Abstract
The localized conversion of inactive doxorubicin prodrug chemotherapeutics to pharmacalogically active forms is difficult to quantify in mouse tumor models because it occurs only in small regions of tissue. The tumor tissue extraction protocol and LC-MS/MS analysis method described here were optimized to obtain a detection limit of 7.8 pg for the activated doxorubicin and 0.36 ng for the doxorubicin prodrug. This method can be useful for determining the biodistribution and activation efficiency for many different doxorubicin prodrugs. It can also be used for quantification of doxorubicin from tumor models that have poor vascularization resulting in low tissue accumulation.
Keywords: doxorubicin, prodrug, tissue extraction, LC-MS/MS
1. Introduction
Doxorubicin (DOX) is one of the frontline drugs currently used for chemotherapy treatment of cancer and is very successful at reducing tumor loads in patients.1 However, the use of DOX has been limited in the clinic due to harmful side effects. The biggest concerns are adverse effects to the gastrointestinal tract 2, damage to the hematopoietic system causing suppression of the immune system 2, and the increased chances of long term cardiomyopathy and congestive heart failure.3-5 These side effects are of particular concern in children.6, 7 This has led to the development of chemically modified versions of chemotherapy drugs called prodrugs. The inactive prodrug is administered systemically with subsequent conversion to active drug in the tumor tissue.5, 8, 9 There are many different mechanisms used to trigger the conversion including environmental factors, such as hypoxia or low pH 10, 11, and metabolic cleavage by enzymes that are secreted by the tumor tissue at higher levels than elsewhere in the body.12-14 A photocleavable biotin (PCB) conjugated DOX prodrug has recently been developed called DOX-PCB (Figure 1).15 This prodrug has been shown to have 200 times less toxicity than DOX with the ability to release pure DOX when exposed to 365 nm light.15 The released DOX was observed to intercalate just like normal DOX into cellular DNA.15
Figure 1.
Chemical structure of DOX-PCB with the three products resulting from photocleavage including pure DOX.
Xenograft mouse tumor models were used to study the biodistribution of DOX-PCB administered by intravascular injection, as well as the biodistribution of the released DOX. The xenograft tumors were mainly vascularized in a thin region on the surface where the DOX-PCB was accumulated. The DOX released after light exposure required a highly sensitive tissue extraction and mass spectrometry analysis because the small volume of tissue resulted in small amounts of DOX. A highly sensitive extraction method was required to extract and quantify both DOX-PCB and the small amount of DOX from these tissue samples.
Both DOX-PCB and DOX are naturally fluorescent preventing fluorescence detection and quantification techniques from being able to discern between the two.16, 17 Methods have been developed to perform simultaneous extractions of different compounds, including nanogram amounts of DOX, from plasma or urine using methanol extracton 18, 19, acetonitrile extraction 20, and solid phase extraction.21 These methods are very useful for determining circulation time of these different compounds. However, one of the main sinks of DOX in tissue is its natural intercalation into DNA.15 These plasma and urine extraction methods efficiently extract DOX but are not designed to remove DOX from DNA since the red blood cells, plasma, and urine are mostly devoid of DNA. DOX-PCB was shown to enter cells where the majority of the released DOX directly intercalated into the DNA.15 Plasma extraction techniques which relied only on methanol or acetonitrile extraction were not able to detect any DOX from probe sonicated homogenates of our tumor tissue samples. It appeared that the intercalated DOX was not being released from the tissue samples in sufficient concentrations.
Methods using carbon nanotubes have been developed to extract very small quantities of DOX from different tissue homogenates by taking advantage of the DOX adsorption to the carbon nanotubes via supramolecular π-π stacking interactions between the aromatic groups of the DOX aglycone and the surface of the nanotubes.22 These carbon nanotubes serve as a stronger sink than the DNA allowing DOX to accumulate preferentially on the nanotubes over time when incubated in a homogenized tissue sample. The nanotubes can be separated using magnetic beads and then treated to release the DOX for subsequent analysis.22 This method works to detect even picogram amounts of DOX. However, the DOX-PCB prodrug has a hairpin conformation where the nitrophenol group and the PEG group sequesters the DOX aglycone aromatic ring structure.23 Sequestering the aglycone is one of the mechanisms by which the DOX-PCB prodrug prevents the DOX portion from intercalating into DNA reducing its toxicity. The carbon nanotubes would not be able to extract both DOX and DOX-PCB at the same time from the same sample and could have problems with other prodrug designs as well.
