Abstract
The incidence of invasive fungal infections is increasing in recent years. The present study mainly investigated glabridin (Gla) alone and especially in combination with fluconazole (FLC) against Cryptococcus neoformans and Candida species (Candida albicans, Candida tropicalis, Candida krusei, Candida parapsilosis and Candida Glabratas) by different methods. The minimal inhibitory concentration (MIC) and the minimal fungicidal concentration (MFC) indicated that Gla possessed a broad-spectrum antifungal activity at relatively high concentrations. After combining with FLC, Gla exerted a potent synergistic effect against drug-resistant C. albicans and C. tropicalis at lower concentrations when interpreted by fractional inhibitory concentration index (FICI). Disk diffusion test and time-killing test confirming the synergistic fungicidal effect. Cell growth tests suggested that the synergistic effect of the two drugs depended more on the concentration of Gla. The cell envelop damage including a significant decrease of cell size and membrane permeability increasing were found after Gla treatment. Together, our results suggested that Gla possessed a synergistic effect with FLC and the cell envelope damage maybe contributed to the synergistic effect, which providing new information for developing novel antifungal agents.
Introduction
Despite recent progress in the clinical management, invasive fungal infections are still a tricky problem and have a high mortality. Candida species are the fourth most important cause of hospital-acquired bloodstream infections. Besides, in developing countries systemic cryptoccocosis remains large and increasing [1]. The most common isolated Candida specie in clinical fungal invasive infection is Candida albicans, followed by Candida tropicalis, Candida parapsilosis and Candida glabrata [2]–[5]. Cryptococcus neoformans is the first or second most common cause of culture-proven meningitis [1]. One most common agent used in clinic is fluconazole (FLC). However, during long-time or repeated treatment, FLC resistance strains are easily developed [6]. The combination of two or more antifungal agents maybe a feasible policy to solve the problem.
Currently, researches on natural products which have potent synergisms with antifungal drugs have been raised. For example, retigeric acid B, a pentacyclic triterpenoid isolated from a lichen called Lobaria kurokawae Yoshim, can increase the susceptibilities of azole-resistant C. albicans strains in combination with azoles [7], [8]. Plagiochin E, a macrocyclic bis (bibenzyl) isolated from the liverwort Marchantia polymorpha, has antifungal activity and resistance reversal effects for C. albicans [9]. Besides, berberine chloride, baicalein, allicin, pure polyphenol curcumin I, pseudolaric acid B, eugenol and methyleugenol were also reported to have synergistic antifungal properties in combination with known antifungals [10]–[15].
Glabridin (Gla) [4-(8,8-dimethyl-2,3,4,8-tetrahydropyrano[2,3-f]chromen-3-yl)-benzene-1,3-diol] is a major active isoflavan isolated from Glycyrrhiza glabra. It has been reported that Gla had numerous beneficial properties, including antioxidant, anticancer, neuroprotective, anti-inflammatory activities, inhibiting fatigue or reversing learning and memory deficits in diabetic rats [16]–[23]. It possessed weak activity against C. albicans, C. krusei, C. neoformans and other filamentous fungi [24], [25]. However, to our knowledge, no study to date has focused on its interaction with FLC.
In this study, synergistic antifungal effect of Gla and FLC against FLC-resistant clinical isolates of C. albicans and other yeast fungi (i.e. C. neoformans, C. tropicalis, C. parapsilosis, C. krusei and C. glabratas) and the possible mechanisms were investigated.
Materials and Methods
Strains and chemicals
25 clinical isolates of FLC-resistant C. albicans, and one C. neoformans 32609, C. tropicalis 2718 and C. parapsilosis ATCC 22019 were kindly provided by the Changhai Hospital, Shanghai, China. C. krusei ATCC2340 and C. glabrata ATCC1182 were kindly provided by professor Changzhong Wang (School of integrated traditional and western medicine, Anhui university of traditional chinese medicine, Hefei, China). The susceptibilities of these strains to FLC were measured by broth microdilution method at advance. Frozen stocks of isolates were stored at −80°C in culture medium supplemented with 40% (vol/vol) glycerol and were subcultured twice at 35°C before each experiment. FLC (sigma Aldrich, St. Louis, MO) was obtained commercially. Gla (purity >98%) was obtained from Yuan Cheng Pharmaceutical Co. Ltd, China, and its initial stored concentration was 6.4 mg/ml in dimethyl sulfoxide (DMSO).
Antifungal susceptibility testing
The minimal inhibitory concentrations (MIC) of Gla and FLC against the yeast strains were determined by broth microdilution method as described previously [10]. The yeast at final concentration of 103 cells/ml in the RPMI 1640 liquid medium with serial (2×) dilutions of each drug were inoculated in 96-well flat-bottomed microtitration plates. After incubation at 35°C for 24 h or 72 h. Optical densities (OD) were measured at 630 nm with a microtitre plate reader (Thermolabsystems Multiskan MK3), and background optical densities were subtracted from that of each well. MIC80 was determined as the lowest concentration of the drugs that inhibited growth by 80% compared with that of drug-free wells. DMSO comprised <1% of the total test volume. The quality control strain, C. parapsilosis ATCC 22019 was included in each susceptibility test to ensure quality control. The MIC range of FLC to C. parapsilosis ATCC 22019 was from 0.5 µg/ml to 4 µg/ml, which meant this test was acceptable.
Checkerboard microdilution assay
Assays were performed on all isolates according to broth microdilution checkerboard method [10]. The initial concentration of fungal suspension in RPMI 1640 medium was 103 cells/ml, and the final combination concentrations ranged from 0.125 to 64 µg/ml for FLC and 1 to 16 µg/ml for Gla. The final concentration for FLC or Gla alone ranged from 0.125 to 64 µg/ml. 96-well flat-bottomed microtitration plates were incubated at 35°C for 24 h or 72 h. OD was measured at 630 nm, MIC was determined as the above.
