Abstract
Extracellular nucleotides are danger signals involved in recognition and control of intracellular pathogens. They are an important component of the innate immune response against intracellular pathogens, inducing the recruitment of inflammatory cells, stimulating secretion of cytokines, and producing inflammatory mediators such as reactive oxygen species (ROS) and nitric oxide (NO). In the case of extracellular ATP, some of the immune responses are mediated through activation of the NLRP3 inflammasome and secretion of the cytokine, interleukin-1β (IL-1β), through a mechanism dependent on ligation of the P2X7 receptor. Here we review the role of extracellular nucleotides as sensors of intracellular bacteria and protozoan parasites, and discuss how these pathogens manipulate purinergic signaling to diminish the immune response against infection.
Keywords: Intracellular pathogens, Danger signals, Extracellular ATP, Purinergic receptors, Inflammasome, Inflammation
1. Introduction
ATP and other nucleotides are released from cells at sites of inflammation of different tissues undergoing different disease states. The nucleotides can be released by active release or as a result of cell death [1–3]. Once in the extracellular space, the nucleotides behave as “danger signals” and can directly activate two families of membrane-bound nucleotide receptors named P2 receptors. The P2X receptors are non-selective cation ion channels [4], while P2Y receptors are G-coupled receptors [5]. Members of both families of P2R have been implicated in modulation of immunity by controlling cell migration [6–8], cytokine release [9,10], maturation of dendritic cells [11], and the immune response during infections with intracellular pathogens [12]. Thus, extracellular ATP can be secreted under a broad range of conditions including antigen presentation and macrophage interaction with bacteria or microbial products such as lipopolysaccharide (LPS) [13]. Once in the extracellular medium, ATP and UTP can mediate migration of macrophages and neutrophils towards inflamed organs [14,15], function as sensors for apoptotic cells and stimulate phagocytosis of apoptotic cells [14,16,17], and promote maturation of dendritic cells shaping the immune response [18].
P2R activation, in turn, is controlled by a family of ectonucleotidases that hydrolyze nucleotides and regulate the degradation of nucleotides into nucleosides, which can activate adenosine receptors named P1 receptors (P1R) [19,20]. Physiologically, the balance between extracellular nucleotide concentrations, and the expression of P2R, ectonucleotidases and P1R on the cell surface, are responsible for the extent to which purinergic signaling will contribute to the immune response [13] and host resistance to infection by microbial pathogens [21].
This review will summarize mechanisms whereby extracellular nucleotides are used to detect infection with intracellular pathogens (bacteria and protozoan) and stimulate an immune response against the infection.
2. The role of extracellular nucleotides during bacterial infection
Humphrey and Dubyak published in 1996 one of the first reports that LPS from bacteria can modify the sensitivity of macrophages to ATP. They demonstrated that pre-treatment with LPS and IFNγ enhances activation of phospholipase D (PLD), plasma membrane permeability, and cytolysis, as indicated by Ca2+ influx, ethidium bromide uptake, and lactate dehydrogenase release triggered by treatment with the P2X7 agonist benzoyl ATP (BzATP) [22]. Moreover, ATP ligation of P2X7 can modulate secretion of the pro-inflammatory cytokine, IL-1β, induced by LPS [23,24]. It was also shown that extracellular ATP could control mycobacterial infection in mice or human macrophages [25,26]. ATP-mediated inhibition of mycobacterial infection involves activation of PLD and increased phagosome-lysosome fusion [27–29].
Subsequently, a number of reports showed an association between gain-of-function or loss-of-function P2X7 single nucleotide polymorphisms (SNPs) with susceptibility or resistance to different diseases [30], including tuberculosis [31,32].
Chlamydiae are obligate intracellular bacteria that infect epithelial cells and macrophages. The bacteria survive within host cells by interfering with development of the vacuole harboring the bacteria (called an inclusion), blocking its fusion with lysosomes and inhibiting its acidification [33,34]. Using different Chlamydia species and strains, we have shown that extracellular ATP can decrease infection in macrophages and epithelial cells [35–38]. The effects are dependent on P2X7 ligation, PLD activation and phagolysosome formation, since PLD activation favors intracellular vesicle fusion [36]. P2X ligation also affects chlamydial infection in vaginally-infected mice [38]. Thus, vaginal infection was more severe in P2X7-deficient mice, which displayed a higher level of acute inflammation in the endocervix, oviduct, and mesosalpingeal tissues than infected wild-type mice [38]. These findings strongly suggest that purinergic signaling is involved in the immune response against Chlamydia infection. More recently, we have expanded this concept by proposing that activation of PLD may be a general mechanism for elimination of parasites that normally reside within intracellular vacuoles that avoid fusion with lysosomes [37].
