Abstract
Mutations in isocitrate dehydrogenase (IDH) 1 and 2, originally discovered in 2009, occur in the vast majority of low grade gliomas and secondary high grade gliomas. These mutations, which occur early in gliomagenesis, change the function of the enzymes, causing them to produce 2-hydroxyglutarate, a possible oncometabolite, and to not produce NADPH. IDH mutations are oncogenic, although whether the mechanism is through alterations in hydroxylases, redox potential, cellular metabolism, or gene expression is not clear. The mutations also drive increased methylation in gliomas. Gliomas with mutated IDH1 and IDH2 have improved prognosis compared to gliomas with wild-type IDH. Mutated IDH can now be detected by immunohistochemistry and magnetic resonance spectroscopy. No drugs currently target mutated IDH, although this remains an area of active research.
Keywords: Isocitrate dehydrogenase, astrocytoma, oligodendroglioma, glioblastoma, 2-hydroxyglutarate, carcinogenesis, prognosis, mutations
Introduction
Diffuse gliomas are the most common primary brain tumor in adults, affecting about 20,000 people in the US each year [1]. Most gliomas are astrocytic (70%) or oligodendroglial tumors (9%), which include classic oligodendrogliomas and mixed oligoastrocytomas [1]. Three pathways of glioma development have been identified: primary glioblastoma that arise de novo without lower grade precursors, astrocytomas that start as grade 2 or 3 and then transform into secondary glioblastoma, or oligodendrogliomas that can transform into anaplastic oligodendroglioma. Infiltrating gliomas are incurable with current treatment modalities: surgery, radiation, and chemotherapy. While several important genetic alterations in gliomas have been known for some time, new technologies have allowed much deeper genetic and epigenetic analyses of larger numbers of glioma samples, leading to a number of novel discoveries in the past few years. One of the most exciting and clinically relevant observations was the discovery that a high percentage of lower grade gliomas (and a lower percentage of higher grade gliomas) harbor mutations in the genes isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2). Growing data indicate that these mutations play a causal role in gliomagenesis, have a major impact on tumor biology, and also have clinical and prognostic importance
Glioma histopathologic and molecular classification
Diffuse gliomas can be categorized according to grade; low grade (grade II), anaplastic (grade III), and glioblastoma (GBM, grade IV). Traditionally, GBMs have been classified as primary or secondary on the basis of clinical presentation [2]. Secondary GBMs display evidence of progression from a lower-grade tumor, whereas primary GBMs present as advanced cancers at diagnosis. Secondary GBMs are predominantly found in younger patients (median age of ~45 years compared with median age of ~60 years for primary GBM) and tend to occur less frequently than primary GBMs, making up ~5% of total GBMs [3]. Despite the differences in their ontology, these high grade tumors are histopathologically indistinguishable [3].
Recently, large scale efforts have been made to identify the major genetic and epigenetic alterations and to define important molecular subtypes in GBM and lower grade gliomas [4, 5]. Publications from the Cancer Genome Atlas (TCGA) effort have identified 4 subgroups of GBM based on dominant gene expression patterns, which were called Proneural, Neural, Mesenchymal, and Classical [5]. Individual gene expression subtypes were associated with specific genetic and epigenetic alterations. Secondary GBMs are virtually always Proneural, while primary GBM can be any of the subtypes.
As discussed below, mutations in one of the IDH genes are tightly linked with the Proneural phenotype and secondary glioblastomas. Virtually all tumors with IDH mutation are of the Proneural gene expression subtype. In addition, IDH mutation was observed to be associated with increased DNA methylation, called G-CIMP for Glioma CpG Island Methylation Phenotype [6]. Thus, both IDH mutation and G-CIMP phenotype are seen almost exclusively in secondary GBMs. Developing data regarding the metabolic and biologic consequences of IDH mutation have led to significant increases in our understanding of the development and clinical behavior of these different molecular subtypes of glioma.
IDH Mutations and Alterations in IDH Function
In 2008, a multi-group collaboration sequenced over 20,000 genes in 22 GBMs and identified a common point mutation in the metabolic gene IDH1 in 12% of the samples analyzed [7]. Further studies found that this mutation is present in ~80% of grade II-III gliomas and secondary GBM [8-14]. Mutations in IDH2 have also been identified in gliomas, although they are much less common and are mutually exclusive with mutations in IDH1 [10, 15, 14]. All mutations identified to date have been a single amino acid missense mutation in IDH1 at arginine 132 (R132) or the analogous residue in IDH2 (R172). Before these observations, mutation of IDH genes had never been linked to cancer. However, subsequent studies have identified IDH mutations in acute myelogenous leukemia, cholangiocarcinoma, cartilaginous tumors, prostate cancer, papillary breast carcinoma, acute lymphoblastic leukemia, angioimmunoblastic T-cell lymphoma, and primary myelofibrosis indicating that these genes may be important players in multiple tumor types [16-23].