The data obtained by the checkerboard microdilution assays were analyzed using the model-fractional inhibitory concentration index (FICI) method based on the Loewe additivity theory. FICI was calculated by the following equation: FICI = FIC A+FIC B, where FIC A is the MIC of the combination/the MIC of drug A alone, and FIC B is the MIC of the combination/the MIC of drug B alone. Among all of the FICIs calculated for each data set, the FICImin was reported as the FICI in all cases unless the FICImax was greater than four, in which case the FICImax was reported as the FICI. Synergy was defined as an FICI value of ≤0.5, while antagonism was defined as an FICI value of >4, addition was defined as an FICI value of 0.5< FICI≤1. An FICI result between 1 and 4 (1< FICI≤4) was considered indifferent [7]. The fractional fungicidal concentration index (FFCI) was calculated the same.
Agar disk diffusion test
C. albicans 103 (one FLC-resistant isolate with a MIC of 32 µg/ml for Gla) and other yeast strains were tested by agar diffusion test [10]. 3 ml of aliquot of 106 cells/ml suspension was spread uniformly onto the yeast peptone dextrose (YPD) agar plate with or without 64 µg/ml FLC. Then, 6 mm paper disks impregnated with Gla alone or in combination with FLC were placed onto the agar surface. There was 5 µl DMSO in control disks. Photos were taken after incubation at 35°C for 48 h.
Time-killing test
C. albicans 103 and other yeast strains were prepared at the starting inoculum of 105 cells/ml. The concentrations were 4, 8, 16 µg/ml for Gla and 8 µg/ml for FLC, DMSO comprised <1% of the total test volume. At predetermined time points (0, 4, 8, 12, 16 and 24 h) after incubation with agitation at 35°C, a 100 µl aliquot was removed from every solution and serially diluted 10-fold in sterile water. A 100 µl aliquot from each dilution was spread on the sabouraud dextrose agar plate. Colony counts were determined after incubation at 35°C for 48 h. Fungicidal activity was defined as a ≥3 log10 reduction from the starting inoculum. Synergism and antagonism were defined as a respective decrease or increase of ≥2 log10 CFU/ml in antifungal activity produced by the combination compared with that by the more active agent alone [26].
Cell growth test
C. albicans 103 was prepared at the starting inoculum of 106 cells/ml in glass tubes. Different concentrations of Gla (2, 4, 8, 16 µg/ml) and FLC (2, 4, 8, 16, 32, 64 µg/ml) alone or the combinations of Gla (2, 4, 8, 16 µg/ml) and FLC (2, 4, 8, 16, 32, 64 µg/ml) were added into tubes. After incubation with agitation at 35°C for 24 h, pictures were taken. Aliquot was removed from each tube and serially diluted 10-fold in sterile water. A 100 µl aliquot from each dilution was spread on the sabouraud dextrose agar plate. Colony counts were determined after incubation at 35°C for 48 h.
Cell membrane permeability
Membrane permeabilization of C. albicans was detected according to a previous study [27]. Briefly, C. albicans 103 (1×107 cells/ml) were incubated with 10 µM calcein acetoxymethyl ester (Fanbo biochemicals, China) for 2 h, The cells were then washed three times and C. albicans (1×107 cells/ml) was transferred to tubes. After treatment with or without FLC (64 µg/ml), MCZ (64 µg/ml) and Gla (16 µg/ml, 32 µg/ml, 64 µg/ml) for 3 h. The cells were washed three times and about 10,000 cells were acquired for flow cytometry analysis (Maflo Astrios flow cytometer). Experiments were repeated at least two times independently on separate days.
Cell wall inhibitors sensitivity test
Congored and calcofluorwhite (CFW) were incorporated into YPD agar plates at 100 µg/ml and 15 µg/ml, respectively. Yeast cells were grown in YPD medium with or without Gla (8, 16 µg/ml) for 12 h and 3 µl drops of serially diluted suspensions were inoculated into plates. After incubation for 48 h at 30°C, pictures were taken.
Results
The combination of Gla and FLC against clinical FLC-resistant C. albicans
The MIC values of Gla tested alone or in combination with FLC in FLC-resistant C. albicans were shown in Table 1. According to the interpretive breakpoints for FLC (<8 µg/ml and ≥64 µg/ml, respectively), 25 clinical FLC-resistant C. albicans were selected. The MICs of Gla against all tested strains ranged from 32 µg/ml to 64 µg/ml. When MIC-like assays were performed for FLC in the presence of fixed subinhibitory concentrations of Gla (4 µg/ml), the median MICs of FLC decreased from 128 µg/ml to 1 µg/ml in resistant strains (32-fold to 512-fold reductions). According to the analysis of FICI method, synergism was observed in all 25 tested strains (FICIs<0.2).
Table 1. MICs and MFCs of Gla alone and in combination with FLC against 25 clinical FLC-resistant C. albicans.
| MIC (μg/ml) | MFC (μg/ml) | |||
| median | range | median | range | |
| FLC | 128 | 64–>256 | >256 | >256 |
| Gla | 32 | 32–64 | 64 | 32–64 |
| FLC/Glaa | 1/4 | 1–1/4–4 | 8/16 | 4–16/8–16 |
| FIC index | 0.13 | 0.04–0.14 | 0.27 | 0.26–0.31 |
| Interaction effect (n/%)b | Syn (25/100) | Syn (25/100) | ||
MIC and MFC in combination expressed as [FLC]/[Gla].
Syn, synergism. The number of strains and percentage for the interaction effect were shown.