Besides directly inhibiting infection, extracellular ATP contributes to the host immune response against intracellular bacteria, through its ability to stimulate P2X7-dependent IL-1β secretion [39,40]. Thus, chlamydial infection stimulates IL-1β or IL-18 secretion through activation of the NLRP3 inflammasome [41,42], and this mechanism may be important for host defense in a model of Chlamydia pneumoniae lung infection [43,44]. Since the activation of P2X7 could control microbial infection through activation of NLRP3 inflammasome and IL-1β or IL-18 secretion, it would not be surprising to observe both pathogen and host adapting to each other through evolution. In this context, it is interesting to note that Chlamydia renders infected cells resistant to ATP-induced host cell apoptosis [35].
Other intracellular bacteria, such as Mycobacterium tuberculosis and Porphyromonas gingivalis, also inhibit P2X7-mediated apoptosis of macrophages or primary gingival epithelial cells (GECs) apoptosis [45–47], which may be mediated by a homolog of nucleoside diphosphate kinase (Ndk) secreted by the bacteria [45,47]. It is possible that the P. gingivalis Ndk may also inhibits inflammation, since ATP treatment induces NLRP3-dependent caspase-1 activation and release of IL-1β in infected GECs cells [48].
Conversely, a leukotoxin, produced by the Gram negative bacterium Aggregatibacter actinomycetemcomitans that is mainly associated with severe forms of periodontitis [49], targets the P2X in human macrophages, inducing cell death through a mechanism known as pyroptosis and IL-1β release [50].
In future studies, it would be interesting to examine whether there is a correlation between the presence of SNPs in P2X7 and periodontal disease or pathogenesis due to vaginal infection with Chlamydia, such as pelvic inflammatory disease, as have been shown for tuberculosis and other inflammatory diseases [30,31].
3. Extracellular nucleotides as sensors for the presence of protozoan parasites
We had originally reported that Trypanosoma cruzi infection can increase the sensitivity of murine thymocytes to the effects of extracellular ATP, which correlates with the thymus involution phase that is typically observed in Chagas’ disease [51]. Moreover, ATP induces P2X7-mediated increase in plasma membrane permeabilization, calcium signaling, and cellular death in CD4+/CD8+ double–positive thymocytes collected from infected mice only during the atrophy phase of disease [51]. However, in vivo assays showing thymus atrophy in P2X7-deficient mice suggested that P2X7 may not be centrally involved in this process [52]. Nonetheless, peritoneal macrophages from infected mice also show decreased expression of P2X7 [52]. There is still little information on how extracellular ATP or P2X receptors contribute to the immune response against T. cruzi infection, but recent studies describing in vivo infection in P2X7-deficient animals showed an effect of P2X7 on migration of mast cells towards the inflamed heart [53], which may reduce the innate immune response. Our data showing reduced mast cell infiltration in the lungs of P2X7-deficient animals following LPS injection [54] support the analysis of T. cruzi infection and suggest that mast cells could play a major role in controlling infection by intracellular parasites through modulation of inflammation, which was not fully appreciated previously in the literature.
Purinergic signaling could also be involved in the immune response against leishmaniasis. Macrophage infection with Leishmania amazonensis positively modulates P2X7 expression, as shown by protein expression of the receptor, and an increase in ATP-induced apoptosis and plasma membrane permeabilization in infected cells [55]. The increase in ATP-induced permeabilization has also been observed in spleen macrophages from Leishmania donovani-infected mice [56]. Furthermore, ligation of P2X7 can control the levels of intracellular Leishmania in macrophages, through a mechanism that is independent of NO secretion but involves ROS production and IL-1β secretion ([55] and unpublished data).
