Abstract
Membranolytic macromolecules are promising vehicles forcytoplasmic drug delivery, but their efficiency and safety remains primary concerns. To address those concerns, membranolytic properties of various poly(β-l-malic acid) (PMLA) copolymers were extensively investigated as a function of concentration and pH. PMLA, a naturally occurring biodegradable polymer, acquires membranolytic activities after substitution of pendant carboxylates with hydrophobic amino acid derivatives. Ruled by hydrophobization and charge neutralization, membranolysis of PMLA copolymers increased as a function of polymer molecular weight and demonstrated a maximum with 50% substitution of carboxylates. Charge neutralization was achieved either conditionally by pH-dependent protonation or permanently by masking carboxylates. Membranolysis of PMLA copolymers containing tripeptide ofleucine, tryptophan and phenylalanine were pH-dependent in contrast to pH-independent copolymers of Leucineethylester and Leu-Leu-Leu-NH2 with permanent charge neutralization. PMLA and tripeptides seemed a unique combination for pH-dependent membranolysis. In contrast to nontoxic pH-dependent PMLA copolymers, pH-independent copolymers were found toxic at high concentration, which is ascribed to their nonspecific disruption of plasma membrane at physiological pH.pH-dependent copolymers were membranolytically active only at acidic pH typical of maturating endosomes, and are thus devoid of cytotoxicity. The PMLA tripeptide copolymers are useful for safe and efficient cytoplasmic delivery routed through endosome.
1. INTRODUCTION
As macromolecules become widely used as drug delivery systems, polymer-membrane interactions have received more and more attention. Polyanions such as poly(malic acid), poly(aspartic acid) or poly(glutamic acid) are attractive platforms for nanoconjugate drug delivery [1–3], because of their favorable properties: a high number of chemically accessible carboxyl groups, a high capacity for payloads of various drugs and biologically functional groups, excellent water solubility and biodegradability. Those polyanions usually do not interact with lipid membrane due to their electric charges and lack of lipophilicity. However, their interaction with membrane is highly desirable for trans membrane delivery of drugs. In order to render them membrane-interactive, polyanions are modified with hydrophobic groups that conceal their charges and enhancing their hydrophobicity. Nucleic acid based therapeutics such as siRNA or other short nucleic acids only functions in cytoplasm and have been rendered cell permeable through the attachments of cell penetrating peptides (CPPs) [4]. CPPs bind independent of pH to membrane phosphates via their net positive charge [5], depending on peptide structure, cargo, and membrane composition. To deliver a bulky cargo, arginine-rich CPP have been used in combination with fusogenic peptides, which contain hydrophobic amino acids for membrane fusion and penetration [6, 7].
Given the membranolytic function of hydrophobic amino acids in fusogenic peptides, polymers unsurprisingly acquire membranolytic activity via built-in stretches of hydrophobic residues. Of particular interest are carboxylated polymers that exhibit pH-responsive membrane disruption with the purpose of endosomolytical drug delivery [8]. At physiological pH, the carboxyl groups are charged, but as the pH decreases below the pKa of its carboxyl groups, these charges are neutralized followed by formation of membranolytic hydrophobic units [9]. The pH-responsiveness is successfully used in endosome-routed receptor-mediated drug delivery.
The pH-responsiveness has two favorable effects. First, it supports exclusively endosomolytic drug delivery with negligible permeation of the cellular membrane at physiological pH. Second, it ensures safe drug delivery without cytotoxicity or side effects that occur by unspecific plasma membrane permeation or membrane damage.
Polymalic acid is a polycarboxylic biopolymer that can be easily chemically derivatized at pendant carboxyl groups to function as a nanoplatform for drug delivery. We have succeeded to target cancer cells and deliver payloads of different drugs to brain and breast tumors [10–14]. To render the polymer membrane permeable, leucine ethyl ester and trileucine have been conjugated resulting in pH-insensitive and pH-sensitive membrane disruption [13]. Here we investigate how the structures of conjugated amino acids and peptides affected membrane disruption and pH-sensitivity.
2. MATERIALS AND METHODS
2.1. Materials
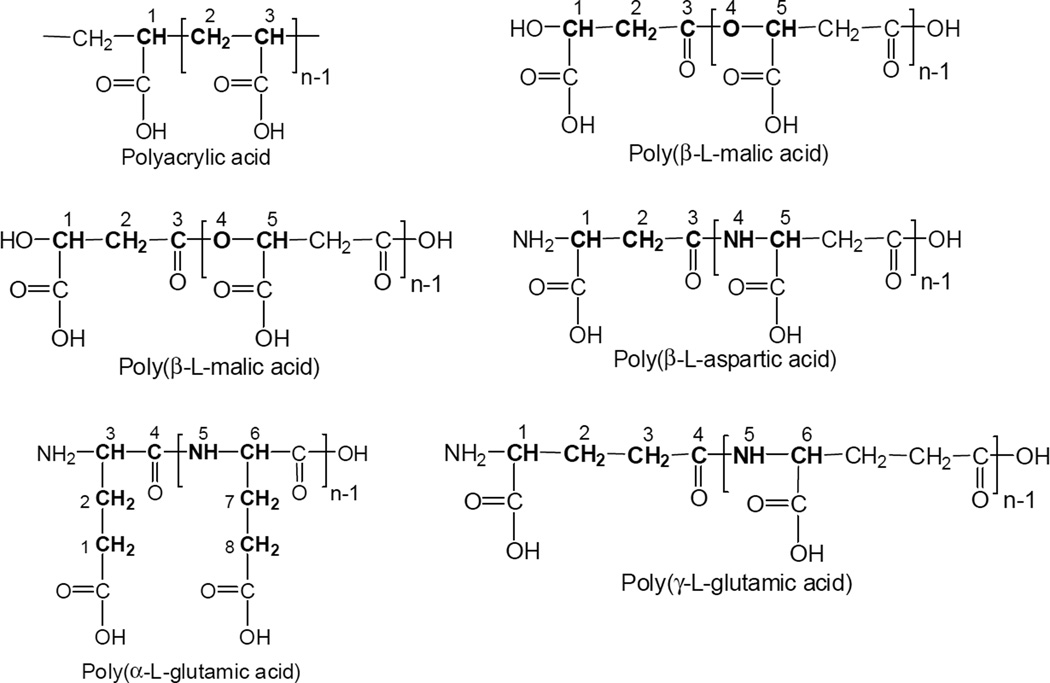
Poly(β-l-malic acid) (PMLA) (unbranched polyester; 100 kDa; polydispersity 1.3) was obtained from culture broth of Physarum polycephalum as described [15, 16]. Poly(γ-l-glutamic acid) (100 kDa) was purchased from Nonstoptec Inc. (Brea, CA, USA), poly(α-l-aspartic acid) (15–50 kDa), poly(α,β-d,l-aspartic acid) (2–10 kDa by thermal polymerization), poly(α-l-glutamic acid) (Mw 50–100 kDa), polyacrylic acid (100 kD) were purchased from Sigma-Aldrich (St. Louis, MO, USA). An overview on the different polymers is found in Fig.1. mPEG5000-amine was purchased from Laysan Bio Inc. (Arab, AL, USA). Tripeptides H-Leu-Leu-Leu-OH (LLL), H-Val-Val-Val-OH (VVV), H-Trp-Trp-Trp-OH (WWW), H-Phe-Phe-Phe-OH (FFF), H-Ile-Ile-Ile-OH (III), and H-Leu-Trp-Leu-OH (LWL) were purchased from Bachem Americas Inc. (Torrance, CA, USA). Calcein and cholesterol were purchased from Sigma-Aldrich (St. Louis, MO, USA) and phosphatidylcholine from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Human glioma cell lines U87MG and T98G were obtained from the American Type Culture Collection (Manassas, VA, USA).
Fig. 1.
Chemical structure of polymers under investigation. Note, the atoms in bold are numbered in order to characterize the space between neighbouring pendant carboxyl groups.
2.2. Synthesis of copolymers
Two categories of copolymers were prepared to study the relationship of polymer structure and membrane destabilization (Table 1). One category consists of copolymers of polymalic acid conjugated with different oligopeptides, such as LLL, WWW, LWL, and FFF, and they were used to investigate the effects of the peptide side chain structure, the degree of polymer loading and the variation of polymer molecular weight. The other category consists of copolymers of poly(α-l-glutamic acid), Poly(γ-l-glutamic acid), poly(α,β-d,l-aspartic acid), and poly(α-l-aspartic acid) conjugated with LLL in order to investigate the structural influence of the polymer platform.
Table 1.
