Abstract
Background
Ultrasound guidance for continuous femoral perineural catheters may be supplemented by electrical stimulation through a needle or through a stimulating catheter. We tested the primary hypothesis that ultrasound guidance alone is noninferior on both postoperative pain scores and opioid requirement and superior on at least one of the two. Secondarily, we compared all interventions on insertion time and incremental cost.
Methods
Patients having knee arthroplasty with femoral nerve catheters were randomly assigned to catheter insertion guided by: 1) ultrasound alone (n=147); 2) ultrasound and electrical stimulation through the needle (n=152); or, 3) ultrasound and electrical stimulation through both the needle and catheter (n=138). Noninferiority between any two interventions was defined for pain as no more than 0.5 points worse on a 0–10 Verbal Response Scale (VRS) scale and for opioid consumption as no more than 25% greater than the mean.
Results
The stimulating needle group was significantly noninferior to the stimulating catheter (difference (95% CI) in mean VRS pain score [stimulating needle versus stimulating catheter] of −0.16 (−0.61, 0.29), P<0.001; percent difference in mean IV morphine equivalent dose of −5% (−25%, 21%), P=0.002) and to ultrasound only (difference in mean VRS pain score of −0.28 (−0.72, 0.16), P<0.001; percent difference in mean IV morphine equivalent dose of −2% (−22%, 25%), P=0.006). In addition, the use of ultrasound alone for femoral nerve catheter insertion was faster and cheaper than the other two methods.
Conclusion
Ultrasound guidance alone without adding either stimulating needle or needle/catheter combination thus appears to be the best approach to femoral perineural catheters.
Introduction
Total knee arthroplasties are common. They cause severe post-operative pain in most patients and moderate pain in the remainder.1 Post-operative pain delays early intensive physical therapy, thereby prolonging rehabilitation.2 Continuous femoral perineural catheters provide excellent postoperative analgesia and are now routinely used after knee arthroplasties.3,4
Continuous femoral perineural catheters often fail,5,6 apparently because the catheter tip is not well positioned adjacent to the femoral nerve.7 Nerve stimulation is the classical method for confirming appropriate catheter or needle placement.6 However, it is now established that ultrasound guidance improves positioning success, speeds catheter insertion, and enhances patient satisfaction.3,8 Whether nerve stimulation improves ultrasound-guided catheter placement and block success remains unclear. In addition to its obvious clinical importance, the question is of economic interest because stimulating needles and stimulating catheters have higher acquisition costs than routine block needles or catheters. Use of stimulation may also affect catheter insertion time.
We therefore compared ultrasound guidance alone with either ultrasound guidance plus needle stimulation or ultrasound guidance plus catheter stimulation for insertion of femoral nerve catheters for total knee arthroplasty under mainly neuraxial anesthesia or general anesthesia. Specifically, we tested the primary hypothesis that ultrasound guidance alone was non-inferior on postoperative pain (as characterized by both pain scores and opioid requirements). Therefore, our secondary hypotheses were to show that ultrasound guidance alone: 1) was non-inferior on block performance time and block failure rate; and, 2) less expensive than either stimulation approach.
Materials and Methods
With approval of the Institutional Review Board at the Cleveland Clinic (Cleveland, Ohio) and written consent, we enrolled 453 patients 18–80 years old from August 13, 2009 to December 28 2012 who were scheduled for total knee arthroplasty under either general or neuraxial anesthesia. We excluded patients who used illicit drugs within six months, were pregnant, or refused a femoral nerve catheter. The study was conducted at the Cleveland Clinic Main Campus and registered at ClinicalTrial.Gov (NCT00927368).
Protocol
Femoral perineural catheters were inserted by staff anesthesiologists experienced in ultrasound or by experienced senior residents or fellows supervised by experienced staff. Most were inserted preoperatively. All catheters were inserted with ultrasound guidance, using a 13–6 MHz linear ultrasound probe (SonoSite, Bothell, WA or GE, Duluth, GA) to provide a short-axis view of the femoral nerve via an out-of-plane approach. Typically, the femoral nerve appeared as a hyperechoic structure ventrally to the iliopsoas hypoechoic muscle, lateral to the pulsating femoral artery.
For study purposes, we used insulated 17-gauge, 10-cm-long Tuohy needles in all patients. We similarly used 19-gauge StimuCath catheters designed for electrical stimulation in all patients (StimuCath, Arrow International, Reading, PA).
Participating patients were randomized to one of three catheter insertion techniques: 1) ultrasound guidance alone (ultrasound alone); 2) ultrasound guidance and needle stimulation (needle stimulation); or, 3) ultrasound guidance and catheter stimulation (catheter stimulation). Randomization (1:1:1 without stratification) was provided by a web-based system that was accessed just before catheter insertion. Allocation was thus concealed until the last practical moment. Treatment assignments were generated in randomly-sized blocks using SAS statistical software (SAS Institute, Cary, NC).
In the ultrasound alone group, the Tuohy needle was inserted in out-plane approach. Needle placement was considered adequate when the tip was visualized beneath the fascia iliaca; the catheter was then introduced 5 cm beyond the needle tip. Electrical stimulation was not used.
In the needle stimulation group, the Tuohy needle was positioned with the tip beneath the fascia iliaca under ultrasound guidance. The needle tip was then adjusted as necessary to obtain a quadriceps muscle response with a stimulating current ≤0.5 mA (2 Hz, pulse width 0.1 msec). Subsequently, the catheter was threaded 5 cm beyond the needle tip without additional electrical stimulation.
In the catheter stimulation group, the Tuohy needle was positioned with the tip beneath the fascia iliaca under ultrasound guidance. The needle tip was then adjusted as necessary to obtain a quadriceps muscle response with a stimulating current ≤0.5 mA. At that point, the peripheral nerve stimulator was then disconnected from the stimulating needle and connected to the proximal end of the catheter. The catheter was then advanced 5 cm past the needle tip. If the motor response disappeared during catheter advancement, the catheter was withdrawn slightly until the response returned. Needle orientation and catheter advancement were adjusted as necessary to elicit quadriceps contractions via the catheter with a stimulating current ≤0.5 mA.
Once the femoral nerve catheter was positioned using the assigned technique, 20 ml of 0.1% ropivacaine was injected. Postoperatively, patients were given an infusion of 0.1% ropivacaine at a rate of 8 ml per hour until the second postoperative day. If the anesthesiologist was unable to elicit nerve stimulation or to maintain quadriceps contractions using stimulating catheter, the catheter was inserted using ultrasound guidance alone.
