Abstract
BACKGROUND
The extent to which diabetes mellitus or hyperglycemia is related to risk of death from cancer or other nonvascular conditions is uncertain.
METHODS
We calculated hazard ratios for cause-specific death, according to baseline diabetes status or fasting glucose level, from individual-participant data on 123,205 deaths among 820,900 people in 97 prospective studies.
RESULTS
After adjustment for age, sex, smoking status, and body-mass index, hazard ratios among persons with diabetes as compared with persons without diabetes were as follows: 1.80 (95% confidence interval [CI], 1.71 to 1.90) for death from any cause, 1.25 (95% CI, 1.19 to 1.31) for death from cancer, 2.32 (95% CI, 2.11 to 2.56) for death from vascular causes, and 1.73 (95% CI, 1.62 to 1.85) for death from other causes. Diabetes (vs. no diabetes) was moderately associated with death from cancers of the liver, pancreas, ovary, colorectum, lung, bladder, and breast. Aside from cancer and vascular disease, diabetes (vs. no diabetes) was also associated with death from renal disease, liver disease, pneumonia and other infectious diseases, mental disorders, nonhepatic digestive diseases, external causes, intentional self-harm, nervous-system disorders, and chronic obstructive pulmonary disease. Hazard ratios were appreciably reduced after further adjustment for glycemia measures, but not after adjustment for systolic blood pressure, lipid levels, inflammation or renal markers. Fasting glucose levels exceeding 100 mg per deciliter (5.6 mmol per liter), but not levels of 70 to 100 mg per deciliter (3.9 to 5.6 mmol per liter), were associated with death. A 50-year-old with diabetes died, on average, 6 years earlier than a counterpart without diabetes, with about 40% of the difference in survival attributable to excess nonvascular deaths.
CONCLUSIONS
In addition to vascular disease, diabetes is associated with substantial premature death from several cancers, infectious diseases, external causes, intentional self-harm, and degenerative disorders, independent of several major risk factors. (Funded by the British Heart Foundation and others.)
The presence of diabetes mellitus approximately doubles the risk of a wide range of vascular diseases.1 Evidence is also emerging that diabetes is associated with nonvascular conditions, including positive associations with certain cancers (e.g., liver cancer) and negative associations with other cancers (e.g., prostate cancer).2-4 However, a joint consensus statement of the American Diabetes Association and the American Cancer Society indicated that it is unclear whether such associations are direct (e.g., due to hyperglycemia) or indirect (e.g., due to diabetes as a marker of underlying biologic factors such as insulin resistance or hyperinsulinemia that alter the risk of cancer) or due to shared risk factors (e.g., obesity) or a combination of these.5-8 Furthermore, many previous reports have considered diabetes in relation to only one or a few selected cancers or other nonvascular conditions. Since diabetes is a multisystem disorder, there is a need for adequately powered, standardized assessment of associations of diabetes with the risk of death from a broad range of causes.9,10
We aimed to provide reliable estimates of any independent associations of baseline diabetes and fasting blood glucose level with the risk of cause-specific death by analyzing data from 820,900 people who were at risk for a total of 12.3 million person-years. We also estimated the effect of diabetes on life expectancy in adults.
METHODS
The study was designed and conducted by the independent academic coordinating center of the Emerging Risk Factors Collaboration (ERFC). Members of the coordinating center vouch for the accuracy and completeness of the data, the data analysis, and the results and made the decision to submit the article for publication. The sponsors had no role in the design, analysis, or interpretation of the study. The study was approved by the Cambridgeshire Ethics Review Committee.
Details of the ERFC have been published previously11 (also see the Supplementary Appendix, available with the full text of this article at NEJM.org). Specifically, we have already published reports on the associations of lipids, lipoproteins, and inflammatory markers with the risk of vas cular disease and cause-specific death12-14 — the foregoing being risk factors that were the initial focus of the ERFC.11 In 2009, the ERFC agreed to extend analyses to diabetes and other metabolic markers in relation to the risk of incident fatal and nonfatal vascular disease outcomes1 and cause-specific death (see the Supplementary Appendix). The current analyses focus on individual-participant data from 97 prospective studies that had information about the diagnosis of diabetes or the fasting blood-glucose level at baseline, that did not select participants on the basis of having previous chronic disease (including vascular disease or diabetes), that included recording of cause-specific deaths classified according to clearly defined criteria, and that had accrued more than 1 year of follow-up data. Study details are presented in Table 1 in the Supplementary Appendix (with references also listed). There were 820,900 participants who had no known preexisting vascular disease at baseline and for whom there was complete information about age, sex, smoking status (current smoker vs. any other status), body-mass index (BMI), history of diabetes or fasting glucose level (measured after ≥8 hours of fasting or overnight fasting), and subsequent cause-specific death recorded during follow-up. The contributing studies classified deaths according to the primary cause (or, in its absence, the underlying cause), on the basis of coding from the International Classification of Diseases, revisions 8 through 10, to at least three digits, or according to study-specific classification systems; ascertainment was based on death certificates. Attribution of death refers to the primary cause provided. A total of 67 of the 97 contributing studies also used medical rec ords, findings on autopsy, and other supplementary sources to help classify deaths. We sought information on a range of risk factors and medication use at baseline (e.g., agents for lowering blood pressure, cholesterol, and glucose). Information on diabetes type (i.e., type 1 or 2) was generally not available, though the age of the participants suggests that the large majority with diabetes would have type 2.