Five genes encode for three human IDH catalytic isozymes: IDH1, IDH2, and IDH3. IDH1 and 2 form homodimers while IDH3 forms a heterotetramer containing two α, one β, and one γ subunit [24]. IDH3 functions in the Kreb cycle to convert isocitrate to α-ketoglutarate (α-KG) and NAD+ to NADH. The IDH1 and IDH2 proteins in the cytosol and mitochondria, respectively, generate reduced nicotinamide adenine dinucleotide phosphate (NADPH) from NADP+ by catalyzing the oxidative decarboxylation of isocitrate to α-KG outside of the Kreb cycle [25]. NADPH is mainly produced by glucose 6-phosphate dehydrogenase (G6PDH), malate dehydrogenase, and IDH. Upon exposure to free radicals and reactive oxygen species (ROS), cells with low levels of IDH became more sensitive to oxidative damage [26]. These studies demonstrated that in addition to being a major enzyme in the citric acid cycle, IDH may also function to maintain the cellular redox state and suggests that IDH plays an important role in cellular defense against oxidative stress.
All mutations in IDH1 in glioma appear to effect amino acid residue 132, with the vast majority (>85%) containing a heterozygous missense mutation of arginine to histidine (R132H) [27]. This residue is located in the active site of the enzyme and is critical for isocitrate binding [28]. The mutation at R132 inactivates the protein’s ability to bind isocitrate and abolishes its normal catalytic activity. The net result is reduced levels of α-KG and NADPH, which is an important cofactor that is necessary to maintain normal levels of reduced glutathione (GSH) to combat ROS [29, 30].
IDH Mutations and Oncogenesis
One question that always arises in studies of gene mutations in cancer is whether that specific gene is a key driver of tumor formation (oncogenesis), whether the mutation arises as a step in tumor progression, or whether it is an incidental consequence of impaired DNA repair in tumors (e.g. a carrier mutation). Two lines of evidence support the concept that IDH mutation is a direct driver of oncogenesis. First, somatic mosaicism for IDH1 or IDH2 at R132 causes the enchondromatosis syndromes, Ollier’s disease and Maffucci syndrome, which are characterized by hemangiomas and cartilaginous tumors and which carry an increased risk for gliomas [18, 31, 32]. Second, introduction of mutated IDH into normal cells causes increased proliferation, increased colony formation, and inability to differentiate [33-35].
Exactly how IDH mutation contributes to oncogenic transformation remains controversial. Initial reports suggested that the mutant protein functions in a dominate-negative fashion by heterodimerizing to wild-type IDH1 and impairing its activity [36-38]; however, more recent in vitro studies have shown that the mutated IDH1 protein acquires the ability to convert α-KG to R(-)-2-hydroxyglutarate (2-HG) [39, 40]. This is supported by the findings that 2-HG levels are elevated in gliomas containing an IDH1 mutation [39]. These findings led to the hypothesis that mutant IDH is an oncogene and 2-HG is an ‘oncometabolite’ [41].
Whether it is 2-HG or mutated IDH or both that promotes cancer remains unclear. An increased risk of gliomas has been observed for patients with inborn errors of metabolism that result in increased levels of S-2-HG but not R-2-HG, although interestingly people with R-2-hydroxyglutaric aciduria can have IDH2 mutations but never IDH1 mutations [42, 43]. Furthermore, It was recently discovered that the wild-type IDH1 can also catalyze the conversion of α-KG to 2-HG, albeit less efficiently than mutant IDH [44].
New evidence suggests that by antagonizing α-KG, 2-HG competitively inhibits the activity of many α-KG-dependent dioxygenases, including but not limited to histone demethylases (e.g. collagen prolyl-4-hydroxylase, prolyl hydroxylases, and the ten-eleven translocation (TET) family of DNA hydroxylases) [45, 35, 46]. As described above, profiling of GBM from the TCGA demonstrated an association between IDH mutation and increased promoter methylation (G-CIMP) [6], which generally results in transcriptional silencing of the associated genes [47].
Two recent independent studies demonstrated that the G-CIMP phenotype was not only correlated with IDH mutation but that IDH mutation alone is actually the cause of the G-CIMP hypermethylation phenotype in diffuse gliomas [48, 49]. In these studies, introduction of mutant IDH1 into immortalized primary human astrocytes was found to be sufficient to cause the hypermethylator phenotype [50]. One possible implication of these findings is that reduced α-KG and increased 2-HG (from IDH mutation) may predispose cells harboring mutant IDH to malignant transformation via genome-wide epigenetic changes. However, further studies are needed to determine the precise link between these effects of IDH mutation and actual oncogenic transformation as development of CIMP phenotype in astrocytes after introduction of IDH mutations did not result in tumor formation.