Patients were given either general or neuraxial anesthesia. Neuraxial block is the preferred method of anesthetic management at the Clinic, and usually performed with 3 ml bupivacaine 0.5%. General endotracheal anesthesia was usually induced with propofol and maintained with sevoflurane.
In the postoperative period, we tested the effectiveness of the femoral perineural catheter to provide the appropriate pain relief. Intravenous patient-controlled analgesia was provided as necessary if the block failed or was insufficient. Morphine sulfate was the default drug, but fentanyl or hydromorphone were substituted if necessary. Oral analgesics consisting of acetaminophen or a combined opioid and non-steroidal anti-inflammatory agent were gradually substituted for the femoral nerve block and patient-controlled opioid.
In cases of severe posterior knee pain unresponsive to pain medications, sciatic nerve blocks were performed either on the day of surgery or on the first postoperative day. Sciatic nerve block was usually performed via a subgluteal approach using ultrasound with nerve stimulation or nerve stimulation alone. Twenty milliliters of 0.1% ropivacaine was injected for the block.
Measurements
Baseline demographic, morphometric, and surgical characteristics were recorded, along with surgical and anesthetic details.
Verbal Response Scale (VRS) pain scores after surgery – which ranged from 0 (no pain) to 10 (maximum intolerable pain) – were assessed every 30 minutes in the recovery area and every 4 hours thereafter up to 48 hours postoperatively. These individual measurements were averaged for each patient using a time-weighted formula. (For a given patient, the observed VRS pain score profile as a function of time was linearly interpolated and integrated using the trapezoidal rule; then, the time-weighted average was calculated as the value of this integral divided by the total monitoring time of 48 hours.) All opioid administrations within the first 48 hours postoperatively were converted to IV morphine equivalent and totaled for each patient.9
Block performance time was characterized by the time elapsed from beginning the block to catheter placement; specifically, from time of the Tuohy needle insertion to the time of its removal.
Statistical Methods
SAS software version 9.3 (SAS Institute, Cary, NC) and R software version 2.15.2 (The R Foundation for Statistical Computing, Vienna, Austria) were used for all data analysis. Our primary analysis was intent-to-treat, such that all randomized patients who received a study intervention (even if crossed over from the randomized intervention) were included. For all analyses we planned to adjust for any baseline characteristics displaying clinically-relevant imbalance between groups.
Primary Outcome: Pain Management
We defined quality of postoperative pain management as a combination of low pain scores and low opioid requirement within the first 48 hours postoperatively. We evaluated these outcomes using joint hypothesis testing10 because differences in pain scores and opioid consumption are difficult to interpret in isolation. We thus deemed a block approach superior only if it were shown to be noninferior on both pain and opioid consumption, and superior on at least one of the two outcomes. Superiority was thus tested only for treatments that were significantly noninferior to a comparator intervention on both outcomes.
Noninferiority was defined by a mean postoperative VRS pain score profile not more than 0.5 points greater and a mean total postoperative morphine equivalent dose not more than 25% greater than the comparison group mean. The overall significance level was 0.025 for assessing noninferiority among the 3 treatments for each of pain and opioid consumption. We made no correction to the significance criterion for testing both pain and opioid consumption since both were required to be significant to claim one treatment better than another (i.e., an intersection-union test). Within an outcome, noninferiority was assessed in both directions for each of the 3 treatment comparisons, for a total of 6 tests. Using the Holm-Bonferroni sequential multiple comparison procedure11 with an overall significance level (alpha) of 0.025, P-values were ordered smallest to largest and compared to significance criteria of 0.025/6, 0.025/5,…,0.025/1.
The primary analysis for pain was a linear regression model comparing two groups on the mean pain score for a patient in the first 48 hours. Noninferiority analysis used the 0.5-point noninferiority delta in a 1-tailed test of the treatment effect regression parameter (i.e., testing the null hypothesis that mean A minus mean B is greater than 0.5 points).
Total opioid requirement during the first 48 postoperative hours generally followed a lognormal distribution (i.e., where the observations are normally distributed after taking their logarithm). Such an analysis changes the interpretation of the effect of catheter insertion technique from that of estimating the difference in arithmetic means to estimating the ratio (or percent difference) of geometric means. We thus used a linear regression model of log-transformed total IV morphine equivalent observations as the response and randomized catheter insertion technique as the treatment of interest.
Given one treatment was found noninferior to another on both outcomes, superiority was assessed with a 1-tailed global test (same direction as noninferiority results) on both outcomes simultaneously, at an overall 0.025 significance level. If significant, superiority was further tested for pain and opioid consumption individually, applying the Holm-Bonferroni approach to adjust for testing 2 outcomes. This step was required because only one superiority test was needed to be significant and thus claim a treatment more effective. Specifically, we used the multiple outcomes nonparametric sum of ranks global test of O’Brien12 to assess superiority over the vector of outcomes (pain, opioids). Patients were ranked on each outcome from largest value (worst) to smallest value (best), and the two ranks were then summed within each patient. Randomized groups were then compared on the mean sum of ranks with a 1-tailed t test or analysis of covariance model. The significance criterion for a particular global test (here called alpha-global) was 0.025 divided by the number of pairs of interventions that passed initial noninferiority testing.
Secondary Outcomes
Block performance time among the three groups was summarized graphically using a three-group boxplot and analyzed for difference using one-way analysis of variance. The overall hypothesis of at least one group exhibiting different average block time from the others was first assessed using an F test. Provided there was a significant overall difference, we then proceeded to pairwise comparisons between the three groups (under the Bonferroni correction for three simultaneous assessments, controlling the nominal Type I error for this secondary outcome at 0.05).
Proportion of patients requiring sciatic nerve block were reported for each group but not formally tested due to limited power for detecting clinically relevant differences in risk.
Interim Analyses
At each of 4 equally-spaced analyses (3 interim plus a final, as needed) we planned to evaluate both efficacy and futility using 1-sided noninferiority and superiority tests (see above methods). We used a group sequential design with a gamma spending function (gamma parameter of −4 for efficacy [alpha] and −2 for futility [beta]). P-values constituting the efficacy boundary at each of the four planned analyses were, respectively, P≤0.000134, P≤0.000390, P≤0.00117, and P≤0.00347; likewise, the P-values constituting the futility boundary were P>0.612, P>0.199, P>0.0359, and P>0.00347.