A new method was needed to release the small amounts of DOX from within the DNA of the tissue samples and still allow DOX-PCB to be recovered from the same sample. A modified salt precipitation protocol was developed here to achieve these goals. High levels of salt can be used to denature and precipitate the DNA from homogenized tissue samples releasing the intercalated DOX from the double helix structure.24-26 However, it was found that the high level of salt interfered with the separation of DOX and the internal standard epirubicin (EPI) on the LC column. EPI is an isomeric form of DOX with similar DNA intercalation properties. Additional steps were added to the protocol to cause precipitation of the DOX from the solvent solution, with subsequent precipitation of the salt. The LC-MS/MS method was optimized using tissue samples spiked with known amounts of DOX, EPI, and DOX-PCB. The optimized LC-MS/MS method was used successfully to quantify DOX in actual in-vivo tumor tissue samples from mice injected with DOX-PCB with subsequent activation to DOX.
2 Experimental
2.1 Materials
Doxorubicin hydrochloride (DOX) was obtained from Qventas (Branford, CT, USA) and Sigma (St. Louis, MO, USA). The Epirubicin (EPI) internal standard was purchased from Sigma (St. Louis, MO, USA). Water soluble photocleavable biotin– N-HydroxySuccinimide (PCB) was purchased from Ambergen (Watertown, MA, USA). HPLC grade acetonitrile and methanol were purchased from Fisher Scientific (Fairlawn, NJ, USA). The athymic nu/nu nude mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The human lung cancer cell line A549 purchased from the American Type Culture Collection (ATCC) (Manassas, VA). The Captisol® was purchased from Ligand Pharmaceuticals (La Jolla, CA).
2.2 Tumor Tissue Samples
The tumor tissue samples used in this study were obtained from xenograft tumor mouse models. The tumors were generated by injecting 200 μl of a (1:1)(v:v) matrigel and PBS mixture containing A549 human lung cancer cells at a concentration of 1 × 108 cells/ml into the left and right flanks of 5 female athymic nu/nu nude mice. The tumors were allowed to grow for 1 month, at which time they reached an approximate size of 1cm3. The administration of DOX-PCB with subsequent light activation is detailed in Ibsen et. al.27 Mice were maintained under standard conditions in the University of California, San Diego Animal Facility. All animal protocols received prior approval by the institutional review board.
2.3 Standard Solution Preparation
Standard solutions of both DOX and the internal standard epirubicin (EPI) were prepared by dissolving 3 mg of each into 30 ml of methanol. A standard solution of DOX-PCB was prepared by dissolving 1 mg of DOX-PCB into 10 ml of acetonitrile (ACN). These standard solutions were then diluted with 5% methanol in water with 0.1% formic acid (mobile phase A from the LC/MS/MS method) and used to calibrate the LC-MS/MS system.
2.4 Tissue Extraction
The following methods were developed and used for two different types of tissue extraction experiments. The first were tissue samples spiked with known amounts of DOX and EPI for the extraction efficiency studies. The second were tissue samples collected from mice that were injected with just the DOX-PCB prodrug and had subsequent activation to pure DOX.