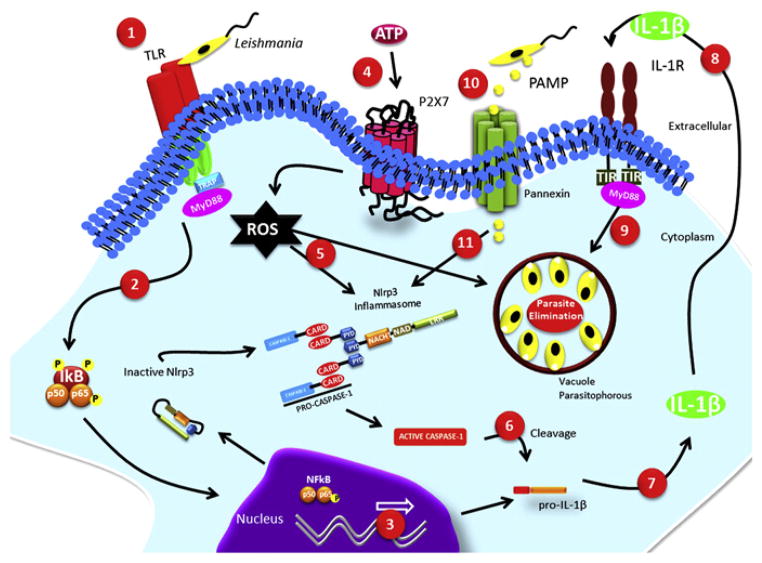
In addition, macrophage infection with L. amazonensis can differentially modulate P2X7-associated dye uptake. While membrane permeabilization for anionic dyes is upregulated, the uptake of cationic dyes is strongly decreased, and the decrease is dependent on active manipulation of the host cell by Leishmania [57]. The positive modulation of anionic dye flow correlates with host defense against parasite infection. P2X7-dependent ion fluxes have been previously associated with pannexin pore formation and NLRP3 inflammasome activation [58,59]. It is possible that the positive modulation of anionic dyes allows pathogen-associated molecular patterns (PAMPs) from the parasite to gain access to the cytoplasm, thereby triggering NLRP3 inflammasome assembly, inducing IL-1β secretion, and culminating with parasite control (Fig. 1) [60].
Fig. 1.
Schematic overview of intracellular parasite elimination following ATP ligation of P2X7. (1) The presence of the parasite triggers a signaling cascade by members of the Toll-like receptor (TLR) family, (2) dephosphorylation of IκB allows NFκB translocation to the nucleous, (3) and expression of genes such as pro-IL-1β. (4) Ligation of P2X7 by extracellular ATP induces ROS production, (5) which can either directly stimulate parasite elimination or activate the NLRP3 inflammasome. (6) Activated caspase-1 cleaves pro-IL-1β, (7) generating the biologically active IL-1β, (8) which acts on its receptor and (9) contributes to the elimination of the parasites. (10) Additionally, PAMPs from parasites can enter the cell through the pannexin pores activated by extracellular ATP, (11) which in turn can also activate the NLRP3 inflammasome.
Interestingly, dead Leishmania also positively modulates uptake of anionic dyes, while having no effect on cationic permeabilization. Therefore, uptake of cationic dyes may not be involved with the host cell response against infection, but it is unclear why the protozoan infection actively down modulates cationic dye fluxes.
In addition to positive modulation of P2X receptor expression, L. amazonensis infection modulates P2Y receptors in infected macrophages. Thus, infected macrophages are more sensitive to UTP-induced intracellular calcium mobilization than uninfected macrophages, and UTP and UDP induce apoptosis only of infected macrophages through a pathway requiring caspase-3 activation. Finally, treatment with the nucleotides reduces the level of parasite infection (Fig. 2), through a mechanism involving production of ROS and NO inflammatory mediators [61].
Fig. 2.
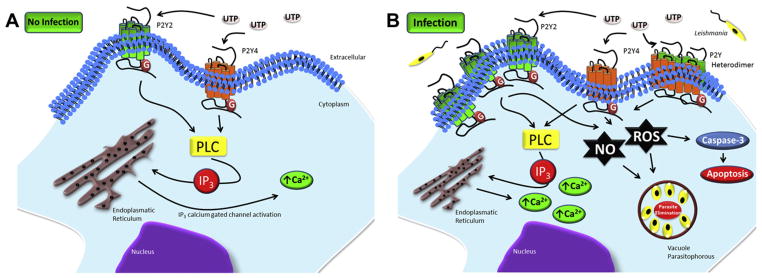
Purinergic signaling cascade due to nucleotide activation of P2Y2 and P2Y4 during Leishmania amazonensis infection. A) There is little signaling through P2Y receptors in uninfected cells. B) In infected cells, enhanced PLC activation and NO and ROS production leads to intracellular calcium mobilization, NO and ROS production, and caspase-3-dependent host cell apoptosis. Taken together, these mechanisms eliminate the parasites or help to contain the infection.
Unlike the effect of P2 receptors, activation of the A2B adenosine receptor seems to contribute to establishment of infection by Leishmania. Thus, inhibition of A2B leads to lower levels of parasitism and fewer lesions in Leishmania-infected mice [62]. In addition, Leishmania infection leads to increased expression of CD39 (ectonucleoside triphosphate diphosphohydrolase) and CD73 (ecto-5′-nucleotidase) on dendritic cells, suggesting that A2B receptor activation may be used by parasites to inhibit dendritic cell function [63].
L. amazonensis infection upregulation of P2X and P2Y receptors in macrophages can therefore be viewed as novel “weapons” used by the host in its fight against infection. While ATP can mediate host immune responses through P2X7-dependent inflammasome activation, the pyrimidinergic nucleotides, through ligation of P2Y, can contribute to host defenses using the well-known inflammatory mediators, ROS and NO [64].
