Summary
Cancer cells that express oncogenic alleles of RAS typically require sustained expression of the mutant allele for survival, but the molecular basis of this oncogene dependency remains incompletely understood. To identify genes that can functionally substitute for oncogenic RAS, we systematically expressed 15,294 open reading frames in a human KRAS-dependent colon cancer cell line engineered to express an inducible KRAS-specific shRNA. We found 147 genes that promoted survival upon KRAS suppression. In particular, the transcriptional co-activator YAP1 rescued cell viability in KRAS-dependent cells upon suppression of KRAS and was required for KRAS-induced cell transformation. Acquired resistance to Kras suppression in a Kras-driven murine lung cancer model also involved increased YAP1 signaling. KRAS and YAP1 converge on the transcription factor FOS and activate a transcriptional program involved in regulating the epithelial-mesenchymal transition (EMT). Together, these findings implicate transcriptional regulation of EMT by YAP1 as a significant component of oncogenic RAS signaling.
Introduction
Mutation of proto-oncogenes, such as KRAS, BRAF, and EGFR, induces a state in which cancers are dependent on signaling from the oncogene for survival (Sharma and Settleman, 2007). Although the mechanisms that lead to this oncogene addiction remain poorly understood, pharmacologic inhibition of such oncogenes results in clinical responses. Furthermore, tumors that are resistant to these therapeutic interventions often exhibit reactivation of the signaling pathways regulated by these oncogenes. For example, EGFR addicted cancers that relapse have been found to harbor MET copy-number amplification or KRAS mutation, which mediate resistance by activating the same downstream effector pathways independent of EGFR (Jänne et al., 2009).
Activating mutations of the KRAS proto-oncogene occur in a substantial fraction of pancreatic, lung and colon cancers (Lau and Haigis, 2009). Oncogenic KRAS activates pleiotropic signaling pathways that contribute to tumor initiation and maintenance including the Mitogen-Activated Protein Kinase (MAPK), Phosphatidylinositol 3-Kinase (PI3K), and Ral Guanine nucleotide Exchange Factor (RalGEF) signaling pathways (Pylayeva-Gupta et al., 2011). Suppression or inhibition of these pathways prevents tumor initiation and slows the growth of established tumors (Ehrenreiter et al., 2009; González-García et al., 2005; Gupta et al., 2007). One consequence of mutant KRAS signaling is aberrant activation of the AP-1 family transcription factors, which promote responses to mitogenic signaling (Karin, 1995). Specifically, KRAS increases FOS and JUN activation through MAPK-dependent and -independent mechanisms (Deng and Karin, 1994).
YAP1 is a transcriptional co-activator that participates in several context-dependent transcriptional programs that regulate organ size and promote cell proliferation (Wang et al., 2009). Recurrent YAP1 amplifications are observed in hepatocellular cancers, where it is an essential oncogene (Zender et al., 2006). In addition, YAP1 is also implicated in the epithelial-to-mesenchymal transition (EMT) and the metastatic potential of mammary epithelial cells (Lamar et al., 2012; Overholtzer et al., 2006). YAP1 serves as an effector of the Hippo (Hpo) kinase cascade and regulates the Transcriptional Enhancer Activator Domain (TEAD) transcription factors (Pan, 2010). Serine phosphorylation of YAP1 by both Hpo-dependent and -independent factors inhibits YAP1 entry into the nucleus, preventing subsequent activation of not only TEAD, but other YAP1 transcriptional partners such as SMAD, RUNX, TBX5, and the ERBB4 internal cytoplasmic fragment (Wang et al., 2009). One Hpo-independent mechanism implicated in cancer involves phosphorylation of YAP1 at tyrosine-357 by YES1 to promote YAP1 interaction with β-catenin and modulation of Wnt signaling (Rosenbluh et al., 2012). These observations suggest that YAP1 interacts with specific transcription factors in particular contexts to promote cell proliferation, organ growth, or survival.
Identification of genes that promote resistance to targeted therapies can provide insight into signaling mechanisms activated by particular oncogenes. Here we applied a similar concept to systematically probe pathways required for cancer cell lines that harbor and are dependent on oncogenic KRAS. Specifically, we performed a genetic screen to identify open reading frames (ORFs) that are able to sustain the survival of KRAS-dependent cancer cell lines in the setting of KRAS suppression.
Results
Systematic identification of genes that rescue the loss of oncogenic KRAS expression
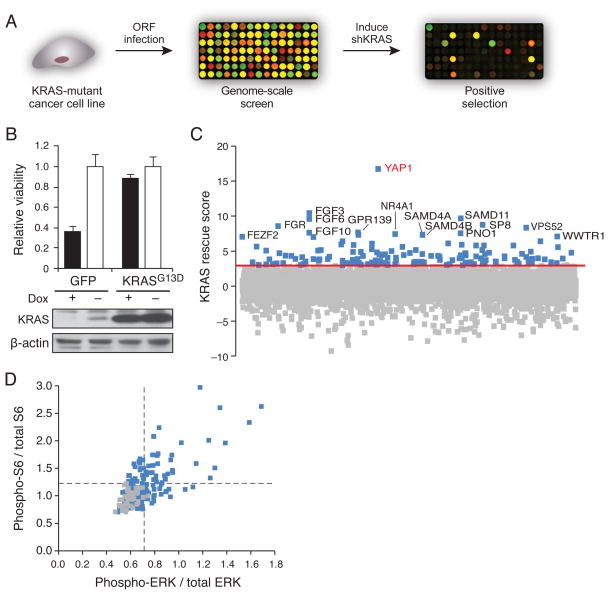
We performed a genome scale genetic rescue screen to identify genes that support the survival of KRAS-dependent cancer cells upon suppression of KRAS. We generated a cell line for screening by stably introducing a doxycycline-inducible shRNA targeting the KRAS 3′ untranslated region (UTR) into the HCT116 KRAS-mutant colon cancer cell line (HCTtetK) and introduced 15,294 ORFs from the Center for Cancer Systems Biology (CCSB)/Broad Institute ORF library (Yang et al., 2011) into these cells in an arrayed format in triplicate under optimized conditions where each well was transduced at high efficiency (98%, Table S1). We induced suppression of KRAS by doxycycline treatment and assessed cell proliferation/survival (Fig. 1A). As a control, we expressed a mutant KRASG13D ORF, which lacks the KRAS 3′UTR and thus cannot be suppressed (Fig. 1B). We considered an ORF a ‘hit’ if it obtained a KRAS rescue score greater than 3, i.e. the viability in that well was at least 3 standard deviations above the mean of negative controls. All of the 150 KRASG13D expressing wells scored above this threshold, and only 1 of the 1,119 negative control wells (0.05%) scored.
Figure 1. Systematic identification of genes that rescue loss of viability induced by KRAS suppression.
(A) Schematic diagram of an arrayed format screen to identify ORFs that rescue loss of cell viability induced by suppression of KRAS in KRAS-dependent cells. (B) Suppression of KRAS in HCTtetK cells, and rescue by KRAS ORF. Data represent mean ± SD normalized to cell viability in untreated conditions. (C) Distribution of scores for all screened genes averaged across 3 replicates. KRAS rescue score indicates SD from mean of negative control wells. Red line, 3 SD. Blue, gene ‘hits.’ (D) Characterization of 147 hits by in-cell western of ERK and S6 phosphorylation. Each point represents average of duplicate wells. Lines indicate 2SD above mean of negative controls. Gray, negative controls. See also Figure S1 and Table S1 and Table S2.
We identified 147 genes that met this criterion (Table S1). The highest scoring candidates included sterile alpha motif (SAM) proteins that function as post-transcriptional regulators (Baez and Boccaccio, 2005), the WW-domain binding proteins YAP1 and WWTR1, and members of the FGF family (Fig. 1C). In a separate screen focused on 597 kinases (CCSB/Broad Kinase ORF Collection), we also identified FGFR1 as a kinase that was able to rescue KRAS suppression (Fig. S1A).
