Abstract
An ovarian follicle is composed of an oocyte and surrounding theca and granulosa cells. Oocytes are stored in an arrested state within primordial follicles until they are signaled to re-initiate development by undergoing primordial to primary follicle transition. Previous gene bionetwork analyses of primordial follicle development identified a number of critical cytokine signaling pathways and genes potentially involved in the process. In the current study, candidate regulatory genes and pathways from the gene network analyses were tested for their effects on the formation of primordial follicles (follicle assembly) and on primordial follicle transition using whole ovary organ culture experiments. Observations indicate that the tyrphostin inhibitor BCI increased follicle assembly significantly, supporting a role for the mitogen activated protein kinase (MAPK) signaling pathway in follicle assembly. The cytokine interleukin 16 (IL16) promotes primordial to primary follicle transition as compared to the controls, where as Delta-like 4 (DLL4) and WNT-3A treatment have no effect. Immunohistochemical experiments demonstrated for both the cytokine IL16 and its receptor CD4 localization to the granulosa cells surrounding each oocyte within the ovarian follicle. The tyrphostin LDN193189 (LDN) is an inhibitor of the bone morphogenic protein receptor 1 (BMPR1) within the TGFB signaling pathway and was found to promote the primordial to primary follicle transition. Observations support the importance of cytokines (i.e. IL16) and cytokine signaling pathways in regulating early follicle development. Insights into regulatory factors affecting early primordial follicle development are provided that may associate with ovarian disease and translate to improved therapy in the future.
Keywords: Ovary, IL16, Primordial Follicle, Growth Factor, Gene Networks
INTRODUCTION
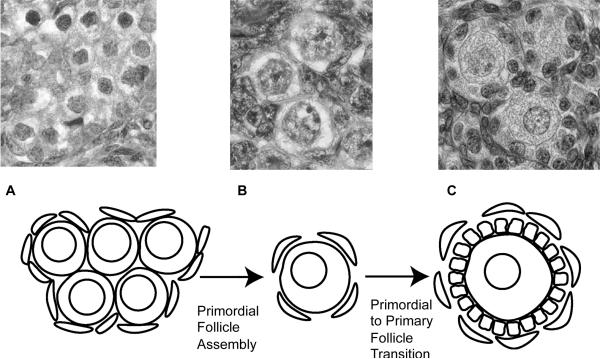
The mammalian ovary is a dynamic organ in which germ cells go through many developmental stages to produce functional gametes. Development through these stages is initiated and regulated by different secreted growth factor and hormonal signals at different developmental points during oocyte maturation. During late fetal development in humans and early postnatal development in rodents, pre-granulosa cells and stromal mesenchymal cells surround the oogonia present in germ cell nests that then undergo follicle assembly to form primordial follicles (Skinner, 2005)(Figure 1). A primordial follicle is composed of a single oocyte surrounded by a single layer of flattened squamous pre-granulosa cells (Skinner, 2005). Primordial follicle assembly creates the pool of primordial follicles available during the reproductive life of the female. If selected for further development the quiescent primordial follicles undergo the primordial to primary follicle transition. This is characterized by an oocyte size increase and by proliferation of the cuboidal pre-granulosa cells which surround each oocyte (Kenngott et al., 2013; McLaughlin and McIver, 2009; Fortune, 2003) (Figure 1B, C). Investigation of the cascade of molecular events involved in ovarian follicle development will provide insight into the etiology of certain ovarian diseases such as primary ovarian insufficiency (POI). POI is characterized by early depletion of the pool of arrested primordial follicles, and by induction of menopause prior to age 40 (Coulam et al., 1986). POI affects approximately 1% of women in the USA (Dixit et al., 2010). Understanding the molecular factors in early follicle development could lead to solutions for some infertilities in humans.
Figure 1.
The process of primordial follicle development. (A) Oocyte nest, with surrounding pregranulosa cells. (B) Primordial follicles. (C) Primary follicles.
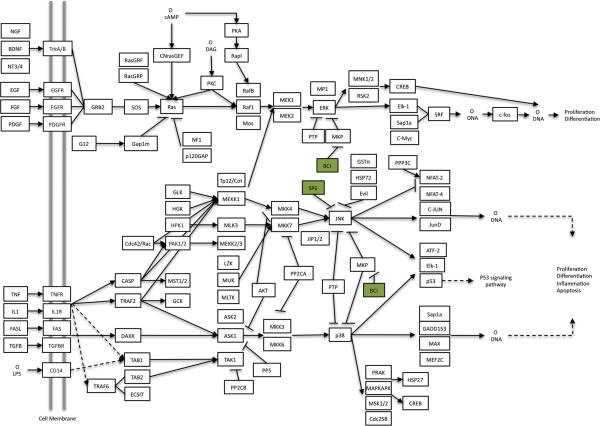
Previous studies have lead to the construction of proposed regulatory gene networks for primordial follicle assembly (Nilsson et al., 2013), and the primordial to primary follicle transition (Nilsson et al., 2010). In the current study several signaling pathways and cytokine growth factors that were identified to potentially regulate primordial follicle development have been chosen to test experimentally. Follicle assembly has previously been shown to be regulated by several cytokines and growth factors that act in part by signaling through the mitogen-activated protein kinase (MAPK) signaling pathway. These include brain-derived neurotrophic factor (BDNF) (Kerr et al., 2009), tumor necrosis factor alpha (TNFa) (Nilsson et al., 2006b; Morrison and Marcinkiewicz, 2002), and kit ligand (KITLG) (Wang and Roy, 2004). The MAPK and focal adhesion pathways were also implicated in gene networks previously proposed to regulate follicle assembly (Nilsson et al., 2013). Certain tyrphostin inhibitors (Levitzki, 1996) are utilized in the current study to test if the MAPK and focal adhesion pathways are involved in early ovarian follicle development. Tyrphostin inhibitor SP600125 inhibits c-Jun N-terminal kinase (JNK) in both the focal adhesion pathway and the MAPK signaling pathway. One would predict that this would lead to inhibited ovarian follicle assembly. (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI) is an allosteric inhibitor of mitogen activated protein kinase phosphatases (MKP), specifically dual specificity phosphatase 6 (Dusp6), in the MAPK signaling pathway. Dusp6 is itself an inhibitor of extracellular-signal-related kinases (ERK1/2) and c-Jun N-terminal Kinase (JNK). BCI treatment can then be expected to stimulate ovarian follicle assembly.