Toxoplasma gondii is an obligate intracellular parasite that infects virtually all nucleated cells. Once inside the host cell, the parasite avoids acidification of the entry vacuole. Recently we and others have reported that ATP ligation of P2X7 leads to fusion between lysosomes and the parasitophorous vacuole, and subsequent elimination of T. gondii tachyzoites in murine or human macrophages [65,66]. Interestingly, ATP has no effect on the parasite burden in infected human macrophages with the 1513A > C loss-of-function polymorphism in P2X7 or murine macrophages from P2X7-deficient mice [66]. Conversely, a gain-of-function polymorphism in P2X7 is associated with resistance to both congenital and ocular toxoplasmosis [32,67]. ATP-induced tachyzoite elimination is NO independent, while ATP induces ROS production and apoptosis in infected macrophages [65,66]. ROS production is an important host defense mechanism against intracellular parasites in general [68], and low levels of ROS lead to NLRP3 inflammasome-mediated caspase-1 activation [40,40,69]. Therefore it is tempting to speculate that ATP-induced ROS production in T. gondii-infected macrophages correlates with NLRP3 inflammasome-mediated caspase-1 activation and IL-1β secretion, as has been observed for other intracellular parasites [68]. Moreover, studies have demonstrated the protective role of IL-1β in mice against infection with intracellular pathogens, including Chlamydia muridarum, Mycobacterium avium and T. gondii [70–72].
4. Concluding remarks
Virtually every known multicellular or unicellular organism has the ability to detect changes in extracellular ATP concentrations, and purinergic signaling represents a primordial form of chemical intercellular signaling [73]. It is therefore reasonable that, during the co-evolution of parasites and humans, the latter have developed different mechanisms for detecting the presence of pathogens through the use of this ancient sensor of nucleotides. Not surprisingly, extracellular nucleotides can rally efficiently various cells of the innate immune system against intracellular parasites, such as macrophages, neutrophils, eosinophils, and mast cells [7,8,14,15,54,74].
On the other hand, different pathogens have evolved the ability to interfere with this important signaling pathway in order to favor infection. Thus, pathogens such as Staphylococcus aureus, Escherichia coli and Bacillus anthracis have developed toxins that target P2X7 and induce cell death of macrophages or lysis of erythrocytes [75–78]. While others, such as M. tuberculosis, Mycobacterium bovis, L. amazonensis, P. gingivalis, and T. gondii secrete Ndk, which scavenge extracellular ATP and act as a virulence factor [45,79–81] (Table 1). Thus, identification of strategies used by different intracellular pathogens to manipulate purinergic signaling, or defense mechanisms relying on purinergic receptors and used by the immune system to eliminate infection, could lead the way to development of future therapeutic interventions targeting purinergic receptors and downstream mediators.
Table 1.
Effects of extracellular nucleotides on intracellular pathogens, and effects of pathogens on purineric receptor signaling.
| Cell type | Pathogen or pathogen toxin | Effect described | Purinergic receptor subtype involved | References |
|---|---|---|---|---|
| Human macrophages | BCG | Mycobacterial clearance | P2X7, P2Y | [25,26] |
| Human macrophages | BCG, M. tuberculosis | Mycobacterial clearance, inhibition of ATP-induced permeabilization | P2X7 | [82] |
| Human macrophages | M. tuberculosis | Mycobacterial clearance, PLD activation | P2X7 + P2(?) | [28] |
| Murine macrophages and J774 cell line | BCG | Mycobacterial clearance, PLD activation, phagosome-lysosome fusion | [27] | |
| Murine macrophages and cell line J774 | Chlamydia caviae | Inhibition of apoptosis induced by ATP, down regulation of P2X7, chlamydial clearance | P2X7 | [35] |
| Murine macrophages | Chlamydia trachomatis | Chlamydial clearance, PLD activation, increase in phagolysosome formation | P2X7 | [36] |
| Human cervical epithelial cells | Chlamydia trachomatis | Chlamydial clearance, PLD activation | P2X7 | [38] |
| Human cervical epithelial cells | Chlamydia trachomatis | Chlamydial retarded growth, increase of cAMP | A2B | [83] |
| Gingival epithelial cells | Porphyromonas gingivalis | Inhibition of apoptosis induced by ATP, P. gingivalis clearance | P2X7 | [45] |
| Gingival epithelial cells | Porphyromonas gingivalis | Caspase-1 activation and IL-1β secretion, P. gingivalis clearance | P2X7 | [48] |