The combination of Gla and FLC against other varied FLC-susceptibility strains
We also tested antifungal effects of Gla alone or in combination with FLC in FLC-sensitive C. albicans and the other yeast strains (C. neoformans, C. tropicalis, C. krusei, C. parapsilosis and C. glabratas) (Table 2). In these strains, the range of MICs of Gla tested alone was from 16 µg/ml to 64 µg/ml, when in combination with FLC the MICs of Gla ranged from 1 µg/ml to16 µg/ml. In C. tropicalis and C. krusei, the MIC of FLC were reduced from >64 µg/ml to 4 µg/ml or 8 µg/ml respectively after in combination with Gla. In C. glabratas, no synergistic effect was observed.
Table 2. MICs and MFCs of Gla alone and in combination with FLC against varied yeast strains.
| Yeast strains | MIC (µg/m | FICI | MFC (µg/m | FFCI | ||||
| FLC | Gla | FLC/Gla | FLC | Gla | FLC/Gla | |||
| C. albicans SC5314 | 1 | 32 | 1/1 | 1.03 | >64 | 32 | 16/16 | 0.63 |
| C. tropicalis 2718 | >64 | 64 | 4/8 | 0.16 | >64 | 64 | 16/16 | 0.38 |
| C. neoformans 32609 | 2 | 16 | ≤0.125/8 | 0.56 | >64 | 32 | 8/16 | 0.56 |
| C. parapsilosis ATCC22019 | 2 | 64 | 2/1 | 1.02 | >64 | 64 | 32/16 | 0.50 |
| C. krusei ATCC2340 | >64 | 64 | 8/16 | 0.31 | >64 | 64 | >64/16 | 1.25 |
| C. glabrata ATCC1182 | >64 | 64 | 64/16 | 0.75 | >64 | 64 | >64/16 | 1.25 |
In combination with FLC, Gla at lower concentrations exhibits fungicidal effect for FLC-resistant C. albicans by different methods
Cells from the microdilution assays after incubation with Gla, FLC or the combination of Gla and FLC at various concentrations were plated on the sabouraud dextrose agar plate to count the colony forming unit (CFU) for determination of the MFC100 (the minimal concentration with complete cell killing, i.e. no CFU counted). As shown in Table 1, the MFC of FLC can be much higher than the MIC and complete cell killing was not achievable. The range of the MFC of Gla was from 32 µg/ml to 64 µg/ml. When in combination with FLC (4 µg/ml or 8 µg/ml), Gla at 16 µg/ml showed fungicidal effect against all strains tested.
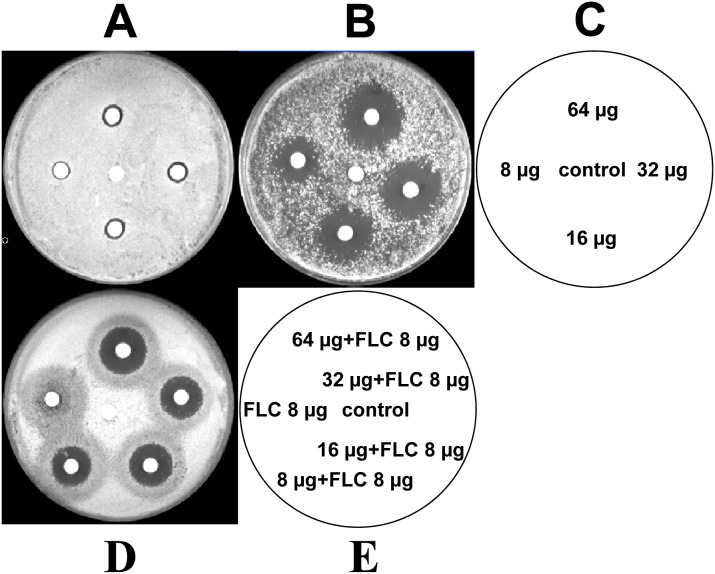
Further to visualize their synergistic fungicidal effect, different concentrations of Gla and the combination with FLC (8 µg/ml) were analyzed by agar disk diffusion assay. Gla alone at 64, 32, 16, 8 µg per disc had minimal fungicidal activity against the FLC-resistant C. albicans 103. While FLC at 8 µg per disc showed weak inhibition effect against C. albicans, the halo surrounding the disc was cloudy with colony (Fig. 1A). Interestingly, when FLC was combined with Gla, the halo surrounding the disc was significantly clearer. The diameters of the zones were clearer and larger than those of either drug alone on the plain agar plate, which was an indication of potent synergistic fungicidal activity (Fig. 1D). Similarly, on the agar plate (containing 64 µg/ml FLC), Gla also yielded significantly clearer and larger zones at 64, 32, 16, 8 µg per disc (Fig. 1B).
Figure 1. Agar disk diffusion assay of different concentrations of Gla combined with FLC against C. albicans 103.
Panels A and D show agar plates, and panel B shows an agar plate containing 64 µg/ml FLC. Panel C describes the images for panels A and B, which containing 64, 32, 16, 8 µg of Gla or DMSO as control per disc. Panel E describes the image for panel D, the combination of Gla (64, 32, 16, 8 µg) with FLC (8 µg) or FLC (8 µg) alone or just DMSO as control were contained in each disc.