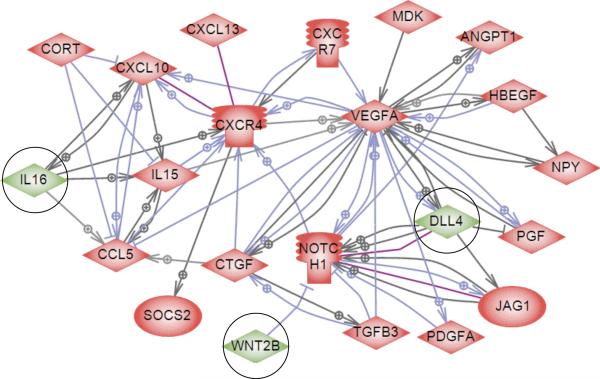
The mRNA expression levels of Interleukin 16 (IL16), Wingless-type MMTV integration site family, member 2B (WNT2B), and Delta-like ligand 4 (Dll4), have been found to change during primordial to primary follicle transition (Nilsson et al., 2010). Interleukin 16 is a cytokine known to act as a chemoattractant for CD4+ T-cells in immune responses throughout the body (Cruikshank and Little, 2008; Kanehisa and Goto, 2000; Mathy et al., 2000). Interleukins have also been found in various ovarian cells including oocytes and supporting follicle cells in mammals and have been shown to be secreted from macrophages in the ovary (Smolikova et al., 2012). WNT2B is known to bind to members of the Frizzled receptor family which are a part of the Wnt signaling pathway. WNT3A is also known to act on this same set of receptors (Kanehisa and Goto, 2000; Lapointe and Boerboom, 2011; Sirotkin, 2011). Members of the Wnt signaling pathway have been found to be expressed in the ovary and to regulate follicle development and steroidogenesis (Richards and Pangas, 2010; Lapointe and Boerboom, 2011; Sirotkin, 2011). DLL4 is known to bind to Notch receptors to activate the Notch signaling pathway (Kanehisa and Goto, 2000; Andrawes et al., 2013). Notch has been shown to influence multiple cellular processes including: cell fate decisions, proliferation, apoptosis, migration, and plasticity, and seems to be especially essential for angiogenesis (Thomas et al., 2013). In the current study, the roles of IL16, WNT3A (as a functional substitute for WNT2B which is not commercially available) and DLL4 in primordial to primary follicle transition are investigated.
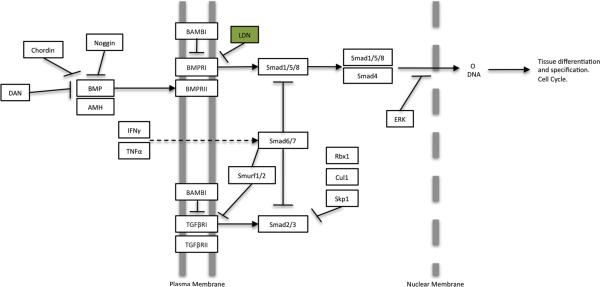
The roles of the MAPK and focal adhesion pathways on the primordial follicle transition are investigated in the current study using the inhibitors SP600125 and BCI. The role of the TGFß signaling pathway is investigated using 4-[6-[4-(1-Piperazinyl)phenyl]pyrazolo [1,5-a]pyrimidin-3-yl]-quinoline dihydrochloride (LDN193189). LDN193189 inhibits bone morphogenetic protein receptor 1 (BMPR1) in the TGFB signaling pathway. BMPR1 is a component of the receptor complex that anti-Müllerian hormone (AMH) and bone morphogenetic proteins (BMP) bind (Nilsson et al., 2011). AMH is a protein hormone previously shown to have inhibitory action on both primordial follicle assembly (Nilsson et al., 2011) and primordial to primary follicle transition (Nilsson et al., 2007). Therefore LDN would be expected to stimulate the primordial follicle transition.
The current study investigates the actions of candidate regulatory factors and selected signaling pathways on ovarian follicle assembly and primordial to primary follicle transition. The objective is to test candidate regulatory pathways controlling follicle assembly with ovarian organ culture experiments using the tyrphostin inhibitors SP600125 and BCI. Candidate regulatory factors and pathways potentially controlling the primordial to primary follicle transition are tested using the cytokines Interleukin 16 (IL16), Delta-like4 (DLL4) and WNT-3A, and tyrphostin inhibitors SP600125, BCI, and LDN193189. In addition, the localization of endogenous IL16 and its receptor CD4 in ovarian follicles are determined using immunohistochemistry.