P-value boundaries were adjusted for six simultaneous one-sided comparisons across two outcomes using a Bonferroni correction; they therefore correspond to the most significant comparison (efficacy) and the least significant comparison (futility) within the Holm-Bonferroni method. Study progress, safety, and results were evaluated at each interim analysis by an Executive Committee (AK, EJM, DIS).
Sample Size Considerations
Sample size was based on having 90% power at the overall 0.025 significance level to detect noninferiority for both primary outcomes for any pair of treatments at the given noninferiority deltas. Using an overall noninferiority significance level of 0.025, with 6 such tests (3 pairs of treatments times 2 directions for each), the lowest significance criterion to be used was 0.00417 (i.e., 0.025/6). Tests for superiority were done at higher significance levels as explained above (Statistical Methods, under “Primary Outcome: Pain Management”) and thus had >90% power for detecting differences at least the size of the pre-specified noninferiority deltas.
Sample size was based on opioid consumption because it required more patients than for VRS pain score. The standard deviation of total IV morphine equivalents for total knee arthroplasty patients receiving a femoral nerve block at 24 hours postoperatively has been estimated at 10.5 mg.13 This standard deviation was 43.4% of the mean of 24.2 mg. We conservatively assumed that the true coefficient of variation (i.e., SD/mean) of 50% of the mean. Under this assumption, a maximum of 453 patients (151 in each catheter insertion technique group, assuming 100% accrual) would provide approximately 90% power to detect noninferiority (using a noninferiority delta of 25% of the reference mean) at the 0.00417 significance level (i.e., 0.025/6).
At the third interim analysis, all four noninferiority tests involving the ultrasound alone group crossed the futility boundary of P>0.0359: in comparing ultrasound alone versus stimulating needle and stimulating catheter, respectively, we observed P=0.18 and P=0.46 for mean VRS pain and P=0.06 and P=0.11 for mean IV morphine equivalent dose. The Executive Committee nonetheless chose to continue randomized allocation through the entire planned enrollment to improve precision of noninferiority tests relative to the ultrasound only group.
Economic Analysis
Using the results of the primary hypothesis, a cost-minimization analysis was conducted from the hospital perspective. This form of economic analyses compares the costs of interventions that are proven to be clinically equivalent. The analysis aims to guide efficient healthcare resource allocation by identifying the intervention that achieves the clinical outcome at the least cost.
Information on clinical equivalency was abstracted from the results of the primary and secondary aims. Cost data were abstracted from Cleveland Clinic Purchasing Department at 2013 values. The incremental cost between strategies was calculated as the additional cost of one strategy to the next less costly strategy. Simplified sensitivity analysis was conducted to determine the robustness of choice of least costly intervention to changes in acquisition costs.
Results
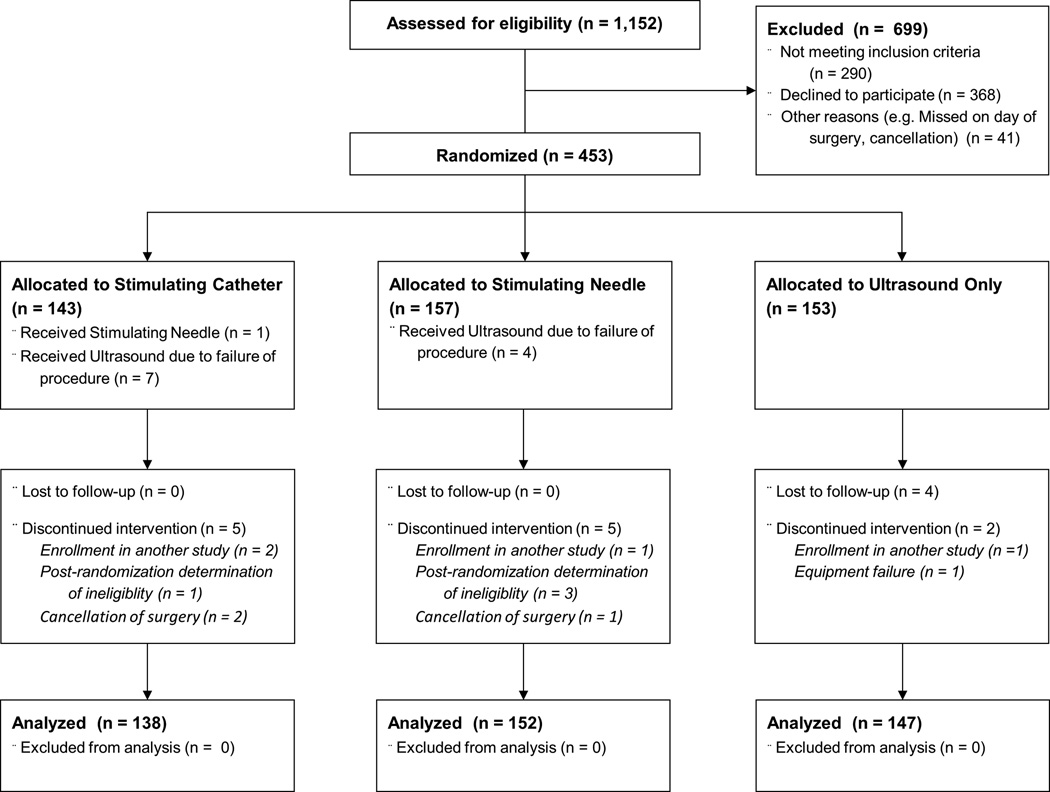
Among 453 enrolled patients, 143 (32%) were randomized to the stimulating catheter, 157 (35%) were randomized to the stimulating needle, and 153 (34%) were randomized to receive ultrasound alone. Among these, 4 had to be removed due to enrollment in a competing study; 4 were removed in light of a post-randomization determination that all inclusion/exclusion were not met; one was removed due to equipment failure; 3 were removed due to cancellation of surgery or change in surgical plan; and 4 were removed due to unavailable outcomes. Four hundred thirty-seven patients were thus available for analysis.
One patient randomized to receive the stimulating catheter actually received a non-stimulating catheter. Additionally, electrical stimulation failed in 7 patients randomized to the simulating catheter and four randomized to the simulating needle. The needle was thus inserted with ultrasound alone in these patients (3.8% of cases randomized to receive either type of stimulation, Fig. 1). Two-thirds of the patients received neuraxial anesthesia for the total knee replacement procedures. All patients were analyzed according to their randomized allocation in accordance with our intention-to-treat plan.
Fig. 1.
Trial diagram.
Baseline patient and surgical characteristics were reasonably balanced among the three groups (Table 1); thus, no baseline variables were included as covariables in the analysis. Table 2 summarizes opioid administration by drug.
