Smad signalling are altered under these conditions as well. The work of Fu and colleagues will stimulate further exploration and application of cell mechanics and mechanotransduction in stem cell biology and regenerative medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chowdhury F, Na S, Li D, Poh YC, Tanaka T, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods. 2010;7:733, 736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester ME, Murtha MJ, Song S, Rao M, Miranda CJ, Meyer K, Tian J, Boulting G, Schaffer DV, Zhu MX, Pfaff SL, Gage FH, Kaspar BK. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol Ther. 2011;19:1905–1912. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh YC, Chen JW, Ying Hong Y, Yi H, Zhang S, Chen JJ, Wu DC, Wang L, Jia Q, Singh R, Yao W, Tan Y, Tajik A, Tanaka TS, Wang N. Generation of organized germ layers from a single mouse embryonic stem cell. Nat Commun. 2014 doi: 10.1038/ncomms5000. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Q, Li D, Louis KR, Li X, Yang H, Sun Q, Crandall SR, Tsang S, Zhou J, Cox CL, Cheng J, Wang F. High-efficiency motor neuron differentiation from human pluripotent stem cells and the function of Islet-1. Nat Commun. 2014;5:3449. doi: 10.1038/ncomms4449. [DOI] [PubMed] [Google Scholar]
- Sun Y, Yong KM, Villa-Diaz LG, Zhang X, Chen W, Philson R, Weng S, Xu H, Krebsbach PH, Fu J. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater. 2014 doi: 10.1038/nmat3945. xx, xxx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–848. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]