| Human macrophages | Aggregatibacter actinomycetemcomitans (leukotoxin) | Caspase-1 activation and IL-1β secretion (pyroptosis) | P2X7 | [50] |
| Murine macrophages and RAW274.6 macrophages | Bacillus anthracis (lethal toxin) | Pannexin pore opening, caspase-1 activation and IL-1β secretion, P38 MAPK inhibition, macrophage death | P2X7 | [75,76] |
| Equine, murine and human erythrocytes | Escherichia coli (α-Hemolysin) | Pannexin pore opening, (hemolysis) | P2X1, P2X7 | [77] |
| Murine and equine erythrocytes | Staphylococcus aureus (α-toxin) | Pannexin pore opening, (hemolysis) | P2X1, P2X7 | [78] |
| Murine thymocytes | T. cruzi | Modulation of thymocytes death | P2X7(?) | [51,52] |
| Murine macrophages | T. cruzi | Inhibition of ATP-induced permeabilization | P2X7 | [52] |
| Murine macrophages | L. amazonensis, L. donovani | Parasite clearance, increased apoptosis and permeabilization induced by ATP, reduced cationic permeabilization | P2X7 | [55,57] |
| Murine macrophages | L. amazonenis | Parasite clearance, increased Ca2+, apoptosis, ROS and NO production | P2Y2, P2Y4, P2Y2/P2Y4 hetero-oligomerization (?) | [61], |
| Murine dendritic cells | L. amazonensis | Modulation of dendritic cell function (reduce T cell proliferation) | A2B | [63] |
| Murine macrophages | L. amazonensis | Increase in parasite and load lesions | A2B | [62] |
| Human and murine macrophages | T. gondii | Parasite clearance, phagolysosome formation, ROS production, host cell apoptosis | P2X7 | [65,66] |
PLD = phospholipase D; ROS = reactive oxygen species; cAMP = cyclic AMP; IL-1β = interleukin-1β; NO = nitric oxide.
Acknowledgments
We would like to thank Ms. Leonardo Campos Monção for help designing the figures. This work was supported by funds from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil (CNPq), Instituto Nacional para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica (INCT-INPeTAm/CNPq/MCT), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Programa de Apoio a Núcleos de Excelência (PRONEX) Brasil, National Institutes of Health grant R01 DE019444, and a Presidential Endowed Chair from the University of California.
References
- 1.Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res. 2010;70:855–858. doi: 10.1158/0008-5472.CAN-09-3566. [DOI] [PubMed] [Google Scholar]
- 2.Burnstock G. Purinergic signalling. Br J Pharmacol. 2006;147(Suppl 1):S172–S181. doi: 10.1038/sj.bjp.0706429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International union of pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kukulski F, Ben YF, Lecka J, Kauffenstein G, Levesque SA, Martin-Satue M, Sevigny J. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46:166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, Robaye B, Conley PB, Kim HC, Sargin S, Schon P, Schwab A, Hanley PJ. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3:ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 8.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi OR, Di Virgilio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 10.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 11.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G, Meyer MP, Clarke JD, Patel K, Townsend-Nicholson A, Burnstock G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho-Silva R, Correa G, Sater AA, Ojcius DM. The P2X(7) receptor and intracellular pathogens: a continuing struggle. Purinergic Signal. 2009;5:197–204. doi: 10.1007/s11302-009-9130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marques-da-Silva C, Burnstock G, Ojcius DM, Coutinho-Silva R. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology. 2011;216:1–11. doi: 10.1016/j.imbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 18.la Sala A, Sebastiani S, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood. 2002;99:1715–1722. doi: 10.1182/blood.v99.5.1715. [DOI] [PubMed] [Google Scholar]
- 19.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 20.Verzijl D, Ijzerman AP. Functional selectivity of adenosine receptor ligands. Purinergic Signal. 2011;7:171–192. doi: 10.1007/s11302-011-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Humphreys BD, Dubyak GR. Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-gamma in the human THP-1 monocytic cell line. J Immunol. 1996;157:5627–5637. [PubMed] [Google Scholar]
- 23.Perregaux D, Gabel CA. Interleukin-1β maturation and release in response to ATP and nigericin. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 24.Perregaux DG, McNiff P, Laliberte R, Conklyn M, Gabel CA. ATP acts as an agonist to promote stimulus-induced secretion of IL-1 beta and IL-18 in human blood. J Immunol. 2000;165:4615–4623. doi: 10.4049/jimmunol.165.8.4615. [DOI] [PubMed] [Google Scholar]