Table 1.
Summary of demographic, morphometric and surgical characteristics.
| Stimulating Catheter |
Stimulating Needle |
Ultrasound Alone |
|
|---|---|---|---|
| (N = 138) | (N = 152) | (N = 147) | |
| Gender – N (%) Female | 76 (55) | 80 (53) | 80 (54) |
| Race – N(%) Caucasian | 128 (93) | 136 (89) | 131 (89) |
| Age – mean ± SD, years | 63 ± 9 | 61 ± 13 | 62 ± 9 |
| ASA Physical Status – N(%) | |||
| I | 0 (0) | 4 (3) | 2 (1) |
| II | 45 (33) | 54 (36) | 58 (39) |
| III | 91 (66) | 90 (59) | 84 (57) |
| IV | 2 (1) | 4 (3) | 3 (2) |
| BMI – median [quartiles], kg/m2 | 33 [29,38] | 31 [27, 36] | 32 [28,36] |
| General Anesthesia – N (%), vs. Spinal | 29 (21) | 38 (25) | 37 (25) |
| Use of Ketorolac – N (%) | 4 (3) | 5 (3) | 8 (5) |
| Intraoperative IV Morphine Equivalent Dose – median [quartiles], mg | 5 [0, 10] | 2 [0, 10] | 2 [0, 10] |
ASA = American Society of Anesthesiologists; BMI = Body mass index; IV = intravenous; SD = standard deviation
Table 2.
Opioid equivalents and total amounts given to study participants within the first 48 hours postoperatively.
| Medication | Route | Equivalent Analgesic Dose (mg) |
Total Amount Administered (mg) |
IV Morphine Equivalent Dose (mg) |
Percent of Total IV Morphine Equivalent Dose Administered |
|---|---|---|---|---|---|
| Morphine | Intravenous | 10 | 1012 | 1012 | 2.9% |
| Oral | 30 | 650 | 217 | 0.6% | |
| Fentanyl | Intravenous | 0.1 | 81 | 8087 | 22.8% |
| Oral | 0.23 | 0 | 9 | 0.0% | |
| Meperidine | Intravenous | 75 | 125 | 17 | 0.0% |
| Oxycodone | Oral | 20 | 37328 | 18664 | 52.6% |
| Hydrocodone | Oral | 30 | 900 | 300 | 0.8% |
| Tramadol | Oral | 150 | 1450 | 97 | 0.3% |
| Hydromorphone | Intravenous | 1.5 | 1011 | 6739 | 19.0% |
| Oral | 7 | 143 | 205 | 0.6% | |
| Methadone | Oral | 20 | 50 | 25 | 0.1% |
| Codeine | Oral | 200 | 30 | 2 | 0.0% |
| Tapentadol | Oral | 75 | 675 | 90 | 0.3% |
IV = intravenous
Analgesia
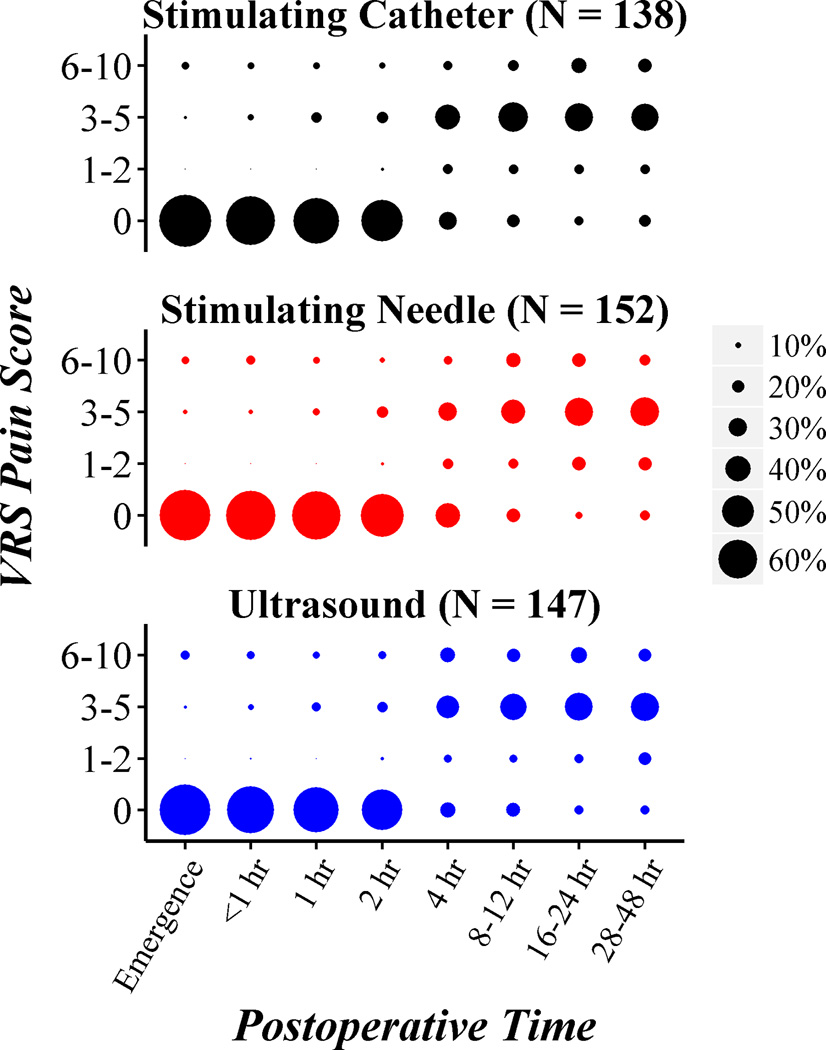