Summary of various synthesized copolymers. Category 1, copolymers based on PMLA and different substituents of amino acid derivatives. Category 2, copolymers with LLL (40%) as substituent and different polymer backbones. aP refers to PMLA backbone. bNo abbreviation will be used for polymer backbone.
| Category 1 | Category 2 | ||
|---|---|---|---|
| amino acid derivatives | Abbreviationa | Other Polymers | Abbreviationb |
| Val | P/V | poly(acrylic acid) | poly(acrylic acid)/LLL |
| Leu | P/L | poly(β-L-malic acid) | poly(β-L-malic acid)/LLL |
| Leucine ethyl ester | P/LOEt | poly(α-L-aspartic acid) | poly(α-L-aspartic acid)/LLL |
| Leu- leu | P/LL | poly(α,β-D,L-aspartic acid) | poly(α,β-D,L-aspartic acid)/LLL |
| Leu-Leu-Leu | P/LLL | poly(α-L-glutamic acid) | poly(α-L-glutamic acid)/LLL |
| Leu-Leu-Leu-Leu | P/LLLL | poly(γ-L-glutamic acid) | poly(γ-L-glutamic acid)/LLL |
| Leu-Leu-Leu-NH2 | P/LLL-NH2 | ||
| Leu-D-Leu-Leu | P/L(D)LL | ||
| Phe-Phe-Phe | P/FFF | ||
| Leu-Trp-Leu | P/LWL | ||
| Trp-Trp-Trp | P/WWW | ||
| Ile-Ile-Ile | P/III | ||
PMLA copolymers containing 40% valine (P/V), leucine (P/L), H-Leu-OEt (P/LOEt), H-Leu-Leu-OH (P/LL), H-Leu-Leu-Leu-OH (P/LLL), H-Leu-Leu-Leu-Leu-OH (P/LLLL), H-Leu-D-Leu-Leu-OH (P/L(d)LL), H-Phe-Phe-Phe-OH (P/FFF), H-Leu-Trp-Leu-OH (P/LWL), H-Trp-Trp-Trp-OH (P/WWW), and Ile-Ile-Ile (P/III) (P refers to PMLA backbone, oligopeptides are represented by one-letter amino acid, and percentage refers to total content (100%) malyl groups, for nomenclatures see Table 1) were all synthesized in analogy to the method for P/LLL 40% as follows: to 1 mL solution of PMLA 73 mg (0.63mmol equivalent of malic acid) in acetone, a mixture of N-hydroxysuccinimide (NHS) and dicyclohexylcarbodiimide (DCC) in 2 mL DMF was added. After 4 hr stirring at room temperature, dicyclohexylurea was filtered and the volume reduced to 0.5 mL by evaporation. Two mL of pyridine were added followed by a solution of H-Leu-Leu-Leu-OH (90 mg, 0.25 mmol equivalent to 40% malyl groups dissolved in 0.4 ml DMF supplemented with 24 µL trifluoacetic acid for solubilization) and kept under stirring for 2 h at room temperature. Triethylamine (20 µL) was added to allow completion of the reaction (usually 30 min followed by thin layer chromatography (TLC) / ninhydrin testing). The degree of loading was varied from 20% to 70%. Additional molecules such as NH2-mPEG5000 were conjugated as described [10].
Polyacrylic acid (100 kD), poly(α-l-aspartic acid) (15–50 kDa) (78 mg in 2 mL DMF), poly(α,β-d,l-aspartic acid) (2–10 kDa) (76 mg in 2 mL DMF), poly(α-l-glutamic acid) (50–100 kDa) (74 mg in 2 mL DMF), Poly(γ-l-glutamic acid) (100 kDa) (64 mg dissolved in 2 mL DMSO under slight warming), were activated as NHS ester and conjugated at 40% of their pendant carboxyls with H-Leu-Leu-Leu-OH following the above protocol for PMLA. Solubility problem of H-Leu-Leu-Leu-OH was solved by including 1.25 equivalent of trifluoroacetic acid and an excess of pyridine in DMF [17]. Remaining unconsumed N-hydroxysuccinimidyl ester was hydrolysed by incubating 30 min with phosphate buffer (50 mM pH 6.8) at room temperature, and products were purified by sieving over PD-10 columns (GE Healthcare, Piscataway, NJ, USA), lyophilized and stored at −80 °C (for nomenclatures of synthesized copolymers see Table 1).
2.3. Polymer fractionation
Copolymer P/LLL of different molecular weights were obtained by size-exclusion chromatography fractionation using a column (2.0 cm × 30 cm) packed with Sephadex G75 (GE Healthcare, Piscataway, NJ, USA). A sample of membranolytic P/LLL (50%) 24 mg dissolved in 1.5 mL of PBS was loaded and fractions of 0.6 mL were collected. Molecular weights were measured by SEC-HPLC (column BioSep-SEC-S 3000, Phenomenex, Torrance, CA, USA) using sodium polystyrene sulfonate as standards (Machery-Nagel, Düren, Germany). The concentration of each fraction was measured by comparing its UV absorption (220 nm wavelength) to standards. Samples of 10 µg P/LLL of a given molecular weight were used for the liposome leakage assay.
2.4. Chemical characterization
Copolymers were subjected to hydrolytic cleavage in sealed ampoules containing 6 M HCl for 12 h at 100 °C. Malic acid in the hydrolysate was quantified by a colorimetric method based on enzymatic reaction using malate dehydrogenase [18]. Amino acids were quantified by the trinitrofluorobenzene (TNBS) method following standard protocols.
2.5. Acid-base titration of peptide polymer conjugates
The pKa values of PMLA, P/LOEt (40%), P/LL (40%), P/LLL (40%), P/LLLL (40%), P/FFF (40%), P/WWW (40%), P/III (40%), and P/LWL (40%) were measured by titration of the copolymers with NaOH. Samples of copolymers containing 0.063 mmol malic acid residue was dissolved in 8 ml of deionized water. The solution was titrated with 0.5 N NaOH in 5 µL aliquots at room temperature. The pH value after each addition was measured after careful mixing and equilibrating and plotted as a function of NaOH equivalents. Equivalence points were identified visually. The pH value obtained midway between neighboring equivalence points referred to 50% neutralization of a titrated acidic group and being equivalent to its pKa value.
2.6. Hydrodynamic diameter and zeta potential
The copolymers were characterized with respect to their absolute molecular weight (Mw), size and ζ potential [19]. Using a Malvern Zetasizer Nano (Malvern Instruments, UK). For the molecular weight determination, 5 solutions of the copolymers in phosphate buffered saline (PBS, pH 7.4) were generated by serial dilution starting with 4mg/mL. For the measurement of the ζ potential, the concentration of the sample was (1–2) mg/mL dissolved in water containing 10 mM NaCl, and the voltage applied was 150 V. For the particle size measurements, the solutions were prepared in PBS at a concentration of 2mg/mL, filtered through a 0.2 µm pore membrane. All the copolymer solutions were prepared immediately before analysis at 25 °C . Means ± standard deviations from three independent measurements are reported.
2.7. Liposome leakage
Membranolysis was measured by the release of fluorescent calcein contained in liposomes upon leakage induced by copolymers. Phosphatidylcholine/cholesterol liposomes containing concentrated calcein were prepared by the extrusion method [20]. Briefly, the mixture of egg yolk phosphatidylcholine 25 mg and cholesterol 6.4 mg (molar ratio, 2:1), dissolved in CHCl3/MeOH (v/v, 2:1), was dried under a stream of nitrogen for 1 h. For hydration, the lipid mixture was hydrated in HBS-buffer 1 ml (5 mM HEPES, 150 mM NaCl, pH 7.4) containing 90 mM calcein and liposomes were prepared by 19 extrusions through a 0.1 µm polycarbonate membrane using a mini-extruder (Avanti Polar Lipids, AL, Alabama). Free calcein was separated from liposome-entrapped calcein by using a Sephadex G-50 column (GE Healthcare, Piscataway, NJ, USA). To measure leakage activity at different pH-values, serially diluted samples were prepared in duplicates of 95 µL of appropriate mixtures of 137 mM HEPES pH 7.4 and 137 mM citrate buffer pH 5.0. Copolymers P/FFF and P/WWW were dissolved in 10 mM phosphate pH 7.4 before dilution because of their low solubility at pH 5.0. Liposomes suspended in 5 µL HBS-buffer (lipid concentration 160 µM) were added to each sample in a 96-well plate and incubated at room temperature for 1 h. Liposomes were 100 nm in diameter [20] and, assuming unilamellar structure, their concentration of 0.2 nM was calculated [21].