Following the example of previous reports from the ERFC,12-15 we assessed whether baseline diabetes status (ascertained on the basis of self-report, medication use, fasting glucose level ≥126 mg per deciliter [7.0 mmol per liter], or a combination of these) and baseline fasting glu- cose level relate to death from any cause and its main components, including deaths from cancer, vascular disease, and nonvascular conditions not attributed to cancer, as well as to further subdivisions of these outcomes (e.g., site-specific cancers) (see definitions in Table 2 in the Supplementary Appendix).
Hazard ratios were calculated with the use of Cox proportional-hazards regression models stratified according to study, sex, and when appropriate, trial group. To minimize potential bias, loge hazard ratios were calculated separately within each study and then pooled across studies by means of a random-effects meta-analysis.16 For the analyses of death from specific causes (cancer and noncancer, nonvascular), a one-step fixed-effect meta-analysis was used. We used methods described previously to characterize shapes of associations with fasting glucose level and to investigate heterogeneity.1,16 Unless otherwise specified, hazard ratios were adjusted for baseline age, sex, smoking status, and BMI. In subsidiary analyses, hazard ratios were also adjusted for other characteristics.
We corrected for regression dilution in subsidiary analyses, using serial measurements in 331,515 participants (mean interval, 2.9 years).17 Participants were included in analyses of death irrespective of the previous occurrence of non-fatal events. For each specific cause of death, participants’ data were censored if the participant was lost to follow-up, died from other causes, or reached the end of the follow-up period. Estimates of cumulative survival from 35 years of age and older among those with and those without diabetes at baseline were calculated by applying hazard ratios (specific to age at risk and sex) for cause-specific mortality associated with diabetes to cause-specific rates of death at 35 years of age and older for residents of the European Union.18,19 The Supplementary Appendix provides further statistical details. Analyses were carried out with Stata software (release 11).
RESULTS
Among the 820,900 participants included in analyses of diabetes status or fasting glucose level, the mean (±SD) age at baseline was 55±9 years; 48% were women. The large majority of participants were enrolled in Europe (58%) or North America (36%). Of the 715,061 participants included in analyses of diabetes status, 40,116 (6%) had diabetes at the time of enrollment (Table 1). The baseline characteristics of participants included in the analyses of fasting glucose are listed in Table 3 in the Supplementary Appendix. During the 12.3 million person-years at risk (median time to death, 13.6 years), 123,205 deaths were recorded: 41,320 from cancer, 44,407 from vascular disease, 27,661 from other causes, and 9817 of unknown or ill-defined cause (Table 4 in the Supplementary Appendix).
Table 1.
Baseline Data Used in Analyses of Diabetes and Cause-Specific Death, According to Participants' Diabetes Status.*
| Characteristic | Diabetes (N = 40,116) | No Diabetes (N = 674,945) |
|---|---|---|
| Demographic factors | ||
| Age — yr | 58±8 | 55±8 |
| Sex — no. (%) | ||
| Male | 21,213 (53) | 337,178 (50) |
| Female | 18,903 (47) | 337,767 (50) |
| Geographic region or country — no. (%) | ||
| North America | 23,844 (59) | 257,818 (38) |
| Europe | 11,883 (30) | 371,717 (55) |
| Japan | 2,835 (7) | 20,170 (3) |
| Other | 1,554 (4) | 25,240 (4) |
| Lifestyle factors | ||
| Smoking status — no. (%) | ||
| Current smoker | 8,894 (22) | 225,331 (33) |
| Other | 31,222 (78) | 449,614 (67) |
| Alcohol use — no./total no. (%) | ||
| Current drinker | 10,177/20,447 (50) | 232,476/365,375 (64) |
| Other | 10,270/20,447 (50) | 132,899/365,375 (36) |
| Physical activity — no./total no. (%)† | ||
| Not active | 4117/8723 (47) | 132,795/258,071 (51) |
| Active | 4606/8723 (53) | 125,276/258,071 (49) |
| Anthropometric markers | ||
| Body-mass index‡ | ||
| No. with data | 40,116 | 674,945 |
| Mean | 28±5 | 26±4 |
| Systolic blood pressure | ||
| No. with data | 36,434 | 504,516 |
| Mean — mm Hg | 144±19 | 135±18 |
| Waist circumference | ||
| No. with data | 10,324 | 127,636 |
| Mean — cm | 97±13 | 89±12 |
| Waist-to-hip ratio | ||
| No. with data | 10,288 | 127,167 |
| Mean | 0.93±0.07 | 0.89±0.08 |
| Lipids | ||
| Total cholesterol | ||
| No. with data | 35,016 | 494,678 |
| Mean — mmol/liter | 5.9±1.2 | 5.9±1.1 |
| Non-HDL cholesterol | ||
| No. with data | 25,906 | 321,267 |
| Mean — mmol/liter | 4.6±1.1 | 4.5±1.1 |
| HDL cholesterol | ||
| No. with data | 25,928 | 321,537 |
| Mean — mmol/liter | 1.2±0.4 | 1.4±0.4 |
| Loge triglycerides | ||
| No. with data | 27,515 | 397,593 |
| Mean — mmol/liter | 0.5±0.6 | 0.3±0.5 |
| Inflammatory markers | ||
| Fibrinogen | ||
| No. with data | 12,598 | 162,820 |
| Mean — μmol/liter | 9.8±2.3 | 9.3±2.1 |
| Loge C-reactive protein | ||
| No. with data | 6,979 | 104,642 |
| Mean — mg/liter | 0.9±1.1 | 0.6±1.1 |
| Metabolic and renal markers | ||
| Fasting glucose | ||
| No. with data | 22,015 | 157,023 |
| Mean — mmol/liter | 8.6±3.6 | 5.2±0.6 |
| Loge insulin | ||
| No. with data | 6,911 | 53,711 |
| Mean — pmol/liter | 4.6±0.7 | 4.0±0.6 |
| Glycated hemoglobin | ||
| No. with data | 4,971 | 48,468 |
| Mean — % | 7.2±2.0 | 5.3±0.5 |
| Loge estimated glomerular filtration rate§ | ||
| No. with data | 22,122 | 216,585 |
| Mean — ml/min/1.73 m2 | 4.3±0.3 | 4.4±0.2 |
Plus–minus values are means ±SD. Data are shown for the 715,061 of the 820,900 participants without known preexisting cardiovascular disease at baseline and for whom there was complete information about age, sex, smoking status (current smoker vs. any other status) and body-mass index. Information about the other characteristics in the table was not available for all participants. Diabetes was ascertained on the basis of self-report, medication use, fasting glucose level of 126 mg per deciliter (7.0 mmol per liter) or more, or a combination of these. To convert values for cholesterol to milligrams per deciliter, divide by 0.02586. To convert values for triglycerides to milligrams per deciliter, divide by 0.01129. To convert values for fibrinogen to grams per deciliter, divide by 29.41. To convert values for insulin to microunits per milliliter, divide by 6.945. To convert values for fasting glucose to milligrams per deciliter, divide by 0.05551. HDL denotes high-density lipoprotein.