IDH Mutations and the Hypoxia Pathway
Prolylhydroxylase domain enzymes (PHD) are important for the hydroxylation and degradation of hypoxia-inducible factor 1α (HIF-1α). These enzymes require α-KG and divalent ferrous iron (Fe2+). One of the effects of IDH mutation is the depletion of α-KG and oxidation of ferrous iron by ROS. Reduced levels of α-KG and Fe2+ in mutant IDH cells may therefore promote cellular accumulation of HIF-1α. Activation of this pathway leads to the induction of HIF-1 target genes that affect angiogenesis, metabolism, growth and differentiation, apoptosis and autophagy, as well as cell motility [51]. Zhao et al. found that overexpression of mutant IDH in U87MG glioma cells increased HIF-1α protein levels and induced HIF-1α target gene expression. This effect was abolished after exogenous administration of α-KG. In addition, HIF-1α protein expression was found to be higher in gliomas with IDH1 mutations compared with those containing wild-type IDH [38]. These observations suggest that IDH mutation can lead to activation of the hypoxia pathways in the presence of normoxia, a process termed “pseudohypoxia. However, other studies by Koivunen et al. found that R-2-HG, could substitute for α- KG in late passage immortalized astrocytes expressing mutant IDH to stimulate the activity of the HIF-1α PHD proteins EGLN1, 2 and 3 that promote the degradation of HIF-1α. Furthermore, analysis of gene expression data from TCGA showed that tumors harboring mutant IDH had reduced expression of HIF target genes relative to tumors containing wild-type IDH [33]. Inconsistencies in these findings suggest that effects of IDH mutation on the hypoxia pathway may be context dependent and further studies are required to determine the association between mutant IDH expression and HIF activity in gliomas.
IDH mutation distribution and significance in human glioma
Several lines of evidence suggest that IDH1 mutations are an early event in glioma development. As described above, IDH1 mutations are seen in a high percentage of grade II and III astrocytomas and oligodendrogliomas and secondary GBM tumors. Mutations in IDH2 have been found in fewer than 3% of glial tumors, with similar distributions related to grade. IDH mutations are rare in primary GBM [14]. In lower grade gliomas with a high percentage of IDH mutation, differences in other tumor mutations and copy number alterations have been found associated with histologic subtypes. IDH1 mutations often occur with a TP53 mutation in astrocytic tumors, and these tumors rarely demonstrate loss of chromosomes 1p and 19q. On the other hand, IDH mutation is seen in virtually all oligodendrogliomas with 1p/19q co-deletion, and these tumors rarely demonstrate p53 mutation [13, 14, 52]. Thus, even though IDH1 is highly associated with both TP53 mutation and loss of 1p/19q, these alterations are generally mutually exclusive in gliomas. These findings suggest that IDH mutation is an early event in glioma formation and occurs prominently in low grade tumors of both astrocytic and oligodendroglial lineage. Indeed, studies of the timing of IDH1 mutations relative to others in gliomas indicate that IDH mutations precede TP53 mutations in 63% of diffuse astrocytomas [13, 30] and 80% of glioblastomas and anaplastic astrocytomas with IDH mutations also had a mutation of TP53 [14]. Mutations in the TP53 tumor suppressor gene were first implicated in gliomagenesis twenty years ago when an increased incidence of gliomas was observed in patients with Li-Fraumeni syndrome, a rare cancer-predisposing disorder caused by mutations in TP53 [53]. Interestingly, IDH mutations found in astrocytomas that developed in Li-Fraumeni syndrome families contained the less common R132C substitution, suggesting a difference in selection in cells that already harbor TP53 mutations [27]. While commonly seen with alterations associated with low grade glioma, IDH mutation is rarely observed with genetic alterations commonly seen in high frequency in primary GBMs (e.g., EGFR), supporting the idea that IDH mutation is not a driver of tumor initiation in these GBM subtypes [13].