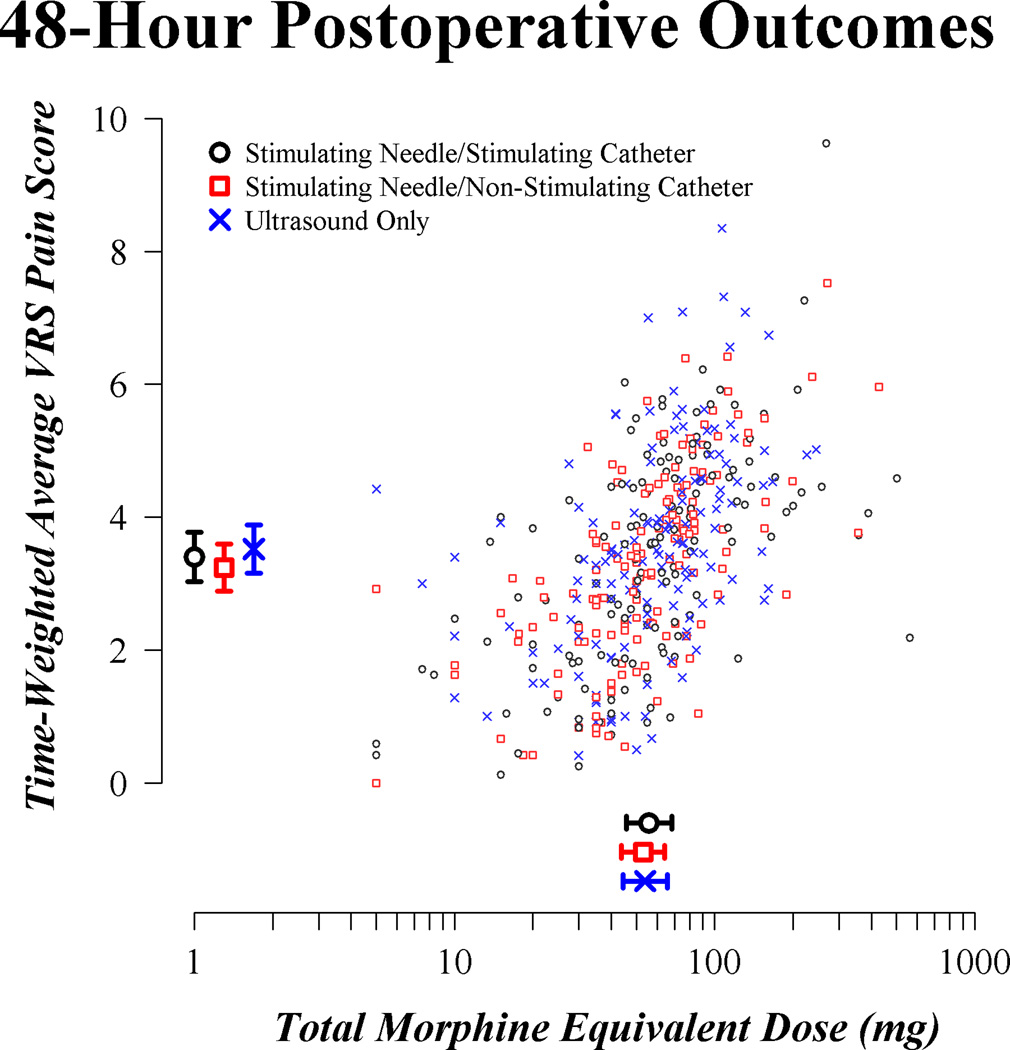
The distribution of VRS pain scores over postoperative time is summarized graphically for each of the three randomized groups in Figure 2. A scatterplot of the two primary outcomes in Figure 3 suggests moderate correlation between postoperative pain score and postoperative opioid requirement, as expected. Estimated means for the three groups, given in the margins of the scatterplot, were largely similar.
Fig. 2.
Distribution of verbal response scale (VRS) pain scores over postoperative time for each of the three randomized catheter insertion technique groups. Plotted points are proportional in size to the percent of patients within a given group for a given time interval.
hr = hour
Fig. 3.
Scatterplot of primary outcomes for the enrolled patients. Estimated means for each outcome and corresponding simultaneous 95% confidence intervals are provided by group in the margins. Confidence intervals used a nominal significance criterion of 0.0023, reflecting the Bonferroni correction for six simultaneous estimates and the adjustment for interim monitoring. VRS = verbal response scale
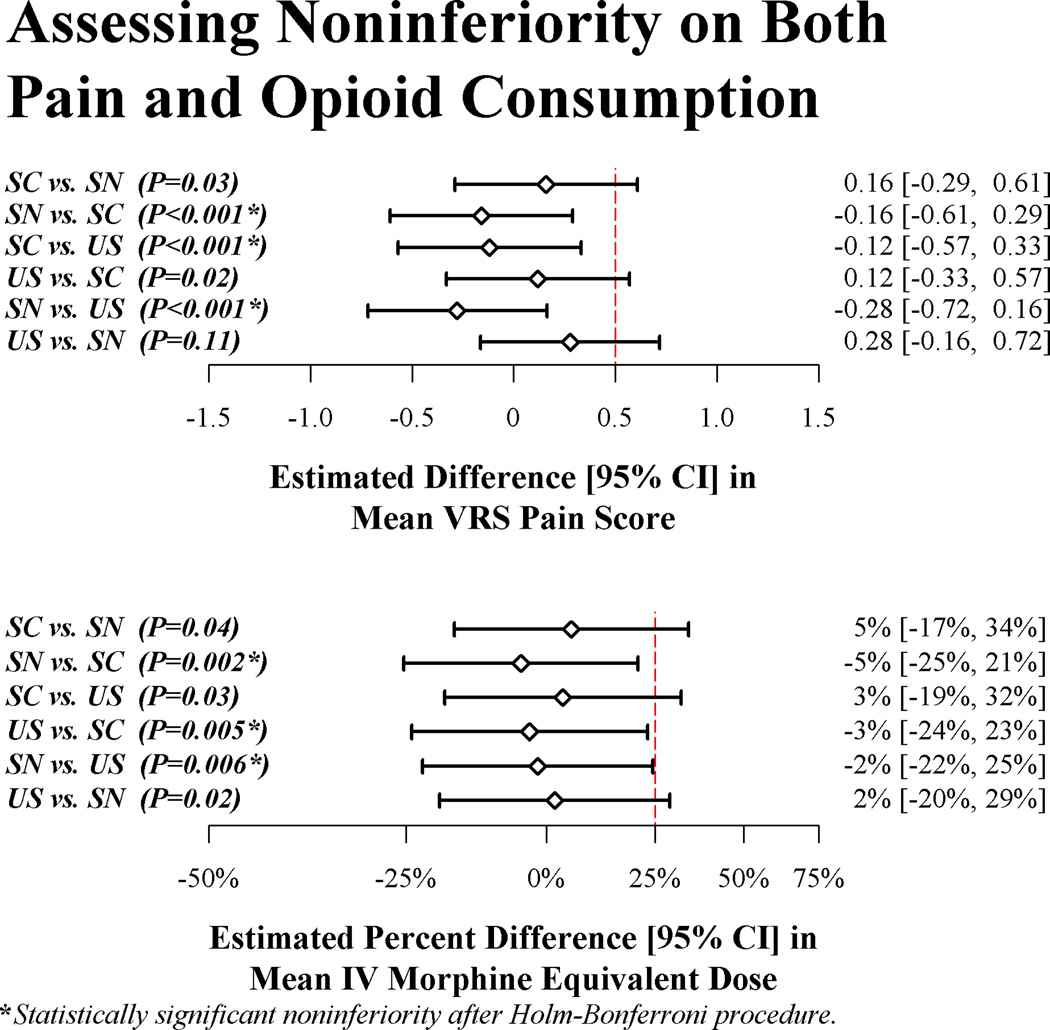
In the primary analysis, the stimulating needle group was significantly noninferior to both the stimulating catheter (difference (95% CI) in mean VRS pain score [stimulating needle versus stimulating catheter] of −0.16 (−0.61, 0.29), P<0.001, and percent difference in mean IV morphine equivalent dose of −5% (−25%, 21%), P=0.002; both statistically significant after application of the Holm-Bonferroni testing procedure) and ultrasound only (difference in mean VRS pain score of −0.28 (−0.72, 0.16), P<0.001, and percent difference in mean IV morphine equivalent dose of −2% (−22%, 25%), P=0.006; both significant after the Holm-Bonferroni procedure) on pain management (Figure 4). For these two comparisons we thus proceeded to assessing superiority.