Physical activity was defined as any form of aerobic or anaerobic exercise.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Estimated glomerular filtration rate was calculated with the use of the Modification of Diet in Renal Disease equation.
DIABETES AND MORTALITY
The crude overall rates of death were higher among participants with diabetes than among those without diabetes: 29 per 1000 person-years versus 12 per 1000 person-years among men, respectively, and 23 per 1000 person-years versus 7 per 1000 person-years among women, respectively. The corresponding cause-specific rates of death were as follows: for cancer deaths, 7 versus 4 per 1000 person-years among men and 4 versus 3 per 1000 person-years among women; for vascular deaths, 13 versus 5 per 1000 person-years among men and 11 versus 2 per 1000 person-years among women; and for noncancer, nonvascular deaths, 6 versus 3 per 1000 person-years among men and 6 versus 2 per 1000 person-years among women.
Hazard ratios for death among participants with diabetes, as compared to those without diabetes, after adjustment for baseline age, sex, smoking status, and BMI, were as follows: 1.80 (95% confidence interval [CI], 1.71 to 1.90) for death from any cause, 1.25 (95% CI, 1.19 to 1.31) for death from cancer, 2.32 (95% CI, 2.11 to 2.56) for death from vascular causes, 1.73 (95% CI, 1.62 to 1.85) for death from nonvascular causes not attributed to cancer, and 1.88 (95% CI, 1.62 to 2.18) for deaths of unknown or ill-defined cause (Fig. 1 in the Supplementary Appendix). These hazard ratios were not appreciably reduced after additional adjustment for systolic blood pressure, lipid levels, C-reactive protein levels, fibrinogen levels, alcohol use, estimated glomerular filtration rate, or indicators of socioeconomic status, when such information was available. However, the hazard ratios were reduced considerably after adjustment for fasting glucose or glycated hemoglobin levels (Table 2).
Table 2.
Hazard Ratios for Death among Participants with Diabetes as Compared with Those without Diabetes at Baseline, after Adjustment for Potential Risk Factors and Mediators, According to Cause of Death.
| Model Variables | Cancer Death | Vascular Death | Noncancer, Nonvascular Death | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total No. of Participants | No. of Deaths | Hazard Ratio (95% CI) | Total No. of Participants | No. of Deaths | Hazard Ratio (95% CI) | Total No. of Participants | No. of Deaths | Hazard Ratio (95% CI) | |
| Progressive adjustment | |||||||||
| Age and sex | 486,807 | 22,399 | 1.20 (1.14–1.27) | 513,951 | 28,354 | 2.38 (2.17–2.60) | 479,601 | 14,481 | 1.67 (1.55–1.79) |
| Plus smoking status | 1.26 (1.19–1.34) | 2.43 (2.22–2.66) | 1.71 (1.59–1.84) | ||||||
| Plus body-mass index | 1.27 (1.20–1.34) | 2.29 (2.10–2.49) | 1.75 (1.64–1.87) | ||||||
| Plus systolic blood pressure | 1.25 (1.18–1.33) | 2.14 (1.96–2.32) | 1.71 (1.60–1.82) | ||||||
| Plus total cholesterol | 1.24 (1.17–1.32) | 2.15 (1.98–2.34) | 1.69 (1.59–1.80) | ||||||
| Additional adjustment* | |||||||||
| Lipids | |||||||||
| Basic model | 242,531 | 8,804 | 1.26 (1.13–1.40) | 260,975 | 10,533 | 2.03 (1.84–2.24) | 235,434 | 6,164 | 1.79 (1.63–1.96) |
| Plus non-HDLcholesterol, HDL cholesterol, and loge triglycerides | 1.24 (1.15–1.34) | 2.00 (1.81–2.21) | 1.75 (1.59–1.93) | ||||||
| Inflammatory markers | |||||||||
| Basic model | 150,758 | 5,803 | 1.26 (1.15–1.38) | 168,997 | 6,344 | 2.23 (1.92–2.59) | 150,758 | 4,251 | 1.75 (1.53–2.00) |