The fluorescence intensity of calcein was enhanced after liposome membrane is disrupted by copolymers and its enhancement was measured using Flexstation (Molecular Devices, Sunnyvale, CA, USA) at 535/485 nm (emission/excitation wavelength). Unless mentioned otherwise, copolymers were assayed at pH 5.0 and pH 7.4 at 22°C. Percentage of liposome leakage was referred to the ratio of fluorescence in the presence of 0.25% (v/v) Triton-X 100 (100% leakage). For a given copolymer, leakage in terms of relative fluorescence intensity Y(%) was evaluated as a function of copolymer concentration. The data curve was fit by a sigmoid dose-response curve (Eq. 1) using GraphPad Prism (3.02) software.
| (Eq. 1) |
Symbols refer to: X, Log concentration of copolymer; Y= % liposome leakage produced by copolymer; Leakage (min), background liposome leakage, usually close to 0; Leakage (max), maximum liposome leakage, usually close to 100; LC50, concentration at 50% liposome leakage; Hill-slope (Hill constant), steepness of the data curve as a function of polymer concentration.
2.8. Cell viability
Two human malignant brain tumor cell lines U87MG or T98G were seeded in a 96-well plate (10,000 cells/well in 100 µL Eagle’s MEM with 10% fetal calf serum, L-glutamine, sodium bicarbonate, nonessential amino acids, antibiotics, and sodium pyruvate) and incubated for 24 h. After a further 24 h incubation with copolymers of different concentrations in 200 µL of media, viable cells were quantified using the CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, USA) by reading the absorbance at 490 nm with a Spectra Max Plus 384 ELISA reader (Molecular Devices, Sunnyvale, CA). SD and mean values were calculated for 3 independent measurements.
3. RESULTS
3.1. Synthesis of copolymers
Published methods were used for the synthesis of amino acid conjugates with pendant carboxylates of PMLA [1, 10–14] and were also employed for other polycarboxylates. Reactions were complete and devoid of side products according to thin layer chromatography/ninhydrin staining, SEC-HPLC and Zetasizer analysis. Amounts of substituents loaded onto the polymer corresponded to the amounts of input reagents as confirmed by quantitation of malic acid and amino acids after hydrolytic copolymer cleavage. Molecular weights (SD ± 10%) of copolymers measured by SEC-HPLC were in agreement with absolute molecular weights measured by light scattering, and are similar to the parent polymers. Polydispersity indices were Mw/Mn = 1.2 – 1.3 (Mw, weight averaged molecular mass, Mn number averaged molecular mass). Stability at 37 °C in PBS corresponded to half-lives of ≥ 6 h, depending on the kind of polymer, structure and number of substituents.
3.2. Liposome leakage
3.2.1. Prescreening of hydrophobic amino acids
Under physiological conditions, PMLA is a highly charged and hydrated polyanion [22]. It cannot bind to neutral or negatively charged membranes for the purpose of membrane leakage. In order to render it membranolytic, charges have to be eliminated and the polymer hydrophobized by covalent attachment of appropriate amino acids or their derivatives at the pendant carboxyl groups of the polymer. The choice of amino acids followed our concept of copolymer biodegradability. In an initial study, we synthesized a variety of PMLA-amino acid copolymers and tested them in our standard liposome leakage assay. It was soon found that single hydrophobic amino acid such as Val, Leu, Phe and Trp, conjugated at their α-amino group with 40% loading, was ineffective. The charge on the amino acid and the degree of hydrophobicity were estimated as inappropriate for the induction of membrane disruption.
3.2.2. Membranolytic activity of copolymers containing amino acid derivatives
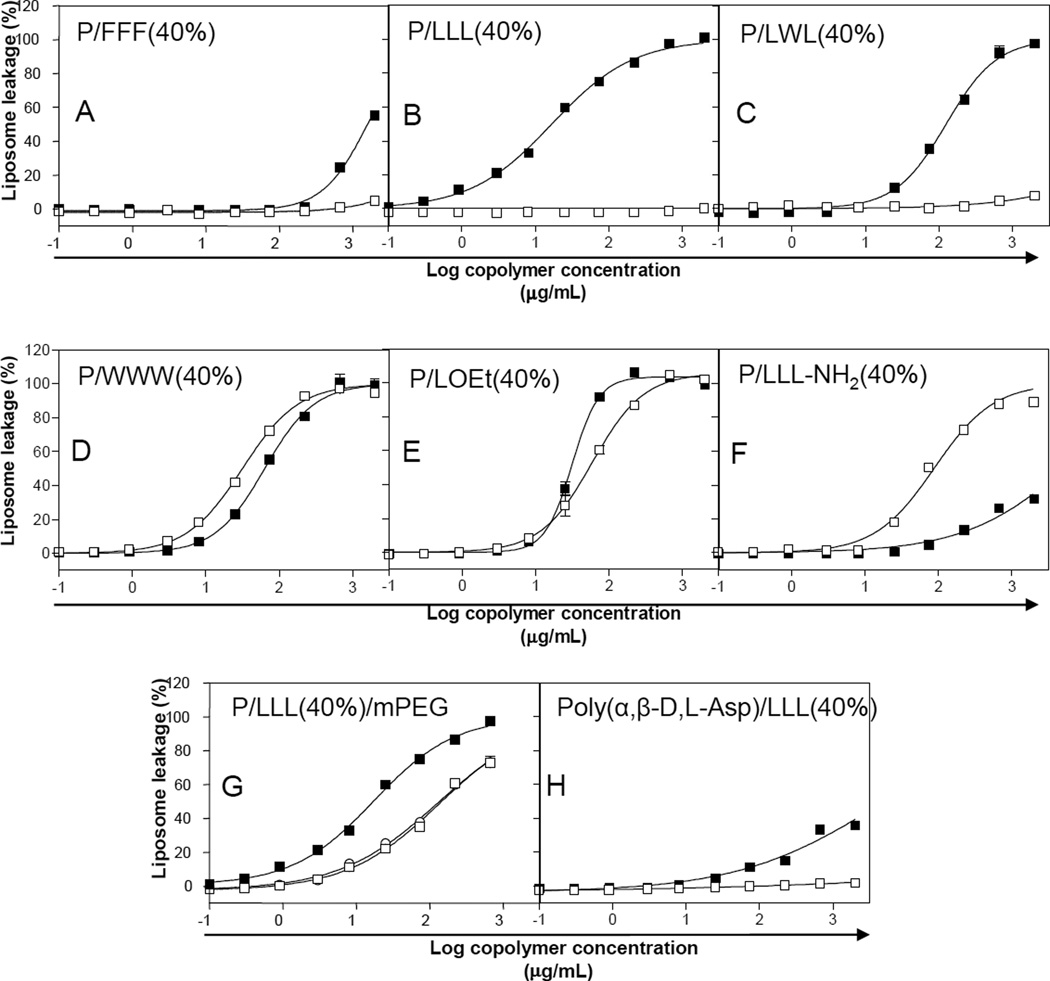
By examining P/FFF(40%), P/LLL(40%), P/LWL(40%), P/WWW(40%), P/LOEt(40%), and PLLL-NH2(40%), we learned that an increase in hydrophobicity and/or elimination of amino acid negative charges resulted in membranolytic activity of PMLA copolymers (Fig. 2A–E, Table 2). Copolymers P/FFF(40%), P/LLL(40%) and P/LWL(40%) were active at pH 5.0 but not at pH 7.4, suggesting that certain carboxylates had been neutralized by protonation which induced membranolytic activity (Fig. 2 A-C). However, copolymer P/WWW(40%) was membranolytically active at pH 7.4, and this result seemed at variance with the promotion of membranolysis by pH-dependent charge neutralization (Fig. 2D). In copolymers P/LOEt(40%) and P/LLL-NH2(40%) the degree of charged carboxylates was permanently eliminated by reaction of 40% of PMLA pendant carboxylates forming neutral ester or amide and pH-independent membranolysis was observed (Fig. 2E, 2F). Moreover, activity could not be observed for copolymers of valine (P/VVV 40%) and isoleucine (P/III 40%), although these amino acids are known as highly hydrophobic [23].
Fig. 2.
Membranolytic activity of various copolymers. Liposome leakage was measured as a function of concentration at pH 7.4 (□) and 5.0 (▪) using the standard liposome leakage assay. Copolymers of PMLA and triphenylalanine (P/FFF) (A), trileucine (P/LLL) (B), and leucyltryptophanylleucine (P/LWL) (C) showed membranolytic activity only at pH 5.0. Copolymers of PMLA and tritryptophan (P/WWW) (D),leucine ethyl ester (P/LOEt) (E) and trileucine amide (PLLLNH2) (F) showed membranolytic activity at both pH 5.0 and 7.4. Copolymers P/LLL(40%) (-▪-) containing mPEG5000 (−○−, 3%) and mPEG5000 (-□-, 5%) (G) showed reduced membranolytic activity at pH 5.0 when mPEG5000 was an additional substituent. Copolymer consisting of poly(α,β-d,l-Asp) and trileucine (H) showed pH- dependent membranolytic activity. Curves in all figures were obtained by fitting with sigmoidal dose-response curves according to GraphPad Prism 3.02. Data are the means ±SEM in triplicate.