2.4.1 Tissue Preparation
30 mg of tumor tissue was frozen in liquid nitrogen and freeze fractured using a liquid nitrogen cooled metal mortar and pestle. 400 μl of a 50 mM ascorbic acid buffer with 2mM D-L Saccharic acid raised to a pH of 4.5 by titration with 1 M NaOH was added to the tissue powder. This buffer solution stopped any enzymatic degradation of DOX or DOX-PCB within the tissue although it was observed that a 15 min incubation with the tissue powder in PBS did not cause any measurable degradation of DOX. The tissue was aspirated 20 times in the buffer to break the compacted powder chunks into a fine suspension. The sample was spiked with 20 ng of the internal standard EPI from a 100× dilution of the standard solution with water. The spiked EPI sample was allowed to incubate for 15 min to allow for tissue integration. 20 ng of DOX and 224 ng of DOX-PCB were also spiked into the tumor tissue at this point for samples used in the extraction efficiency study. A greater amount of DOX-PCB was used for subsequent light activation studies.
2.4.2 DNA Salt Precipitation
The DNA was denatured from its double helix form to single stranded form to cause the release of any intercalated DOX by addition of 50μl of 3M AgNO3 which caused the suspension to turn dark black. The samples were aspirated 20 times and vortexed for 5 min. The excess Ag+ ions were precipitated by the addition of 50 μl of 3M NaCl upon which the solution turned light grey. The sample was aspirated 20 times and vortexed for 5 min. To precipitate out proteins and other water soluble compounds 1.25 mL of acetonitrile/methanol (2:1)(v:v) was added. The sample was aspirated 20 times, vortexed for 10 min and then bath sonicated for 10 min. The sample was then centrifuged for 5 min at 3186 × g. The supernatant was transferred to 2 ml microcentrifuge tubes and centrifuged again at 17,500 × g for 5 min to remove any possible particulate residues. The sample was then dried in a centrifugal evaporator for approximately 3 hours to a volume of 100 μl to remove the acetonitrile, methanol, and most of the water. A large amount of precipitation occurred. It was determined that the vast majority of the DOX and DOX-PCB was contained within the precipitation. The sample was centrifuged at 17,500 × g for 5 min and the water removed.
2.4.3 DOX Extraction from the Precipitant
200 μl of acetonitrile was added to the precipitant and bath sonicated for 5 min. A white salt precipitant was left behind. Precipitation of the salt was necessary to achieve good separation between the DOX and EPI compounds on HPLC. The sample was concentrated in a centrifugal evaporator to a final volume of 75 μl. The sample was centrifuged again at 17,500 × g for 5 min and the supernatant transferred to a new 1.5 ml microcentrifuge tube for LC-MS\MS analysis. 6.0 μl of the final concentrated 75μl extraction supernatant was injected into the LC column for LC-MS/MS analysis.