| Plus fibrinogen | 1.23 (1.12–1.34) | 2.18 (1.89–2.52) | 1.67 (1.47–1.90) | ||||||
| Basic model | 84,172 | 4,402 | 1.26 (1.11–1.42) | 101,681 | 6,513 | 1.99 (1.76–2.25) | 82,127 | 3,479 | 1.63 (1.43–1.85) |
| Plus loge CRP | 1.25 (1.10–1.41) | 1.95 (1.73–2.20) | 1.62 (1.42–1.84) | ||||||
| Lifestyle factors | |||||||||
| Basic model | 338,476 | 12,704 | 1.25 (1.17–1.34) | 353,614 | 14,548 | 2.16 (1.95–2.39) | 332,487 | 8,530 | 1.69 (1.55–1.85) |
| Plus alcohol use | 1.23 (1.14–1.32) | 2.11 (1.91–2.33) | 1.65 (1.52–1.79) | ||||||
| Basic model | 247,831 | 12,291 | 1.33 (1.20–1.49) | 255,535 | 13,671 | 2.34 (2.05–2.67) | 246,565 | 7,034 | 1.91 (1.72–2.13) |
| Plus physical activity | 1.30 (1.18–1.43) | 2.29 (2.01–2.62) | 1.90 (1.71–2.12) | ||||||
| Basic model | 242,977 | 10,699 | 1.19 (1.10–1.29) | 254,215 | 14,560 | 2.08 (1.87–2.32) | 237,603 | 8,029 | 1.69 (1.57–1.83) |
| Plus educational level† | 1.19 (1.10–1.28) | 2.08 (1.87–2.31) | 1.68 (1.56–1.81) | ||||||
| Metabolic markers | |||||||||
| Basic model | 204,609 | 11,130 | 1.25 (1.16–1.34) | 222,376 | 13,888 | 2.10 (1.86–2.37) | 203,841 | 7,907 | 1.66 (1.51–1.82) |
| Plus loge estimated GFR‡ | 1.25 (1.16–1.34) | 2.11 (1.86–2.37) | 1.65 (1.50–1.82) | ||||||
| Basic model | 155,049 | 9,943 | 1.25 (1.16–1.35) | 176,288 | 13,078 | 1.98 (1.76–2.22) | 152,550 | 7,051 | 1.71 (1.58–1.85) |
| Plus fasting glucose | 1.08 (0.98–1.19) | 1.61 (1.44–1.81) | 1.46 (1.33–1.62) | ||||||
| Basic model | 47,456 | 1,464 | 1.27 (1.09–1.48) | 48,295 | 1,480 | 1.91 (1.42–2.58) | 47,456 | 1,306 | 1.65 (1.44–1.89) |
| Plus glycated hemoglobin | 1.10 (0.91–1.33) | 1.41 (1.07–1.86) | 1.63 (1.38–1.93) | ||||||
| Basic model | 48,361 | 3,363 | 1.21 (1.08–1.37) | 59,350 | 4,483 | 2.08 (1.71–2.54) | 48,361 | 2,402 | 1.71 (1.52–1.92) |
| Plus loge insulin | 1.19 (1.05–1.35) | 1.94 (1.58–2.38) | 1.52 (1.35–1.72) | ||||||
All basic models were adjusted for age, sex, smoking status (current smoker vs. any other status), body-mass index, systolic blood pressure, and total cholesterol. Total cholesterol was not included in the analysis that further adjusted for high-density lipoprotein (HDL) cholesterol, non-HDL cholesterol, and triglycerides. Participants with known preexisting cardiovascular disease at baseline were excluded from all analyses. CRP denotes C-reactive protein.
Educational level was categorized as no education, completion of primary school, completion of secondary school, or completion of vocational school or university. Hazard ratios adjusted for occupation were similar (data not shown).
The estimated glomerular filtration rate (GFR) was calculated with the use of the Modification of Diet in Renal Disease equation.
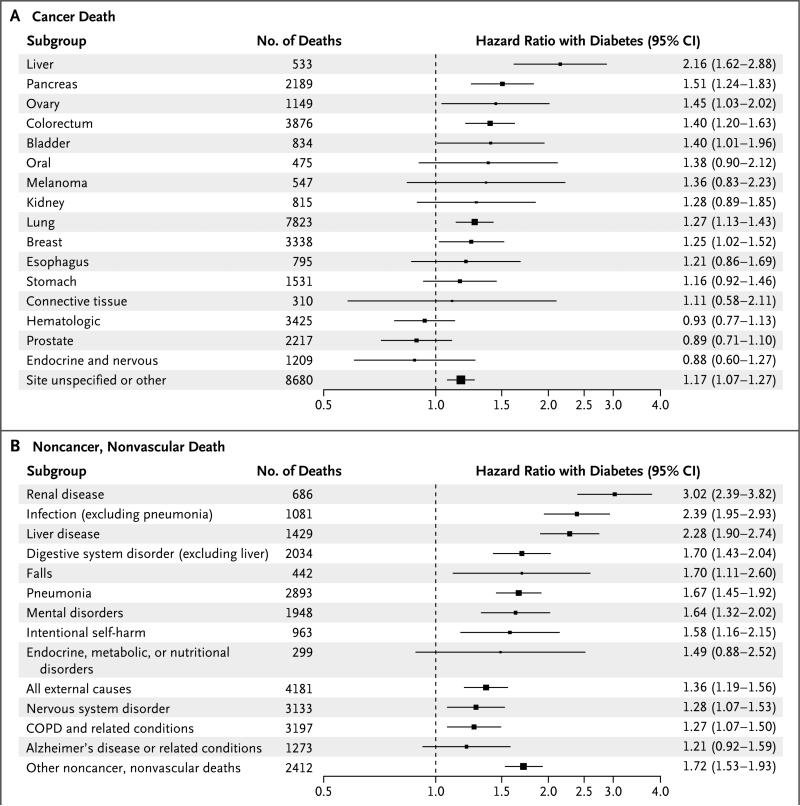
Hazard ratios for death among participants with diabetes, as compared to those without diabetes, were significantly higher at younger ages and among women, except for death from any cancer (Fig. 2 in the Supplementary Appendix). Hazard ratios appeared to decline somewhat with increasing calendar decade of study enrollment (Fig. 3 in the Supplementary Appendix). Diabetes was moderately associated with deaths from cancers of the liver, pancreas, ovary, colorectum, lung, bladder, and breast (Fig. 1A, and Fig. 4 in the Supplementary Appendix). Aside from cancer and vascular disease, diabetes was also associated with deaths from renal disease, liver disease, pneumonia, other infectious diseases, mental disorders, nonhepatic digestive diseases, external causes, intentional self-harm, nervous system disorders, and chronic obstructive pulmonary disease (Fig. 1B).