- 25.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 26.Stober CB, Lammas DA, Li CM, Kumararatne DS, Lightman SL, McArdle CA. ATP-mediated killing of Mycobacterium bovis Bacille calmette-guerin within human macrophages is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J Immunol. 2001;166:6276–6286. doi: 10.4049/jimmunol.166.10.6276. [DOI] [PubMed] [Google Scholar]
- 27.Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X(7)-dependent process inducing bacterial death by phagosomelysosome fusion. J Immunol. 2001;167:3300–3307. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 28.Kusner DJ, Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J Immunol. 2000;164:379–388. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 29.Kusner DJ, Barton JA. ATP stimulates human macrophages to kill intracellular virulent Mycobacterium tuberculosis via calcium-dependent phagosomelysosome fusion. J Immunol. 2001;167:3308–3315. doi: 10.4049/jimmunol.167.6.3308. [DOI] [PubMed] [Google Scholar]
- 30.Fuller SJ, Stokes L, Skarratt KK, Gu BJ, Wiley JS. Genetics of the P2X7 receptor and human disease. Purinergic Signal. 2009;5:257–262. doi: 10.1007/s11302-009-9136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singla N, Gupta D, Joshi A, Batra N, Singh J. Genetic polymorphisms in the P2X7 gene and its association with susceptibility to tuberculosis. Int J Tuberc Lung Dis. 2011 doi: 10.5588/ijtld.11.0076. [DOI] [PubMed] [Google Scholar]
- 32.Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM, Wiley JS, Smith NC. The role of the P2X(7) receptor in infectious diseases. PLoS Pathog. 2011;7:e1002212. doi: 10.1371/journal.ppat.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193:137–152. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- 34.Wyrick PB. Intracellular survival by Chlamydia. Cell Microbiol. 2000;2:275–282. doi: 10.1046/j.1462-5822.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 35.Coutinho-Silva R, Perfettini J-L, Persechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol. 2001;280:C81–C89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 36.Coutinho-Silva R, Stahl L, Raymond M-N, Jungas T, Verbeke P, Burnstock G, Darville T, Ojcius DM. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19:403–412. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 37.Coutinho-Silva R, da Cruz CM, Persechini PM, Ojcius DM. The role of P2 receptors in controlling infections by intracellular pathogens. Purinergic Signal. 2007;3:83–90. doi: 10.1007/s11302-006-9039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darville T, Welter-Stahl L, Cruz C, Sater AA, Andrews CW, Jr, Ojcius DM. Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol. 2007;179:3707–3714. doi: 10.4049/jimmunol.179.6.3707. [DOI] [PubMed] [Google Scholar]
- 39.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice Lacking P2X7 receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 40.Said-Sadier N, Ojcius DM. Alarmins. Inflammasomes Immun. 2012 doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdul-Sater AA, Said-Sadier N, Padilla EV, Ojcius DM. Chlamydial infection of monocytes stimulates IL-1beta secretion through activation of the NLRP3 inflammasome. Microbes Infect. 2010;12:652–661. doi: 10.1016/j.micinf.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagarajan UM, Sikes JD, Yeruva L, Prantner D. Significant role of IL-1 signaling, but Limited role of inflammasome activation, in oviduct Pathology during Chlamydia muridarum genital infection. J Immunol. 2012;188:2866–2875. doi: 10.4049/jimmunol.1103461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, Vergnes L, Ojcius DM, Arditi M. Caspase-1 dependent IL-1beta secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One. 2011;6:e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He X, Mekasha S, Mavrogiorgos N, Fitzgerald KA, Lien E, Ingalls RR. Inflammation and fibrosis during Chlamydia pneumoniae infection is regulated by IL-1 and the NLRP3/ASC inflammasome. J Immunol. 2010;184:5743–5754. doi: 10.4049/jimmunol.0903937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, Lamont RJ, Ojcius DM. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 2008;10:863–875. doi: 10.1111/j.1462-5822.2007.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spooner R, Yilmaz O. Nucleoside-diphosphate-kinase: a pleiotropic effector in microbial colonization under interdisciplinary characterization. Microbes Infect. 2012;14:228–237. doi: 10.1016/j.micinf.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dar HH, Prasad D, Varshney GC, Chakraborti PK. Secretory nucleoside diphosphate kinases from both intra- and extracellular pathogenic bacteria are functionally indistinguishable. Microbiology. 2011;157:3024–3035. doi: 10.1099/mic.0.049221-0. [DOI] [PubMed] [Google Scholar]