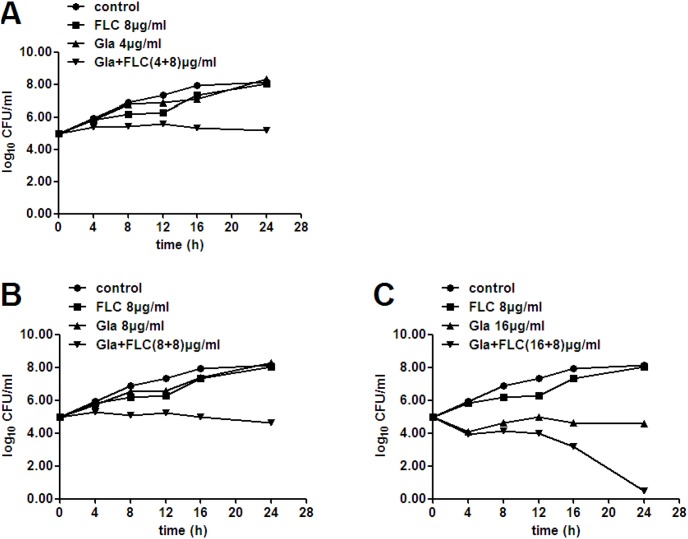
In addition, their synergistic fungicidal effect was confirmed by time-killing test (Fig. 2). Gla alone at 16 µg/ml showed fungicidal effect and led to a decrease of 3.57–log10 CFU/ml at 24 h. No appreciable antifungal activity of FLC alone at 8 µg/ml was observed, but the combination of FLC (8 µg/ml) and Gla (4, 8 or 16 µg/ml) yielded 3.14, 3.62 or 4.10–log10 CFU/ml reductions compared with Gla alone at 24 h (Table 3). Besides, the combination of Gla at 16 µg/ml and FLC at 8 µg/ml almost resulted in a complete cell-killing at 24 h (Fig. 2C).
Figure 2. Time killing curves of C. albicans 103 treated with different concentrations of Gla and FLC.
FLC-resistant C. albicans 103 were treated with FLC (8 µg/ml), Gla (4 µg/ml) and FLC+Gla (4+8) µg/ml (A), Gla (8 µg/ml) and FLC+Gla (8+8) µg/ml (B) or Gla (16 µg/ml) and FLC+Gla (8+16) µg/ml (C) for 24 h. Aliquots were obtained at the indicated time points and serially dilutions were spreaded on agar plates. Colony counts were determined after 48 h incubation.
Table 3. Decrease in log10 CFU/ml of yeast strains using different concentrations of Gla combining with FLC at 24 h.
| FLC+Gla (µg/ml) | Mean (±SD) decrease in log10 CFU/ml compared with Gla alone | |||
| C. albicans 103 | C. albicans SC5314 | C. tropicalis 2718 | C. neoformans 32609 | |
| 8+4 | 3.14 (0.08) | |||
| 8+8 | 3.62 (0.11) | 1.51 (0.08) | 1.64 (0.09) | 4.42 (0.12) |
| 8+16 | 4.10 (0.30) | 1.82 (0.09) | 3.16 (0.11) | 4.50 (0.11) |
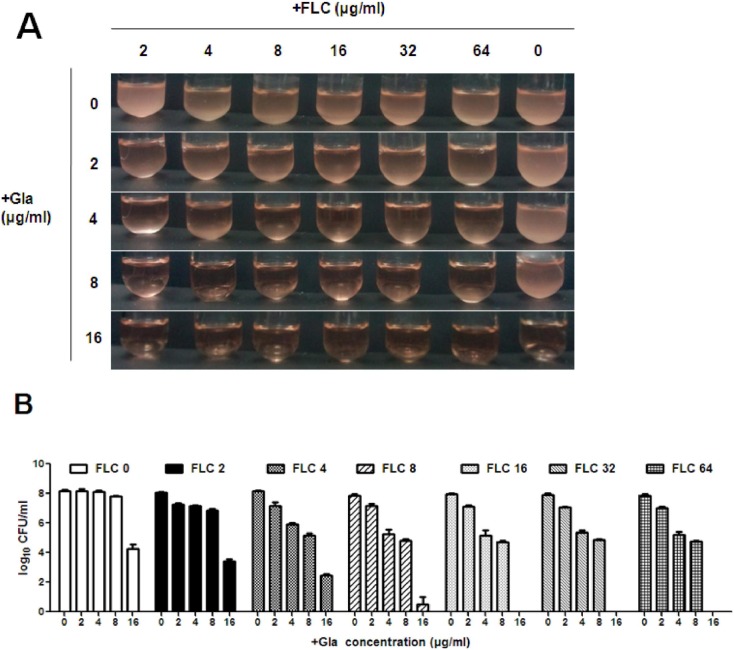
In order to determine the relationship between the synergistic effect and the dosage of Gla and FLC, different concentrations of Gla (2 µg/ml–16 µg/ml) and FLC (2 µg/ml–64 µg/ml) were used in the cell growth test. Our results indicated that the synergism of the two drugs depended more on the concentration of Gla than FLC (Fig. 3). 4 µg/ml and 8 µg/ml Gla alone had no antifungal effect, while 64 µg/ml FLC had a weak antifungal activity. The antifungal effect was improved significantly after the two drugs used together at different concentrations except when FLC used at the concentration of 2 µg/ml. More specifically, 16 µg/ml Gla alone had an antifungal effect, while after combining with FLC (4 µg/ml–64 µg/ml), significantly synergistic effects were observed, and even complete cell killing activities were found when the concentration of FLC were above 16 µg/ml. Interestingly, the synergistic effects of the two drugs were unchanged when the dose of FLC declined from 64 µg/ml to 16 µg/ml, but when the combination concentration of FLC was below 16 µg/ml, the synergistic effect was lessened with the doseage of FLC decreasing.
Figure 3. Growth condition of C. albicans 103 treated with different concentrations of Gla and FLC.
Exponetially growing FLC-resistant C. albicans 103 were treated with or without different concentrations of Gla (2, 4, 8, 16 µg/ml) and FLC (2, 4, 8, 16, 32, 64 µg/ml) alone or the combinations of Gla and FLC in a shaking incubator. (A) Pictures of the growth condition of C. albicans were taken after 24 h incubation. (B) Aliquots from each tube were obtained at 24 h and serially dilutions were spread on agar plates. The number of C. albicans in each tube was determined by counting colonies after 48 h incubation.