Fig. 4.
Results of simultaneous noninferiority hypothesis tests among randomized femoral nerve catheter insertion treatments (SC = stimulating catheter, SN = stimulating needle, and US = ultrasound alone), given as estimates and 95% confidence intervals (confidence intervals adjusted for simultaneous inference and interim monitoring). One treatment (say, SN) was declared noninferior to another treatment (say, US) on mean VRS pain score when the estimated difference in means was significantly less than the pre-specified noninferiority delta of 0.5 points (given as the dashed vertical line). Likewise, SN was declared noninferior to US on mean total IV morphine equivalent dose if the mean for SN was significantly not more than 25% greater than the mean for US. Significant noninferiority (as assessed by the Holm-Bonferroni step-down method) is given by asterisks next to the raw P-values. VRS = verbal response scale
Global superiority tests (on both pain and opioid use) were not significant. Specifically, the P value was 0.32 for global superiority test of the stimulating needle group over the stimulating catheter group; and the P value was 0.20 for global superiority of the stimulating needle group over the ultrasound only group.
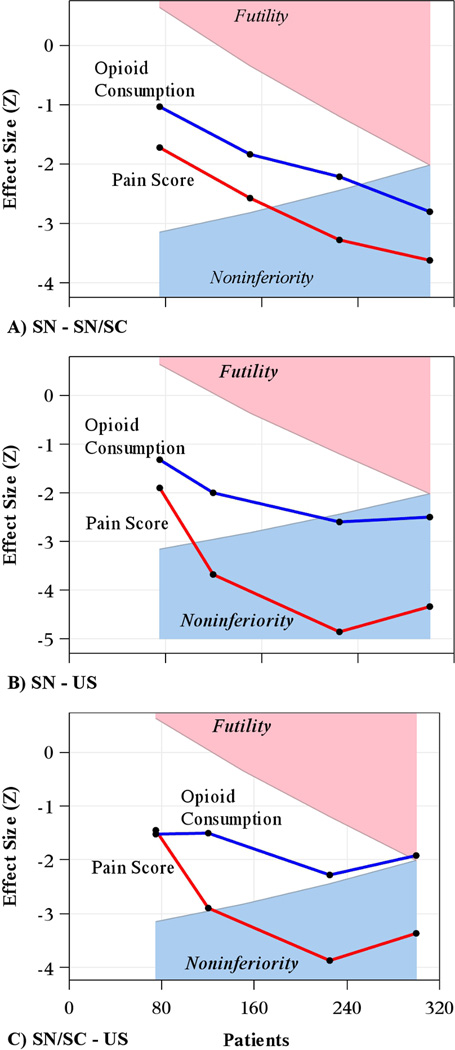
Interim analyses on the primary outcomes of on VRS pain score and opioid consumption for efficacy and futility as outlined above did not result in the Executive Committee stopping the study early. Figure 5 shows the interim monitoring results for noninferiority testing over time on both outcomes for each comparison of interest.
Fig. 5.
Results of group sequential monitoring assessing noninferiority on VRS pain score and opioid consumption across the 4 planned interim analyses.
Y-axis is the observed z-statistic representing the treatment effect at the given interim analysis or final analysis, while x-axis is the cumulative enrollment over time. Crossing into the blue region indicates a finding of noninferiority of one group over the other, while crossing into the pink region indicates an acceptance of the null hypothesis (i.e., intervention at least “delta” worse than comparator) and no claim of noninferiority. We used the gamma spending function with gamma = −4 for efficacy and −2 for futility, with alpha=0.025 overall for noninferiority and 90% power.
Panel A: Stimulating needle (SN) minus stimulating catheter (SC). SN was noninferior to its comparator on both pain and opioid consumption.
Panel B: SN minus ultrasound (US). Panel C: SN minus US. SN was noninferior to its comparator on both pain and opioid consumption.
Panel C: SC minus US. SC was noninferior to US on pain score, but not on opioid consumption. Superiority tests were done for comparisons in panels A and B but were not significant.
SC = stimulating catheter; SN = stimulating needle; US = ultrasound alone; VRS = verbal response scale
Secondary Outcomes
Estimated mean block performance time [Bonferroni-adjusted 95% confidence interval] was 177 (151, 202) seconds for the stimulating catheter group, 150 (126, 175) seconds for the stimulating needle group, and 110 [85, 135] seconds for the ultrasound only group. Mean block performance time for the ultrasound-alone group was significantly lower than both other groups. Specifically, the difference in means compared to the stimulating catheter group was 67 (31, 102) seconds (P<0.001, Wald test) and the difference in means compared to the stimulating needle group was 40 (6, 75) seconds (P=0.01). In contrast, the two stimulation groups did not differ significantly on block performance time (P=0.11).
Sciatic nerve blocks were required in 18 (13.0%) patients randomized to stimulating catheter, 20 (13.2%) patients randomized to stimulating needle, and 15 (10.2%) patients randomized to ultrasound alone.