2.4.4 LC-MS\MS Analysis
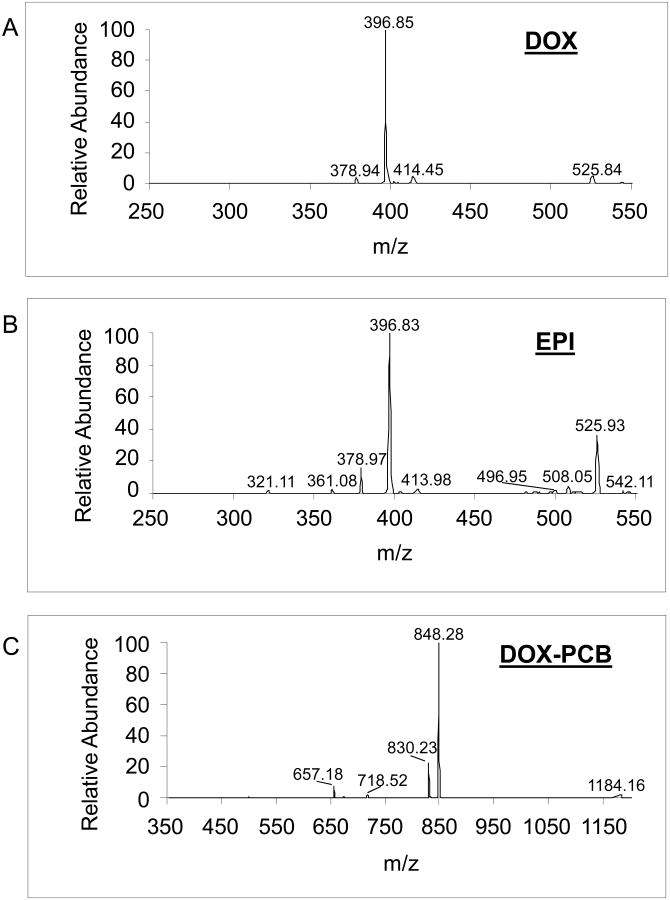
An Agilent 1260 liquid chromatograph (LC) system coupled with a Thermo LCQdeca mass spectrometer (MS) was used to perform the LC-MS/MS analysis using positive ion mode electrospray ionization (ESI) as the ion source with source voltage of 5 kV, sheath gas flow rate of 80 units, auxiliary gas flow rate of 20 units, and capillary temperature of 250 °C. A CAPCELL MG III C-18 column (Catalog number 92744, ID 2.0mm × length 50mm, particle size 3 μm) with guard column was employed for LC separation using 5% methanol in water with 0.1% formic acid as the mobile phase A and pure methanol with 0.1% formic acid as the mobile phase B. The LC flow rate was set at 0.20 ml/min. The LC gradient increased from 30% mobile phase B to 95% mobile phase B in 10 minutes, held at 95% B for 5 minutes, returned to 30% B in 1 minute, and then held at 30% B for 6 minutes. With above-mentioned LC conditions, DOX, EPI, and the two isomers of DOX-PCB were eluted separately from the LC column with baseline separation at a retention time of ∼9.8 for DOX, ∼10.1 for EPI, and ∼13.2 and 13.7 minutes for the two DOX-PCB isomers (Figure 2). Molecular ion peaks were observed for both DOX and EPI at m/z 544 ([M+H]+). Under positive ion mode ESI-MS/MS analysis, a major fragment peak of both DOX and EPI ([M+H]+) was observed at m/z 396.8 with a normalized collision energy of 30%. Molecular ion peaks at m/z 1244.4 ([M+Na]+) were observed for the DOX-PCB isomers. Under positive ion mode ESI-MS/MS analysis a major fragment peak ([M+Na]+) was observed at m/z 848.3 with a normalized collision energy of 35%. LC-ESI-MS/MS analysis was performed on DOX, EPI, and DOX-PCB spiked mixture controls along with the actual unknown samples. Selected reaction monitoring (SRM) mode was used to acquire the m/z 396.8 and m/z 848.3 fragment ion peaks. The SRM peak area ratio (DOX/EPI and DOX-PCB/EPI) related to the amount of drug loaded (DOX or DOX-PCB) and was used for the quantification of DOX and DOX-PCB.
Figure 2.
LC-MS/MS chromatograms from tumor tissue showing that both DOX and DOX–PCB were successfully extracted from samples of tumor tissue spiked with 20 ng of DOX, 20 ng of EPI, and 224 ng of DOX-PCB. 6.0 μl of the final concentrated 75μl extraction volume was injected into the LC column for a total loading of 1.6 ng of DOX, 1.6 ng of EPI, and 17.9 ng of DOX-PCB. The two isomeric forms of DOX-PCB were separated from one another.
3. Results and Discussion
The LC-MS/MS method described above resulted in complete separation of DOX and EPI on the LC column as shown in Figure 2. This prevented any interference between the spiked EPI internal standard and the DOX. DOX-PCB was separated into its two isomeric forms.