Table 2.
Parameters of liposome leakage induced by the copolymers. Concentrations for half maximum leakage (LC50) and Hill-slopes are calculated by the method of sigmoidal dose response curve-fitting using equation Eq. 1. Fitting curves are shown in Fig 2 for best fits to the experimental data (R2, correlation coefficient). ND, membrane leakage not detectable.
| pH 5.0 | pH 7.4 | ||||||
|---|---|---|---|---|---|---|---|
| Copolymers | Loading (%) |
Hill-slope | LC50 (µg/ml) |
R2 | Hill-slope | LC50 (µg/ml) |
R2 |
| P/LLL | 30 | 0.88 | 190.2 | 0.990 | ND | ND | ND |
| P/LLL | 40 | 0.78 | 16.9 | 0.995 | ND | ND | ND |
| P/LLL | 50 | 0.91 | 10.8 | 0.990 | ND | ND | ND |
| P/LLL | 60 | 0.96 | 9.3 | 0.978 | ND | ND | ND |
| P/LLL | 70 | 0.74 | 531.3 | 0.992 | ND | ND | ND |
| P/LLL-NH2 | 40 | 0.61 | 5538.0 | 0.956 | 1.08 | 88.1 | 0.989 |
| P/FFF | 40 | 1.43 | 1574.0 | 0.974 | ND | ND | ND |
| P/LWL | 40 | 1.26 | 126.2 | 0.994 | ND | ND | ND |
| P/WWW | 40 | 1.28 | 63.6 | 0.996 | 1.02 | 30.7 | 0.995 |
| P/LOEt | 30 | 1.34 | 185.8 | 0.964 | 0.72 | 864.0 | 0.965 |
| P/LOEt | 40 | 2.01 | 34.2 | 0.991 | 1.10 | 62.9 | 0.992 |
| P/LOEt | 50 | 1.41 | 3.4 | 0.973 | 1.06 | 9.3 | 0.978 |
| P/LOEt | 60 | 2.24 | 28.6 | 0.993 | 1.43 | 42.0 | 0.994 |
| P/LOEt | 70 | 1.38 | 39.9 | 0.990 | 1.30 | 44.3 | 0.992 |
| P/LLL/mPEG(3%) | 40 | 0.74 | 148.1 | 0.994 | ND | ND | ND |
| P/LLL/mPEG(5%) | 40 | 0.70 | 138.0 | 0.985 | ND | ND | ND |
| P/L(D)LL | 40 | 1.78 | 9.8 | 0.985 | ND | ND | ND |
| Poly(α,β-D,L-Asp)/LLL | 40 | 0.44 | 4358.0 | 0.962 | ND | ND | ND |
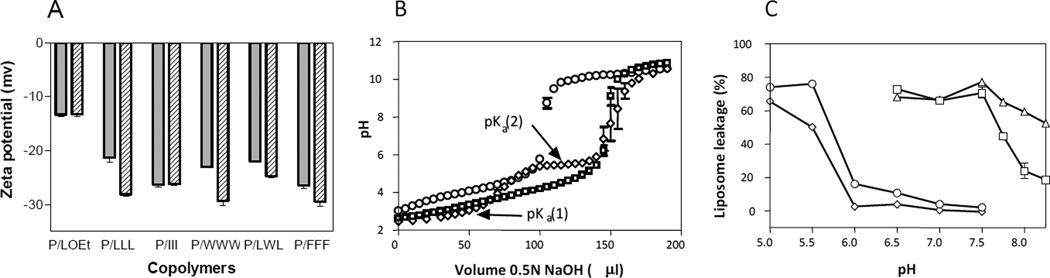
pH-dependent membranolytic activity was correlated with an increase in the zeta potential (Fig. 3A). Zeta potentials of P/FFF(40%), P/LLL(40%), P/LWL(40%), P/WWW(40%) changed from low negative (> −23 mV) at pH 5.0 to high negative (< −28 mV) at pH 7.4. Among them, copolymer P/FFF(40%) had the lowest zeta potential (−27 mV) at pH 5.0 and was the one with the highest concentration requirement to achieve membranolysis (Fig. 3A). Thus, membranolytic activity was inversely correlated with magnitude of negative zeta potential. Copolymer P/LOEt(40%), which was membranolytically active at all pH, had a pH-independent zeta potential of −13 mV ([13] and Fig. 3A). Inactive copolymer P/III(40%), had a pH-independent, highly negative zeta potential of −26 mV.
Fig. 3.
pH-Dependent properties of copolymers. A, zeta potential of copolymers, P/LOEt, P/LLL,P/III,P/WWW,PLWL, and P/FFF at pH 5.0 (gray bar) and 7.4 (striped bar). B, representative base titration of PMLA, P/LOEt(40%) and P/LLL (40%) with 0.5 N NaOH. pKa values were obtained from the titration curves as described under Methods. For the titration of PMLA (□) and P/LOEt (o) only single pKa(1)s were identified, while for P/LLL (◊) pKa(1) and pKa(2) were indicated. C, pH-dependence of membranolysis induced by copolymers P/LOEt(40%) (Δ), P/LLL (40%) (◊), P/L(d)LL(40%) (○), and P/WWW (40%) (□) at 50 µg/ml. Data represent means +SEM in triplicate.
Acid-base titration of copolymers P/FFF(40%), P/LLL(40%), P/LWL(40%), and P/WWW(40%) exhibited two kinds of ionizing groups corresponding to pKa(1) and pKa(2) (Fig. 3B, Table 3). It was assumed that charge elimination corresponding to pKa(2) promoted membranolytic activity. Values of pKa(2) were in the range pH 4.6 – 5.8 (Fig. 3B and Table 3), where membranolytic activity and a decrease in negative zeta potential was observed. In contrast, PMLA and copolymer P/III(40%) exhibited only a single ionization corresponding to pKa(1) (Table 2) falling in the low pH-range considered outside of physiological relevance. For copolymer P/LOEt(40%) with carboxylates permanently masked by esterification, a single pKa(1) and an invariant zeta potential between pH 5 and pH 7.4 that corresponded to pKa(1) of PMLA in the low pH-region (< pH 5). Although not measured, it was assumed that amidation of P/LLL(40%) as in P/LLL-NH2(40%) would eliminate ionization with pKa ∼ 5 in agreement with initiating membranolytic activity at pH 7.4 (Fig. 2F). Although the existence of pKa(2) correlated well the effects of permanent charge neutralization in P/LOEt and P/LLL-NH2 and with the pH-dependent membranolysis of P/FFF(40%), and P/LLL(40%) (Fig. 3C), the pH-activity profile for P/WWW(40%) did not agree with its measured pKa(2) = 5.8 (Fig. 3C; Table 2). This suggested that the pH-dependence of membranolytic activity of this copolymer was strongly influenced by a particularly favorable binding to the liposomal membranes.
Table 3.
pKa of copolymers measured by acid-base titration (Fig. 3B and Methods).
| Copolymers | pKa(1) | pKa(2) |
|---|---|---|
| PMLA | 3.5 | none |
| P/LLL | 3.0 | 5.5 |
| P/LWL | 3.6 | 5.6 |
| P/FFF | 3.6 | 4.6 |
| P/WWW | 5.0 | 5.8 |
| P/III | 4.2 | none |
| P/LOEt | 4.0 | none |
3.2.3. Size of peptides, spacing of carboxylates in the polymer, polymer loading, and molecular weight of PMLA
Several other criteria besides copolymer charge and hydrophobicity were considered. One was the configuration of amino acids, usually in the l-enantiomer form. We probed for the effect of replacing middle L-Leu of the tripeptide by the d-enantiomer using P/L(d)LL(40%) (Table 2). The effect was not substantial, showing that a strict orientation of the tripeptide side chain in this position was not required for membranolysis.