Cost
For our cost-minimization analysis, the source of cost variability between strategies was identified. All the consumables required for the strategies are supplied in kit form, specific for femoral nerve blocks. The kits differ between strategies only by the type of needle and catheter they contain. The contractual price of standard kit, containing a standard needle and standard catheter is $30. The contractual price of a stimulating kit and stimulating catheter is $80. As a contractual price was not available for a kit containing a stimulating needle but a standard catheter, the item price of a stimulating needle ($14) was added to the contractual price of a standard kit, giving a total cost of $44. All other costs were not different between strategies. Because the cost analysis only considers cost differences, including nonvariable costs was not necessary, therefore only the cost of the kits was considered.
The incremental costs between the kits required for each strategy were as follows: ultrasound alone and stimulating needle, $14; stimulating needle and stimulating needle/catheter, $36; ultrasound alone and stimulating needle/catheter, $50. As contractual prices can vary between hospitals, a sensitivity analysis was performed to see how robust the choice strategy was to changes in price. The sensitivity analysis demonstrated that only if the price of a standard kit increased by 48% or the price of a stimulating needle kit decreased by 32% would the stimulating needle strategy become the choice strategy. Due to the high relative cost of a stimulating catheter/needle kit, it was dominated in price by both alternate strategies. In addition, the cost analysis was conservative, including only consumable costs. If the failure rate of the stimulating needle and stimulating needle/catheter strategies were included, the additional costs of consumables and time would only increase the cost difference between a standard kit and a stimulating kit. This would further strengthen ultrasound only as the choice strategy.
Our study indicates that use of ultrasound alone for femoral perineural catheter is noninferior to the use of ultrasound plus stimulating needle or to ultrasound combined with both a stimulating needle and stimulating catheter. Furthermore, use of ultrasound alone was both faster and less expensive.
Discussion
Most1,14,15 but not all6 studies report no analgesic benefit for stimulating versus non-stimulating catheters when inserted without ultrasound guidance. Post-operative analgesia is also similar with stimulating and non-stimulating catheters under ultrasound guidance for femoral nerve blocks.16 Furthermore, ultrasound alone for femoral nerve catheters provides comparable postoperative analgesia to the combination of ultrasound guidance and nerve stimulation, and involved fewer manipulations and less procedure-related pain.8,17 Our study, though, is the first adequately powered trial comparing clinically important outcomes — pain and opioid consumption — among the three techniques.3,8,16,18
The results were clear: use of ultrasound alone for continuous femoral perineural catheter was significantly noninferior to ultrasound combined with a stimulating needle or ultrasound combined with stimulating nerve catheter. Furthermore, adding stimulation prolonged block time and outright failed in 3.8% of the cases.
Superiority of one intervention versus another on pain management is sometimes assessed using either pain scores or opioid consumption in isolation. However, better pain scores with more opioid consumption, or vice versa, would not usually be accepted as better management. It thus seems reasonable to define a more effective regimen as being better on either pain or opioid consumption, but not worse on either. We therefore used a joint hypothesis testing methodology to assess whether one treatment for pain management was more effective than another.10
To be considered more effective, a treatment needed to be noninferior (i.e., not worse) on both outcomes, and superior on at least one of them. This is much preferable to simply requiring superiority on one or both outcomes, since a non-significant test for superiority could be consistent with inferiority or lack of power. Instead, we defined noninferiority margins for both outcomes a priori (25% worse for opioids and 0.5 points worse for pain), and then claimed noninferiority versus a comparator if the upper limit of the treatment effect confidence interval was no larger than the stated margin. Superiority of one treatment over another was assessed only if noninferiority was found on both outcomes.
Besides providing meaningful assessment of pain management, our joint hypothesis framework used the intersection-union principle to efficiently control the type I error at 0.025 across all of the noninferiority and superiority testing. For example, since for a particular comparison noninferiority was required on both outcomes before claiming “noninferiority,” the significance criterion for a particular test did not need to be adjusted for testing multiple outcomes (i.e., intersection-union test). Likewise, since both noninferiority and superiority were required before claiming one intervention better than another, no adjustment for testing both noninferiority and superiority was needed. We did adjust for multiple testing due to comparing 3 groups and for assessing noninferiority in both directions. However, we conserved alpha by using the Holm-Bonferroni method,11 which was less conservative than the traditional Bonferroni multiple comparison procedure. Finally, we used a flexible nonparametric global test to assess superiority across a vector of outcomes which could be on quite different scales with very different distributions (e.g., pain and opioid consumption).12
Ultrasound alone had the lowest cost and was significantly noninferior to the stimulating needle or stimulating catheter and stimulating needle. Ultrasound alone was thus the preferable intervention in that it achieved the desired outcome at the lowest cost. Our cost-minimization analysis was conservative in that it included only the cost of consumables. Not included was the cost of additional time required for the stimulating needle or stimulating catheter and needle or the additional time and consumables required when stimulation fails and the ultrasound only strategy must be implemented. If these additional costs were included, results would only further support ultrasound only being the choice strategy.
Available literature suggest that some sort of stimulation in addition to ultrasound is used for most femoral perineural catheters.19 Our results and analysis, showing no benefit, thus presents a cost-saving opportunity for hospitals. The potential savings on consumables is relatively small ($14–$83), but can be realized for every patient having a femoral nerve block. Furthermore, avoiding stimulation is actually easier for physicians. Our results suggest that stimulation, whether via needle or catheter, should be reserved for special cases and only after the additional cost and clinical benefit have been considered.
The clinicians who inserted the blocks in our patients varied from being highly experienced attendings to moderately experienced senior residents. And while there was no obvious difference among the techniques as a function of experience, individual clinicians may have found that electrical nerve stimulation, either with the needle alone or needle and catheter, improved femoral perineural catheters outcomes of ultrasound-guided insertion. It is also possible that individuals will find that nerve stimulation can be substituted for ultrasound guidance — a combination that we did not evaluate. We did not evaluate motor block and it remains possible that one technique or another produced more motor block and consequent risk of falls. And while most of our patients were given spinal anesthesia, some received general anesthesia which might have affected immediate postoperative pain.
In summary, ultrasound guidance alone for femoral perineural catheter insertion was significantly non-inferior to ultrasound combined with electrical stimulation of the needle and to ultrasound combined with electrical stimulation of the needle and catheter. That is, ultrasound guidance alone produced comparable pain scores and opioid requirements — and was faster. Because there is an incremental cost associated with using insulated needles and an even greater incremental cost when using insulated needles and stimulating catheters, using ultrasound alone results in realizable cost savings. We thus conclude that ultrasound alone is both a clinically sufficient and cost efficient choice for successful placement of femoral perineural catheters.
Summary.