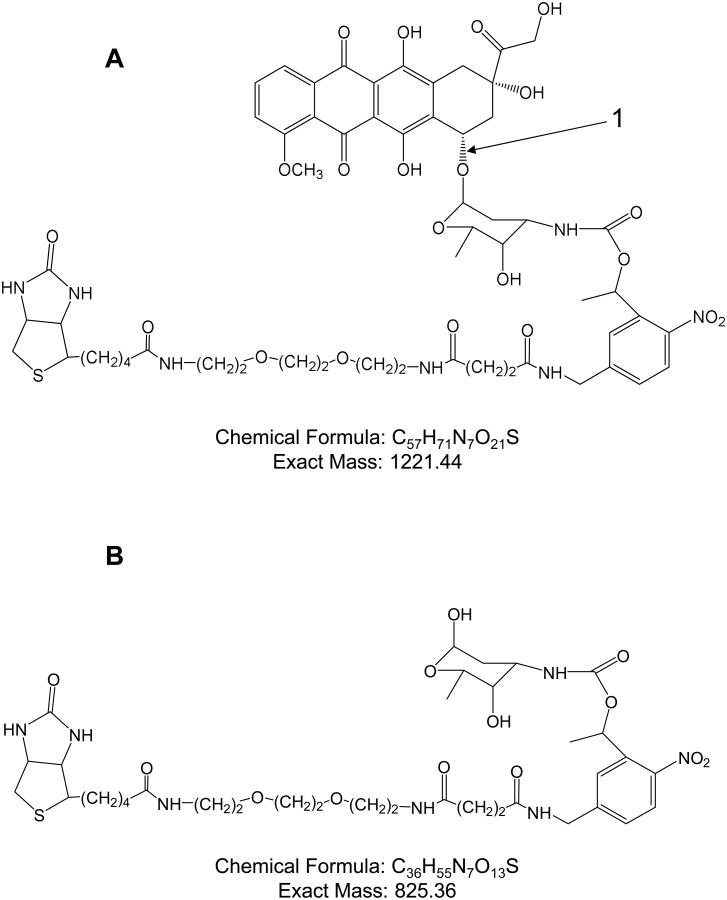
The MS/MS spectra for DOX, EPI, and DOX-PCB are shown in Figure 3. EPI is the 4′-epimer of doxorubicin at the number 1 site labeled in Figure 4A.28 The fragmentation pathways were the same for both DOX and EPI and are shown in Figure 4. 29 The fragmentation pathway for DOX-PCB is shown in Figure 5.
Figure 3.
Representative MS/MS spectra for A. DOX, B. EPI, and C. DOX-PCB. For DOX and EPI, the 396.8 peak is the [M+H]+ ion of the fragments shown in Figure 4. For DOX-PCB, the 848.3 peak is the [M+Na]+ ion of the fragment shown in Figure 5.
Figure 4.
Fragmentation pathway for DOX and EPI. A. EPI is the 4′-epimer of DOX at site 1. Both EPI and DOX have the same fragmentation pathway that can take two possible directions. Fragmentation can occur at site 3 resulting in the neutral fragment shown in B or fragmentation can occur at sites 2 and 4 resulting in the neutral fragment shown in C.29 The ion peaks for both of these fragments ([M+H]+) are shown in Figure 3A and 3B.
Figure 5.
Fragmentation pathway of DOX-PCB. A. The fragmentation site 1 results in a fragment with mass 825 shown in B. The ion peak for this fragment ([M+Na]+) is shown in Figure 3C.
The linear dynamic range of the method was determined by spiking 30 mg samples of tumor tissue with 0.01, 0.5, 10, 50 and 200 ng of DOX. Each tumor sample was also spiked with 20 ng of the EPI internal standard. The extraction process was conducted as described in the methods section. The final extraction samples were all diluted to the same final volume of 200 μL using 30% mobile phase B/70% mobile phase A. One fifth of this final sample volume was used for the LC/MS/MS analysis. The peak area for both DOX and EPI were determined and the DOX/EPI peak area ratios were calculated. These ratio values were plotted as a function of the mass of DOX loaded into the LC column as shown in Figure 6. This gives a linear range for the LC-MS/MS method using 30 mg tumor tissue samples of 0.01-200 ng of DOX, which is well within the range that would be physiologically expected in-vivo. A total of 1.2 mg of DOX-PCB was injected into the mouse and it was shown to have an α circulation half life of 10 min. This was a high enough dose to achieve an accumulation in the tumor that was detectable and resulted in enough released DOX to be detectable. The A549 tumor that developed here, like many tumors, had a very poor blood supply. The only real vascularization was on the very periphery of the tumor. This meant that only a very thin layer around the outside edge of the tumor was able to accumulate the DOX-PCB prodrug since this was the only part that had significant vascularization. No DOX was found in the serum after 30 min of 365nm light exposure in the tumor, only DOX-PCB. This means that 1.9 × 10-6% to 3.7 × 10-2% of the injected dose would have to be converted to DOX inside the periphery of the tumor to fall within the linear range.