- 48.Yilmaz O, Sater AA, Yao L, Koutouzis T, Pettengill M, Ojcius DM. ATP-dependent activation of an inflammasome in primary gingival epithelial cells infected by Porphyromonas gingivalis. Cell Microbiol. 2010;12:188–198. doi: 10.1111/j.1462-5822.2009.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 50.Kelk P, Abd H, Claesson R, Sandstrom G, Sjostedt A, Johansson A. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis. 2011;2:e126. doi: 10.1038/cddis.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantuano MB, Henriques-Pons A, Araujo-Jorge TC, Di Virgilio F, Coutinho-Silva R, Persechini PM. Extracellular ATP induces cell death in CD4+/CD8+ double-positive thymocytes in mice infected with Trypanosoma cruzi. Microbes Infect. 2003;5:1363–1371. doi: 10.1016/j.micinf.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Cascabulho CM, Menna-Barreto RF, Coutinho-Silva R, Persechini PM, Henriques-Pons A. P2X7 modulatory web in Trypanosoma cruzi infection. Parasitol Res. 2008;103:829–838. doi: 10.1007/s00436-008-1063-8. [DOI] [PubMed] [Google Scholar]
- 53.Meuser-Batista M, Correa JR, Carvalho VF, de Carvalho Britto CF, da Cruz MO, Batista MM, Soares MJ, Filho FA, ESPM, Lannes-Vieira J, Coutinho-Silva R, Henriques-Pons A. Mast cell function and death in Trypanosoma cruzi infection. Am J Pathol. 2011;179:1894–1904. doi: 10.1016/j.ajpath.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moncao-Ribeiro LC, Cagido VR, Lima-Murad G, Santana PT, Riva DR, Borojevic R, Zin WA, Cavalcante MC, Rica I, Brando-Lima AC, Takiya CM, Faffe DS, Coutinho-Silva R. Lipopolysaccharide-induced lung injury: role of P2X7 receptor. Respir Physiol Neurobiol. 2011 doi: 10.1016/j.resp.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Chaves SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B. Modulation of P2X(7) purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009;11:842–849. doi: 10.1016/j.micinf.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Torres-Santos EC, Mantuano MB, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B. Enhanced macrophage permeabilization by ATP upon Leishmania infection. Drug Dev Res. 2000;50(1):61. [Google Scholar]
- 57.Marques-da-Silva C, Chaves MM, Rodrigues JC, Corte-Real S, Coutinho-Silva R, Persechini PM. Differential modulation of ATP-Induced P2X7-Associated Permeabilities to cations and Anions of macrophages by infection with Leishmania amazonensis. PLoS One. 2011;6:e25356. doi: 10.1371/journal.pone.0025356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 59.Pelegrin P, Surprenant A. The P2X(7) receptor-pannexin connection to dye uptake and IL-1beta release. Purinergic Signal. 2009;5:129–137. doi: 10.1007/s11302-009-9141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;19(140):821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 61.Marques-da-Silva C, Chaves MM, Chaves SP, Figliuolo VR, Meyer-Fernandes JR, Corte-Real S, Lameu C, Ulrich H, Ojcius DM, Rossi-Bergmann B, Coutinho-Silva R. Infection with Leishmania amazonensis upregulates purinergic receptor expression and induces host-cell susceptibility to UTP-mediated apoptosis. Cell Microbiol. 2011;13:1410–1428. doi: 10.1111/j.1462-5822.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 62.de Almeida Marques-da-Silva E, de Oliveira JC, Figueiredo AB, de Souza Lima JD, Carneiro CM, Rangel Fietto JL, Crocco Afonso LC. Extracellular nucleotide metabolism in Leishmania: influence of adenosine in the establishment of infection. Microbes Infect. 2008;10:850–857. doi: 10.1016/j.micinf.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Figueiredo AB, Serafim TD, Marques-da-Silva EA, Meyer-Fernandes JR, Afonso LC. Leishmania amazonensis impairs DC function by inhibiting CD40 expression via A(2B) adenosine receptor activation. Eur J Immunol. 2012;42(5):1203–1215. doi: 10.1002/eji.201141926. [DOI] [PubMed] [Google Scholar]
- 64.Mukbel RM, Patten C, Jr, Gibson K, Ghosh M, Petersen C, Jones DE. Macrophage killing of Leishmania amazonensis amastigotes requires both nitric oxide and superoxide. Am J Trop Med Hyg. 2007;76:669–675. [PubMed] [Google Scholar]