Synergistic effect of FLC and Gla against other yeast strains
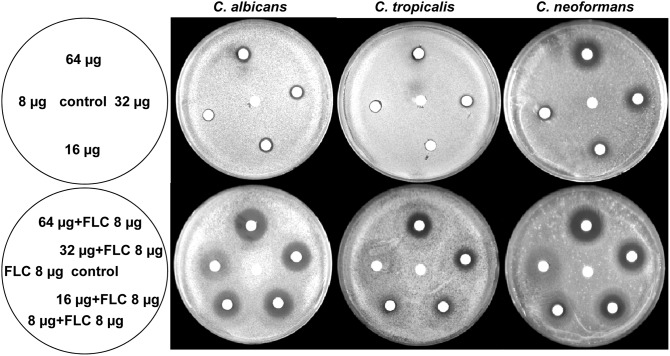
The interactions of FLC and Gla against the other yeast strains (i.e. FLC-senstive C. albicans, C. tropicalis, C. neoformans, C. parapsilosis, C. krusei and C. glabrata) were investigated by MFCs, agar disk diffusion assay and time-killing test. As shown in Table 2, the strains showed varied susceptibility to Gla and FLC, and there was no apparent correlation between the susceptibility towards Gla and the susceptibility towards FLC. Consistent with results from the disc diffusion assays, the C. krusei and C. glabrata were highly resistant to FLC. The range of MFC of Gla for each strain tested was from 32 µg/ml to 64 µg/ml, supporting its fungicidal property. Synergistic fungicidal interactions between Gla and FLC were also observed in C. tropicalis by counting cells from the microdilution assay. The halo surrounding the discs with FLC and Gla was significantly clearer (Fig. 4) and the diameters of the zones were larger than those of either drug alone on the plain agar plate for FLC-senstive C. albicans, C. tropicalis and C. neoformans. Besides, the FLC+Gla combination yielded a decreased CFU compared with Gla alone in FLC-senstive C. albicans, C. tropicalis and C. neoformans, and even greater reductions were observed in C. neoformans and C. tropicalis (>3 Log10 CFU/ml decrease, fungicidal effect can be defined) (Table 3). However, the synergistic fungicidal effect of FLC and Gla was not observed in C. krusei and C. glabrata by agar disk diffusion assay and time-killing test (results not shown), which was consistent with FFCI values of the previous tests.
Figure 4. Agar disk diffusion assay of Gla alone or in combination with FLC against C. albicans SC5314, C. tropicalis and C. neoformans.
Upper agar plates of disks were impregnated with 64, 32, 16 and 8 µg of Gla or 5 µl of DMSO as control disk. In lower agar plates, disks were impregnated with FLC+Gla (64+8) µg, FLC+Gla (32+8) µg, FLC+Gla (16+8) µg, FLC+Gla (8+8) µg, FLC (8 µg) or 5 µl of DMSO as control disk. Left sketch panels describe the images for the right agar plates.
Effect of Gla on the cell envelope
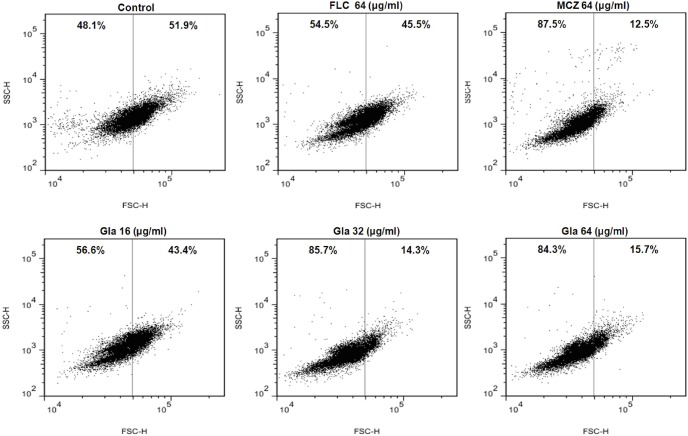
Flow cytometry analysis (side scatter [SSC]-forward light scatter [FSC]) showed that C. albicans 103 treated with FLC underwent weak cell shrinkage, while the cells treated with MCZ exhibited a significant decrease in cell size, as evidenced by the decrease in forward light scattering. Interestingly, changes in cell size were also observed after exposure to Gla, especially to Gla at the concentration of 32 µg/ml and 64 µg/ml (Fig. 5).
Figure 5. Changes in cell size (forward scatter-side scatter) in the presence of Gla.
FLC-resistant C. albicans 103 were treated with or without Gla (16, 32, 64 µg/ml), FLC (64 µg/ml), MCZ (64 µg/ml) for 3 h. Then the cells were analyzed by flow cytometry.