We then assessed the ability of each ORF to activate MAPK or PI3K signaling. Specifically, we expressed the 147 ORFs in HCTtetK cells in an arrayed format and quantified the activity of the MAPK and PI3K pathways by measuring the ratio of phospho-ERK to total-ERK levels, and the ratio of phospho-S6 ribosomal protein to total S6 ribosomal protein levels, respectively (Fig. 1D, Table S2). We found that 55.1% of the candidates activated at least one of the two pathways (16.1% MAPK only, 13.4% PI3K only, and 25.6% both pathways). A number of candidate genes failed to activate either pathway, suggesting that MEK and PI3K-independent mechanisms may also play a role in KRAS-dependent tumors. The observation that a large proportion of these candidates indeed activated KRAS effector pathways increased our confidence in the biological relevance of the rescue screen.
YAP1 substitutes for oncogenic KRAS in KRAS-dependent cancer cell lines
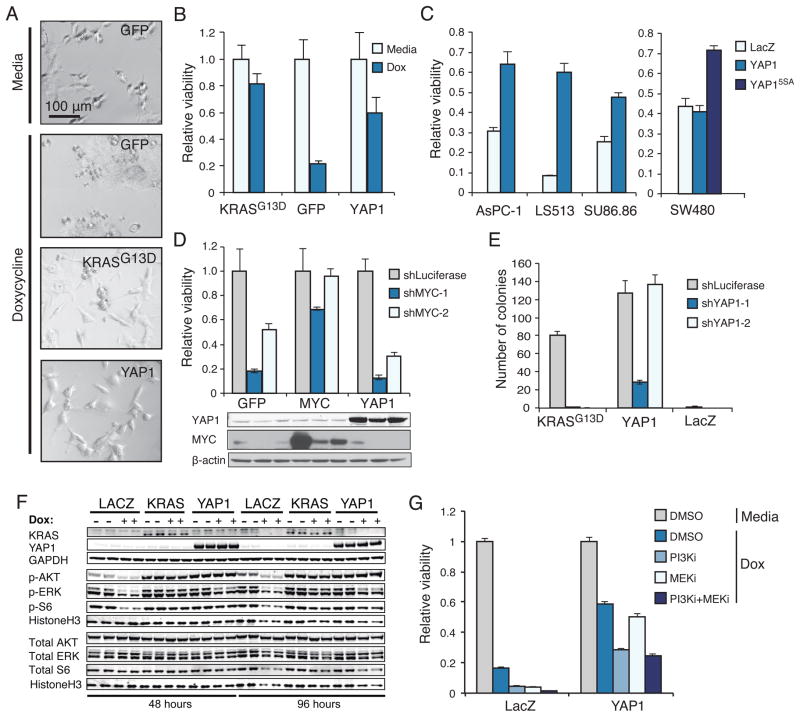
We focused on understanding YAP1, the highest scoring gene in the screen. Expression of YAP1-2γ (hereafter referred to as YAP1)(Sudol, 2012) in HCTtetK cells prevented the morphological changes observed after suppression of KRAS (Fig. 2A) and rescued the loss of viability induced by suppressing KRAS (Fig. 2B). To ensure that the effects were not HCT116-specific, we tested the ability of YAP1 to complement loss of KRAS function in 4 additional KRAS-mutant colon and pancreatic cancer cell lines (SW480, LS513, SU86.86, AsPC-1). We found that wild-type YAP1 rescued LS513, SU86.86, and AsPC-1 cell lines from KRAS suppression, and that a constitutively active version of YAP1, which lacks 5 serine phosphorylation sites (YAP15SA)(Zhao et al., 2007), rescued loss of viability in SW480 cells (Fig. 2C). We concluded that YAP1 signaling functionally replaces KRAS in KRAS-dependent cancer cells, although YAP1 itself may be differentially regulated in specific cell lines. In consonance with these findings, tumors that escape suppression of Kras in Kras-driven murine pancreatic ductal adenocarcinomas exhibit Yap1 amplifications (Kapoor et al., this issue).
Figure 2. YAP1 rescues KRAS mutant cancer cells in vitro.
(A) Morphology of HCTtetK cells expressing the indicated vectors at 20x magnification. The indicated ORFs were expressed and cells were treated with doxycycline (KRAS suppressed). (B) Viability of HCTtetK cells upon KRAS suppression in cells expressing the indicated genes, normalized to cell viability in media condition. (C) Consequences of expressing YAP1 in KRAS-mutant cell lines after KRAS suppression. Viability of shKRAS normalized to shLuciferase in the presence of each indicated ORF. (D) Response of HCT116 cells to MYC suppression in cells that express the indicated ORFs. (E) Effect of YAP1 suppression on anchorage independent growth of HA1E transformed with KRASG13D or YAP1 ORF. (F) Effect of doxycycline-induced KRAS suppression on activation of ERK, AKT, and S6 in HCTtetK cells expressing LacZ, KRAS, or YAP1. (G) Effect of a PI3K inhibitor (PI3Ki; GDC-0941) or a MEK inhibitor (MEKi; AZD-6244) on the ability of YAP1 to rescue KRAS suppression. Cells were treated with 1uM of GDC-0941, 1uM of AZD-6244, both, or DMSO. Data normalized to viability of cells without KRAS suppression (media) with DMSO treatment. (B–E, G) Mean ± SD of at least 3 replicates in a representative experiment shown. See also Figure S2.
To assess the specificity of the YAP1 phenotype to KRAS suppression, we tested whether YAP1 expression rescued suppression of additional oncogenes, c-MYC and PIK3CA, which are also activated in HCT116 cells. Suppression of c-MYC using two c-MYC-specific shRNAs reduced cell viability. Expression of c-MYC but not YAP1 rescued this phenotype (Fig. 2D). Furthermore, exposure to the PI3K inhibitor GDC-0941 arrested proliferation, and this effect was not rescued by YAP1 expression (Fig. S2A). Together, these observations support the conclusion that rescue of KRAS suppression by YAP1 was not due to a general effect on survival by YAP1.
To interrogate the functional relationship between KRAS and YAP1 further, we assessed whether YAP1 is required for KRAS-induced cell transformation. We expressed KRASG13D or YAP1 cDNAs in immortalized HA1E cells (Hahn et al., 1999) and confirmed that either KRASG13D or YAP1 induced anchorage-independent colony formation when a control shRNA was expressed. We found that expression of two YAP1-specific shRNAs abrogated KRAS-driven anchorage independent colony formation (Fig. 2E, Fig. S2B). Expression of shYAP1-2 targets the YAP1 3′UTR and, as expected, failed to suppress YAP1-driven anchorage independent colony formation.
We next examined the effect of manipulation of KRAS on the regulation of YAP1. Manipulating KRAS expression did not affect phosphorylation of YAP1 serine-127, a site implicated in regulation of YAP1 by Hpo signaling (Zhao et al., 2007), nor phosphorylation of components of the Hpo cascade such as LATS-1/2 and MST2 (Fig. S2C). We concluded that KRAS does not alter YAP1 phosphorylation or activation.
In HCTtetK cells expressing the negative control LacZ, treatment with doxycycline for 2 days to induce KRAS suppression led to decreased levels of phosphorylated ERK, AKT and S6. We found that YAP1 expression restored AKT and S6 phosphorylation to baseline levels, and increased ERK phosphorylation (Fig. 2F). These observations are in consonance with prior reports (Zhang et al., 2009; Overholtzer et al., 2006) that showed that YAP1-regulated expression of the EGFR ligand amphiregulin led to activation of ERK and AKT. However, expression of ERK, MEK, or AKT failed to rescue HCTtetK cells upon suppression of KRAS in our original screen (Table S1) and YAP1 expression had no measureable effect on the activation of KRAS, as assessed by GTP-bound KRAS levels after pull-down with the Ras-binding domain of RAF1 (Fig. S2D).
To determine if re-activation of MAPK and PI3K signaling was necessary for the ability of YAP1 to rescue KRAS suppression, we treated HCTtetK cells expressing YAP1 with the PI3K inhibitor GDC-0941 or the MEK inhibitor AZD-6244 (Fig. 2G, S2E). We found that YAP1 rescued HCTtetK cells from KRAS suppression to the level that we observed previously when treated with the vehicle (DMSO, Fig. 2B). Treatment with either MEK or PI3K inhibitor decreased, but failed to fully suppress, the ability of YAP1 to rescue KRAS inhibition as compared to cells expressing LacZ (Fig. 2G). Furthermore, combined treatment with both inhibitors did not further decrease viability. Thus although YAP1 partially restores the activity of PI3K and MEK pathways after KRAS suppression, activation of these pathways fails to fully account for the ability of YAP1 to promote survival in KRAS dependent cells after KRAS suppression.