MATERIALS AND METHODS
Organ culture and data collection
Ovaries were removed from 0-day old Sprague-Dawley rat pups (for ovary follicle assembly studies) and 4-day old rat pups (for ovary follicle transition studies). Animals were obtained from the Washington State University Center for Reproductive Biology Animal Core breeding colonies. All experimental protocols for the procedures with rats were pre-approved by the Washington State University Animal Care and Use Committee (IACUC approval # 02568-026). These ovaries were then placed into a whole organ culture system as previously described (Nilsson et al., 2011; Nilsson et al., 2007; Nilsson et al., 2002). The whole organ culture system allows for modeling of multi-tissue biological processes. The culture medium contains 10 mg/ml gentamycin, 40 units/ml penicillin, and 10 mg/ml streptomycin. Micro-dissected ovaries were placed in groups of two or three in a culture well on a Teflon filter (0.2 μm Biopore membrane; Millipore Corp., Billerica, MA, USA) floating on 500μL culture medium (control), or culture medium including specific treatments of Interleukin 16 (IL16; recombinant human, PeproTech cat# 200-16A, USA) (20ng/ml, 100ng/ml, 500ng/ml), Delta-like 4 (DLL4; R&D Systems, cat# 1389-D4, USA) (100ng/ml, 500ng/ml), Wnt3a (R&D Systems, cat# 1324-WN, USA) (20ng/ml, 100ng/ml), SP600125 (LC Laboratories, cat# S-7979, USA) (10μM and 50μM), anti-Müllerian hormone (AMH; R&D Systems, cat# 1737-MS, USA) (50ng/ml), BCI (Sigma-Aldrich, cat# B4313, USA) (0.5μM, 1μM, 5μM), or LDN193189 (Axon Medchem, cat# 1509, the Netherlands) (0.003μg/ml, 0.03μg/ml), along with positive controls KITLG (50ng/ml) plus FGF2 (50ng/ml). Wnt3a was used as a functional substitute for Wnt2b, as Wnt2b was not commercially available. Positive controls have been shown to affect follicle assembly or primordial follicle transition in previous studies (Nilsson et al., 2011; Nilsson et al., 2007). Ovaries were cultured for two days in the case of follicle assembly studies (Nilsson et al., 2011), and for ten days in the case of primordial to primary follicle transition studies (Nilsson et al., 2002). At the completion of the culture period, ovaries were processed as previously described (Nilsson et al., 2011; Nilsson et al., 2007; Nilsson et al., 2010).
After fixation, sectioning at 3μM thickness and staining, the ovaries and follicles in each ovarian cross-section were categorized as either oocytes un-assembled in nests, primordial follicles (stage 0), early primary (1), primary (2), transitional (3) or pre-antral follicles (4) (Figure 1B,1C) as previously described (Nilsson et al., 2011; Nilsson et al., 2010). Oocytes and follicles from two adjacent sections from the largest cross section of each ovary were counted and categorized, and then the counts in each category were averaged. In this way two almost identical ovarian sections are evaluated, and an average number of un-assembled oocytes, primordial follicles and developing follicles are determined across those sections. Only oocytes with visible nuclei are counted. The total number of follicles was determined for each ovary cross section for each treatment, as well as the percent of follicles at each stage of development. These results were analyzed with ANOVA, Student's t-tests, and Dunnet's post-hoc tests for specific comparisons.
Immunohistochemistry
Immunohistochemical procedures were performed for IL16 and its known receptor CD4 in order to localize expression in the developing ovary. Formalin-fixed tissues from 14-day old and 19-day old rat pups were used for immunohistochemical staining as previously described (Nilsson et al., 2002). The ovary sections were incubated with a blocking buffer [10% goat serum (normal goat serum) or 10% rabbit serum (normal rabbit serum)], followed by the primary antibody, either anti- IL16 (Santa Cruz Biotechnology Inc., cat# sc-7902, USA) (made in rabbit, 0.2 μg/ml, 0.8 μg/ml, or 4 μg/ml) or anti CD4 (Santa Cruz Biotechnology Inc., cat# sc-1140, USA) (made in goat, 0.2 μg/ml, 0.8 μg/ml, or 4 μg/ml) for antigen binding. Secondary antibody was biotinylated anti-rabbit IgG made in goat (Vector Laboratories Inc., cat# BA-1000, USA) or biotinylated anti-goat IgG made in rabbit (Vector Laboratories Inc., cat# BA-5000, USA). The ovary sections were incubated with Streptavidin Peroxidase Complex (Invitrogen, cat# 50-420Z, USA) for enzyme coupling. A color reaction was performed using a DAB peroxidase substrate kit (Vector Laboratories Inc., cat# SK-4100, USA). Images were taken of the stained sections and the results were qualitatively analyzed.
RESULTS
Ovarian organ culture experiments were used to investigate the potential role of candidate regulatory factors and selected signaling pathways on ovarian follicle assembly and/or primordial to primary follicle transition. Follicle assembly used ovaries removed from 0-day old rats and cultured for two days before being fixed and evaluated (Figure 1). These 0-day old rat ovaries contain predominately nests of oocytes not yet assembled into follicles (Nilsson et al., 2011). Primordial to primary follicle transition studies used ovaries removed from 4-day old rats and then cultured for ten days with replacement of organ culture medium every other day. These 4-day old rat ovaries contain predominately assembled primordial follicles (Nilsson et al., 2007) (Figure 1). After culture and treatment, ovaries were then fixed, sectioned and evaluated for their proportions of un-assembled oocytes, primordial follicles and developing follicles as described in Methods. Regulatory factors and pathways were selected for further investigation from previous system biology gene bionetwork studies (Nilsson et al., 2013; Nilsson et al., 2010).
Primordial Follicle Assembly
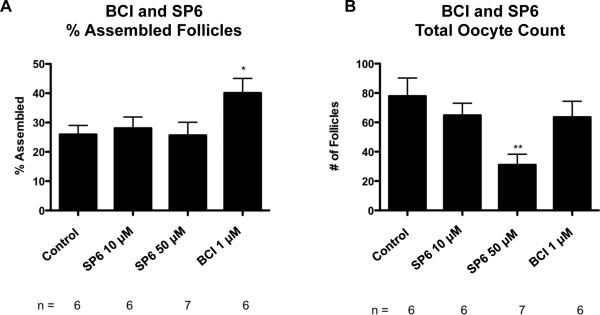
In order to test the importance of the MAPK signaling pathway to the follicle assembly process, zero day old ovaries were cultured for two days with or without the tyrphostin inhibitor compounds BCI and SP600125 (see Methods). BCI acts indirectly to stimulate ERK 1/2 and JNK. Both ERK and JNK play prominent roles in the MAPK pathway (Figure 2). After two days of culture untreated control ovaries had 26% +/− 3.0% assembled follicles. Ovaries treated with BCI (1 μM) had a significant increase in assembled follicles as compared to the control with 40% +/− 5.0% assembled into primordial follicles (Figure 3A). There was no significant difference in the total oocyte count between control and BCI-treated ovaries, indicating that oocyte death was not a confounding factor in these studies (Figure 4B). These results indicate that stimulation of the MAPK pathway promotes the follicle assembly process.