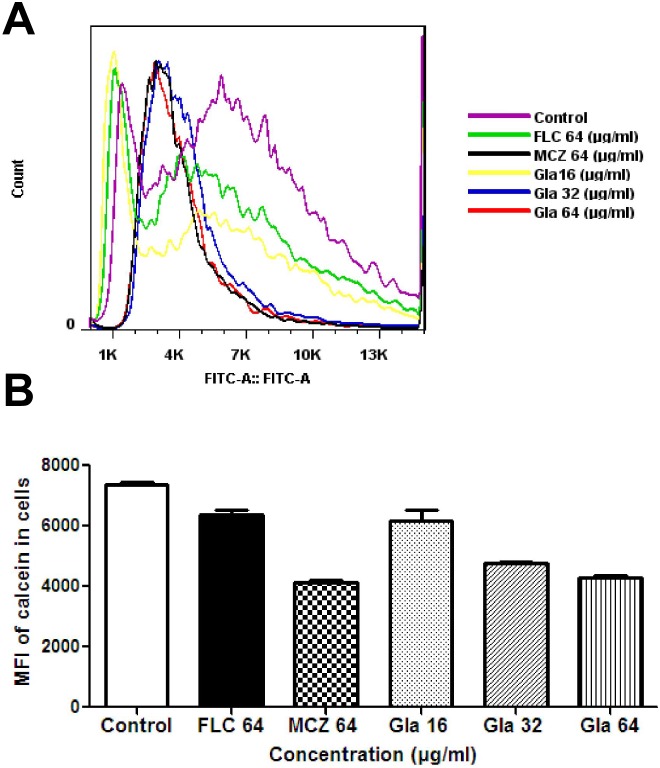
Calcein AM is a non-fluorescent derivative of calcein that can readily diffuse across membranes. Once within the cytoplasms of target cells, calcein AM is hydrolyzed by cytoplasmic esterases, yielding membrane-impermeable calcein which could be loaded into intact cells. After incubation with calcein AM, the cellular fluorescence of calcein was detected and quantified by flow cytometry to evaluate the effect of Gla on the cell membrane permeabilization. Results showed that cellular calcein was markedly decreased by treatment of Candida cells with different concentrations of Gla (16 µg/ml, 32 µg/ml, 64 µg/ml), while by FLC a slight reduction of cellular calcein was observed (Fig. 6).
Figure 6. The influence of Gla on cell membrane permeability.
Candida cells (1×107 cells/mL) were cultured in the presence of 10 µM calcein acetoxymethylester for 2 h. After washing four times, the cells were cultured in the presence or absence of Gla (16, 32, 64 µg/ml), FLC (64 µg/ml), MCZ (64 µg/ml) for 3 h. Cellular fluorescence intensities of calcein in the cells were analyzed by flow Cytometry.
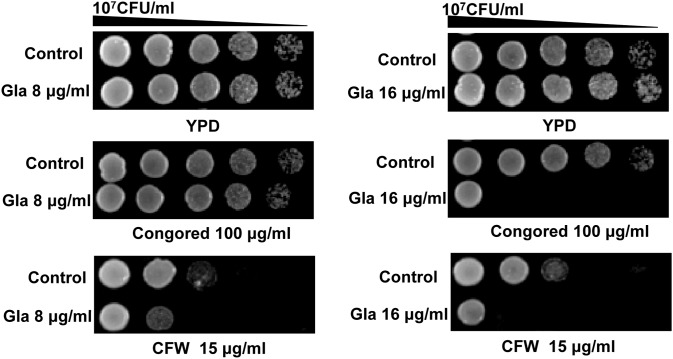
We also investigated the effect of Gla on the cell wall carbohydrates. Spot assays indicated that 16 µg/ml Gla treatment made C. albicans become more sensitive to both cell wall inhibitors (CFW and congored) compared with the control cells (Fig. 7). We used concanavalin A, calcofluorwhite (CFW) and specific anti-β-glucan primary antibody to stain the carbohydrates (mannan, chitin and glucan). However, fluorescence micrographs did not show obvious change in the three cell wall layers (Fig. S1).
Figure 7. Susceptibilities of C. albicans 103 to cell wall inhibitors after treated with or without Gla.
Exponetially growing FLC-resistant C. albicans 103 were treated with or without Gla (8, 16 µg/ml) for 12 h in YPD. Then they were diluted to 1×107 CFU/ml and 3 µl from 1∶5 serial dilutions were spotted onto YPD agar plates containing 15 µg/ml CFW or 100 µg/ml congored.
Discussions
A large number of natural products from plants are reported to possess potent antifungal properties in recent years, such as terpene derivatives, flavans, nucleosides, peptides, alkaloids, saponins and sterols [28]. Gla is an isoflavan from G. glabra root. Previous studies have been reported that the alcohol or ethanol extract of the roots of G. glabra possessed weak antifungal activity against C. albicans, Arthrinium sacchari, Chaetonmium funicola and various filamentous fungi [24], [29], [30]. Synergistic activity of Gla and nystatin against oral C. albicans has been demonstrated previously [25]. But to our knowledge, there was no investigation on the combination of Gla and FLC against fungi.
Here, we demonstrated that Gla had weak antifungal activity against different fungi such as Candida species and C. neoformans, consisting with the previous reports [24], [25],[29],[30]. When combining with FLC, Gla exerted a strong synergistic antifungal effects at lower concentrations. Different assays indicated a potent synergistic effect of Gla and FLC against FLC-resistant C. albicans, C. neoformans and C. tropicalis. The synergistic effects depended more on the concentration of Gla. According to Celine Messier’s study on the toxic effect of Gla for oral epithelial cells (67% cell viability at 10 µg/ml), the concentration of Gla required for reducing the MIC of FLC ranged from 1 µg/ml to 4 µg/ml, below the concentration which significantly reduced cell viability [25]. In order to determine whether this property of glabridin is specific to other isoflavans, we selected a second isoflavan equol and tested the interaction of equol and FLC against FLC-resistant C. albicans by checkerboard microdilution assay. The results showed a weak synergistic effect between equol and FLC. The MIC of equol alone was >1280 µg/ml, only when the concentration of equol was at 32 µg/ml a synergism was observed between equol and FLC, the concentration of FLC was reduced from >64 µg/ml to 4 µg/ml. This may suggested that there were synergistic effects between isoflavans and FLC, but the antifungal activities and synergisms of isoflavans and FLC were different for their distinct chemical structures.