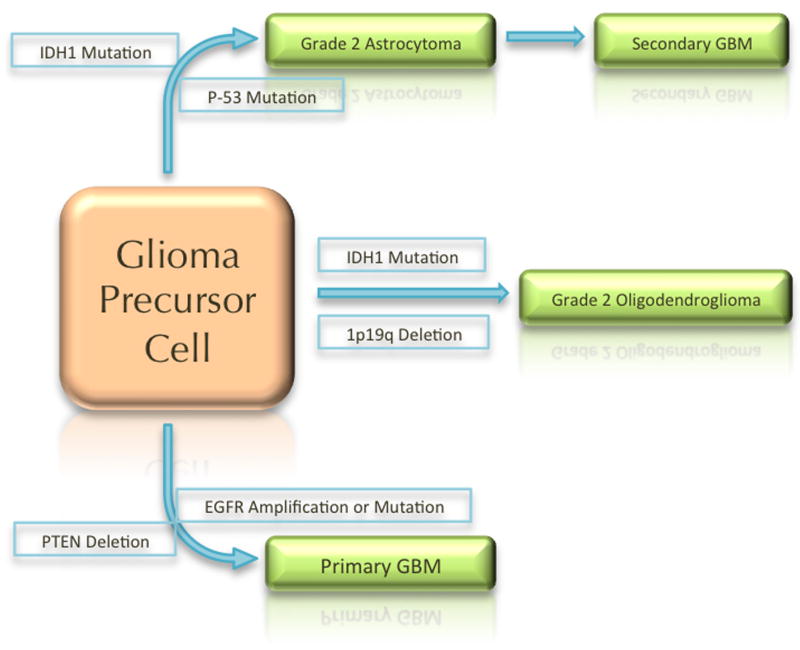
Thus, there appear to be three pathways to the development of glioma. One pathway starts with IDH mutation followed by TP53 mutation and results in astrocytic lineage tumors. These gliomas start as grade 2 astrocytomas, have the G-CIMP phenotype, and presumably then acquire other genetic alterations that result in progression to higher grade tumors. Another pathway starts with IDH mutation followed by loss of 1p/19q, which is associated with mutation in the Capicua (CIC) gene. These alterations result in development of grade 2 oligodendrogliomas, which can then acquire other genetic alterations to become anaplastic oligodendrogliomas. The third pathway includes those gliomas that are wild-type for IDH. These gliomas appear to rapidly acquire multiple complex genetic alterations, including amplification or mutation of EGFR, and loss of the PTEN gene, and become glioblastomas very early in their development (See figure 1). It should be noted that the cell of origin for these tumors remains unknown, and it also remains unclear whether there is a common cell of origin, or different cells of origin for the different lineage and molecular subtypes.
Figure 1.
Schematic of the three possible paths of gliomagenesis based on IDH mutation status.
People with gliomas with mutated IDH are, on average, several years younger than people with gliomas with wild-type IDH. On the other hand, IDH mutations are rare in gliomas in young children, although they are common in gliomas in adolescents 14 years-old or older [54, 55]. About 20-25% of people with IDH-mutated gliomas carry the rs55705857 single nucleotide polymorphism (SNP) on chromosome 8q24, compared with 5% of the general population. [56]In a prospective analysis, grade II-IV glioma patients whose tumors harbored mutant IDH1 or IDH2 had significantly longer overall survival than patients without IDH mutation [12]. This finding has been confirmed independently by others [57, 58, 14]. The prognostic importance of IDH mutation is independent of other known prognostic factors, including age, grade, and MGMT methylation status [12].
However, it remains to be determined whether IDH mutation is a prognostic factor only or whether it is predictive of outcome to specific treatments or mechanistically related to treatment response. In vitro, induction of mutated IDH increased the sensitivity of gliomas to radiation [59]. Small retrospective series have suggested that the response rate to alkylating chemotherapy is also higher in IDH mutated grade 2 tumors than in wild-type tumors and that progression free survival after radiation or alkylating chemotherapy is higher for people with IDH-mutated tumors than for people with wild type tumors [60-62]. In the German Glioma Group retrospective study, IDH mutation influenced survival only in those patients who received radiation or chemotherapy immediately after surgery [63]. IDH mutation does not predict progression free survival for temozolomide treatment in low grade astrocytomas that had previously received radiation [64]. However, their retrospective nature and lack of control groups limits the conclusions that can be drawn from these studies.
In EORTC 26951, a randomized trial comparing the addition of chemotherapy with procarbazine, CCNU, and vincristine to adjuvant radiation in anaplastic oligodendrogliomas, the presence of IDH mutation predicted prolonged survival in the chemotherapy group, but the presence of IDH mutation nearly completely overlapped with 1p/19q deletion, so the independent effect of these genetic alterations in grade 3 oligondendrogliomas cannot be determined [65]. The NOA-04 trial compared radiation with alkylating chemotherapy as up front treatment of grade 3 gliomas. IDH mutation was associated with improved PFS in both treatment groups approximately equally [66]. The ongoing CATNON trial should provide important additional data to address the question of the predictive association with IDH mutation and specific treatments in non-1p/19q deleted grade 3 gliomas.