Figure 2.
SP600125 and BCI Inhibition in MAPK Signaling Pathway adapted from KEGG 04010. MKP (mitogen activated protein kinase phosphatase) abbreviation. (www.genome.jp/kegg/pathway.html)
Figure 3.
(A) Primordial follicle assembly after 2 days of treatment represented as % Assembled Follicles with SP600125 and BCI treatments with statistical significance indicated as (*)(P<0.05). (B) Oocyte number per cross section after the same treatments. The Mean ±SEM from a minimum of five different experiments performed in replicate with statistical significance indicated as (**) (P<0.01).
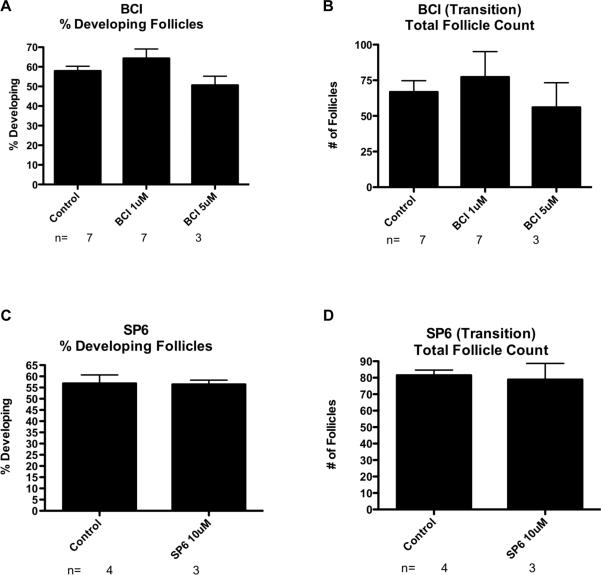
Figure 4.
(A) Primordial to primary follicle transition after 10 days of treatment with BCI represented as % Developed Follicles. (B) Oocyte number per cross section after the BCI treatments. The Mean ±SEM from a minimum of three different experiments performed in replicate. (C) Primordial follicle transition after 10 days of treatment with SP600125 represented as % Developed Follicles. (D) Oocyte number per cross section after SP600125 treatments. The Mean ±SEM from a minimum of three different experiments performed in replicate.
The tyrphostin inhibitor SP600125 inhibits JNK in the MAPK and focal adhesion pathways (Figure 2), and so decreases activity of these pathways. Treatment of ovaries from zero-day old rats with SP600125 did not have a significant effect on follicle assembly as compared to the untreated controls. A 10 μM SP600125 treatment resulted in 28% +/− 3.8% assembled follicles, while 50 μM treatment with SP600125 resulted in 26% +/− 4.5% assembled follicles (Figure 3A). This indicates that inhibition of the focal adhesion and MAPK pathways did not affect the follicle assembly process. This would also indicate that inhibition of JNK in the MAPK pathway has no effect on ovarian follicle assembly. However, treatment with SP600125 at 50 μM concentration did result in a significant decrease in number of follicles per cross section (31 +/− 7.2), as compared to controls, (78 +/− 12.3) (Figure 3B). SP600125 treatment at 50 μM was also observed to result in disorganized follicles and deteriorating cell nuclei (data not shown). This toxicity only occurs at the higher levels of the inhibitor. Together these observations indicate that increased MAPK signaling promoted follicle assembly, but that decreased MAPK and focal adhesion pathway signaling had little effect.
Primordial to Primary Follicle Transition
Previous studies suggest that the MAPK signaling pathway is a component of the gene network that regulates primordial follicle transition (Nilsson et al., 2010). In order to test this hypothesis, ovaries from 4-day old rats were cultured for ten days in the presence or absence of BCI or SP600125. BCI treatment resulted in no significant change in the proportion of developing follicles (1 μM: 64% +/− 4.8%, 5 μM: 51% +/− 4.6%) as compared to the untreated controls (58% +/− 2.4%) (Figure 4A). There were also no significant differences in total follicle count between these treatment groups (Figure 4B), indicating that oocyte death was not a confounding factor in these studies. Similarly, treatment with SP600125 resulted in no significant change in the proportion of developing follicles compared to the untreated controls (Figure 4C). There was also no significant difference in total follicle count (Figure 4D). These observations indicate that the MAPK and focal adhesion signaling pathways may not play prominent roles in regulating primordial to primary follicle transition, or that compensatory pathways exist. Therefore, growth factors known to affect follicle transition may act through additional parallel signaling pathways.
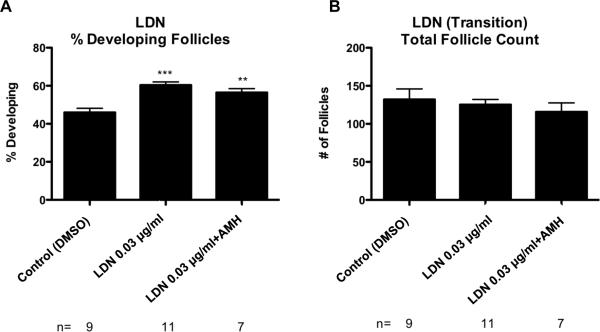
Previous research has shown that AMH produced by larger developing follicles will inhibit the primordial to primary follicle transition (Nilsson et al., 2007; Visser and Themmen, 2005). Conversely, BMP4 and BMP7 are shown to stimulate primordial follicle transition (Nilsson and Skinner, 2003; Lee et al., 2001). AMH and BMP growth factors can act through the bone morphogenetic protein receptor 1 (Bmpr1) branch of the TGFB family signaling pathway (Figure 5). In the current study, ovaries were treated with the tyrphostin inhibitor LDN, which inhibits bone morphogenetic protein receptor I (BMPRI), in order to determine how disruption of this TGFB family signaling pathway affects ovarian follicle development (Figure 5). The control ovaries for these experiments were treated with dimethylsulfoxide (DMSO) vehicle. Control ovaries were found to have 46% +/− 2.2% developing follicles. LDN treatment at a concentration of 0.03 μg/ml resulted in a significant increase (p<0.05) in the proportion of developing follicles, with 60% +/− 1.7% of the follicles in the developing stages. Treatment with LDN plus 50 ng/ml AMH also resulted in a significant increase in developing follicles with 56% +/− 2.1% developing (Figure 6A). There were no significant differences between treatment groups in the total number of follicles per ovarian cross section (Figure 6B). The results for this treatment indicate that the BMP/AMH portion of the TGFB signaling pathway plays a role in primordial to primary follicle transition, and that the inhibitory actions of AMH are blocked by LDN treatment.