Figure 1. Hazard Ratios for Death from Cancer and from Noncancer, Nonvascular Causes among Participants with Diabetes as Compared with Those without Diabetes at Baseline.
Panel A shows hazard ratios for deaths from cancer, and Panel B shows hazard ratios for deaths from noncancer, nonvascular causes. With the exception of the classifications “site unspecified or other” in Panel A and “other noncancer, nonvascular deaths” in Panel B, causes of death are presented in descending order according to their estimated hazard ratios. All analyses were stratified on the basis of study, sex, and trial group (where applicable) and adjusted for baseline age, smoking status (current smoker vs. any other status), and body-mass index. There was evidence of heterogeneity in hazard ratios among cancer sites and among the noncancer, nonvascular causes of death (P<0.001 for both comparisons). Participants with known preexisting cardiovascular disease at baseline were excluded from all analy ses. The sizes of the data markers are proportional to the inverse of the variance of the loge hazard ratios. In Panel A, risk estimates for cancer of the colorectum were broadly similar to those for cancer at subsites (i.e., colon cancer vs. cancer of the rectosigmoid and anus). In Panel B, death from endocrine disorders does not include death coded as being due to diabetes. Other noncancer, nonvascular deaths are those that could not be attributed to a major organ or system. COPD denotes chronic obstructive pulmonary disease.
FASTING GLUCOSE AND MORTALITY
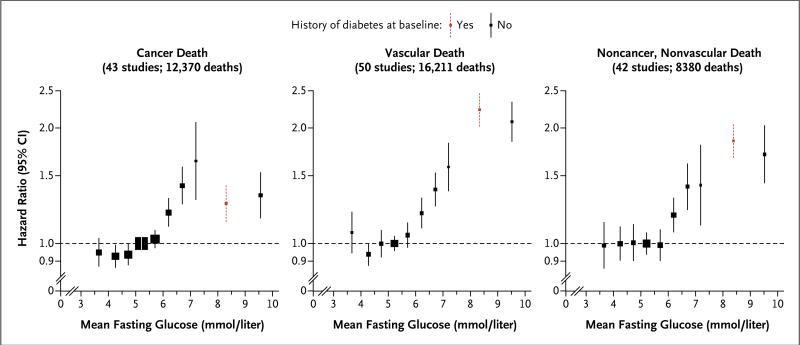
Fasting glucose level was nonlinearly related to the risk of death (Fig. 2). Fasting glucose levels exceeding 100 mg per deciliter (5.6 mmol per liter) but not levels of 70 to 100 mg per deciliter (3.9 to 5.6 mmol per liter) were associated with excess risk (Fig. 5 and 6 in the Supplementary Appendix). At fasting glucose levels above 100 mg per deciliter, hazard ratios for higher levels of glucose, assessed in increments of 18 mg per deciliter (1 mmol per liter), were 1.05 (95% CI, 1.03 to 1.06) for cancer deaths, 1.13 (95% CI, 1.11 to 1.15) for vascu- lar deaths, 1.10 (95% CI, 1.07 to 1.12) for non-cancer, nonvascular deaths, and 1.10 (95% CI, 1.09 to 1.11) for death from any cause, assuming the existence of log-linear relationships above the threshold of 100 mg per deciliter (although associations with cancer deaths tended to plateau at higher levels).
Figure 2. Hazard Ratios for Major Causes of Death, According to Baseline Levels of Fasting Glucose.
History of diabetes at baseline was defined according to a self-reported history of diabetes or treatment for diabetes. Glucose levels for participants without a known history of diabetes at baseline were classified as less than 4.0, 4.0 to less than 4.5, 4.5 to less than 5.0, 5.0 to less than 5.5, 5.5 to less than 6.0, 6.0 to less than 6.5, 6.5 to less than 7.0, 7.0 to less than 7.5, and 7.5 mmol per liter or higher. Hazard ratios were plotted against the mean fasting glucose level in each group (reference category, 5.0 to <5.5 mmol per liter). The sizes of the data markers are proportional to the inverse of the variance of the loge hazard ratios. All analyses were stratified or adjusted for sex and adjusted for baseline age, smoking status (current smoker vs. any other status), and body-mass index. Participants with known preexisting cardiovascular disease at baseline were excluded from all analyses. To convert values for fasting glucose to milligrams per deciliter, divide by 0.05551.