Functions of YAP1 required for the survival of KRAS-dependent cells
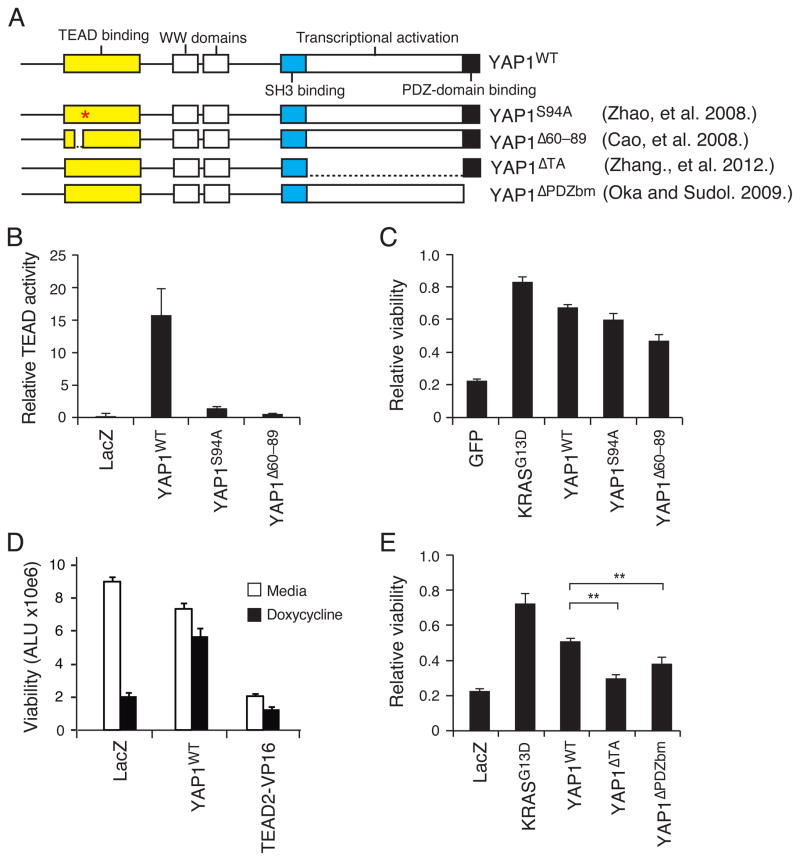
YAP1 is regulated by multiple signaling pathways and regulates the function of several transcription factors. YAP1 is composed of a TEAD-binding domain, an SH3 binding motif, two WW domains and a transcription activation domain (Fig. 3A), and YAP1 nuclear localization is mediated by a C-terminal PDZ domain-binding motif (Oka and Sudol, 2009). To identify regions of YAP1 necessary to promote survival after KRAS suppression, we expressed previously described YAP1 domain-specific mutants in HCTtetK cells to assess their effect on YAP1 function (Fig. 3A).
Figure 3. Structure-function analysis of YAP1.
(A) YAP1 domain structure and YAP1 mutants. (B) Effects of expressing YAP1 TEAD-defective mutants on the activity of a TEAD reporter in 293T cells. (C) Viability after KRAS suppression in HCTtetK cells expressing YAP1 mutants defective in TEAD activation. (D) Viability, in arbitrary luminescence units (ALU), after KRAS suppression in HCTtetK cells expressing a constitutively active TEAD2-VP16 fusion. (E) Effects of expressing the YAP1 mutants defective in transcriptional activation or nuclear localization in HCTtetK cells after KRAS suppression. **P-value <0.01. (B–E) Mean ± SD of 6 replicates of a representative experiment shown. (C–E) Viability of doxycycline treated relative to untreated samples displayed. See also Figure S3.
First, we explored the role of TEAD transcription factors for the KRAS-related functions of YAP1. Prior work identified YAP1 TEAD-binding domain mutants that disrupt binding to TEAD transcription factors (YAP1S94A and YAP1Δ60–89) (Zhao et al., 2008b; Cao et al., 2008). Expression of these mutants indeed abolished the ability of YAP1 to activate a TEAD-specific reporter (Fig. 3B)(Ota and Sasaki, 2008). However, these YAP1 mutants rescued the proliferation effects of KRAS suppression similar to wild-type YAP1 (YAPWT; Fig. 3C), suggesting that TEAD-specific effects are dispensable for YAP1 rescue. These findings differ from those of Kapoor et al. (this issue), who identified TEAD2 as one mediator of YAP1 function by showing that a constitutively active fusion of the VP16 domain to the DNA-binding region of TEAD2 (TEAD2-VP16)(Cao et al., 2008) was able to rescue KRAS suppression. We tested the ability of this construct to rescue in the HCTtetK model, and found that TEAD2-VP16 activated a TEAD-specific reporter (Fig. S3A), but failed to rescue the effect of KRAS suppression (Fig. 3D, Fig. S3B). TEAD2-VP16 expression slowed the proliferation of HCTtetK cells (Fig. 3D) and resulted in a change in cell morphology different from what we observed upon expression of YAP1 or expression of KRAS. Together, these results suggest that the TEAD family is not the primary mediator of the ability of YAP1 to complement loss of KRAS in this model system.
Since we recently reported a role for YES1 phosphorylation of YAP1 for β-catenin signaling (Rosenbluh et al., 2012), we investigated whether YES1 played a role in KRAS signaling. We expressed two YAP1 mutants: YAP1ΔSH3bm, which disrupts the interaction of YES1 with YAP1 (Vassilev et al., 2001; Sudol, 1994), and YAPY357F, which prevents YES1 phosphorylation of YAP1 (Rosenbluh et al., 2012). Expression of either YAP1ΔSH3bm or YAP1Y357F rescued the loss of cell viability observed after KRAS suppression to the level of YAP1WT (Fig. S3C, S3D). Thus, YES1 modulation of β-catenin signaling also does not contribute to YAP1 activity in the context of KRAS suppression.
We then tested whether YAP1-induced transcriptional activation was required to rescue KRAS suppression. Expression of YAP1 mutants that harbor a deletion of the transcriptional activation domain (YAPΔTA; Zhang et al., 2012) disrupted the ability of YAP1 to rescue cells from KRAS suppression in HCTtetK cells, as did expression of YAP1 mutants that harbored a deletion of the 5 amino acid PDZ domain-binding motif (YAPΔPDZbm), reported to disrupt YAP1 nuclear localization (Fig. 3E, S3E). These two YAP1 mutants also prevented YAP1-induced anchorage-independent growth (Fig. S3F). These observations show that YAP1 mediates survival after suppression of KRAS through interactions with transcription factors in the nucleus.
YAP1 regulates AP-1 family transcription factors and EMT
Since transcriptional activation was important for the ability of YAP1 to rescue KRAS suppression, we analyzed transcriptional profiles in HCTtetK cells in which we manipulated KRAS or YAP1 expression. We first identified genes that were downregulated after 30 h of doxycycline (KRAS suppressed) treatment compared to untreated cells. Of those, we identified genes whose expression was rescued by the expression of both YAP1 and KRAS (Fig. 4A; Table S3). Separately, we analyzed the transcriptional profiles of cells expressing the YAP1 TEAD-binding defective mutant YAP1S94A, compared to KRASG13D and YAP1WT as positive controls, or to LACZ and YAP1ΔTA as negative controls. We noted that the expression signature induced by YAP1S94A overlapped but was distinct from that of YAP1WT, suggesting differences in specific transcriptional programs regulated by these alleles. Yet, we found that YAP1S94A rescued expression of genes that decreased upon KRAS suppression (Fig. S4A), as expected.