The synergistic antifungal effect of Gla has not been characterized. Similar studies on the mode of synergism as follows: increasing reactive oxygen species (ROS) to accelerate apoptosis [31], inhibiting drug efflux pumps to increase intracellular drug concentration [11], [32], targeting the ergosterol biosynthesis pathway to increase the fluidity for the resulted ergosterol depletion [32]. We tested the membrane sterols of C. albicans after treated with Gla alone or in combination with FLC by GC/MS, but none obvious sterols change was observed in Gla treatment cells (data not shown). This suggested that the synergism of Gla and FLC may not be related to the inhibition of membrane sterols synthesis. Previous study have displayed that isoflavan equol was capable of changing Candida cell membrane integrity by formation of membrane lesions and cell surface abnormalities against C. albicans [33]. Besides, flavans catechin hydrate and epigallocatechin gallate were also identified to have synergistic effect with FLC against C. tropicalis, cell shrinkage and plasma membrane damage were observed in the combination [34]. In our study, similar cell envelope changes were found in the Candida cells treated with Gla. A significant decrease in cell size and an increase of cell membrane permeability were observed in C. albicans after the treatment with Gla. However, Candida cells treated with Gla became more sensitive to cell wall inhibitors. We further stained the carbohydrate of the cell wall (mannan, chitin and glucan), while no obvious change of cell wall after Gla treatment was observed (Fig. S1).
In conclusion, the present study first demonstrated that Gla could enhance the antifungal effect of FLC, especially showed strong synergistic effect against C. albicans, C. neoformans and C. tropicalis. Their synergism maybe related to the effect of Gla on the cell envelope. Gla may serve as a pro-natural product for fungal infection treatment. Further studies should be carried out to identify its relationship of activity and structures.
Supporting Information
Fluorescence micrographs of the cell wall structures of Candida albicans by the treatment of Gla. Exponentially growing cells treated with or without 32 µg/ml Gla were stained by 50 µg/ml Concanavalin A alexa fluor 488 conjugate for mannan, 30 µg/ml Calcofluorwhite for chitin, or specific anti-β-glucan primary antibody and Cy3-labeled goat-anti mouse secondary antibody for glucan. Then cells were scanned under a Leica confocal laser scanning microscope and micrographs were acquired.
(TIF)
Acknowledgments
We thank Professor Changzhong Wang (School of Integrated Traditional and Western Medicine, Anhui University of Traditional Chinese Medicine, Hefei, China) for providing C. krusei ATCC2340 and C. glabrata ATCC1182.
Funding Statement
This study was supported by National Key Basic Research Program of China (No 2013CB531602), the National Science Foundation of China (81202563, 81330083), National Science and Technology Major Project for the Creation of New Drugs (No 2012ZX09103101-003), and China posdoctoral Science foundation grant (No 2012M511141). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pukkila-Worley R, Mylonakis E (2008) Epidemiology and management of cryptococcal meningitis: developments and challenges. Expert Opin Pharmacother 9: 551–560. [DOI] [PubMed] [Google Scholar]
- 2. Warnock DW (2007) Trends in the epidemiology of invasive fungal infections. Nihon Ishinkin Gakkai Zasshi 48: 1–12. [DOI] [PubMed] [Google Scholar]
- 3. Morace G, Borghi E (2010) Fungal infections in ICU patients: epidemiology and the role of diagnostics. Minerva Anestesiol 76: 950–956. [PubMed] [Google Scholar]
- 4. Yapar N, Pullukcu H, Avkan-Oguz V, Sayin-Kutlu S, Ertugrul B, et al. (2011) Evaluation of species distribution and risk factors of candidemia: a multicenter case-control study. Med Mycol 49: 26–31. [DOI] [PubMed] [Google Scholar]
- 5. Zirkel J, Klinker H, Kuhn A, Abele-Horn M, Tappe D, et al. (2012) Epidemiology of Candida blood stream infections in patients with hematological malignancies or solid tumors. Med Mycol 50: 50–55. [DOI] [PubMed] [Google Scholar]
- 6. Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, et al. (2009) Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48: 1695–1703. [DOI] [PubMed] [Google Scholar]
- 7. Sun L, Sun S, Cheng A, Wu X, Zhang Y, et al. (2009) In vitro activities of retigeric acid B alone and in combination with azole antifungal agents against Candida albicans . Antimicrob Agents Chemother 53: 1586–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang W, Li Y, Zhang L, Cheng A, Liu Y, et al. (2012) Retigeric acid B enhances the efficacy of azoles combating the virulence and biofilm formation of Candida albicans . Biol Pharm Bull 35: 1794–1801. [DOI] [PubMed] [Google Scholar]
- 9. Guo XL, Leng P, Yang Y, Yu LG, Lou HX (2008) Plagiochin E, a botanic-derived phenolic compound, reverses fungal resistance to fluconazole relating to the efflux pump. J Appl Microbiol 104: 831–838. [DOI] [PubMed] [Google Scholar]
- 10. Quan H, Cao YY, Xu Z, Zhao JX, Gao PH, et al. (2006) Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob Agents Chemother 50: 1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang S, Cao YY, Dai BD, Sun XR, Zhu ZY, et al. (2008) In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol Pharm Bull 31: 2234–2236. [DOI] [PubMed] [Google Scholar]