What we already know
Ultrasound guided placement of femoral perineural catheters is sometimes supplemented with the use of electrical stimulation through the needle or catheter, but whether this improves catheter function is not known
What this tells us that is new
In a controlled study of nearly 450 patients, electrical stimulation through the needle or needle and catheter was noninferior to ultrasound guidance alone in terms of pain score and IV opioid requirement.
Additionally, the use of ultrasound alone was both faster and less expensive.
Acknowledgments
Support was provided solely from institutional and/or departmental sources Dr. Dalton’s effort was supported by the Clinical and Translational Science Collaborative of Cleveland, KL2TR000440 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health (NIH) and National Institutes of Health Roadmap for Medical Research (Bethesda, MD). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
None of the authors has a personal financial interest in this research. The authors declare no competing interests.
References
- 1.Morin A, Eberhart L, Behnke H, Wagner S, Koch T, Wolf U, Nau W, Kill C, Geldner G, Wulf H. Does femoral nerve catheter placement with stimulating catheters improve effective placement? A randomized, controlled, and observer-blinded trial. Anesth Analg. 2005;100:1503–1510. doi: 10.1213/01.ANE.0000151160.93288.0A. [DOI] [PubMed] [Google Scholar]
- 2.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Aveline C, Le Roux A, Le Hetet H, Vautier P, Cognet F, Bonnet F. Postoperative efficacies of femoral nerve catheters sited using ultrasound combined with neurostimulation compared with neurostimulation alone for total knee arthroplasty. Eur J Anaesthesiol. 2010;27:978–984. doi: 10.1097/EJA.0b013e32833b34e1. [DOI] [PubMed] [Google Scholar]
- 4.Ilfeld BM, Le LT, Meyer RS, Mariano ER, Vandenborne K, Duncan PW, Sessler DI, Enneking FK, Shuster JJ, Theriaque DW, Berry LF, Spadoni EH, Gearen PF. Ambulatory continuous femoral nerve blocks decrease time to discharge readiness after tricompartment total knee arthroplasty: A randomized, triple-masked, placebo-controlled study. Anesthesiology. 2008;108:703–713. doi: 10.1097/ALN.0b013e318167af46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capdevila X, Biboulet P, Morau D, Bernard N, Deschodt J, Lopez S, d'Athis F. Continuous three-in-one block for postoperative pain after lower limb orthopedic surgery: Where do the catheters go? Anesth Analg. 2002;94:1001–1006. doi: 10.1097/00000539-200204000-00042. [DOI] [PubMed] [Google Scholar]
- 6.Salinas F, Neal J, Sueda L, Kopacz D, Liu S. Prospective comparison of continuous femoral nerve block with nonstimulating catheter placement versus stimulating catheter-guided perineural placement in volunteers. Reg Anesth Pain Med. 2004;29:212–220. doi: 10.1016/j.rapm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Ganapathy S, Wasserman R, Watson J, Bennett J, Armstrong K, Stockall C, Chess D, MacDonald C. Modified continuous femoral three-in-one block for postoperative pain after total knee arthroplasty. Anesth Analg. 1999;89:1197–1202. [PubMed] [Google Scholar]
- 8.Fredrickson M, Danesh-Clough T. Ambulatory continuous femoral analgesia for major knee surgery: A randomised study of ultrasound-guided femoral catheter placement. Anaesth Intensive Care. 2009;37:758–766. doi: 10.1177/0310057X0903700514. [DOI] [PubMed] [Google Scholar]
- 9.Turan A, Atim A, Dalton JE, Keeyapaj W, Chu W, Bernstein E, Fu A, Ho LJ, Saager L, Sessler DI. Preoperative angiotensin-converting enzyme inhibitor use is not associated with increased postoperative pain and opioid use. Clin J Pain. 2013;29:1050–1056. doi: 10.1097/AJP.0b013e318287a258. [DOI] [PubMed] [Google Scholar]
- 10.Mascha E, Turan A. Joint hypothesis testing and gatekeeping procedures for studies with multiple endpoints. Anesth Analg. 2012;114:1304–1317. doi: 10.1213/ANE.0b013e3182504435. [DOI] [PubMed] [Google Scholar]
- 11.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 12.O'Brien PC. Procedures for Comparing Samples with Multiple Endpoints. Biometrics. 1984;40:1079–1087. [PubMed] [Google Scholar]
- 13.Sites BD, Beach M, Gallagher JD, Jarrett RA, Sparks MB, Lundberg CJ. A single injection ultrasound-assisted femoral nerve block provides side effect-sparing analgesia when compared with intrathecal morphine in patients undergoing total knee arthroplasty. Anesth Analg. 2004;99:1539–1543. doi: 10.1213/01.ANE.0000136470.51029.52. [DOI] [PubMed] [Google Scholar]
- 14.Barrington M, Olive D, McCutcheon C, Scarff C, Said S, Kluger R, Gillett N, Choong P. Stimulating catheters for continuous femoral nerve blockade after total knee arthroplasty: A randomized, controlled, double-blinded trial. Anesth Analg. 2008;106:1316–1321. doi: 10.1213/ane.0b013e318164efd1. [DOI] [PubMed] [Google Scholar]
- 15.Jack N, Liem E, Vonhögen L. Use of a stimulating catheter for total knee replacement surgery: Preliminary results. Br J Anaesth. 2005;95:250–254. doi: 10.1093/bja/aei161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi K, Lindenmuth D, Hadzic A, Xu D, Patel V, Maliakal T, Gadsden J. The effect of stimulating versus conventional perineural catheters on postoperative analgesia following ultrasound-guided femoral nerve localization. J Clin Anesth. 2011;23:626–631. doi: 10.1016/j.jclinane.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Sites B, Beach M, Chinn C, Redborg K, Gallagher J. A comparison of sensory and motor loss after a femoral nerve block conducted with ultrasound versus ultrasound and nerve stimulation. Reg Anesth Pain Med. 2009;34:508–513. doi: 10.1097/AAP.0b013e3181ae7306. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Ngeow J, Yadeau J. Ultrasound-guided regional anesthesia and analgesia: A qualitative systematic review. Reg Anesth Pain Med. 2009;34:47–59. doi: 10.1097/AAP.0b013e3181933ec3. [DOI] [PubMed] [Google Scholar]
- 19.Ilfeld BM. Continuous peripheral nerve blocks: A review of the published evidence. Anesth Analg. 2011;113:904–925. doi: 10.1213/ANE.0b013e3182285e01. [DOI] [PubMed] [Google Scholar]