The number of aminoacyl groups in the series P/L, P/LL, P/LLL, and P/LLLL dramatically affected membranolytic activity. Only P/LLL(40%) was active, while P/L(40%), P/LL(40%), PLLLL(40%) were totally inactive. This all or none response to seemingly small structural variations suggested that hydrophobization only functioned in concert with a unique structure of the peptide substituent to achieve membranolytic activity. Further it was discovered that this structural demand had a correspondence on polymers as well. Among copolymers poly(acrylic acid)/LLL(40%), poly(β-l-malic acid)/LLL(40%), poly(α-l-aspartic acid)/LLL(40%), poly(α,β-d,l-aspartic acid)/LLL(40%), poly(α-l-glutamic acid)/LLL(40%), and poly(γ-l-glutamic acid)/LLL(40%), only poly(β-l-malic acid)/LLL(40%) and poly(α,β-d,l-aspartic acid)/LLL(40%) acquired membranolytic activity (Fig. 2B,H; Table 2). The structures of these polycarboxylic acids are shown in Fig. 1, where the distance between neighboring carboxylates is indicated in numbers of atoms between them. By comparison, the distance is shorter in polyacrylic acid (3 atoms) than in poly(β- malic acid) (5 atoms) or longer in poly(α-aspartic acid) (6 atoms), poly(α-glutamic acid) (8 atoms), and poly(γ-glutamic acid) (6 atoms). The distance in poly(β-malic acid) and poly(β-aspartic acid) is equally 5 atoms. The distance in poly(α,β-d,l-aspartic acid)/LLL(40%) varies between 5 and 6 atoms. It thus appeared that copolymers of tripeptides supported membranolysis when nearest carboxyl groups were five atoms apart, but those with narrower or wider spacing were inactive.
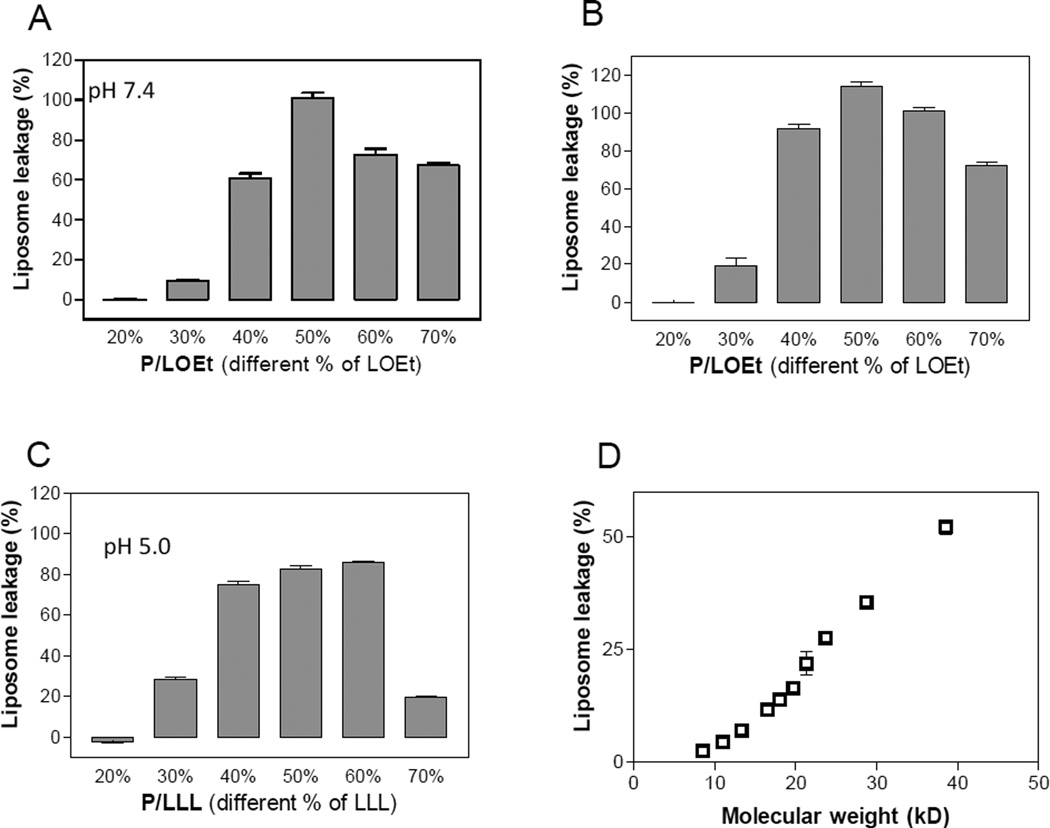
Another structural variation that affected membranolysis was the density of substituents on the polymer chain, i.e. their number per polymer chain. To this end, P/LOEt or P/LLL copolymers in a substitution density varying between 20% and 70% were tested for membranlysis. As a result, at a fixed molar concentration of copolymers membranolytic activity increased more than linearly in the low percentage loading range, namely from not detectable at 10%, to barely detectable at 20%, high at 40%, and maximum efficacy at 50% (Fig. 4A–C). A corresponding dependence on loading was also seen by the LC50 values in Table 2. In another experiment, we have studied the effect of copolymer molecular weight on the membranolytic activity of P/LLL(50%). Again an exponential increase of membranolysis was observed in the low range of molecular weight (Fig. 4D). Thus, both experiments revealed an increase in membranolytic activity depending markedly on the total number of conjugated residues per molecule of polymer.
Fig. 4.
Effects of substituent loading density (%) and molecular weight of the polymer platform. Membranolytic activities were measured using the standard liposome leakage assay. A – C, copolymers P/LLL and P/LOEt with varying amounts of loaded LLL (pH 5.0) or LOEt (pH 5.0 and pH 7.4) at a constant concentration of polymer platform 75 µg/mL. Optimal liposome leakage is seen for ∼ 50% substitution of carboxyl groups. D, membranolyticacitivity of copolymer P/LLL(50%) as a function of varied molecular weight at the copolymer concentration of 0.25 µM. Data represent means ± SEM in triplicate.
We further tested whether membranolytic activity of P/LLL could be adversely affected by the additional conjugation of polyethylene glycol (PEG), a hydrophilic polyether widely used to protect nanoparticles from biodegradation and resorption by the reticuloendothelial system (RES) [24, 25]. At 1% PEG, the effect was marginal, however at 3% and 5%, a 10-fold increase in LC50 was observed (Table 2, Fig. 2G). This indicated that the copolymer had a manageable capacity for carrying hydrophilic ligands. In the case of PMLA with molecular weight of 100 kDa, 5% would amount to approximately 50 residues of PEG5000.
3.2.4. A simplistic membrane interaction mechanism
In order to evaluate the parameters LC50 and Hill-slope in Table 2, a simplistic membranolysis mechanism of polymer was assumed involving binding of copolymer to membrane (indicated by LC50), followed by copolymer intrusion and irreversible reorganization of membrane and the lytic event. In this model, the affinity for binding and intrusion will reflect the hydrophobicity among several other criteria and the pH-dependent or permanent neutralization of pendant carboxylates. Obviously, P/LOEt has high overall affinity for lipid membrane independent of pH probably involving substantial membrane intrusion and rearrangement as visualized by confocal microscope [13]. Significant amounts of labeled P/LOEt(40%) are seen sticking to lipid membrane of giant unilamellar vesicles at both pH 7.4 and 5.0 [13]. In contrast, P/LLL(40%), although having similar values of LC50 (Table 2), could not be detected, suggesting that membrane intrusion and rearrangement were not substantial.
The membranolytic event could either involve binding of a single molecule copolymer to liposome or binding of several molecules simultaneously. This type could give rise to cooperativity indicated by the curvature (value of Hill-slopes) in the concentration dependences shown in Fig. 2. The generated curves with best fits (R2-values close to unity) to experimental data points allowed to generate half-maximum leakage concentration LC50, and Hill-slopes (Table 2). According to the definition of the Hill-slope, values close to unity were interpreted as binding of a single molecule to liposome induced its leakage, and values higher than unity to indicate two or more molecules functioning cooperatively [26]. Inspection of Table 1 reveals that Hill-slopes for P/LOEt (pH 5.0) are significantly higher than unity while those for P/LLL (pH 5.0) are close or below unity. Values for other copolymers (pH 5.0), such as of P/LLL-NH2(40%) or poly(α,β-d,l-aspartic acid)/LLL(40%) could be significantly and reproducibly lower than unity suggesting anti-cooperativity, or higher than unity as in the case of P/FFF(40%), P/L(D)LL(40%) and P/WWW(40%). The variation suggested that the value of the Hill-slope reflected structural features of the copolymer.