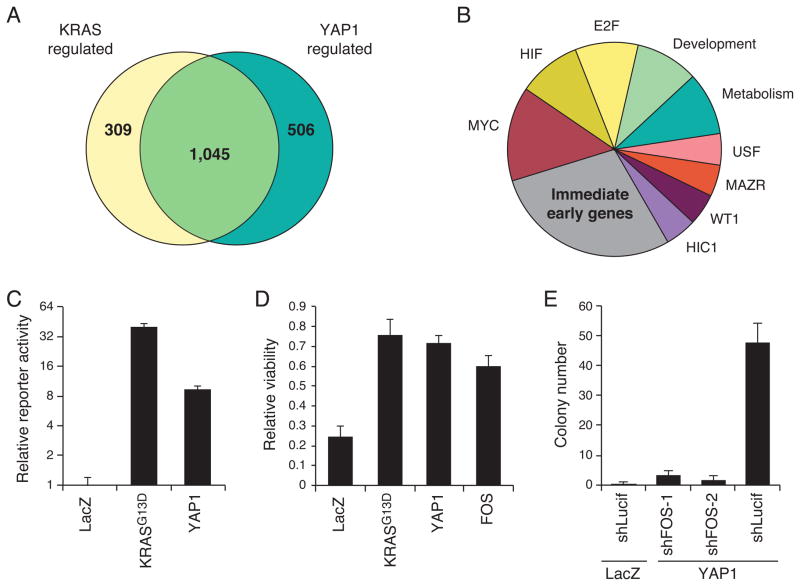
Figure 4. YAP1 and KRAS converge to regulate the AP-1 transcription factor FOS.
(A) Genes rescued by YAP1, KRAS, or both, in the context of KRAS suppression. (B) Categories of transcription factor motifs enriched amongst genes rescued by both KRAS and YAP1. (C) AP-1 reporter activity in 293T cells expressing YAP1. Arbitrary luminescence units normalized to LacZ condition. (D) Expression of FOS rescues suppression of KRAS in HCTtetK cells. Viability of doxycycline treated relative to untreated samples displayed. (E) Effects of suppressing FOS on YAP1-mediated cell transformation of HA1E cells. (C–E) Mean ± SD of at least 3 replicates in a representative experiment shown. See also Figure S4 and Table S3.
Using the 1,045 genes that were rescued by both YAP1WT and KRAS (Fig. 4A; Table S3), we searched for co-regulated transcription factor motifs and co-regulated gene sets. We used TransFind motif analysis (Kielbasa et al., 2010) to identify transcription factor motifs enriched in promoter regions of genes rescued by both YAP1 and KRAS compared to motifs rescued by KRAS alone. Enriched motifs (p-values <0.05) included transcriptional factors involved in the immediate early gene response, c-MYC, HIF, and E2F (Fig. 4B, S4B). Kapoor et al. (this issue) showed that E2F1 is required for Yap1 to permit Kras-independent tumor growth in a murine pancreatic ductal adenocarcinoma model. Depending on the context, multiple transcription factors may play roles in YAP1 function.
We focused on the largest category, transcription factors involved in the immediate early gene response, which are regulated by both growth factor stimulation and Ras signaling (O’Donnell et al., 2012). Specifically, this category includes gene families such as activating transcription factor (ATF), early growth response protein (EGR), and specificity protein (SP) (Fig. S4B). To determine which of these genes play a role in YAP1 function, we systematically suppressed members of these gene families using multiple independent shRNAs and assessed the effect on YAP1-driven anchorage independent growth (Fig. S4C). Suppression of several SP family and ATF family transcription factors repressed YAP1-mediated cell transformation. Genes in the SP family are required for development and are ubiquitously involved in maintaining cell survival (Suske, 1999). We were particularly interested in the role of the ATF genes since they are members of the AP-1 transcription factor family, previously shown to be regulated by KRAS-MAPK signaling (Mechta et al., 1997). Further supporting the role of AP-1, we found that expression of YAP1 or KRAS activated an AP-1 luciferase reporter driven by a consensus AP-1 binding element (Fig. 4C).
From our genome-scale screening data, we noted that FOS was the only member of the AP-1 transcription factor family that scored (3.75 SD above controls; Table S1). We confirmed that expression of FOS rescued HCTtetK cells upon suppression of KRAS (Fig. 4D). To test whether FOS expression was necessary for YAP1 function, we assessed whether suppression of FOS affected YAP1-induced transformation. Depletion of FOS with two FOS-specific shRNAs reduced YAP1-driven anchorage-independent colony formation in HA1E cells (Fig. 4E). RAS-induced transformation in vivo requires FOS (Ledwith et al., 1990; Saez et al., 1995), and as expected, FOS suppression reduced colony formation by HCT116 cells or HA1E cells expressing KRASG13D (Fig. S4D, S4E). These observations support the role of AP-1 family transcription factors as effectors of YAP1 and KRAS.
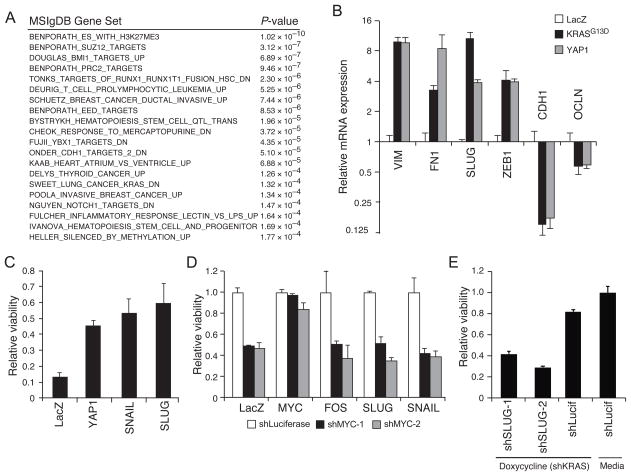
We then looked for transcriptional programs regulated by both KRAS and YAP1 in our microarray data (Fig. 4A; Table S3). Using the Molecular Signatures Database (MSigDB)(Subramanian et al., 2005), the top gene sets, enriched to p-value < 10−3, included several related to differentiation and development (Fig 5A). In many cancers, normal cell differentiation is perturbed by the interruption of differentiation steps or by the aberrant activation of EMT programs. Furthermore, EMT has been implicated in resistance to therapies targeting receptor tyrosine kinases (Witta et al., 2006), and FOS has been shown to regulate EMT directly (Reichmann et al., 1992). When we analyzed transcriptional profiles of the YAP1 mutants (Fig. S4A), we found that YAP transcriptional signature (YAP_DUPONT) and EMT signatures (SARRIO_EPITHELIAL_MESENCHYMAL_TRANSITION_UP) were enriched among genes rescued by YAP1WT (Fisher exact, p-values 0.005 and <10−16, respectively; threshold log(−0.5) compared to LacZ), and neither signature was enriched among the genes rescued by functionally deficient YAP1ΔTA mutant (Fisher exact, p-values 0.48 and 0.53, respectively). Thus we hypothesized that EMT induced by YAP1 contributed to the survival of cells after suppression of KRAS.
Figure 5. YAP1 regulates the epithelial-mesenchymal transition (EMT).
(A) Enriched gene sets rescued by both YAP1 and KRAS. (B) qRT-PCR validation of EMT regulation by KRAS and YAP1 in HCT116 cells. Data represent mean +/− SD of four replicates relative to LacZ control. (C) Viability of doxycycline treated relative to untreated HCTtetK cells expressing Slug and Snail. (D) Effects of suppressing MYC after expressing FOS, Slug, and Snail in HCT116 cells. Viability of shMYC normalized to shLuciferase control in the presence of each indicated ORF. (E) Effect of Slug on the ability of YAP1 to rescue KRAS suppression. Viability of doxycycline-treated HCTtetK cells expressing YAP1 after expression of each indicated shRNA, normalized to media-treated shLuciferase control. (B–E) Mean ± SD of at least 3 replicates in a representative experiment shown. See also Figure S5.
We found that both KRAS and YAP1 expression strongly induced expression of mesenchymal genes such as Vimentin (VIM), Fibronectin (FN1), Slug (SNAI2), and Zinc-finger E-box-binding homeobox 1 (ZEB1) and reduced the expression of epithelial genes such as E-cadherin (CDH1) and Occludin (OCLN) (Fig. 5B). Although only a subset of genes implicated in EMT were regulated by KRAS or YAP1 (Fig. S5A), we found that KRAS and YAP1 regulated a similar set of EMT markers in both HCTtetK cells (Fig. 5B, S5A) and SU86.86 (Fig. S5B).