Figure 6.
Linear dynamic range of the LC-MS/MS method. The equation for the linear curve fitted to the data is Peak Area Ratio = 0.7629 × Mass Loaded + 0.1736 with an R2 = 0.999.
To determine the precision of the method, 30 mg samples of tumor were spiked each with 2.5 ng of DOX and 20 ng of the EPI internal standard. The final extraction samples were all diluted to the same final volume of 200 μL using 30% mobile phase B/70% mobile phase A. One tenth of this final sample volume was used for the LC/MS/MS analysis. The peak area for both DOX and EPI were determined and the DOX/EPI peak area ratios were calculated for 4 different samples. The intersample Coefficient of Variation (CV%) was determined to be 1.3%.
The limits of detection (LOD) of the DOX and DOX-PCB extracted from the tumor tissues were calculated to be 7.8 pg and 0.36 ng, respectively. The limit of quantitation (LOQ) was defined as three times the standard deviation of the background intensity divided by the intensity factor observed for the injected DOX (1.6 ng loaded) and DOX-PCB (17.9 ng loaded) mixture. The limit of quantitation is 13 pg for DOX and 0.6 ng for DOX-PCB.
The extraction efficiency for DOX from tissue samples spiked with 2.5 ng of DOX was 80% ± 12%. Other extraction methods have had similar efficiencies with samples that contained significantly higher amounts of DOX. The 80% extraction efficiency reported here is significant for the small amounts of DOX in this application and allowed DOX to be detected from tissue extractions of tumors collected from mice injected with DOX-PCB with subsequent activation to DOX as shown in Figure 7. DOX and DOX-PCB were clearly detectable in the tumor tissue. The amount of DOX in the 30 mg tissue sample was estimated to be ∼425 picograms based on the standard solutions and the tissue extraction efficiency. With this same method DOX-PCB was found to have an extraction efficiency of about 60% allowing it to be extracted with a strong signal from the same tissue samples used to study the DOX concentration.
Figure 7.
LC-MS/MS chromatograms of extractions from tumor tissue collected from mice injected with DOX-PCB with subsequent conversion to DOX. The tumor tissue was spiked with 20 ng of EPI. 6.0 μl of the final concentrated 75μl extraction volume was injected into the LC column for a total loading of 1.6 ng of EPI.
The methods developed here can be used to efficiently extract and quantitatively analyze small amounts of DOX and the DOX-PCB prodrug down into the picogram range even from thinly vascularized tumor margins and poorly vascularized bulk tumor tissue. This extraction method can be adapted to other prodrug forms of DOX allowing it to benefit studies that require the determination of prodrug and activated DOX biodistribution.
Acknowledgments
The authors are grateful for the support of Howard Cottam in the collection and interpretation of the data. The authors also wish to thank Ambergen Inc, for clarification of the structural details of the PCB diastereoisomers. The study was supported by the National Cancer Institute grant # 5 U54 CA119335.