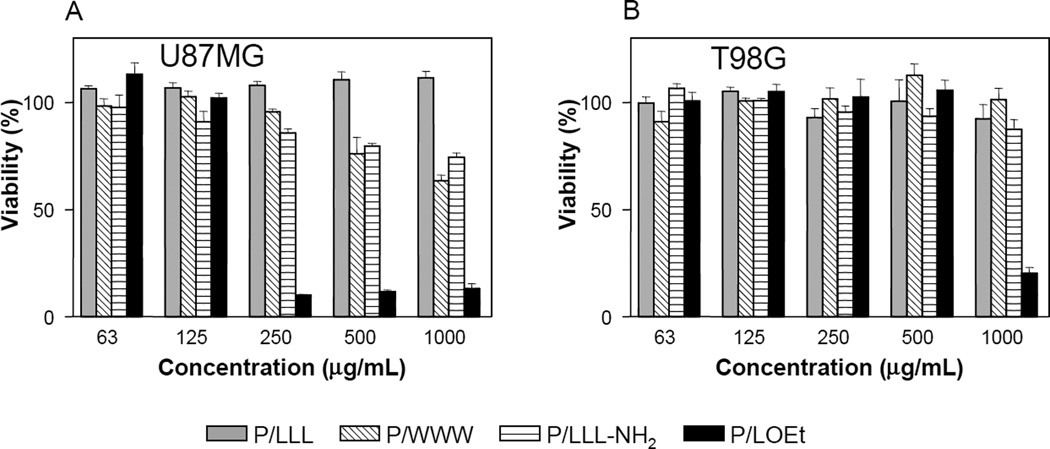
3.3. Cell viability
Incubation of copolymers with human glioma cells from U87MG and T98G cell lines, showed effects on cell viability at physiological pH depending on whether or not copolymers were membranolytically active at this pH (Fig. 5). All copolymers P/WWW, P/LLL-NH2 and P/LOEt active at pH 7.4 suppressed cell viability of U87MG cells in a concentration dependent manner. In contrast, P/LLL, membranolytically inactive at physiological pH, did not suppress cell viability of both cell lines. Suppression was cell line dependent, U87MG being more sensitive than T98G cells. The viability of U87MG dropped dramatically after treatment with 250 µg/ml of P/LOEt leading to a significant change in cell morphology [13]. Such dramatic concentration dependent cytotoxicity was in line with membranolysis by P/LOEt in a cooperative event that involved an extensive membrane rearrangement. Differently, P/LLL-NH2 and P/WWW suppressed cell viability mildly without obvious morphological change.
Fig. 5.
Viability of two human glioma cell lines, U87MG (A) and T98G (B) after treatment with PMLA copolymers, P/LLL(40%), P/WWW(40%), P/LLL-NH2(40%), and P/LOEt(40%). 100% viability refers to PBS instead of copolymers. Data are the means ± SD in triplicate.
4. DISCUSSION
4.1. Hydrophobization and charge neutralization
Targeted delivery of nanoconjugates involves endosomal pathway through receptor mediated endocytosis. Synthetic anionic polymers such as poly(methacrylic acid) and poly(2-ethylacrylic acid) reinforce this pathway by their pH dependent membranolytic activity during acidification of endosomes [27–29]. Since these non- biodegradable polymers may give rise to storage diseases or other adverse responses because of prolonged cycling, naturally occurring biodegradable polyanions became of great interest. Naturally occurring poly(β-l-malic acid) (PMLA) is highly water soluble, nontoxic and non-immunogenic [15], and it contains abundant carboxyl groups readily accessible for chemical modification. It has been proven useful as a platform for drug delivery [1, 11, 13, 14, 30]. Unlike poly(methacrylic acid) and poly(2-ethylacrylic acid), PMLA is not membranolytically active without proper chemical modification, due to its abundant negative charges in the pH-range of interest and its insufficient hydrophobicity. First attempts to accomplish membrane permeation involved hydrophobization and permanent charge neutralization using esterification of pendant carboxyl groups with octyl alcohol (5 mol% pendant carboxyls substituted); however, membranolysis was achieved only at acidic condition, pH < 4 [31], which was not reconcilable with drug delivery. Yet hydrophobization and charge neutralization turned out to be the key concept for achieving membranolysis. Following this concept, we substituted carboxylates with hydrophobic amino acid derivatives while keeping the principle of biodegradability.
In our systematic approach using derivatives of amino acids, we identified five key determinants: (i) hydrophobicity of amino acid substituents, (ii) charge neutralization either permanently or by pH-dependently, (iii) structural compatibility between polymer and substituents, (iv) discrete spatial distance between pendant carboxylates of the polymer, and (v) density of substitution and size (molecular weight) of the polymer. Unique combination of polymer and its substituents for membranolysis was found, thereby imposing structural determinants (iii) and (iv).
Hydrophobicity was introduced by substituting pendant carboxylates with amino acids derivatives of Leu, Trp, Phe, Ile and Val. Only copolymers of Leu, Trp, and Phe in the form of leucine ethyl ester or the tripeptides were membranolytically active, whereas tripeptides of Ile and Val with comparable hydrophobicity were not active. Val and Ile have side chains branching in Cβ- position. Beta branching is known of disfavoring the formation of alpha-helices [32], and Val and Ile constitute their own cluster of amino acids in the Ramachandran backbone torsion angle space [33], suggesting that membranolytically active clusters by trileucine copolymers are sterically permitted unlike tripeptides of Val and Ile.
Negative charges of carboxylates prevent copolymers from forming hydrophobic clusters and from intruding into lipid bilayers to ultimately provoke membrane lysis. Charge hydration and charge-charge repulsion between carboxylates of the same copolymer molecule restrain the formation of such hydrophobic clusters. Simple charge neutralization by methylation of pendant carboxylates rendered pH-independent membranolysis fo PMLA [34]. Similarly, permanent charge neutralization of copolymers P/LLL-NH2 and P/LOEt evoked pH-independent liposome leakage. In contrast, conditional charge neutralization by protonation at low pH resulted in pH-dependent membranolysis shown in this work. This mechanism applies perfectly to pH dependent neutralization of PMLA copolymers with pendant Leu, Trp and Phe tripeptides. It offers the strategy to activate membranolysis in maturating endosomes. Membranolysis of copolymers P/LLL, P/LWL, and P/FFF closely followed their pKa except of P/ WWW (Table 2). pKa values up-shifted by 2 units for tripeptides to pKa 4 – 6 for their copolymers due to the spontaneous formation of hydrophobic clusters that buried and stabilized neutralized carboxyl groups [35]. Similar pKa-shifts have been well documented elsewhere [36–38]. Membranolysis of P/WWW was pH dependent at pH 7.8 without following its pKa of 5.8. resulting in liposome leakage activity at pH 7.4 (Fig. 2D), unexpectedly at variance with pKa-membranolysis relationship of most copolymers. Tryptophan has been reported to localize preferentially at the membrane-water interface [39], to stabilize membrane proteins [40], and to interact most favorably with zwitterionic membranes by forming the WWW peptidyl residue of an antimicrobial peptide tritrpticin (VRRFPWWWPFLRRPFLRR) [41, 42]. In addition to the assistance inferred by the copolymer clusters, neutralized carboxyl group of P/WWW side chain could be further facilitated by strong hydrophobic interaction of tryptophan residues with lipids of liposome membranes, which accounted for its pH dependent membranolysis at pH 7.8 (Fig. 3a), two units higher than its pKa.
4.2. Geometry and charge distribution as structural determinants
β-branching Val and Ile was suggested incompatibile for cluster formation rendering copolymers P/VVV and P/III membranolytically inactive. Another stringency was the number of aminoacyl residues in the peptide. Of the copolymers of LL, LLL and LLLL only the LLL, but neither the dimer nor the tetramer of leucine were active. Energy minimization of copolymers using MM2 force field of Chem 3D Pro 11.0 showed that trimer of leucine LLL gave the best geometrical fit to PMLA backbone allowing a maximum of H-bonding and hydrophobic contact, while Leu and LL were too short and LLLL was too long to fit. The fit between PMLA backbone and pedant LLL was best accounted for by molecular geometry, preferential angle of peptide bonds and charge distribution. The spacing of PMLA is five atoms between neighboring carboxylates. The spacing of other polyanions was either too wide for poly(α-l-aspartic acid), poly(α-l-glutamic acid), and poly(γ-l-glutamic acid) or too narrow for polyacrylic acid, thus not suitable for geometrical fit of LLL cluster formation (Fig.1). Poly(α,β-d,l-aspartic acid) contained stretches of poly(β-d,l-aspartic acid) [43], with a spacing of 5 atoms comparable with that of PMLA (Fig. 1). The fact that poly(α,β-d,l-aspartic acid)/LLL(40%) showed pH dependent membranolysis similar to P/LLL further supported the geometry and charge distribution as an important structural determinant (Fig.2H).