- 12. An M, Shen H, Cao Y, Zhang J, Cai Y, et al. (2009) Allicin enhances the oxidative damage effect of amphotericin B against Candida albicans . Int J Antimicrob Agents 33: 258–263. [DOI] [PubMed] [Google Scholar]
- 13. Sharma M, Manoharlal R, Negi AS, Prasad R (2010) Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res 10: 570–578. [DOI] [PubMed] [Google Scholar]
- 14. Guo N, Ling G, Liang X, Jin J, Fan J, et al. (2011) In vitro synergy of pseudolaric acid B and fluconazole against clinical isolates of Candida albicans . Mycoses 54: e400–406. [DOI] [PubMed] [Google Scholar]
- 15. Ahmad A, Khan A, Khan LA, Manzoor N (2010) In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J Med Microbiol 59: 1178–1184. [DOI] [PubMed] [Google Scholar]
- 16. Belinky PA, Aviram M, Fuhrman B, Rosenblat M, Vaya J (1998) The antioxidative effects of the isoflavan glabridin on endogenous constituents of LDL during its oxidation. Atherosclerosis 137: 49–61. [DOI] [PubMed] [Google Scholar]
- 17. Carmeli E, Harpaz Y, Kogan NN, Fogelman Y (2008) The effect of an endogenous antioxidant glabridin on oxidized LDL. J Basic Clin Physiol Pharmacol 19: 49–63. [DOI] [PubMed] [Google Scholar]
- 18. Hasanein P (2011) Glabridin as a major active isoflavan from Glycyrrhiza glabra (licorice) reverses learning and memory deficits in diabetic rats. Acta Physiol Hung 98: 221–230. [DOI] [PubMed] [Google Scholar]
- 19. Shang H, Cao S, Wang J, Zheng H, Putheti R (2010) Glabridin from Chinese herb licorice inhibits fatigue in mice. Afr J Tradit Complement Altern Med 7: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsai YM, Yang CJ, Hsu YL, Wu LY, Tsai YC, et al. (2011) Glabridin inhibits migration, invasion, and angiogenesis of human non-small cell lung cancer A549 cells by inhibiting the FAK/rho signaling pathway. Integr Cancer Ther 10: 341–349. [DOI] [PubMed] [Google Scholar]
- 21. Kwon HS, Oh SM, Kim JK (2008) Glabridin, a functional compound of liquorice, attenuates colonic inflammation in mice with dextran sulphate sodium-induced colitis. Clin Exp Immunol 151: 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsu YL, Wu LY, Hou MF, Tsai EM, Lee JN, et al. (2011) Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol Nutr Food Res 55: 318–327. [DOI] [PubMed] [Google Scholar]
- 23. Yu XQ, Xue CC, Zhou ZW, Li CG, Du YM, et al. (2008) In vitro and in vivo neuroprotective effect and mechanisms of glabridin, a major active isoflavan from Glycyrrhiza glabra (licorice). Life Sci 82: 68–78. [DOI] [PubMed] [Google Scholar]
- 24. Fatima A, Gupta VK, Luqman S, Negi AS, Kumar JK, et al. (2009) Antifungal activity of Glycyrrhiza glabra extracts and its active constituent glabridin. Phytother Res 23: 1190–1193. [DOI] [PubMed] [Google Scholar]
- 25. Messier C, Grenier D (2011) Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans . Mycoses 54: e801–806. [DOI] [PubMed] [Google Scholar]
- 26. Roling EE, Klepser ME, Wasson A, Lewis RE, Ernst EJ, et al. (2002) Antifungal activities of fluconazole, caspofungin (MK0991), and anidulafungin (LY 303366) alone and in combination against Candida spp. and Crytococcus neoformans via time-kill methods. Diagn Microbiol Infect Dis 43: 13–17. [DOI] [PubMed] [Google Scholar]
- 27. Tanida T, Okamoto T, Ueta E, Yamamoto T, Osaki T (2006) Antimicrobial peptides enhance the candidacidal activity of antifungal drugs by promoting the efflux of ATP from Candida cells. Journal of Antimicrobial Chemotherapy 57: 94–103. [DOI] [PubMed] [Google Scholar]
- 28. Di Santo R (2010) Natural products as antifungal agents against clinically relevant pathogens. Nat Prod Rep 27: 1084–1098. [DOI] [PubMed] [Google Scholar]
- 29. Motsei ML, Lindsey KL, van Staden J, Jager AK (2003) Screening of traditionally used South African plants for antifungal activity against Candida albicans . J Ethnopharmacol 86: 235–241. [DOI] [PubMed] [Google Scholar]
- 30. Hojo H, Sato J (2002) Antifungal activity of licorice (Glycyrrhiza Glabra) and potential applications to production of beverages. Foods Food Ingredients J Jpn 203: 27–33. [Google Scholar]
- 31. Fu Z, Lu H, Zhu Z, Yan L, Jiang Y, et al. (2011) Combination of baicalein and Amphotericin B accelerates Candida albicans apoptosis. Biol Pharm Bull 34: 214–218. [DOI] [PubMed] [Google Scholar]
- 32. Sun LM, Cheng AX, Wu XZ, Zhang HJ, Lou HX (2010) Synergistic mechanisms of retigeric acid B and azoles against Candida albicans . J Appl Microbiol 108: 341–348. [DOI] [PubMed] [Google Scholar]
- 33. Lee JA, Chee HY (2010) In Vitro Antifungal Activity of Equol against Candida albicans . Mycobiology 38: 328–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. da Silva CR, de Andrade Neto JB, de Sousa Campos R, Figueiredo NS, Serpa Sampaio L, et al. (2014) Synergistic Effect of the Flavonoid Catechin, Quercetin, or Epigallocatechin Gallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole. Antimicrobial Agents and Chemotherapy 58: 1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence micrographs of the cell wall structures of Candida albicans by the treatment of Gla. Exponentially growing cells treated with or without 32 µg/ml Gla were stained by 50 µg/ml Concanavalin A alexa fluor 488 conjugate for mannan, 30 µg/ml Calcofluorwhite for chitin, or specific anti-β-glucan primary antibody and Cy3-labeled goat-anti mouse secondary antibody for glucan. Then cells were scanned under a Leica confocal laser scanning microscope and micrographs were acquired.
(TIF)