We then tested whether key transcriptional regulators of EMT such as Slug and Snail were necessary and sufficient to rescue KRAS dependence. Expression of either Slug or Snail in HCTtetK cells rescued the loss of viability induced by suppressing KRAS (Fig. 5C). To assess the specificity of this effect to KRAS, we suppressed MYC and expressed FOS, Slug, or Snail (Fig. S5C). Neither expression of EMT transcriptional regulators nor FOS rescued loss of viability upon MYC suppression (Fig. 5D), suggesting that induction of EMT did not broadly rescue oncogenic suppression. Moreover, we tested whether Slug was required for YAP1 rescue of KRAS suppression. Expression of two Slug-specific shRNAs reduced Slug expression and decreased the ability of YAP1 to rescue suppression of KRAS (Fig. 5E, S5D).
FOS and YAP1 coordinately regulate downstream targets
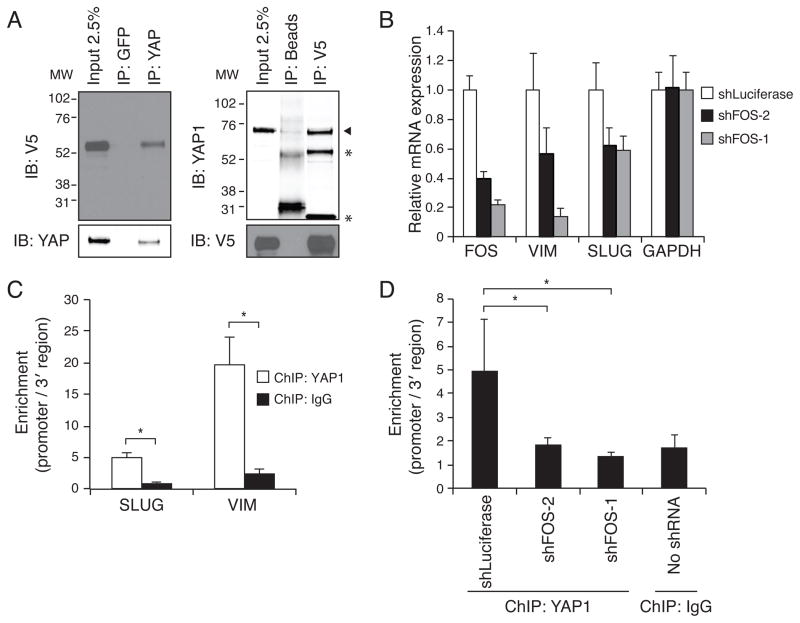
Because FOS expression was required for YAP1-induced cell transformation, we examined whether FOS and YAP directly interact. We expressed a V5-epitope tagged FOS in HCT116 cells and isolated YAP1 or control (anti-GFP) immune complexes. In YAP1 complexes but not control immune complexes, we detected FOS (Fig. 6A), and in V5 immune complexes (FOS), we found endogenous YAP1 (Fig. 6A). In contrast, co-immunoprecipitation experiments with a V5-epitope tagged version of JUN, another AP-1 transcription factor, failed to show an interaction between YAP1 and JUN (Fig. S6A).
Figure 6. YAP1 and FOS interact at promoter regions to regulate EMT.
(A) Co-immunoprecipitation using control antibody or target-specific antibody for YAP1 and FOS in lysates from HCT116 cells expressing YAP1 and V5-tagged FOS. Binding of the reciprocal protein was assessed by immunoblotting. YAP1 indicated by arrowhead. *, IgG heavy and light chains. MW, molecular weight in kDa. (B) mRNA expression of Vimentin (VIM) and Slug after FOS suppression. Mean ± SD of four replicates relative to shLuciferase control in HCT116 cells shown. (C) Chromatin immunoprecipitation in HCT116 to assess YAP1 DNA binding at promoter regions of SLUG (SNAI2) and Vimentin (VIM). (D) Chromatin immunoprecipitation in HCT116 to assess YAP1 binding at SLUG promoter after FOS suppression. (C,D) Bars represent enrichment of promoter compared to 3′ region of each gene. Mean ± SD of 3 replicates shown. *P-value <0.05. See also Figure S6 and Table S4.
We hypothesized that FOS and YAP1 regulate common downstream target genes that are important for KRAS dependence. Using transcription factor ChIP-seq data from the Encyclopedia of DNA Elements (ENCODE)(ENCODE Project Consortium et al., 2012), we found that YAP1-regulated genes such as VIM and Slug (SNAI2) harbor FOS binding sites in their promoter regions. We confirmed that FOS suppression using FOS-specific shRNA decreased VIM and Slug expression levels (Fig. 6B). To determine whether FOS and YAP1 bind at the same loci, we performed chromatin immunoprecipitation using antibodies specific to YAP1, FOS, or control IgG in HCT116 cells. As reported by ENCODE, FOS was enriched at the promoter regions of VIM and Slug (compared to a region 3′ of each gene)(Fig. S6B). We found that YAP1 binding was also enriched at the same loci, whereas binding of IgG was not (Fig. 6C). We confirmed the specificity of the YAP1 antibody by showing decreased YAP1 binding at those loci when YAP1-specific shRNAs were expressed (Fig. S6C). Notably, suppression of FOS also decreased YAP binding at the VIM promoter (Fig. 6D), suggesting that YAP may function through FOS as a transcriptional co-activator.
We then interrogated ChIP-on-Chip and ChIP-Seq data to identify FOS and YAP1 binding sites across the genome. Specifically, we looked for genes co-occupied by YAP1 and FOS by using Ingenuity Pathway Analysis on a list of YAP1-occupied genes in MCF10A cells (Zhao et al., 2008b) and corresponding FOS-occupied genes (1 kb upstream, 0.3kb downstream of transcription start site in MCF10A-ER-Src cells; ENCODE Project Consortium, 2012). As a control, we used a list of androgen receptor (AR) occupied genes (Zhao et al., 2008b). We found that the EMT canonical pathway was enriched in the YAP1-FOS overlap set (p<0.05) but was not enriched in the AR-FOS overlap set (Table S4). This observation provides additional support that YAP1 and FOS regulate EMT to rescue viability upon KRAS suppression.
Yap1 mediates resistance to Kras suppression in a KrasG12D-driven lung cancer mouse model
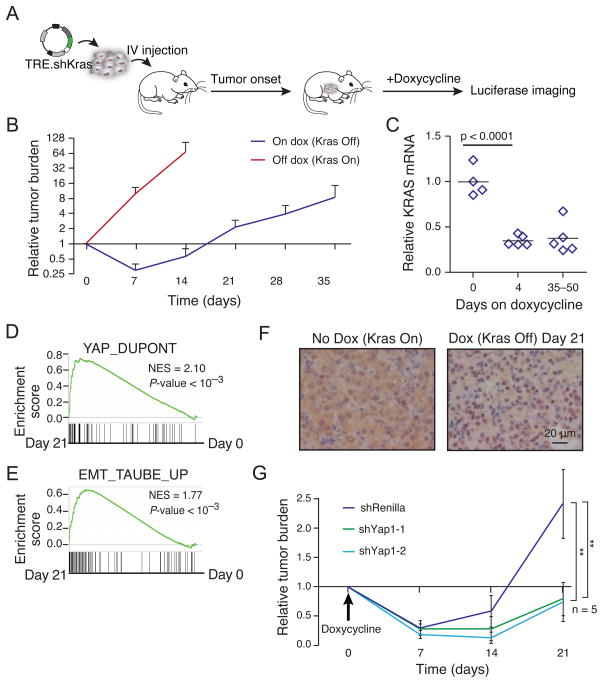
We then examined the consequences of Kras inhibition in a well-studied mouse model of lung adenocarcinoma (Tuveson et al., 2004; DuPage et al., 2009). We first used primary lung adenocarcinoma cells derived from the KrasLox-STOP-Lox-G12D;p53flox/flox (KP) mouse lung cancer model into which we introduced a doxycycline-inducible shRNA targeting Kras expressed from the 3′UTR of GFP (KP-KrasA cells). In this system, doxycycline treatment activates the GFP reporter as well as shKras, resulting in suppression of endogenous wild-type Kras and mutant KrasG12D. After intravenous injection of tumor cells, tumor burden in the lung was monitored weekly by a constitutively expressed luciferase construct in the tumor cells (Fig. 7A). Seven days post-transplantation, the mice were fed a doxycycline-containing diet, which resulted in rapid lung tumor regression within 7 days (Fig. 7B), confirming that these lung tumors depend on ongoing oncogenic Kras signaling. However, tumors recurred over the course of the next 2 weeks even though Kras remained suppressed in tumor tissue, as confirmed by imaging of GFP reporter and by qRT-PCR of Kras mRNA from microdissected tumors (Fig. 7C, S7A). Thus, in this model some Kras-driven tumor cells can continue to proliferate in a Kras-independent manner after prolonged suppression of oncogenic Kras.