- 65.Correa G, Marques da SC, de Abreu Moreira-Souza AC, Vommaro RC, Coutinho-Silva R. Activation of the P2X(7) receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, Zakrzewski AM, Mui EJ, Witola WH, Coyne JJ, Hargrave AC, Jamieson SE, Blackwell JM, Wiley JS, Smith NC. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J Immunol. 2010;184:7040–7046. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix LA, Mui EJ, Boulter NR, Miller EN, Fuller SJ, Wiley JS, Castellucci L, Boyer K, Peixe RG, Kirisits MJ, Elias LS, Coyne JJ, Correa-Oliveira R, Sautter M, Smith NC, Lees MP, Swisher CN, Bahia-Oliveira LM, McLeod R, Blackwell JM. Evidence for associations between the purinergic receptor P2X(7) (P2RX7) and toxoplasmosis. Genes Immun. 2010;11:374–383. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spooner R, Yilmaz O. The role of reactive-oxygen-species in microbial persistence and inflammation. Int J Mol Sci. 2011;12:334–352. doi: 10.3390/ijms12010334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abdul-Sater AA, Said-Sadier N, Ojcius DM, Yilmaz O, Kelly KA. Inflammasomes bridge signaling between pathogen identification and the immune response. Drugs Today (Barc) 2009;45(Suppl B):105–112. [PMC free article] [PubMed] [Google Scholar]
- 70.Denis M, Ghadirian E. Interleukin-1 is involved in mouse resistance to Mycobacterium avium. Infect Immun. 1994;62:457–461. doi: 10.1128/iai.62.2.457-461.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown CR, Hunter CA, Estes RG, Beckmann E, Forman J, David C, Remington JS, McLeod R. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology. 1995;85:419–428. [PMC free article] [PubMed] [Google Scholar]
- 72.Prantner D, Darville T, Sikes JD, Andrews CW, Jr, Brade H, Rank RG, Nagarajan UM. Critical role for interleukin-1beta (IL-1beta) during Chlamydia muridarum genital infection and bacterial replication-independent secretion of IL-1beta in mouse macrophages. Infect Immun. 2009;77:5334–5346. doi: 10.1128/IAI.00883-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf) 2009;195:415–447. doi: 10.1111/j.1748-1716.2009.01957.x. [DOI] [PubMed] [Google Scholar]
- 74.McCloskey MA, Fan Y, Luther S. Chemotaxis of rat mast cells toward adenine nucleotides. J Immunol. 1999;163:970–977. [PubMed] [Google Scholar]
- 75.Moayeri M, Wickliffe KE, Wiggins JF, Leppla SH. Oxidized ATP protection against anthrax lethal toxin. Infect Immun. 2006;74:3707–3714. doi: 10.1128/IAI.00051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ali SR, Timmer AM, Bilgrami S, Park EJ, Eckmann L, Nizet V, Karin M. Anthrax toxin induces macrophage death by p38 MAPK inhibition but leads to inflammasome activation via ATP leakage. Immunity. 2011;35:34–44. doi: 10.1016/j.immuni.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skals M, Jorgensen NR, Leipziger J, Praetorius HA. Alpha-hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. Proc Natl Acad Sci USA. 2009;106:4030–4035. doi: 10.1073/pnas.0807044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skals M, Leipziger J, Praetorius HA. Haemolysis induced by alpha-toxin from Staphylococcus aureus requires P2X receptor activation. Pflugers Arch. 2011;462:669–679. doi: 10.1007/s00424-011-1010-x. [DOI] [PubMed] [Google Scholar]
- 79.Kolli BK, Kostal J, Zaborina O, Chakrabarty AM, Chang KP. Leishmania-released nucleoside diphosphate kinase prevents ATP-mediated cytolysis of macrophages. Mol Biochem Parasitol. 2008;158:163–175. doi: 10.1016/j.molbiopara.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaborina O, Li X, Cheng G, Kapatral V, Chakrabarty AM. Secretion of ATP-utilizing enzymes, nucleoside diphosphate kinase and ATPase, by Mycobacterium bovis BCG: sequestration of ATP from macrophage P2Z receptors? Mol Microbiol. 1999;31:1333–1343. doi: 10.1046/j.1365-2958.1999.01240.x. [DOI] [PubMed] [Google Scholar]
- 81.Nakaar V, Beckers CJ, Polotsky V, Joiner KA. Basis for substrate specificity of the Toxoplasma gondii nucleoside triphosphate hydrolase. Mol Biochem Parasitol. 1998;97:209–220. doi: 10.1016/s0166-6851(98)00153-4. [DOI] [PubMed] [Google Scholar]
- 82.Gan H, He X, Duan L, Mirabile-Levens E, Kornfeld H, Remold HG. Enhancement of antimycobacterial activity of macrophages by stabilization of inner mitochondrial membrane potential. J Infect Dis. 2005;191:1292–1300. doi: 10.1086/428906. [DOI] [PubMed] [Google Scholar]
- 83.Pettengill MA, Lam VW, Ojcius DM. The danger signal adenosine induces persistence of chlamydial infection through stimulation of A2b receptors. PLoS One. 2009;4:e8299. doi: 10.1371/journal.pone.0008299. [DOI] [PMC free article] [PubMed] [Google Scholar]