Figure 5.
LDN Inhibition in TGFB Signaling Pathway adapted from KEGG 04350. (www.genome.jp/kegg/pathway.html)
Figure 6.
(A) Primordial follicle transition after 10 days of treatment represented as % Developing Follicles after LDN, and LDN combined with AMH treatments with statistical significance indicated as (**)(P<0.01). (***)(P<0.005). (B) Oocyte number per cross section. The Mean ±SEM from a minimum of five different experiments performed in replicate.
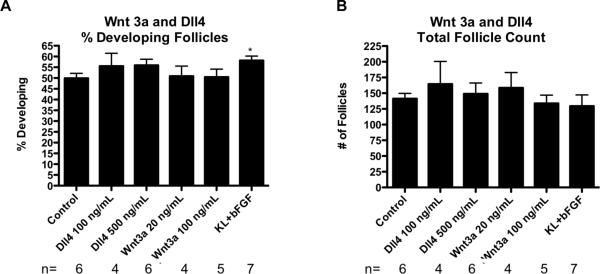
Previous research had suggested that specific secreted growth factors and cytokines might have a role in regulating the primordial to primary follicle transition (Nilsson et al., 2010) (Figure 7). Three of these candidate regulatory factors, WNT3A (as a functional substitute for WNT2B), DLL4 and IL16, were chosen for evaluation. After the culture period the untreated control ovaries had an average of 50% +/− 2.3% developing follicles. Neither WNT3A nor DLL4 treatment resulted in any significant difference in the proportion of primordial to developing follicles as compared to the controls (Figure 8A). There was also no significant difference in total follicle count between any of these treatment groups and the controls (Figure 8B). In these experiments positive control treatments of KITLG plus FGF2 were performed, which stimulated primordial to primary follicle transition, as expected. This indicates that experimental conditions supports follicle transition, even though WNT3A and DLL4 did not stimulate this.
Figure 7.
Scheme of direct connections between candidate regulatory growth factors and receptors previously found to be differentially expressed during primordial follicle transition. Circles around genes identify the candidate genes investigated. Nodes represent secreted extracellular signaling molecules and receptors that change in expression level during primordial to primary follicle transition (except Notch1, which does not change in expression). Node shapes code: diamond – ligand; ice cream cone – receptor. Green color represents the regulatory factors investigated in this paper. Connecting arrows represent relationships between genes present in published literature. Arrows with plus sign show positive regulation/activation, arrows with minus sign – negative regulation/inhibition, purple connections represent direct binding. Adapted from Nilsson, et al. 2010.
Figure 8.
(A) Primordial follicle transition after 10 days of treatment represented as % Developed Follicles after Wnt3a, Dll4, and KL+ bFGF treatments with statistical significance indicated as (*)(P<0.05). (B) Oocyte number per cross section. The Mean ±SEM from a minimum of three different experiments performed in replicate.
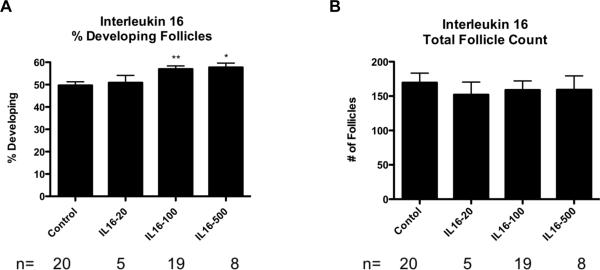
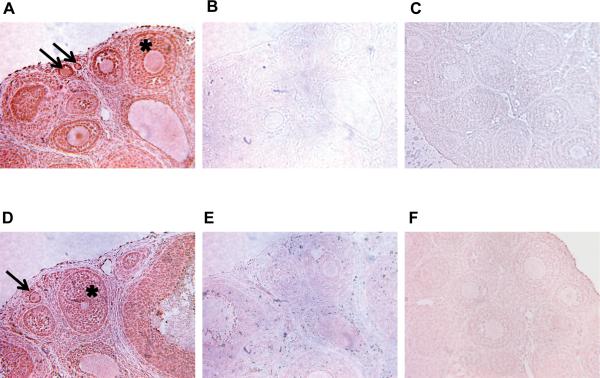
Ovaries treated with 100 ng/ml IL16 had 57% +/− 1.4% developing follicles, which was a significant increase (p<0.01) compared to the corresponding control group (50% +/− 1.6%). Ovaries treated with 500 ng/ml IL16 had a mean of 58% +/− 1.9% developing follicles, which was also a significant increase as compared to the control (Figure 9A). IL16 treatment at 20 ng/ml had no significant effect. None of the IL16 treatments had a significant effect on the total counts of ovarian follicles as compared to the controls (Figure 9B). These data suggest the cytokine IL16 has a role in regulating the primordial to primary ovarian follicle transition. This supports the importance of IL16 as part of a proposed network of genes regulating the primordial follicle transition (Figure 7). Since IL16 treatment had a significant effect on the primordial to primary follicle transition, immunohistochemical experiments were performed to localize IL16 and its receptor CD4 within the ovary. Both IL16 and CD4 were found to be at highest levels in the granulosa cells of the developing follicle structures, including developing primordial follicles (Figure 10). Negative controls using either non-specific IgG as primary antibody, or using no primary antibody, showed no differential staining of granulosa cells compared to other ovarian cell types (Figure 10). Observations suggest IL16 is an active part of the network of genes regulating the primordial follicle transition, and that an apparent autocrine signaling loop between IL16 and CD4 is maintained in the granulosa cells of developing follicles.