Figure 7. Yap1 activity is required for endogenously acquired KRAS resistance in vivo.
(A) Schematic of mouse transplant model of KRAS-driven lung cancer. KrasG12D;p53fl/fl lung adenocarcinoma cells were infected with retroviral vectors expressing rtTA3, luciferase and a tet-on shKras. Cells were transplanted into recipient mice by tail vein injection. 7 d later, mice were fed a doxycycline diet to induce shKras in tumor cells (D0). (B) Time course of tumor regression and relapse after Kras suppression. Mean ± SD shown. N=3 off dox and N=10 on dox. (C) Suppression of Kras in tumor tissue. Kras mRNA was measured by qRT-PCR in microdissected lung tumors after the indicated days of doxycycline treatment. (D) Enrichment of a published YAP1 signature (Dupont et al., 2011) after 21 d doxycycline treatment versus untreated cells. (E) Enrichment of a published EMT signature (Taube et al., 2010) after 21 d doxycycline treatment versus untreated cells. (F) Yap1 localization in tumors that escape Kras suppression. Immunohistochemistry was performed with Yap1 antibody on frozen tissue sections from tumors which developed after Kras suppression (dox on) for 21 d and tumors which formed with continued Kras expression (dox off). (G) Tumor response to suppression of Kras in combination with Yap1 or control suppression. Mean ± SD shown. See also Figure S7 and Table S5.
To assess the molecular basis of this Kras-independent process, KP-KrasA cells were cultured in the presence of doxycycline, resulting in cells that continued to proliferate despite suppression of Kras. RNA sequence profiling (RNA-seq) of these cells after 21 days on doxycycline compared to cells without exposure to doxycycline showed significant up-regulation of a published Yap1 gene signature (Fig. 7D; Table S5) (Dupont et al., 2011). Since we showed that EMT played a critical role in mediating resistance to KRAS suppression in human cells (Fig. 5), we also assessed whether EMT was involved in the Kras resistance observed in the murine cancer cell system. Using a published EMT gene signature (Taube et al., 2010), we noted a significant enrichment of the signature in Kras-independent cells compared to parental cells (Fig. 7E). In addition, EMT-associated markers including Fn1, Cdh2, Snai1, Snai2, Zeb1, and Ocln were altered in RNA samples from Kras-independent cells (Fig. S7B). We confirmed by qRT-PCR that expression of several of these markers was upregulated in tumor cells that had escaped Kras suppression in vivo as compared to tumors in which Kras expression was maintained (Fig. S7C). Notably, we found that Yap1 expression was not significantly different in these two groups. Thus, we examined Yap1 localization by immunohistochemistry from tumor tissue. We found that Yap1 showed increased nuclear localization in tumors that escaped Kras suppression (Fig. 7F), which may explain the observed upregulation of genes involved in EMT. Together, these observations suggest that Kras-independent mouse lung cancer cells exhibit signatures similar to those that we observed when we expressed YAP1 or KRAS in human cancer cell lines.
To explore whether Yap1 signaling was involved in Kras oncogenic addiction in vivo, we performed both gain-of-function and loss-of-function experiments in this mouse model. We observed that forced expression of YAP1 partially prevented the tumor regression observed upon initial suppression of Kras (Fig. S7D, S7E). To test whether relapse from Kras suppression requires Yap1 signaling, we generated KP cells in which two shRNAs are expressed simultaneously. Specifically, in addition to the Kras-specific shRNA, we expressed a doxycycline inducible construct that drives the expression of red fluorescent protein (RFP) carrying shRNAs targeting either Yap1 or Renilla luciferase within the 3′UTR (Zuber et al., 2011). While tumors with Kras suppression alone relapsed over time, concurrent Yap1 suppression delayed tumor relapse while the expression of the Renilla luciferase shRNA did not (Fig. 7G). We found that the residual tumors that formed in the presence of the Yap1-specific shRNA after 28 days no longer suppressed Yap1 as assessed by qRT-PCR (Fig. S7F), suggesting that Kras-independent proliferation in vivo occurs in cells with higher Yap1 activity. Together, the observations from this murine model confirm that the upregulation of Yap1 signaling correlates with the expression of an EMT-like transcriptional program and plays a compensatory role in vivo upon loss of Kras signaling.
Discussion
Using a systematic functional approach, we identified YAP1 as a gene whose expression rescued cell death induced by suppression of KRAS in KRAS-dependent cancer cells. In a murine model of Kras-driven lung cancer, we found that tumors that escaped Kras suppression in vivo also exhibited increased YAP1 activity. Through transcriptional profiling, we described a YAP1-driven transcriptional program that recapitulates the oncogenic signals in KRAS-driven cancers through involvement of AP-1 family transcription factors, specifically FOS, and the regulation of EMT.
Complementation screening in cancer cells to elucidate KRAS signaling
We found that a substantial proportion of genes identified through our rescue screen activated known KRAS downstream MAPK and PI3K pathways, in line with previous observations that MAPK and PI3K signaling provide a general mechanism to substitute for Ras signaling (Lim and Counter, 2005). Although we focused on YAP1 and FOS herein, these observations suggest that other genes that scored in this screen may represent novel components of KRAS-regulated signaling pathways.
Models of resistance to RAS oncogenic addiction
Withdrawal of oncogenic Ras results in rapid tumor regression in mouse models which used tetracycyline-inducible overexpression of oncogenic Ras (Fisher et al., 2001; Chin et al., 1999; Jechlinger et al., 2009). Relapse was often associated with mutations in the tetracycline transactivator (Podsypanina et al., 2008), resulting in reactivation of the oncogene. Here, we used inducible in vivo RNAi in order to model Kras inhibition in mouse lung adenocarcinoma cells driven by KrasG12D expressed from its endogenous promoter, and observed tumor relapse through Kras-independent mechanisms. This model of relapse thus provided strong evidence that increased activity of YAP1 is a physiologically relevant mechanism to bypass loss of KRAS signaling.
We found that YAP1 replaces oncogenic KRAS signaling at least in part by regulating an EMT-like transcriptional program. Singh et al. (2009) previously showed a correlation between KRAS dependency and epithelial morphology amongst KRAS-mutant cell lines and argued that induction of EMT makes cells insensitive to KRAS suppression. However, RAS itself activates EMT (Wong et al., 2013), and genes involved in EMT, such as Slug, are essential in KRAS mutant cells (Wang et al., 2010). Furthermore, our observation that YAP1 and FOS cooperate to regulate portions of the EMT program is in consonance with a prior report that FOS itself drives an EMT phenotype (Eger et al., 2000). We report here that sustaining the established EMT phenotype is necessary to rescue KRAS suppression. Based on the available evidence, it is clear that the correlation between KRAS dependency, morphology and EMT is complex and that the interplay of YAP1, KRAS and EMT regulators will be context dependent.