Detection of IDH mutation in glioma
Initial studies of IDH mutations used direct sequencing [14]. Pyrosequencing has remained clinically valuable for detection of IDH mutations in hematologic malignancies, particularly AML. While sequencing remains the gold standard for IDH mutation testing, more rapid and easy to apply methods have been developed for initial screening in gliomas. The development of a monoclonal antibody against IDH1 R132H allows the detection of the most common mutation seen in gliomas by immunohistochemistry from paraffin embedded sections, and this method is being used with increasing frequency for clinical classification of tumors [67].
In addition to detection of IDH mutation in tissue, the increased levels of 2-hydroxyglutarate in IDH mutated tumors have led several groups to develop non-invasive imaging methods for detection of IDH-mutated gliomas. Indeed, 2-hydroxyglutarate has a distinctive magnetic resonance spectrum that is detectable by in vivo magnetic resonance spectroscopy (MRS) [68]. MRS may therefore be useful for distinguishing gliosis from low grade gliomas, potentially avoiding biopsy in ambiguous cases. However, additional prospective studies are needed to further define the sensitivity and specificity of these imaging approaches.
Models of IDH-mutated glioma
Study of IDH-mutated gliomas has been limited by a lack of appropriate mouse models. There are no models of spontaneous IDH-mutated glioma-producing mice. Expression of mutated IDH1 in the brains of transgenic mice causes death soon after birth due to intercerebral hemorrhage [46]. In vitro cell lines with IDH mutation can be transiently produced but do not persist in non-immortalized cells. In standard cell culture conditions, IDH-mutated gliomas cells from IDH-mutated patient tumors generally do not grow well [69]. Moreover, there may be a selection pressure against IDH-mutated glioma cells in culture because, in contrast to the effect on normal cells, introduction of mutated IDH into IDH wild-type glioma cells decreases the proliferation rate [70]. Despite these challenges, IDH-mutated primary glioma neurospheres have been isolated from a human tumor and propagated in stem cell conditions by at least one group [71].
Targeting IDH mutations
The discovery of IDH mutation in glioma has resulted in a number of novel ideas for therapeutic approaches. The concept of 2-HG being an “oncometabolite” has led several investigators to try to devise strategies to either restore normal IDH function or block production or downstream effects of 2-HG. Indeed, in an in vitro model, suppression of expression of mutated IDH decreases growth and clone formation in an IDH-mutated fibrosarcoma cell line [72]. While there are currently no direct IDH inhibitors in clinical trials, a number of investigators and drug companies are working on bringing specific inhibitors to the clinic. In vitro, the production of 2-HG by mutated IDH2 can be inhibited by metabolites such as oxaloacetate [73].
Another approach that is being considered is targeting the downstream signaling pathway alterations and epigenetic changes that are induced by IDH mutation. For example, IDH-mutated glioblastomas have increased activation of the hedgehog pathway, so use of a hedgehog inhibitor is of potential interest in these tumors [74]. Alternatively, hypomethylating agents or other epigenetic modifiers may be rational to try to target the G-CIMP hypermethylation phenotype induced by mutated IDH [75]. One potential caveat of this approach is that the general activation of expression of many genes through a decrease in methylation could also increase expression of deleterious ones such as oncogenes.
Conclusion
The story of IDH mutations in gliomas is one of the successes of mapping the human genome. In the three years since their discovery, our understanding of the biochemistry, genetics, and epigenetics of IDH mutations has grown. It is now clear that mutations in IDH1 and IDH2 are driver mutations in low grade gliomas, likely through 2-HG production. These mutations lead to a hypermethylation phenotype as well as changes in cellular metabolism and response to hypoxic and oxidative stress. IDH mutations have a definite effect on prognosis and may be predictive of response to radiation and/or alkylating chemotherapy. Our ability to target gliomas with IDH mutations remains limited, however. Thus, while our understanding of IDH mutation and its biology has increased dramatically in a relatively short time period, it is clear more investigation is necessary to determine optimal therapeutic strategies to target the IDH mutated subsets of gliomas.
Acknowledgments
The authors thank Rowan Arave for assistance with preparation of the figure.