4.3. Number of substituents and polymer size
The loading of substituents on the copolymer had a strong impact on liposome leakage as shown for P/LOEt and P/LLL (Fig. 4A–C, Table 1). Understandably, liposome leakage increased highly depending on loading % at low levels of substitution, because of enhanced hydrophobicity of copolymer itself and simultaneously enhanced hydrophobic contact area with lipid membrane. However, liposome leakage declined after it reached maximum with a loading between 40%-60%. Maximum liposome leakage of both P/LOEt and P/LLL was achieved at about 50% of loading suggesting that further loading was considered unfavorable probably because of molecular crowding after substitution of neighboring carboxyl groups and/or because of copolymer aggregation.
Hypothetically, higher molecular weight of copolymer or a larger polymer size possesses more number of side chain substitutions, giving rise to larger hydrophobic surface and resulting in enhanced hydrophobic interaction with lipid membrane. Actually this phenomenon was exactly as observed: increase of P/LLL (50%) molecular weight gave rise to dramatic increase in membranolyic activity especially when molecular weight is below 30 kD (Fig. 4D).
4.4. Biological implications and drug delivery
The leakage of artificial membrane of liposome was used to measure the membranolytic activity of various copolymers. The lipid membrane of liposome consists of phosphatidylcholine and cholesterol (67%:33%), where cholesterol is known to increase membrane stability [20]. In comparison, lower amounts of cholesterol were found in cell plasma membranes and endosome membranes, 20 – 25% and 0 – 5% respectively [44, 45], which rendered biological membranes less stable than artificial liposome membrane. On this basis, copolymers active for liposome leakage at pH 7.4 are also considered membranolytically active for less stable membranes of both plasma and endosome. Similarly, copolymers with liposome leakage active only at pH 5.0, sufficiently disrupt endosome membrane without affecting plasma membrane because of their different pH environment.
To assure complete biocompatibility of PMLA copolymers, highly water soluble PMLA was converted into a membrane disruptive copolymer by hydrophobization and charge elimination with substitution of amino acid derivatives. Similarly, this potential of amino acids has been recently verified in another case of pH dependent membranolysis implicative of charge neutralization [46]. However, only structurally tailored and derivatized amino acids/peptides were able to convert PMLA into membranolytically active copolymers.
PMLA copolymers containing LOEt and LLL have been used for in vitro and in vivo drug delivery for treatment of xenogenic mouse models of human brain and breast tumors [1, 10–14, 30]. The multifunctional drug delivery systems contained several key components with LOEt and LLL as endosome escape unit in addition to PEG5000, antibodies and Morpholino antisense oligonucleotides (AONs). In particular, pH dependent delivery system based on P/LLL was able to successfully deliver AONs through endothelial cell layers of the vascular system, in particular blood-brain tumor-barrier (BTB), finally escaping from endosome and reaching cytoplasm of glioma cells [13]. Assumedly, cytoplasmic delivery by pH-dependent P/LLL copolymer exclusively is routed through the receptor mediated endosomal pathway, whereas pH-independent delivery by P/LOEt copolymer is routed through both endosome membranes and plasma membranes. Delivery of AONs was successful using P/LOEt copolymer, but pH-independent P/LOEt is cytotoxic at high concentrations for certain cell lines due to apoptosis (Fig. 6) [13]. Cytotoxicity of pH-independent copolymers including P/LOEt, P/WWW and P/LLL-NH2 was cell-line dependent, e.g. less toxicity for T98G cells than for U87MG. In addition, pH-independent copolymers nonspecifically interact with normal cells and thus less available for tumor targeting [13]. Therefore, pH-dependent copolymers are more suitable for cytoplasmic delivery in terms of safety and efficiency.
Great efforts have been made for safe and efficient cytoplasmic delivery using pH-sensitive nanoconjugates [29, 47, 48]. PMLA has emerged as a platform for the successful delivery of various anticancer agents [1, 10–14, 30]. We have recently shown the advantage of pH-dependent P/LLL over pH-independent P/LOEt in the endosome-routed cytoplasmic delivery of Morpholino antisense oligos [13]. The choice of P/LLL for exclusively endosomal membranolysis will greatly reduce the risk of damaging healthy tissue, thus minimizing risks of side effects in clinical treatment. Starting from P/LLL, this work extensively studied dozens of modified anionic copolymer for their membranolytic activity, explored the structural determinants for pH dependent membranolysis, and verified the biological importance of pH-dependent membranolytic copolymers for cytoplasmic delivery.
5. CONCLUSION
Anionic poly(β-l-malic acid) (PMLA) was strategically transformed into a membranolytic agent through hydrophobization and charge neutralization of pendant carboxyl groups with a variety of hydrophobic amino acid derivatives. Permanent charge neutralization renders PMLA usually pH-independent membranolytic. In contrast, conditional charge neutralization by protonation of pendant carboxyl groups renders PMLA membranolytically pH-dependent, that is governed by a few distinct structural determinants, including the charge distribution of polymer backbone and geometrical fit of the amino acid derivatives. For safe and efficient cytoplasmic delivery, copolymers of pH-dependent membranolysis are of primary interest. In addition to P/LLL, more pH dependent PMLA copolymers, P/LWL, P/FFF, and P/L(D)LL were identified, which are suitable platforms for bona fide drug delivery through the endosomal pathway. The pH-independent copolymers P/LLL-NH2, and P/WWW might be applicable for other cases of trans membrane drug delivery under certain circumstances. Furthermore, our studies implied that anionic poly(β-l-aspartic acid) with same charge distribution as PMLA may act as an pH-dependent cytoplasmic delivery platform.
ACKNOWLEDGEMENTS
This work was supported by grants from NIH (R01 CA123495, R01 CA 136841 and U01 CA151815 to JYL), and Winnick Family Foundation clinical grant (to JYL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ljubimova JY, Fujita M, Ljubimov AV, Torchilin VP, Black KL, Holler E. Poly(malic acid) nanoconjugates containing various antibodies and oligonucleotides for multitargeting drug delivery. Nanomedicine. 2008;3:247–265. doi: 10.2217/17435889.3.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pratesi G, Savi G, Pezzoni G, Bellini O, Penco S, Tinelli S, et al. Poly-L-aspartic acid as a carrier for doxorubicin: a comparative in vivo study of free and polymer-bound drug. Br J Cancer. 1985;52:841–848. doi: 10.1038/bjc.1985.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li C. Poly(L-glutamic acid)--anticancer drug conjugates. Adv Drug Deliv Rev. 2002;54:695–713. doi: 10.1016/s0169-409x(02)00045-5. [DOI] [PubMed] [Google Scholar]
- 4.Fonseca SB, Pereira MP, Kelley SO. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev. 2009;61:953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Juliano RL, Alam R, Dixit V, Kang HM. Cell-targeting and cell-penetrating peptides for delivery of therapeutic and imaging agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:324–335. doi: 10.1002/wnan.4. [DOI] [PubMed] [Google Scholar]
- 6.El-Sayed A, Futaki S, Harashima H. Delivery of macromolecules using arginine-rich cell-penetrating peptides: ways to overcome endosomal entrapment. AAPS J. 2009;11:13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endoh T, Ohtsuki T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Adv Drug Deliv Rev. 2009;61:704–709. doi: 10.1016/j.addr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Bulmus V. Biomembrane-active molecular switches as tools for intracellular drug delivery. Aust J Chem. 2005;58:411–422. [Google Scholar]
- 9.Vial F, Oukhaled AG, Auvray L, Tribet C. Long-living channels of well defined radius opened in lipid bilayers by polydisperse, hydrophobically-modified polyacrylic acids. Soft Matter. 2007;3:75–78. doi: 10.1039/b613003h. [DOI] [PubMed] [Google Scholar]