References
- 1.Paridaens R, Biganzoli L, Bruning P, et al. Paclitaxel Versus Doxorubicin as First-Line Single-Agent Chemotherapy for Metastatic Breast Cancer: A European Organization for Research and Treatment of Cancer Randomized Study With Cross-Over. Journal of Clinical Oncology. 2000;18(4):724–733. doi: 10.1200/JCO.2000.18.4.724. [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, Cesne AL, Chawla SP, et al. First-line treatment of metastatic or locally advanced unresectable soft tissue sarcomas with conatumumab in combination with doxorubicin or doxorubicin alone: A Phase I/II open-label and double-blind study. European Journal of Cancer. 2012 doi: 10.1016/j.ejca.2011.12.008. Online. [DOI] [PubMed] [Google Scholar]
- 3.Pawan K, Singal NI. Doxorubicin-Induced Cardiomyopathy. The New England Journal of Medicine. 1998 Sep 24;339(13):900–905. doi: 10.1056/NEJM199809243391307. [DOI] [PubMed] [Google Scholar]
- 4.Olson RD, M P. Doxorubicin cardiotoxicity: analysis of prevailing hypotheses. FASEB J. 1990;4(13):3076–3086. [PubMed] [Google Scholar]
- 5.Minotti Giorgio, M P, Salvatorelli Emanuela, Cairo Gaetano, Gianni Luca. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 6.Ewer MS, Jaffe N, Ried H, Zietz HA, Benjamin RS. Doxorubicin cardiotoxicity in children: Comparison of a consecutive divided daily dose administration schedule with single dose (rapid) infusion administration. Medical and Pediatric Oncology. 1998;31(6):512–515. doi: 10.1002/(sici)1096-911x(199812)31:6<512::aid-mpo8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Mertens AC, Yasui Y, Neglia JP, et al. Late Mortality Experience in Five-Year Survivors of Childhood and Adolescent Cancer: The Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2001;19(13):3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 8.Denny WA. Prodrug strategies in cancer therapy. Eur J Med Chem. 2001;36:577–595. doi: 10.1016/s0223-5234(01)01253-3. [DOI] [PubMed] [Google Scholar]
- 9.Denny WA. Tumor-activated Prodrugs—A New Approach to Cancer Therapy. Cancer Investigation. 2004;22(4):604–619. doi: 10.1081/cnv-200027148. [DOI] [PubMed] [Google Scholar]
- 10.Tietze Lutz F, N M, Mollers Thomas, Fischer Roland, Glusenkamp Karl-Heinz, Rajewsky Manfred F, Jahde Eckhard. Proton-mediated Liberation of Aldophosphamide from a Nontoxic Prodrug: A Strategy for Tumor-selective Activation of Cytocidal Drugs. Cancer Research. 1989;49:4179–4184. [PubMed] [Google Scholar]
- 11.Martin Brown J, W WR. Exploiting Tumor Hypoxia in Cancer Treatment. Nature Reviews Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 12.Breistol K, H HR, Berger DP, Langdon SP, Fiebig HH, Fodstad O. The Antitumour Activity of the Prodrug N-l-leucyl-doxorubicin and its Parent Compound Doxorubicin in Human Tumour Xenografts. European Journal of Cancer. 1998;34(10):1602–1606. doi: 10.1016/s0959-8049(98)00152-x. [DOI] [PubMed] [Google Scholar]
- 13.Gopin A, Ebner S, Attali B, Shabat D. Enzymatic Activation of second-generation dendritic prodrugs: Conjugation of self-immolative dendrimers with PEG via click chemistry. Bioconjugate Chem. 2006;17:1432–1440. doi: 10.1021/bc060180n. [DOI] [PubMed] [Google Scholar]
- 14.Shamis M, Lode HN, Shabat D. Bioactivation of self-immolative dendritic prodrugs by catalytic antibody 38C2. JACS. 2004;126:1726–1731. doi: 10.1021/ja039052p. [DOI] [PubMed] [Google Scholar]