Literature Cited
- 1.Dolecek T, Propp J, Stroup N, Kruchko C. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2005-2009. Neuro Oncol. 2012;15(suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scherer HJ. A Critical Review: The Pathology of Cerebral Gliomas. J Neurol Psychiatry. 1940;3(2):147–77. doi: 10.1136/jnnp.3.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–9. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 4.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–73. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noushmehr H, Weisenberger DJ, Diefes K, et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510–22. doi: 10.1016/j.ccr.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–12. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 9.Bleeker FE, Lamba S, Leenstra S, et al. IDH1 mutations at residue p.R132 (IDH1(R132)) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30(1):7–11. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- •10.Hartmann C, Meyer J, Balss J, et al. Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol. 2009;118(4):469–74. doi: 10.1007/s00401-009-0561-9. This paper is the largest series of gliomas typed for IDH mutations. It established the distribution of IDH mutations by histology and grade. [DOI] [PubMed] [Google Scholar]
- 11.Kang MR, Kim MS, Oh JE, et al. Mutational analysis of IDH1 codon 132 in glioblastomas and other common cancers. Int J Cancer. 2009;125(2):353–5. doi: 10.1002/ijc.24379. [DOI] [PubMed] [Google Scholar]
- •12.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4. doi: 10.1200/JCO.2009.21.9832. This paper reinforced the clinical relevance of IDH mutation by validating that it is prognostic independent of age, grade, and MGMT status. [DOI] [PubMed] [Google Scholar]
- ••13.Watanabe T, Nobusawa S, Kleihues P, Ohgaki H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174(4):1149–53. doi: 10.2353/ajpath.2009.080958. This paper established that IDH mutations occur before other known genetic changes, including TP53 mutation and 1p/19q deletion, during the course of gliomagenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••14.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. doi: 10.1056/NEJMoa0808710. This was the first paper to identify IDH mutations in gliomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sonoda Y, Kumabe T, Nakamura T, et al. Analysis of IDH1 and IDH2 mutations in Japanese glioma patients. Cancer Sci. 2009;100(10):1996–8. doi: 10.1111/j.1349-7006.2009.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361(11):1058–66. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17(1):72–9. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amary MF, Bacsi K, Maggiani F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224(3):334–43. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 19.Mauzo SH, Lee M, Petros J, et al. Immunohistochemical Demonstration of Isocitrate Dehydrogenase 1 (IDH1) Mutation in a Small Subset of Prostatic Carcinomas. Appl Immunohistochem Mol Morphol. 2012 doi: 10.1097/PAI.0b013e3182649d1c. [DOI] [PubMed] [Google Scholar]
- 20.Ang D, Vansandt AM, Beadling C, et al. Biphasic Papillary and Lobular Breast Carcinoma With PIK3CA and IDH1 Mutations. Diagn Mol Pathol. 2012;21(4):221–4. doi: 10.1097/PDM.0b013e31826ddbd1. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wei H, Tang K, et al. Mutation analysis of isocitrate dehydrogenase in acute lymphoblastic leukemia. Genet Test Mol Biomarkers. 2012;16(8):991–5. doi: 10.1089/gtmb.2011.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghiam AF, Cairns RA, Thoms J, et al. IDH mutation status in prostate cancer. Oncogene. 2012;31(33):3826. doi: 10.1038/onc.2011.546. [DOI] [PubMed] [Google Scholar]
- 23.Tefferi A, Jimma T, Sulai NH, et al. IDH mutations in primary myelofibrosis predict leukemic transformation and shortened survival: clinical evidence for leukemogenic collaboration with JAK2V617F. Leukemia. 2011 doi: 10.1038/leu.2011.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloosterhof NK, Bralten LB, Dubbink HJ, French PJ, van den Bent MJ. Isocitrate dehydrogenase-1 mutations: a fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011;12(1):83–91. doi: 10.1016/S1470-2045(10)70053-X. [DOI] [PubMed] [Google Scholar]
- 25.Pollard PJ, Ratcliffe PJ. Cancer. Puzzling patterns of predisposition. Science. 2009;324(5924):192–4. doi: 10.1126/science.1173362. [DOI] [PubMed] [Google Scholar]
- 26.Lee SM, Koh HJ, Park DC, et al. Cytosolic NADP(+)-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med. 2002;32(11):1185–96. doi: 10.1016/s0891-5849(02)00815-8. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe T, Vital A, Nobusawa S, Kleihues P, Ohgaki H. Selective acquisition of IDH1 R132C mutations in astrocytomas associated with Li-Fraumeni syndrome. Acta Neuropathol. 2009;117(6):653–6. doi: 10.1007/s00401-009-0528-x. [DOI] [PubMed] [Google Scholar]
- 28.Xu X, Zhao J, Xu Z, et al. Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem. 2004;279(32):33946–57. doi: 10.1074/jbc.M404298200. [DOI] [PubMed] [Google Scholar]