- 10.Lee BS, Fujita M, Khazenzon NM, Wawrowsky KA, Wachsmann-Hogiu S, Farkas DL, et al. Polycefin, a new prototype of a multifunctional nanoconjugate based on poly(beta-L-malic acid) for drug delivery. Bioconjug Chem. 2006;17:317–326. doi: 10.1021/bc0502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita M, Khazenzon NM, Ljubimov AV, Lee BS, Virtanen I, Holler E, et al. Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis. Angiogenesis. 2006;9:183–191. doi: 10.1007/s10456-006-9046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patil R, Portilla-Arias J, Ding H, Inoue S, Konda B, Hu J, et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(beta-L-malic acid) Pharm Res. 2010;27:2317–2329. doi: 10.1007/s11095-010-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding H, Inoue S, Ljubimov AV, Patil R, Portilla-Arias J, Hu J, et al. Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release. Proc Natl Acad Sci U S A. 2010;107:18143–18148. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue S, Ding H, Portilla-Arias J, Hu J, Konda B, Fujita M, et al. Polymalic acid-based nanobiopolymer provides efficient systemic breast cancer treatment by inhibiting both HER2/neu receptor synthesis and activity. Cancer Res. 2011;71:1454–1464. doi: 10.1158/0008-5472.CAN-10-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BS, Vert M, Holler E. Water-soluble aliphatic polyesters: poly(malic acid)s. In: Y Doi, SteinbüA chel., editors. Biopolymers. Volume 3a: Polyesters. Weinheim, Germany: Wiley-VCH; 2002. pp. 75–103. [Google Scholar]
- 16.Lee BS, Holler E. Beta-poly(L-malate) production by non-growing microplasmodia of Physarum polycephalum. Effects of metabolic intermediates and inhibitors. FEMS Microbiol Lett. 2000;193:69–74. doi: 10.1111/j.1574-6968.2000.tb09404.x. [DOI] [PubMed] [Google Scholar]
- 17.Mitin YV. An effective organic solvent system for the dissolution of amino acids. Int J Pept Protein Res. 1996;48:374–376. doi: 10.1111/j.1399-3011.1996.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 18.Bergmeyer HU. Methoden der enzymatischen analyse. Weinheim: Verlag Chemie; 1974. pp. 1632–1639. [Google Scholar]
- 19.Hiemenz PC. Polymer Chemistry: The Basic Concepts. New York: Marcel Decker Inc; 1984. Light scattering by polymer solutions; pp. 659–722. [Google Scholar]
- 20.Van Rossenberg SM, Sliedregt-Bol KM, Meeuwenoord NJ, Van Berkel TJ, Van Boom JH, Van Der Marel GA, et al. Targeted lysosome disruptive elements for improvement of parenchymal liver cell-specific gene delivery. J Biol Chem. 2002;277:45803–45810. doi: 10.1074/jbc.M203510200. [DOI] [PubMed] [Google Scholar]
- 21.Pidgeon C, Hunt CA. Calculating number and surface area of liposomes in any suspension. J Pharm Sci. 1981;70:173–176. doi: 10.1002/jps.2600700215. [DOI] [PubMed] [Google Scholar]
- 22.Nagata N, Nakahara T, Tabuchi T. Fermentative production of poly(β-L-malic acid), a polyelectronic biopolyester, by Aureobasidium sp. Biosci Biotech Biochem. 1993;57:638–642. [Google Scholar]
- 23.White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 24.van Vlerken LE, Vyas TK, Amiji MM. Poly(ethylene glycol)-modified nanocarriers for tumor-targeted and intracellular delivery. Pharm Res. 2007;24:1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- 25.Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145:178–181. doi: 10.1016/j.jconrel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its oxygen dissociation curve. J Physiol. 1910;40 [Google Scholar]
- 27.Cheung CY, Murthy N, Stayton PS, Hoffman AS. A pH-sensitive polymer that enhances cationic lipid-mediated gene transfer. Bioconjug Chem. 2001;12:906–910. doi: 10.1021/bc0100408. [DOI] [PubMed] [Google Scholar]
- 28.Lackey CA, Press OW, Hoffman AS, Stayton PS. A biomimetic pH-responsive polymer directs endosomal release and intracellular delivery of an endocytosed antibody complex. Bioconjug Chem. 2002;13:996–1001. doi: 10.1021/bc010053l. [DOI] [PubMed] [Google Scholar]
- 29.Jones RA, Cheung CY, Black FE, Zia JK, Stayton PS, Hoffman AS, et al. Poly(2-alkylacrylic acid) polymers deliver molecules to the cytosol by pH-sensitive disruption of endosomal vesicles. Biochem J. 2003;372:65–75. doi: 10.1042/BJ20021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujita M, Lee BS, Khazenzon NM, Penichet ML, Wawrowsky KA, Patil R, et al. Brain tumor tandem targeting using a combination of monoclonal antibodies attached to biopoly(beta-L-malic acid) J Control Release. 2007;122:356–363. doi: 10.1016/j.jconrel.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki K, Tirrell DA, Braud C, Vert M. pH-Dependent structural modification of dipalmitoylphosphatidylcholine vesicle membranes by a degradable poly(carboxylic acid) of pharmacological importance. Makromol Chem Rapid Commun. 1984;5:187–190. [Google Scholar]
- 32.Cornish VW, Kaplan MI, Veenstra DL, Kollman PA, Schultz PG. Stabilizing and destabilizing effects of placing beta-branched amino acids in protein alpha-helices. Biochemistry. 1994;33:12022–12031. doi: 10.1021/bi00206a003. [DOI] [PubMed] [Google Scholar]
- 33.Dahl DB, Bohannan Z, Mo Q, Vannucci M, Tsai J. Assessing side-chain perturbations of the protein backbone: a knowledge-based classification of residue Ramachandran space. J Mol Biol. 2008;378:749–758. doi: 10.1016/j.jmb.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Portilla-Arias J, Patil R, Hu J, Ding H, Black KL, García-Alvarez M, et al. Nanoconjugate platforms development based on poly(β,L-malic acid) methyl esters for tumor drug delivery. J Nanomater. 2010:1–9. doi: 10.1155/2010/825363. Article ID 825363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lide DR. Handbook of Chemistry and Physics. CRC Press; 1991. [Google Scholar]
- 36.Urry DW, Peng SQ, Parker TM. Hydrophobicity-induced pK shifts in elastin protein-based polymers. Biopolymers. 1992;32:373–379. doi: 10.1002/bip.360320413. [DOI] [PubMed] [Google Scholar]
- 37.Urry DW, Gowda DC, Peng S, Parker TM, Jing N, Harris RD. Nanometric design of extraordinary hydrophobic-induced pKa shifts for aspartic acid: relevance to protein mechanisms. Biopolymers. 1994;34:889–896. doi: 10.1002/bip.360340708. [DOI] [PubMed] [Google Scholar]
- 38.Mehler EL, Fuxreiter M, Simon I, Garcia-Moreno EB. The role of hydrophobic microenvironments in modulating pKa shifts in proteins. Proteins. 2002;48:283–292. doi: 10.1002/prot.10153. [DOI] [PubMed] [Google Scholar]
- 39.Killian JA, von Heijne G. How proteins adapt to a membrane-water interface. Trends Biochem Sci. 2000;25:429–434. doi: 10.1016/s0968-0004(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 40.Yau WM, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–14718. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 41.Schibli DJ, Hwang PM, Vogel HJ. Structure of the antimicrobial peptide tritrpticin bound to micelles: a distinct membrane-bound peptide fold. Biochemistry. 1999;38:16749–16755. doi: 10.1021/bi990701c. [DOI] [PubMed] [Google Scholar]
- 42.Schibli DJ, Epand RF, Vogel HJ, Epand RM. Tryptophan-rich antimicrobial peptides: comparative properties and membrane interactions. Biochem Cell Biol. 2002;80:667–677. doi: 10.1139/o02-147. [DOI] [PubMed] [Google Scholar]
- 43.Wolk SK, Swift G, Paik YH, Yocom KM, Smith RL, Simon ES. One-and two-dimensional nuclear magnetic resonance characterization of poly(aspartic acid) prepared by thermal polymerization of L-aspartic acid. Macromolecules. 1994;27:7613–7620. [Google Scholar]
- 44.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4th edition. New York: Garland Science; 2002. Intracellular Vesicular Traffic. [Google Scholar]
- 45.Kobayashi T, Yamaji-Hasegawa A, Kiyokawa E. Lipid domains in the endocytic pathway. Semin Cell Dev Biol. 2001;12:173–182. doi: 10.1006/scdb.2000.0234. [DOI] [PubMed] [Google Scholar]
- 46.Chen R, Khormaee S, Eccleston ME, Slater NK. Effect of L-leucine graft content on aqueous solution behavior and membrane-lytic activity of a pH-responsive pseudopeptide. Biomacromolecules. 2009;10:2601–2608. doi: 10.1021/bm900534j. [DOI] [PubMed] [Google Scholar]
- 47.Stayton PS, El-Sayed ME, Murthy N, Bulmus V, Lackey C, Cheung C, et al. ‘Smart’ delivery systems for biomolecular therapeutics. Orthod Craniofac Res. 2005;8:219–225. doi: 10.1111/j.1601-6343.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 48.Roux E, Francis M, Winnik FM, Leroux JC. Polymer based pH-sensitive carriers as a means to improve the cytoplasmic delivery of drugs. Int J Pharm. 2002;242:25–36. doi: 10.1016/s0378-5173(02)00183-7. [DOI] [PubMed] [Google Scholar]