Figure 9.
(A) Primordial follicle transition after 10 days of IL16 treatment represented as % Developed Follicles. Statistical significance indicated as (*)(P<0.05). (**)(P<0.01). (B) Oocyte number per cross section after the same treatments. The Mean ±SEM from a minimum of five different experiments performed in replicate.
Figure 10.
Immunohistochemical analysis of Interleukin 16 and its receptor, CD4. Brown stain indicates presence of the specified protein. (A) Localization of IL16 in the ovary, particularly in granulosa cells of follicles (*). (B) Negative control experiment conducted without the use of primary antibody. (C) Negative control experiment conducted with non-specific Rabbit IgG. (D) Localization of CD4 receptor in the ovary, particularly in granulosa cells of follicles (*). (E) Negative control experiment conducted without the use of primary antibody. (F) Negative control experiment conducted with non-specific Goat IgG. Arrows point to developing primordial and primary follicles.
DISCUSSION
Previous studies have used a systems biology approach to identify gene networks that potentially regulate primordial follicle assembly (Nilsson et al., 2013) and the primordial to primary follicle transition (Nilsson et al., 2010). In these studies, the MAPK and focal adhesion signaling pathways were implicated as mediating some of the actions of these proposed regulatory networks for both follicle assembly and the primordial follicle transition. In addition, examination of the proposed regulatory network for primordial follicle transition suggested that the TGFB signaling may be important, as well as WNT, Delta/Notch and interleukin cytokine signaling. In the current study these proposed regulatory gene networks are experimentally evaluated.
Primordial Follicle Assembly
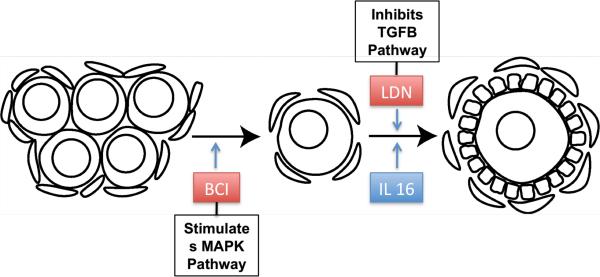
In order to evaluate the roles of the MAPK and focal adhesion pathways in follicle assembly, 0-day rat ovaries were cultured and treated with the tyrphostin inhibitors BCI and SP600125. BCI has been shown to directly and specifically inactivate Dusp6 within the MAPK signaling pathway (Molina et al., 2009). Dusp6 is itself an inhibitor of extracellular signal regulated kinases 1/2 (ERK 1/2) and c-Jun N-terminal kinase (JNK) (Figure 2), so the end result of BCI treatment is to increase MAPK activity. MAP kinases control many cellular events from embryogenesis, cell differentiation, cell proliferation, and cell death, to short-term changes required for homeostasis and acute hormonal responses (Chen et al., 2001). ERK1 and ERK2 are two specific MAP kinases that are co-expressed in all mammalian tissues and implicated as key regulators of cell proliferation and differentiation as well as oocyte maturation in culture (Su et al., 2002). Ovaries treated with BCI showed a significant increase in assembled follicles as compared to the controls. Observations indicate that increasing the activity of the MAPK pathway promotes the follicle assembly process (Figure 11). This supports the previously proposed gene network for regulation of follicle assembly (Nilsson et al., 2013).
Figure 11.
Primordial follicle developmental summary.
In order to test the effect of inhibiting the MAPK pathway during primordial follicle assembly these 0-day ovaries were cultured with SP600125. SP600125 is a small-molecule inhibitor of JNK (Bennett et al., 2001) that plays a role in both the focal adhesion and MAPK signaling pathways. Both JNK and c-Jun have been shown to be essential for cell cycle progression and cell proliferation, and previous research has shown that inhibition of JNK via SP600125 halts in vitro growth of mouse preantral follicles (Oktem et al., 2011). In the present study, SP600125 treatment had no affect on the percentage of assembled ovarian follicles as compared to the controls. These results suggest that inhibiting JNK in either the focal adhesion pathway or the MAPK pathway does not have an effect on follicle assembly. Because of the significant decrease in total follicle number in ovaries treated with high concentrations of SP600125, the results also suggest that the SP600125 inhibitor has a toxic effect at higher concentrations. Ovaries treated with high concentrations of SP600125 have disorganized morphology and signs of necrotic cell death (data not shown).
An explanation is needed as to why BCI had an effect on follicle assembly via the MAPK pathway whereas SP600125 did not. Inhibition of Dusp6 with BCI amplifies the proliferative effect of c-Jun and JNK signaling (Figure 2). SP600125 decreases activity in this pathway by inhibiting JNK. This would mean that a decrease of activity in the MAPK pathway does not have an effect on follicle assembly, but an increase of activity at the JNK site does in fact affect development. This suggests that MAPK activity is sufficient to stimulate increased follicle assembly, but that the activity of the pathway is not necessary for follicle assembly to occur, perhaps because of compensation with other signaling pathways. Further investigation is needed to clarify the molecular mechanisms underlying the differences in response to BCI and SP600125 treatments.