- 29.Bayley JP, Devilee P. Warburg tumours and the mechanisms of mitochondrial tumour suppressor genes. Barking up the right tree? Curr Opin Genet Dev. 2010;20(3):324–9. doi: 10.1016/j.gde.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Yan H, Bigner DD, Velculescu V, Parsons DW. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res. 2009;69(24):9157–9. doi: 10.1158/0008-5472.CAN-09-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pansuriya TC, van Eijk R, d’Adamo P, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011;43(12):1256–61. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranger A, Szymczak A. Do intracranial neoplasms differ in Ollier disease and maffucci syndrome? An in-depth analysis of the literature. Neurosurgery. 2009;65(6):1106–13. doi: 10.1227/01.NEU.0000356984.92242.D5. discussion 13-5. [DOI] [PubMed] [Google Scholar]
- •33.Koivunen P, Lee S, Duncan CG, et al. Transformation by the (R)-enantiomer of 2- hydroxyglutarate linked to EGLN activation. Nature. 2012;483(7390):484–8. doi: 10.1038/nature10898. This paper demonstrated that 2-HG is an oncometabolite that can transform normal astrocytes into a malignant phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu C, Ward PS, Kapoor GS, et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012 doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Figueroa ME, Abdel-Wahab O, Lu C, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang B, Zhong C, Peng Y, Lai Z, Ding J. Molecular mechanisms of “off-on switch” of activities of human IDH1 by tumor-associated mutation R132H. Cell Res. 2010;20(11):1188–200. doi: 10.1038/cr.2010.145. [DOI] [PubMed] [Google Scholar]
- 37.Zhao S, Guan KL. IDH1 mutant structures reveal a mechanism of dominant inhibition. Cell Res. 2010;20(12):1279–81. doi: 10.1038/cr.2010.160. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S, Lin Y, Xu W, et al. Glioma-derived mutations in IDH1 dominantly inhibit IDH1 catalytic activity and induce HIF-1alpha. Science. 2009;324(5924):261–5. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••39.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–44. doi: 10.1038/nature08617. This paper changed the paradigm for understanding IDH mutations by demonstrating that in addition to losing the normal funciton of IDH, the mutants gained the ability to convert alphaketoglutarate to 2-HG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin G, Reitman ZJ, Spasojevic I, et al. 2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations. PLoS One. 2011;6(2):e16812. doi: 10.1371/journal.pone.0016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garber K. Oncometabolite? IDH1 discoveries raise possibility of new metabolism targets in brain cancers and leukemia. J Natl Cancer Inst. 2010;102(13):926–8. doi: 10.1093/jnci/djq262. [DOI] [PubMed] [Google Scholar]
- 42.Kranendijk M, Struys EA, Salomons GS, Van der Knaap MS, Jakobs C. Progress in understanding 2-hydroxyglutaric acidurias. J Inherit Metab Dis. 2012;35(4):571–87. doi: 10.1007/s10545-012-9462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patay Z, Mills JC, Lobel U, et al. Cerebral neoplasms in L-2 hydroxyglutaric aciduria: 3 new cases and meta-analysis of literature data. AJNR Am J Neuroradiol. 2012;33(5):940–3. doi: 10.3174/ajnr.A2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pietrak B, Zhao H, Qi H, et al. A tale of two subunits: how the neomorphic R132H IDH1 mutation enhances production of alphaHG. Biochemistry. 2011;50(21):4804–12. doi: 10.1021/bi200499m. [DOI] [PubMed] [Google Scholar]
- 45.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19(1):17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki M, Knobbe CB, Munger JC, et al. IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature. 2012 doi: 10.1038/nature11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christensen BC, Smith AA, Zheng S, et al. DNA methylation, isocitrate dehydrogenase mutation, and survival in glioma. J Natl Cancer Inst. 2011;103(2):143–53. doi: 10.1093/jnci/djq497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laffaire J, Everhard S, Idbaih A, et al. Methylation profiling identifies 2 groups of gliomas according to their tumorigenesis. Neuro Oncol. 2011;13(1):84–98. doi: 10.1093/neuonc/noq110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••50.Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012 doi: 10.1038/nature10866. This paper both linked the hypermethylated phenotype in gliomas to IDH mutation but showed that IDH mutations are necessary and sufficient for establishing the methylator phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu Y, Zheng S, Zheng Y, et al. Glioma derived isocitrate dehydrogenase-2 mutations induced up-regulation of HIF-1alpha and beta-catenin signaling: Possible impact on glioma cell metastasis and chemo-resistance. Int J Biochem Cell Biol. 2012;44(5):770–5. doi: 10.1016/j.biocel.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Labussiere M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010;74(23):1886–90. doi: 10.1212/WNL.0b013e3181e1cf3a. This paper showed that 1p/19q deletion, the hallmark of oligodendrogliomas, never occurs without first having IDH mutation. [DOI] [PubMed] [Google Scholar]