There were generally too few deaths to characterize the shapes of associations of fasting glucose level with more specific causes of death. To avoid potential bias, we examined hazard ratios for various fasting glucose categories after excluding participants with a known history of diabetes at enrollment. As compared with the reference group (with a fasting glucose level of 70 to 100 mg per deciliter), hazard ratios for those with a fasting glucose level of 126 mg per deciliter (7.0 mmol per liter) or more were 1.39 (95% CI, 1.22 to 1.59) for cancer deaths, 1.89 (95% CI, 1.69 to 2.10) for vascular deaths, and 1.54 (95% CI, 1.31 to 1.81) for noncancer, nonvascular deaths. As compared with the same reference group, hazard ratios for participants with impaired fasting glucose levels (100 to <126 mg per deciliter [5.6 to <7.0 mmol per liter]) were 1.13 (95% CI, 1.06 to 1.20) for cancer deaths, 1.17 (95% CI, 1.08 to 1.26) for vascular deaths, and 1.12 (95% CI, 1.07 to 1.18) for noncancer, nonvascular deaths; whereas hazard ratios for participants with fasting glucose levels less than 70 mg per deciliter were 1.01 (95% CI, 0.93 to 1.10) for cancer deaths, 1.32 (95% CI, 1.12 to 1.56) for vascular deaths, and 1.05 (95% CI, 0.89 to 1.24) for noncancer, nonvascular deaths (Fig. 5 in the Supplementary Appendix). Among people with a history of diabetes at baseline, hazard ratios for death were higher among those who had a fasting glucose level of 126 mg per deciliter or more as compared with those who had a level below 126 mg per deciliter (hazard ratio, 2.16 vs. 1.51) (Fig. 5 in the Supplementary Appendix).
Qualitatively similar findings to those reported here were observed in a range of subsidiary analyses, such as those that included 75,195 participants with a history of cardiovascular disease at baseline (Fig. 7 in the Supplementary Appendix), excluded the initial 5 years of follow-up, excluded current smokers (Table 5 in the Supplementary Appendix), corrected for regression dilution in the fasting glucose level and in potential confounders (Fig. 8 and Table 6 in the Supplementary Appendix), assessed interactions with sex and age, excluded 56,766 participants known to be taking lipid-lowering or blood pressure–lowering medication at baseline (Table 5 in the Supplementary Appendix), standardized glucose values in studies that did not involve plasma measurements, assessed fasting glucose levels while ignoring a history of diabetes at baseline (Fig. 9 in the Supplementary Appendix), focused solely on the 60 cohort studies that recruited participants from population registries or general-practice lists (Fig. 10 in the Supplementary Appendix), and explored the effect of plausible degrees of potential misclassification of baseline diabetes status, new-onset diabetes, or attribution of cause of death (Table 7 and Fig. 11 in the Supplementary Appendix).
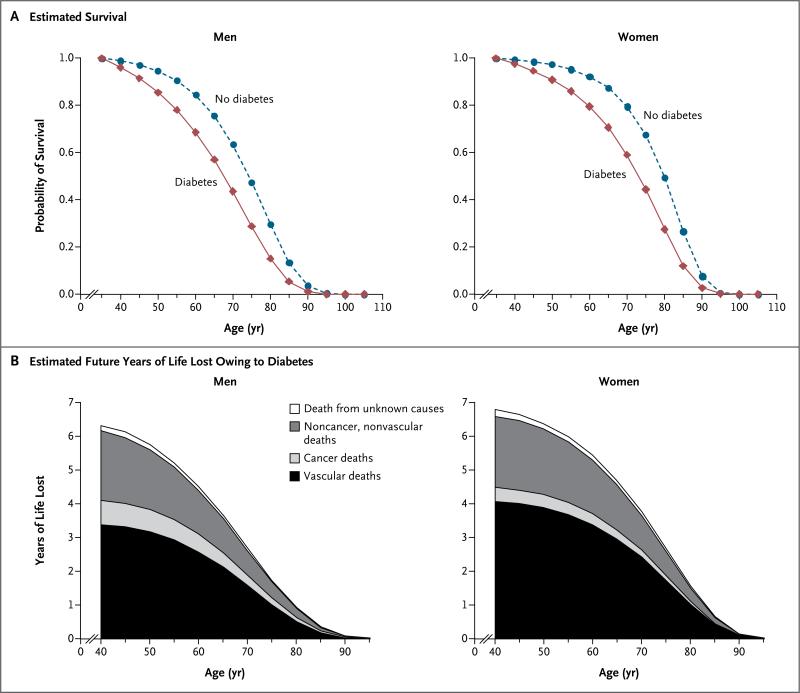
On average, middle-aged adults with diabetes but without known vascular disease at the time of enrollment died about 6 years younger than people without diabetes (Fig. 3). At 40, 50, and 60 years of age, men with diabetes but without a history of vascular disease would incur about 6.3, 5.8, and 4.5 years of life lost, respectively. The corresponding years of life lost for women with diabetes in middle age were 6.8, 6.4, and 5.4 years, respectively. About 58%, 9%, and 30% of this survival difference at 50 years of age can be attributed to excess vascular, cancer, and non-cancer, nonvascular deaths, respectively.
Figure 3. Diabetes and Survival, According to Sex and Diabetes Status.
Panel A shows estimated survival curves that were plotted by applying hazard ratios for death from any cause (specific for sex and age at risk) from the present analyses to mortality data for the European Union in 2000. Panel B shows the estimated numbers of years of life lost owing to diabetes. Participants with known preexisting cardiovascular disease at baseline were excluded from both analyses.
DISCUSSION
In addition to the excess risk of vascular disease, our data show that diabetes is associated with substantial premature mortality from several cancers, infectious diseases, external causes, intentional self-harm, and degenerative disorders, independent of several major risk factors. Our results suggest that, on average, a 50-year old with diabetes but with no history of vascular disease is about 6 years younger at the time of death than a counterpart without diabetes; for comparison, the reduction in life expectancy from long-term cigarette smoking is about 10 years.20 About 40% of the years of life lost from diabetes can be attributed to nonvascular conditions, including about 10% attributable to death from cancer.