In consonance with our observations, Kapoor, et al. (this issue) used a model of murine pancreatic adenocarcinoma in which Kras is expressed from an inducible promoter. After Kras suppression, some tumor cells spontaneously relapsed through a mechanism involving amplifications of a genomic locus containing Yap1 and a gene signature associated with EMT. They showed that TEAD2 and E2F1 cooperate with YAP1 in these Kras-independent tumors. We also observed that E2F motifs were enriched in genes regulated by both KRAS and YAP1 (Fig. 4B), but did not find a role for TEAD2. Since YAP1 engages different transcription factors in a context-dependent manner, TEAD2 involvement may be related to contextual differences of species or cell lineage, the method of gene suppression, or to functional differences between suppression of both mutant and wild-type KRAS (as occurs with shRNA) compared to suppression of mutant KRAS alone. We also observed that YAP1 re-activated MAPK and PI3K signaling in our experimental model, while Kapoor et al. observed baseline activation of these pathways by Yap1 in their system. This may be attributed to different baseline levels of MAPK/PI3K signaling in the two systems, or the influence of wild-type KRAS (To et al., 2013). Despite these differences, the observation that YAP1 can rescue KRAS suppression in three independent experimental models underscores the ability of YAP1 to promote survival in KRAS-dependent cancers.
KRAS and YAP1 converge at the transcriptional level
RAS signaling has been linked to YAP1 in D. melanogaster imaginal wing discs, in which YAP1 was required for EGFR and RAS activity (Reddy and Irvine, 2013). We showed that YAP1 is required for KRAS-driven transformation, but did not observe biochemical modulation of YAP1 activity by KRAS signaling. Although downstream effectors of KRAS such as RASSF1 and AKT have been shown to regulate YAP1 phosphorylation in specific contexts (Zhao et al., 2008a), their influence on YAP1 may be context-specific.
Recurrent amplifications of YAP1 have been observed in liver, breast, and esophageal cancer (Overholtzer et al., 2006; Muramatsu et al., 2011; Zender et al., 2006). As an oncogene, YAP1 has been shown to drive development of hepatocellular cancer and to induce colonic adenomas in mouse models (Zender et al., 2006; Camargo et al., 2007), although the gene programs regulated by YAP1 to induce transformation may differ from that engaged by YAP1 to mediate survival in the setting of KRAS suppression. In the context of KRAS suppression, we found that YAP1-regulation of FOS was required for rescue and that YAP1 and FOS interact at promoter sites of regulated genes to activate an EMT-like program in order to mediate survival.
EMT phenotypes are upregulated in cancer cells resistant to chemotherapy (Fuchs et al., 2002; Kajiyama et al., 2007; Cheng et al., 2007), and modulation of EMT influences cellular dependency on receptor tyrosine kinases (Witta et al., 2006). We found that YAP1-induced regulation of EMT was specific for oncogenic KRAS, but not other oncogenes. The specificity of this mechanism to KRAS suggests resistance associated with EMT might be attributed to bypass of specific oncogenic pathways. Understanding the role of YAP1 and FOS in regulating EMT will not only provide further insights into the transcriptional programs regulated by oncogenic KRAS, but as therapeutic strategies to target KRAS are developed, will also elucidate potential mechanisms by which KRAS-driven cancers escape these interventions.
Experimental Procedures, see also Extended Experimental Procedures
KRAS rescue screen
HCTtetK cells were seeded at 300 cells per well in 50 ul in 384-well plates. The next day, cells were infected using 1 ul of virus (1.4x108 infectious particles/ml using the RNAi Consortium virus tittering protocol; http://www.broadinstitute.org/rnai/public/resources/protocols) in 25 ul media supplemented with 8 ug/mL polybrene. The media was changed the following day. Two days after infection, 500 ng/mL doxycycline was added. For 10% of plates, additional replicates received treatment with blasticidin or no treatment to confirm overall infection efficiency. 7 d after infection, viability of each well was determined by CellTiterGlo (Promega). B-score adjustment was performed for each plate (Brideau et al., 2003), and final score for each ORF was normalized to approximately 40 negative control values on each plate (uninfected wells and wells infected with HcRed, eGFP, BFP, LacZ, or Luciferase).
Rescue experiments in vitro
ORFs were introduced by lentiviruses followed by selection with 10ug/mL Blasticidin for ≥ 4 d. For cell lines harboring a tet-inducible shRNA, cells expressing each ORF were seeded in 96-well plates and treated with media supplemented with doxycycline or media alone for 5 d. Viability was quantified by CellTiterGlo (Promega). For cell lines that did not harbor a tet-inducible shRNA, the desired shRNA was introduced by lentiviral delivery and selected with puromycin for 48 h before replating at 10,000 cells/well in 12-well plates. Results were quantified by Vi-Cell Cell Viability Analyzer (Beckman Coulter).
Generation of KP-KrasA cells and derivative lines
KrasG12D;p53fl/fl lung adenocarcinoma cells were infected with retroviral vectors TRE-GFP-miR30 shKras-PGK-Puro (Zuber et al., 2011), rtTA3-PGK-Hygro and MSCV-luciferase-IRES-GFP. GFP+ cells were sorted into single cell clones to screen for cells showing efficient doxycycline-inducible Kras knockdown, resulting in KP-KrasA, KP-KrasB, and KP-KrasC lines from independent clones. KP-KrasA were stably infected with TRE-dsRed-miR30 shYap1-PGK-Venus-IRES-NeoR (Zuber et al., 2011) to simultaneously express shKras and shYap1 from both TRE promoters upon doxycycline treatment.
Mouse lung transplant model
5x104 cells were transplanted into NCr-nu/nu recipient mice (Taconic) by tail vein injection and mice were treated with Doxycycline diet (Harlan Laboratories). Bioluminescence imaging was performed as described (Xue et al., 2011). Luciferase signal in the lung was quantified using Xenogen software and normalized to tumors on Day 0, before doxycycline treatment.
Supplementary Material
Highlights.
YAP1 substitutes for loss of oncogenic KRAS in human and murine cancers.
YAP1 expression is required for KRAS-induced cell transformation.
KRAS and YAP1 converge on FOS to regulate the epithelial-mesenchymal transition.
YAP1 and FOS co-localize at promoters of genes involved in EMT.
Acknowledgments
We thank Y. Kwon, M. Muzumdar, P. Chen, D.A. Barbie, N. Perrimon, K. Haigis, D. Sabatini, and P. Park for discussions and advice. We thank D. Lam, L. Hamm, and F. Izzo for screening support. We thank L. Gaffney for help with figure illustration. Snail and Slug plasmids were generously provided by B. Bierie and R. Weinberg, and TRE.shKras shRNA by S.W. Lowe. This project was supported by R01 CA140545 (W.C.H.), U01 CA176058 (W.C.H.), P01 CA050661 (W.C.H., T.M.R.), K99 CA169512 (W.X.), and T32 GM007753 (D.S.) from the U.S. National Institutes of Health; and Koch Institute Support Grant P30-CA14051 (T.J.) from the National Cancer Institute. Additional support was provided by Howard Hughes Medical Institute (T.J.), Daniel K. Ludwig Fund for Cancer Research (T.J.), American Association for Cancer Research (W.X.), the Leukemia Lymphoma Society (W.X.), the Svenson Family Foundation (J.R.), fellowships W81XWH-10-1-0062 (X.W.) and W81XWH-12-1-0115 (Y.Z.) from the Department of Defense, and fellowship HFCR-11-03-03 from Hope Funds for Cancer Research (E.B.K.). T.M.R. and W.C.H. are consultants for Novartis.
Footnotes
D.D.S., W.X., E.B.K., X.W., J.R., J.W.K., Y.Z., D.E.R., T.J., and W.C.H designed experiments.
D.D.S., F.P., A.C.S. designed and performed screening assays.
D.D.S. and E.B.K. performed in vitro experiments.
W.X. and S.S. performed mouse experiments.
D.D.S. and A.B. performed computational analyses.
D.D.S. and W.C.H. wrote the manuscript.