Primordial to Primary Follicle Transition
A previous systems biology investigation showed that mRNA expression levels of the secreted growth factors WNT2B, DLL4 and IL16 changed in ovaries during primordial to primary follicle transition (Figure 7) (Nilsson et al., 2010). In the current study, organ culture experiments were performed to directly test if these signaling molecules regulate follicle transition. WNT3A acts on the same receptor as WNT2B and is assumed to have the same physiological effects, so WNT3A was used as a functional substitute treatment factor to determine effects on ovarian follicle primordial to primary transition. The WNT2B is not commercially available. In this study WNT3A had no significant effect on the primordial to primary follicle transition as compared to the controls. Even though WNT2B is known to be present in the ovary, it has been shown that when the WNT2B gene was knocked out in mice, the mice were viable, fertile, and had a normal reproductive lifespan (Tsukiyama and Yamaguchi, 2012). This indicates that WNT2B is not essential for follicle transition despite its presence in the ovary and/or that compensatory factors exist. Previous studies suggest that WNT2B mRNA expression in the ovary is localized to the rat ovarian surface epithelium (Ricken et al., 2002). This would support the idea that WNT2B may have an indirect supporting role within the ovary with regard to follicle development.
DLL4 is known to bind to Notch receptors to activate the Notch signaling pathway (Musse et al., 2012). Notch has been shown to influence multiple cellular processes including: cell fate decisions, proliferation, apoptosis, migration, and plasticity, and seems to be especially essential for processes such as angiogenesis (Thomas et al., 2013). In previous studies, DLL4 was shown to be expressed in ovaries (Nilsson et al., 2010), but in this study DLL4 treatment resulted in no significant changes in the percentage of follicle transition as compared to the controls (Figure 8). This may indicate a non-essential supporting role of DLL4 within the ovary. Alternatively, DLL4 expression at the time of follicle transition may prepare the way for vascular development that occurs later as follicle develop. In previous studies, loss of DLL4 expression or Notch signaling has lead to abnormal development of the vascular network, but was not shown to be directly related to the process of angiogenesis (Mettouchi, 2012).
IL16 is a cytokine often associated with immune response, especially for immunoregulatory responses (Mahindra and Anderson, 2012). IL16 is produced by CD8-positive T cells and its known receptor is the CD4 molecule (Cruikshank et al., 1996). Previous studies showed that mRNA expression levels of IL16 changed in ovaries during primordial to primary follicle transition (Nilsson et al., 2010) (Figure 7). Based on these data, organ culture experiments were conducted to test the activity of IL16 within the ovary, and immunohistochemical experiments were then preformed to specifically locate both IL16 and its receptor CD4 in developing ovaries. The organ culture experiments demonstrated a moderate increase in primordial to primary follicle transition as compared to the controls. The immunohistochemical experiments showed that both IL16 and CD4 proteins were located within the granulosa cells of ovarian follicles, suggesting an autocrine signaling loop. Therefore, IL16 appears to promote the primordial follicle transition process (Figure 11).
A systems biology investigation of the primordial to primary follicle transition predicted that MAPK signaling was involved in this process (Nilsson et al., 2010). The importance of MAPK signaling in regulating the primordial to primary follicle transition was investigated in the current studies by using the tyrphostin inhibitors BCI and SP600125 to treat 4-day old rat ovaries cultured for ten days. Ovaries treated with BCI at this stage of development showed no significant difference compared to the controls. This suggests that stimulating the MAPK pathway may not be an important mode of regulating the ovarian primordial to primary follicle transition, even though it did seem to have an effect earlier in the developmental process. Ovaries treated with SP600125 at this stage also showed no significant difference between untreated controls and the treated ovaries, at either treatment concentration. This indicates that neither directly inhibiting JNK within the MAPK pathway nor inhibiting the focal adhesion pathway affects development at this stage and therefore neither is vital for regulating the primordial follicle transition. Several growth factors that can act in part by signaling through the MAPK pathway have been shown to regulate the primordial to primary follicle transition, including PDGF (Nilsson et al., 2006a), FGF2 (Chaves et al., 2012; Nilsson et al., 2001; Jin et al., 2005), NTF3 (Nilsson et al., 2009) and KITLD (Parrott and Skinner, 1999). Therefore, these factors likely affect follicle transition by acting through other parallel signaling pathways, rather than MAPK exclusively. C-erbB2 signaling has been shown to promote primordial follicle development in rats, and this increased follicle transition was blocked by treatment with the MAPK inhibitor PD98059 (Li-Ping et al., 2010), suggesting an active role for MAPK signaling in this process. In addition, IL16 signaling through the CD4 receptor has been shown to activate MAP kinases in immune B-cells, as well as NFkB and P27 signaling pathways (Yang et al., 2013). Cross-talk between several intracellular pathways is likely when regulating the primordial to primary follicle transition, including MAPK, NFkB, PI3K and PTEN (Jin et al., 2005; Serafica et al., 2005; Liu et al., 2006; Morohaku et al., 2013; Adhikari et al., 2013; Jagarlamudi et al., 2009). Therefore, the signaling that occurs within the MAPK pathway may have effects that are distributed across other pathways, such as PI3K. It is possible that inhibiting JNK with SP600125 allows cross-talk to other pathways and so has little effect on follicle transition, while inhibiting MEK in the MAPK pathway with PD98059 does slow follicle transition when ovaries from 2-day old rats are treated. In the current experiments it was demonstrated that a completely intact MAPK signaling pathway, including fully functional JNK, is not necessary for the primordial to primary follicle transition. Increasing MAPK pathway activity with BCI also did not result in increased follicle transition. Therefore, IL16 treatment appears to increase follicle transition through additional parallel signaling pathways, rather than MAPK alone.