We have also found that there are generally continuous associations between fasting glucose levels greater than 100 mg per deciliter and risk of death, supporting the view that hyperglycemia (or some factor closely related to it) may be directly relevant. This possibility is also consistent with substantial attenuation observed in hazard ratios for death from diabetes after adjustment for markers of glycemia (though interpretation of such analyses is complicated because glucose levels are used to define diabetes status). In con- trast, we did not observe appreciable alteration in the associations between diabetes and mortality after adjustment for several other risk factors (e.g., systolic blood pressure, measures of adiposity, inflammation biomarkers, insulin, or renal function), even after using serial measurements to adjust for their long-term average levels. These findings reduce the likelihood that such risk factors are major mediators of the excess risk of death associated with diabetes in our study.
Our study indicates that diabetes is moder ately associated with death from cancers of the liver, pancreas, ovary, colorectum, lung, bladder, and breast (although, because multiple disease outcomes were studied, any marginally significant findings should be evaluated further). Although causality has not been established for these associations, systemic21 and local22,23 factors have been proposed to explain them. For pancreatic cancer, however, diabetes can be a consequence of the cancer, rather than a cause,24 although the exclusion of initial follow-up data did not weaken the hazard ratios in this study. In contrast with some previous studies of the incidence of prostate cancer, however, we did not find a significant inverse association of diabetes with the incidence of death from prostate cancer, although our confidence intervals were compatible with the risk estimates reported previously.25
Aside from cancers, we observed strong positive associations of diabetes with deaths from renal and digestive diseases and infectious diseases. These results may reflect associated nephropathy, fatty liver disease, and suppression of cellular immunity, respectively.26-28 Furthermore, diabetes is associated with death from injuries, which could be related to end-organ complications such as neuropathy and eye disease or to episodes of hypoglycemia. Associations observed between diabetes and deaths due to mental and neurologic diseases should be studied further, including disaggregation into more specific conditions and investigation of the possible link between diabetes and the onset of depression,29 particularly since we found that among people with diabetes there was a substantial excess of deaths due to intentional self-harm. Collectively, therefore, our findings broaden and intensify the need for efforts to prevent and understand diabetes and encourage detailed study of a broader range of disease outcomes than has been customary in randomized trials of diabetes prevention and treatment.30 These results also reinforce the need for people with diabetes to consider cancer screening appropriate for their age and sex.
Our study has several strengths. These include the large sample size (over 123,000 deaths recorded during more than 12 million person-years at risk), standardized approaches to adjustment for several potential confounding factors, serial assessment of risk factors in 331,515 participants, an extended follow-up period, and information about cause-specific deaths from a variety of conditions. Furthermore, we studied several factors proposed to mediate associations of diabetes and cancer.31
The generalizability of our findings to populations in economically developed Western countries is supported by broadly consistent results across 97 prospective cohorts in 25 countries. Even though the 6% overall prevalence of diabe tes in our study was somewhat lower than the prevalence currently reported for some Western populations, this difference would not have influenced hazard ratios. To enhance the validity of our findings, we conducted a range of sensitivity analyses, including a focus on population-based cohorts, adjustment for several major risk factors, and exclusion of the initial years of the follow-up period. We investigated mortality rather than the incidence of nonfatal disease, which should have reduced the likelihood of finding artifactual associations due to preferential diagnosis of certain conditions in people with diabetes. Indeed, we observed qualitatively similar results among people with no history of diabetes but a fasting glucose level of 126 mg per deciliter or more and among people with a history of diabetes at baseline. However, our study cannot determine whether diabetes might increase the incidence of such diseases or reduce survival (or both) among persons with such illnesses.
Despite the strengths of our study, residual bias could persist owing to unmeasured or imprecisely measured potential confounding factors (e.g., dietary intake and physical activity, respectively). Certain glucose-lowering agents have been reported to increase32 or decrease33 the risk of cancer, but our observational study cannot reliably address these questions. Because our study did not include comprehensive recording of use of aspirin (an agent that is known to prevent vascular disease and may also prevent colorectal cancer34,35), we may have underestimated the hazard ratios for death from colorectal cancer. Although our data suggest that hazard ratios for death in patients with diabetes may have declined somewhat in recent decades,36 this finding may be subject to confounding, because cohort studies initiated in earlier decades may differ in several ways from more recent cohort studies. Were the decline to be confirmed, however, then it would imply that our study has slightly overestimated the contemporary effect of diabetes on death. Future studies are warranted to investigate additional (and potentially more specific) risk factors that may link diabetes and chronic diseases, to study non-Western populations,37 and to explain associations observed between very low glucose levels and vascular death in people without diabetes.
In conclusion, in addition to vascular disease, diabetes is associated with substantial premature death from several cancers, infectious diseases, external causes, intentional self-harm, and degenerative disorders, independent of major risk factors. These findings highlight the need to better understand and prevent the multisystem consequences of diabetes.
Supplementary Material
Acknowledgments
Supported by grants from the British Heart Foundation (RG/08/014), the U.K. Medical Research Council, and Pfizer (to the ERFC Coordinating Centre), as well as the Gates Cambridge Trust Scholarship, an Overseas Research Studentship Award, and an Addenbrooke's Charitable Trust Clinical Research Fellowship (to Dr. Kondapally Seshasai). Various sources have supported recruitment, follow-up, and laboratory measurements in the cohorts contributing to the ERFC. Investigators of several of these studies have contributed to a list (http://ceu.phpc.cam.ac.uk/ research/erfc/studies) naming relevant funding sources.