- 53.Srivastava S, Zou ZQ, Pirollo K, Blattner W, Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990;348(6303):747–9. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- 54.Paugh BS, Qu C, Jones C, et al. Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. J Clin Oncol. 2010;28(18):3061–8. doi: 10.1200/JCO.2009.26.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pollack IF, Hamilton RL, Sobol RW, et al. IDH1 mutations are common in malignant gliomas arising in adolescents: a report from the Children’s Oncology Group. Childs Nerv Syst. 2011;27(1):87–94. doi: 10.1007/s00381-010-1264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jenkins RB, Xiao Y, Sicotte H, et al. A low-frequency variant at 8q24.21 is strongly associated with risk of oligodendroglial tumors and astrocytomas with IDH1 or IDH2 mutation. Nat Genet. 2012;44(10):1122–5. doi: 10.1038/ng.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Combs SE, Rieken S, Wick W, et al. Prognostic significance of IDH-1 and MGMT in patients with glioblastoma: one step forward, and one step back? Radiat Oncol. 2011;6:115. doi: 10.1186/1748-717X-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27(34):5743–50. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Chou AP, Chen W, et al. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol. 2012 doi: 10.1093/neuonc/nos261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Juratli TA, Kirsch M, Robel K, et al. IDH mutations as an early and consistent marker in lowgrade astrocytomas WHO grade II and their consecutive secondary high-grade gliomas. J Neurooncol. 2012 doi: 10.1007/s11060-012-0844-1. [DOI] [PubMed] [Google Scholar]
- 61.SongTao Q, Lei Y, Si G, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci. 2012;103(2):269–73. doi: 10.1111/j.1349-7006.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 62.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75(17):1560–6. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 63.Hartmann C, Hentschel B, Tatagiba M, et al. Molecular markers in low-grade gliomas: predictive or prognostic? Clin Cancer Res. 2011;17(13):4588–99. doi: 10.1158/1078-0432.CCR-10-3194. [DOI] [PubMed] [Google Scholar]
- 64.Taal W, Dubbink HJ, Zonnenberg CB, et al. First-line temozolomide chemotherapy in progressive low-grade astrocytomas after radiotherapy: molecular characteristics in relation to response. Neuro Oncol. 2011;13(2):235–41. doi: 10.1093/neuonc/noq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant Procarbazine, Lomustine, and Vincristine Chemotherapy in Newly Diagnosed Anaplastic Oligodendroglioma: Long-Term Follow-Up of EORTC Brain Tumor Group Study 26951. J Clin Oncol. 2012 doi: 10.1200/JCO.2012.43.2229. [DOI] [PubMed] [Google Scholar]
- 66.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–80. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- •67.Capper D, Zentgraf H, Balss J, Hartmann C, von Deimling A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009;118(5):599–601. doi: 10.1007/s00401-009-0595-z. The description of this monoclonal antibody allowed for the detection of the most common IDH mutation using immunohistochemistry, allowing for easy detection for both research and clinical purposes. [DOI] [PubMed] [Google Scholar]
- ••68.Choi C, Ganji SK, Deberardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012 doi: 10.1038/nm.2682. This description of MRS detection of 2-HG allows for the first pathognomonic sign of gliomas that can be detected by imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Piaskowski S, Bienkowski M, Stoczynska-Fidelus E, et al. Glioma cells showing IDH1 mutation cannot be propagated in standard cell culture conditions. Br J Cancer. 2011;104(6):968–70. doi: 10.1038/bjc.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bralten LB, Kloosterhof NK, Balvers R, et al. IDH1 R132H decreases proliferation of glioma cell lines in vitro and in vivo. Ann Neurol. 2011;69(3):455–63. doi: 10.1002/ana.22390. [DOI] [PubMed] [Google Scholar]
- 71.Luchman HA, Stechishin OD, Dang NH, et al. An in vivo patient-derived model of endogenous IDH1-mutant glioma. Neuro Oncol. 2012;14(2):184–91. doi: 10.1093/neuonc/nor207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin G, Pirozzi CJ, Chen LH, et al. Mutant IDH1 is required for IDH1 mutated tumor cell growth. Oncotarget. 2012;3(8):774–82. doi: 10.18632/oncotarget.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kranendijk M, Salomons GS, Gibson KM, et al. A lymphoblast model for IDH2 gain-offunction activity in d-2-hydroxyglutaric aciduria type II: novel avenues for biochemical and therapeutic studies. Biochim Biophys Acta. 2011;1812(11):1380–4. doi: 10.1016/j.bbadis.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 74.Gerardo Valadez J, Grover VK, Carter MD, et al. Identification of Hedgehog pathway responsive glioblastomas by isocitrate dehydrogenase mutation. Cancer Lett. 2013;328(2):297–306. doi: 10.1016/j.canlet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fathi AT, Abdel-Wahab O. Mutations in epigenetic modifiers in myeloid malignancies and the prospect of novel epigenetic-targeted therapy. Adv Hematol. 2012;2012:469592. doi: 10.1155/2012/469592. [DOI] [PMC free article] [PubMed] [Google Scholar]