- 15.Ibsen S, Zahavy E, Wrasdilo W, Berns M, Chan M, Esener S. A Novel Doxorubicin Prodrug with Controllable Photolysis Activation for Cancer Chemotherapy. Pharmaceutical Research. 2010;27(9):1848–1860. doi: 10.1007/s11095-010-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laginha KM, Verwoert S, Charrois GR, Allen T. Determination of Doxorubicin Levels in Whole Tumor and Tumor Nuclei in Murine Breast Cancer Tumors. Clin Cancer Res. 2005;11(19):6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- 17.Hong RL, Huang CJ, Tseng YL, et al. Direct Comparison of Liposomal Doxorubicin with or without Polyethylene Glycol Coating in C-26 Tumor-bearing Mice: Is Surface Coating with Polyethylene Glycol Beneficial? Clinical Cancer Research. 1999;5:3645–3652. [PubMed] [Google Scholar]
- 18.Jong JD, Guerand WS, Schoofs PR. Simple and sensitive quantification of anthracyclines in mouse atrial tissue using high-performance liquid chromatography and fluorescence detection. Journal of Chromatography. 1991;570:209–202 I206. doi: 10.1016/0378-4347(91)80218-2. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh YJ, Chang CH, Huang SP, et al. Effect of cyclosporin A on the brain regional distribution of doxorubicin in rats. International Journal of Pharmaceutics. 2008;350:265–271. doi: 10.1016/j.ijpharm.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Arnold RD, Slack JE, Straubinger RM. Quantification of Doxorubicin and metabolites in rat plasma and small volume tissue samples by liquid chromatography/electrospray tandem mass spectroscopy. Journal of Chromatography B. 2004;808:141–152. doi: 10.1016/j.jchromb.2004.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DiFrancesco R, Griggs JJ, Donnelly J, DiCenzo R. Simultaneous analysis of cyclophosphamide, doxorubicin and doxorubicinol by liquid chromatography coupled to tandem mass spectrometry. Journal of Chromatography B. 2007;852(1):545–553. doi: 10.1016/j.jchromb.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Shen S, Ren J, Chen J, Lu X, Deng C, Jiang X. Development of magnetic multiwalled carbon nanotubes combined with near-infrared radiation-assisted desorption for the determination of tissue distribution of doxorubicin liposome injects in rats. Journal of Chromatography A. 2011;1218(29):4619–4626. doi: 10.1016/j.chroma.2011.05.060. [DOI] [PubMed] [Google Scholar]
- 23.Ibsen SD. ProQuest Dissertations and Theses. 2011. Energy deposition in the body from external sources to chemically trigger cellular responses in desired localized regions. [Google Scholar]
- 24.Murdter TE, Sperker B, Bosslet K, Fritz P, Kroemer HK. Simultaneous high-performance liquid chromatographic determination of a glucuronyl prodrug of doxorubicin, doxorubicin and its metabolites in human lung tissue. Journal of Chromatography B. 1998;709:289–295. doi: 10.1016/s0378-4347(98)00079-6. [DOI] [PubMed] [Google Scholar]
- 25.Maessen PA, Pinedo HM, Mross KB, Vijh WJFVD. New Method for the Determination of Doxorubicin, 4′-Epidoxorubicin and all known metabolites in Cardiac Tissue. Journal of Chromatography. 1988;424:103–110. doi: 10.1016/s0378-4347(00)81080-4. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Cedron L, Sayalero ML, Lanao JM. High-performance liquid chromatographic validated assay of doxorubicin in rat plasma and tissues. Journal of Chromatography B. 1999;721:271–278. doi: 10.1016/s0378-4347(98)00475-7. [DOI] [PubMed] [Google Scholar]
- 27.Ibsen S, Zahavy E, Wrasidlo W, et al. Localized In-Vivo Activation of a Photoactivatable Doxorubicin Prodrug in Deep Tumor Tissue. In submission. doi: 10.1111/php.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaklamani VG, Gradishar WJ. Epirubicin Versus Doxorubicin: Which Is the Anthracycline of Choice for the Treatment of Breast Cancer? Clinical Breast Cancer. 2003;4:26–33. doi: 10.3816/cbc.2003.s.012. [DOI] [PubMed] [Google Scholar]
- 29.Sleno L, Campagna-Slater Vr, Volmer DA. Dissociation reactions of protonated anthracycline antibiotics following electrospray ionization-tandem mass spectrometry. International Journal of Mass Spectrometry. 2006;255:130–138. [Google Scholar]