We thank Jill Boreham for information on mortality rates in the European Union and Paul Pharoah for helpful comments on a draft of the manuscript.
Footnotes
The members of the writing committee of the Emerging Risk Factors Collaboration assume responsibility for the overall content and integrity of this article.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Sreenivasa Rao Kondapally Seshasai, University of Cambridge, Cambridge, United Kingdom
Stephen Kaptoge, University of Cambridge, Cambridge, United Kingdom
Alexander Thompson, University of Cambridge, Cambridge, United Kingdom
Emanuele Di Angelantonio, University of Cambridge, Cambridge, United Kingdom
Pei Gao, University of Cambridge, Cambridge, United Kingdom
Nadeem Sarwar, University of Cambridge, Cambridge, United Kingdom
Peter H. Whincup, St. George's University of London, London
Kenneth J. Mukamal, Harvard University, Boston
Richard F. Gillum, Centers for Disease Control and Prevention, Atlanta
Ingar Holme, Ullevål University Hospital, Oslo
Inger Njølstad, University of Tromsø, Tromsø, Norway
Astrid Fletcher, London School of Hygiene and Tropical Medicine, London
Peter Nilsson, Lund University, Lund, Sweden
Sarah Lewington, University of Oxford, Oxford, United Kingdom
Rory Collins, University of Oxford, Oxford, United Kingdom
Vilmundur Gudnason, Icelandic Heart Association and the University of Iceland, Reykjavik
Simon G. Thompson, Medical Research Council Biostatistics Unit, Cambridge, United Kingdom
Naveed Sattar, University of Glasgow, Glasgow, United Kingdom
Elizabeth Selvin, Johns Hopkins University, Baltimore
Frank B. Hu, Harvard University, Boston
John Danesh, University of Cambridge, Cambridge, United Kingdom
References
- 1.The Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepato-cellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–80. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Huxley R, Ansary-Moghaddam A, Berrington de González A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Bio-markers Prev. 2006;15:2056–62. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 5.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 6.Stocks T, Rapp K, Bjorge T, et al. Blood glucose and risk of incident and fatal cancer in the Metabolic syndrome and Cancer project (Me-Can): analysis of six prospective cohorts. PLoS Med. 2009;6(12):e1000201. doi: 10.1371/journal.pmed.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parr CL, Batty GD, Lam TH, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11:741–52. doi: 10.1016/S1470-2045(10)70141-8. [Erratum, Lancet Oncol 2010;11:721.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atchison EA, Gridley G, Carreon JD, Leitzmann MF, McGlynn KA. Risk of cancer in a large cohort of U.S. veterans with diabetes. Int J Cancer. 2011;128:645–53. doi: 10.1002/ijc.25362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batty GD, Shipley MJ, Marmot M, Smith GD. Diabetes status and post-load plasma glucose concentration in relation to site-specific cancer mortality: findings from the original Whitehall study. Cancer Causes Control. 2004;15:873–81. doi: 10.1007/s10552-004-1050-z. [DOI] [PubMed] [Google Scholar]
- 10.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–7. doi: 10.1093/aje/kwh161. [DOI] [PubMed] [Google Scholar]
- 11.The Emerging Risk Factors Collaboration Analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur J Epidemiol. 2007;22:839–69. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 12.Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. Idem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. Idem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. Idem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Fibrinogen Studies Collaboration Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 16.Thompson S, Kaptoge S, White I, Wood A, Perry P, Danesh J. Statistical methods for the time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010;39:1345–59. doi: 10.1093/ije/dyq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Fibrinogen Studies Collaboration Correcting for multivariate measurement error by regression calibration in meta-analyses of epidemiological studies. Stat Med. 2009;28:1067–92. doi: 10.1002/sim.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO statistical information system (WHOSIS) World Health Organization; Geneva: 2007. [Google Scholar]
- 19.United Nations Population Division . World population prospects (2004 revision: ST/ESA/SER.A/244) United Nations; New York: 2005. [Google Scholar]
- 20.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–80. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 22.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 23.Chen SL, Jackson SL, Boyko EJ. Diabe tes mellitus and urinary tract infection: epidemiology, pathogenesis and proposed studies in animal models. J Urol. 2009;182(Suppl):S51–S56. doi: 10.1016/j.juro.2009.07.090. [DOI] [PubMed] [Google Scholar]
- 24.Pannala R, Leibson CL, Rabe KG, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104:2318–25. doi: 10.1038/ajg.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters KM, Henderson BE, Stram DO, Wan P, Kolonel LN, Haiman CA. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol. 2009;169:937–45. doi: 10.1093/aje/kwp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jawa A, Kcomt J, Fonseca VA. Diabetic nephropathy and retinopathy. Med Clin North Am. 2004;88:1001–36. doi: 10.1016/j.mcna.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 28.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 29.Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dormandy J, Bhattacharya M, van Troostenburg de Bruyn AR. Safety and tolerability of pioglitazone in high-risk patients with type 2 diabetes: an overview of data from PROactive. Drug Saf. 2009;32:187–202. doi: 10.2165/00002018-200932030-00002. [DOI] [PubMed] [Google Scholar]
- 31.Zhou XH, Qiao Q, Zethelius B, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53:1867–76. doi: 10.1007/s00125-010-1796-7. [DOI] [PubMed] [Google Scholar]
- 32.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–44. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–6. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–60. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–50. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 36.Gulliford MC, Charlton J. Is relative mortality of type 2 diabetes mellitus decreasing? Am J Epidemiol. 2009;169:455–61. doi: 10.1093/aje/kwn342. [DOI] [PubMed] [Google Scholar]
- 37.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.