D.D.S., W.X., E.B.K., X.W., Y.Z., J.W.K., T.J., and W.C.H. edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baez MV, Boccaccio GL. Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. J Biol Chem. 2005;280:43131–43140. doi: 10.1074/jbc.M508374200. [DOI] [PubMed] [Google Scholar]
- Brideau C, Gunter B, Pikounis B, Liaw A. Improved Statistical Methods for Hit Selection in High-Throughput Screening. Journal of Biomolecular Screening. 2003;8:634–647. doi: 10.1177/1087057103258285. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang L-H. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O’Hagan R, Pantginis J, Zhou H, et al. Essential role for oncogenic Ras in tumour maintenance. Nature. 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]
- Deng T, Karin M. c-Fos transcriptional activity stimulated by H-Ras-activated protein kinase distinct from JNK and ERK. Nature. 1994;371:171–175. doi: 10.1038/371171a0. [DOI] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Eger A, Stockinger A, Schaffhauser B, Beug H, Foisner R. Epithelial mesenchymal transition by c-Fos estrogen receptor activation involves nuclear translocation of beta-catenin and upregulation of beta-catenin/lymphoid enhancer binding factor-1 transcriptional activity. J Cell Biol. 2000;148:173–188. doi: 10.1083/jcb.148.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreiter K, Kern F, Velamoor V, Meissl K, Galabova-Kovacs G, Sibilia M, Baccarini M. Raf-1 Addiction in Ras-Induced Skin Carcinogenesis. Cancer Cell. 2009;16:149–160. doi: 10.1016/j.ccr.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Dunham I, Kundaje A, Aldred SF, Collins PJ, Davis CA, Doyle F, Epstein CB, Frietze S, Harrow J, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, Whitsett JA, Koretsky A, Varmus HE. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs IB, Lichtenegger W, Buehler H, Henrich W, Stein H, Kleine-Tebbe A, Schaller G. The prognostic significance of epithelial-mesenchymal transition in breast cancer. Anticancer Res. 2002;22:3415–3419. [PubMed] [Google Scholar]
- González-García A, Pritchard CA, Paterson HF, Mavria G, Stamp G, Marshall CJ ENCODE Project Consortium. RalGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell. 2005;7:219–226. doi: 10.1016/j.ccr.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ramjaun AR, Haiko P, Wang Y, Warne PH, Nicke B, Nye E, Stamp G, Alitalo K, Downward J. Binding of Ras to Phosphoinositide 3-Kinase p110α Is Required for Ras- Driven Tumorigenesis in Mice. Cell. 2007;129:957–968. doi: 10.1016/j.cell.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements: Article: Nature. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Jänne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- Jechlinger M, Podsypanina K, Varmus H. Regulation of transgenes in three-dimensional cultures of primary mouse mammary cells demonstrates oncogene dependence and identifies cells that survive deinduction. Genes Dev. 2009;23:1677–1688. doi: 10.1101/gad.1801809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama H, Shibata K, Terauchi M, Yamashita M, Ino K, Nawa A, Kikkawa F. Chemoresistance to paclitaxel induces epithelial-mesenchymal transition and enhances metastatic potential for epithelial ovarian carcinoma cells. Int J Oncol. 2007;31:277–283. [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kielbasa SM, Klein H, Roider HG, Vingron M, Bluthgen N. TransFind--predicting transcriptional regulators for gene sets. Nucleic Acids Research. 2010;38:W275–W280. doi: 10.1093/nar/gkq438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar JM, Stern P, Liu H, Schindler JW, Jiang Z-G, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 2012;109:E2441–50. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KS, Haigis KM. Non-Redundancy within the RAS Oncogene Family: Insights into Mutational Disparities in Cancer. Molecules and Cells. 2009;28:315–328. doi: 10.1007/s10059-009-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledwith BJ, Manam S, Kraynak AR, Nichols WW, Bradley MO. Antisense-fos RNA causes partial reversion of the transformed phenotypes induced by the c-Ha-ras oncogene. Mol Cell Biol. 1990;10:1545–1555. doi: 10.1128/mcb.10.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KH, Counter CM. Reduction in the requirement of oncogenic Ras signaling to activation of PI3K/AKT pathway during tumor maintenance. CCELL. 2005;8:381–392. doi: 10.1016/j.ccr.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Mechta F, Lallemand D, Pfarr CM, Yaniv M. Transformation by ras modifies AP1 composition and activity. Oncogene. 1997;14:837–847. doi: 10.1038/sj.onc.1200900. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Imoto I, Matsui T, Kozaki KI, Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T, Inazawa J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389–398. doi: 10.1093/carcin/bgq254. [DOI] [PubMed] [Google Scholar]
- O’Donnell A, Odrowaz Z, Sharrocks AD. Immediate-early gene activation by the MAPK pathways: what do and don’t we know? Biochem Soc Trans. 2012;40:58–66. doi: 10.1042/BST20110636. [DOI] [PubMed] [Google Scholar]
- Oka T, Sudol M. Nuclear localization and pro-apoptotic signaling of YAP2 require intact PDZ-binding motif. Genes Cells. 2009;14:607–615. doi: 10.1111/j.1365-2443.2009.01292.x. [DOI] [PubMed] [Google Scholar]
- Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng C-X, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The Hippo Signaling Pathway in Development and Cancer. Developmental Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsypanina K, Politi K, Beverly LJ, Varmus HE. Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci USA. 2008;105:5242–5247. doi: 10.1073/pnas.0801197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nature Publishing Group. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BVVG, Irvine KD. Regulation of Hippo Signaling by EGFR-MAPK Signaling through Ajuba Family Proteins. Developmental Cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez E, Rutberg SE, Mueller E, Oppenheim H, Smoluk J, Yuspa SH, Spiegelman BM. c-fos is required for malignant progression of skin tumors. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- Sharma SV, Settleman J. Oncogene addiction: setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–3231. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, Settleman J. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15:489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M. YAP1 oncogene and its eight isoforms. Oncogene. 2012 doi: 10.1038/onc.2012.520. [DOI] [PubMed] [Google Scholar]
- Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145–2152. [PubMed] [Google Scholar]
- Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. PNAS. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To MD, Rosario RD, Westcott PMK, Banta KL, Balmain A. Interactions between wild-type and mutant Ras genes in lung and skin carcinogenesis. Oncogene. 2013;32:4028–4033. doi: 10.1038/onc.2012.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. CCELL. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–1241. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Degerny C, Xu M, Yang X-J. YAP, TAZ, and Yorkie: a conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol. 2009;87:77–91. doi: 10.1139/O08-114. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ngo VN, Marani M, Yang Y, Wright G, Staudt LM, Downward J. Critical role for transcriptional repressor Snail2 in transformation by oncogenic RAS in colorectal carcinoma cells. Oncogene. 2010;29:4658–4670. doi: 10.1038/onc.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witta SE, Gemmill RM, Hirsch FR, Coldren CD, Hedman K, Ravdel L, Helfrich B, Dziadziuszko R, Chan DC, Sugita M, et al. Restoring E-cadherin expression increases sensitivity to epidermal growth factor receptor inhibitors in lung cancer cell lines. Cancer Res. 2006;66:944–950. doi: 10.1158/0008-5472.CAN-05-1988. [DOI] [PubMed] [Google Scholar]
- Wong CE, Yu JS, Quigley DA, To MD, Jen KY, Huang PY, Del Rosario R, Balmain A. Inflammation and Hras signaling control epithelial-mesenchymal transition during skin tumor progression. Genes Dev. 2013;27:670–682. doi: 10.1101/gad.210427.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W, Meylan E, Oliver TG, Feldser DM, Winslow MM, Bronson R, Jacks T. Response and Resistance to NF-kappaB Inhibitors in Mouse Models of Lung Adenocarcinoma. Cancer Discov. 2011;1:236–247. doi: 10.1158/2159-8290.CD-11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Boehm JS, Yang X, Salehi-Ashtiani K, Hao T, Shen Y, Lubonja R, Thomas SR, Alkan O, Bhimdi T, et al. A public genome-scale lentiviral expression library of human ORFs. Nat Methods. 2011;8:659–661. doi: 10.1038/nmeth.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ji J-Y, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–1450. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Grusche FA, Harvey KF. Control of Tissue Growth and Cell Transformation by the Salvador/Warts/Hippo Pathway. PLoS ONE. 2012;7:e31994. doi: 10.1371/journal.pone.0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL. The Hippo-YAP pathway: new connections between regulation of organ size and cancer. Current Opinion in Cell Biology. 2008a;20:638. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang C-Y, Chinnaiyan AM, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008b;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, McJunkin K, Fellmann C, Dow LE, Taylor MJ, Hannon GJ, Lowe SW. Toolkit for evaluating genes required for proliferation and survival using tetracycline-regulated RNAi. Nat Biotechnol. 2011;29:79–83. doi: 10.1038/nbt.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.