Previous studies suggested that TGFB pathway signaling was another mechanism regulating the primordial to primary follicle transition (Nilsson et al., 2010). In order to determine if disruption of a specific branch of the TGFB signaling pathway affects primordial follicle transition, a third tyrphostin inhibitor was selected for use in organ culture experiments. The tyrphostin inhibitor LDN inhibits bone morphogenetic protein receptor I (BMPRI) within the TGFB signaling cascade (Cuny et al., 2008) (Figure 5). BMPs induce many different cellular effects ranging from stem cell maintenance, migration, differentiation, and proliferation to apoptosis depending on context (Sieber et al., 2009). AMH also binds to BMPR1 (bone morphogenetic protein receptor 1) (Cuny et al., 2008), and has been shown in previous studies to inhibit primordial follicle assembly and transition (Nilsson et al., 2011; Nilsson et al., 2007; Visser and Themmen, 2005; Durlinger et al., 1999). In the current study, LDN was shown to have a significant effect on ovarian primordial to primary follicle transition. These previous observations suggest LDN treatment would increase the percentage of developing follicles. This was verified by the results of this study. When combined with AMH, there was still a significant increase in the percentage of primary follicles as compared to the controls, likely indicating LDN is blocking the activity of AMH at the receptor. Together, these data indicate that signaling through the BMPR1 branch of the TGFB pathway regulates the primordial to primary follicle transition (Figure 11). This is consistent with previous studies showing that the growth factors AMH and BMP4 affect primordial follicle transition (Nilsson et al., 2007; Buratini and Price, 2011; van Houten et al., 2010; Nilsson and Skinner, 2003) and helps validate the predictions of previous systems biology investigations (Nilsson et al., 2010) that suggested the TGFB signaling pathway helped regulate this process.
CONCLUSIONS
In summary, specific signaling networks and candidate regulatory factors were tested for their roles in regulating early follicle development. Based on inhibitor experimentation in this study, the MAPK signaling pathway was shown to be important for regulation of primordial follicle assembly. Interleukin 16 has now been shown to play an active role in regulating transition from primordial follicles with arrested oocytes into primary follicles with developing oocytes and proliferating granulosa cells. IL16 and its receptor CD4 were also specifically localized to the granulosa cells of developing follicles. Both WNT2B and DLL4 treatment could have indirect roles with regard to primordial follicle transition within the ovary, but while mRNA expression of these cytokines was regulated within the ovary (Nilsson et al., 2010) (Figure 7), neither WNT2B nor DLL4 treatment had a direct effect on primordial to primary follicle transition in cultured ovaries. The TGFB signaling pathway through BMPR1 was shown to be important for regulating primordial to primary follicle transition. Therefore IL16, the MAPK pathway and the TGFB pathway were experimentally confirmed to be a part of the network of signaling molecules and pathways that regulate early ovarian follicle development (Figure 11).
The results of these studies support the utility of the previously proposed gene networks in identifying candidate regulatory points to control follicle development (Nilsson et al., 2010; Nilsson et al., 2011). A systems biology approach to studying developmental processes can be a tool that is complementary to the more reductionist experiments such as performed in the current investigation. Some findings from this study supported the predictions from the systems biology investigations, and some did not. This emphasizes the importance of using both experimental approaches.
Oocyte maturation and follicle development are complicated processes and the pathways and factors regulating primordial follicle assembly and primordial to primary follicle transition are still being determined. Understanding the regulators of early follicle development could lead to solutions for certain types of infertility in mammals, including humans. Primary ovarian insufficiency (POI) leading to early menopause (Dixit et al., 2010) is increasingly becoming a detriment to female reproductive success. Further investigation of the cytokine and hormonal signaling cascades involved in ovarian development will help to advance and improve treatments to combat ovarian disease.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Ms. Alison Durand and Ms. Heather Johnson for assistance in preparation of the manuscript.
FUNDING
This research was supported by NIH grants to MKS.
Footnotes
DECLARATION OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Adhikari D, Risal S, Liu K, Shen Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS ONE. 2013;8:e53810. doi: 10.1371/journal.pone.0053810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrawes MB, Xu X, Liu H, Ficarro SB, Marto JA, Aster JC, Blacklow SC. Intrinsic Selectivity of Notch 1 for Delta-like 4 over Delta-like 1. J Biol Chem. 2013 doi: 10.1074/jbc.M113.454850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratini J, Price CA. Follicular somatic cell factors and follicle development. Reprod Fertil Dev. 2011;23:32–39. doi: 10.1071/RD10224. [DOI] [PubMed] [Google Scholar]
- Chaves RN, de Matos MH, Buratini J, Jr., de Figueiredo JR. The fibroblast growth factor family: involvement in the regulation of folliculogenesis. Reprod Fertil Dev. 2012;24:905–915. doi: 10.1071/RD11318. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. MAP kinases. Chem Rev. 2001;101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- Cruikshank W, Kornfeld H, Berman J, Chupp G, Keane J, Center D. Biological activity of interleukin-16. Nature. 1996;382:501–502. doi: 10.1038/382501b0. [DOI] [PubMed] [Google Scholar]
- Cruikshank W, Little F. lnterleukin-16: the ins and outs of regulating T-cell activation. Crit Rev Immunol. 2008;28:467–483. doi: 10.1615/critrevimmunol.v28.i6.10. [DOI] [PubMed] [Google Scholar]
- Cuny GD, Yu PB, Laha JK, Xing X, Liu JF, Lai CS, Deng DY, Sachidanandan C, Bloch KD, Peterson RT. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit H, Rao L, Padmalatha V, Raseswari T, Kapu AK, Panda B, Murthy K, Tosh D, Nallari P, Deenadayal M, Gupta N, Chakrabarthy B, Singh L. Genes governing premature ovarian failure. Reprod Biomed Online. 2010;20:724–740. doi: 10.1016/j.rbmo.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78:135–163. doi: 10.1016/s0378-4320(03)00088-5. [DOI] [PubMed] [Google Scholar]
- Jagarlamudi K, Liu L, Adhikari D, Reddy P, Idahl A, Ottander U, Lundin E, Liu K. Oocyte-specific deletion of Pten in mice reveals a stage-specific function of PTEN/PI3K signaling in oocytes in controlling follicular activation. PLoS ONE. 2009;4:e6186. doi: 10.1371/journal.pone.0006186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Han CS, Zhang XS, Yuan JX, Hu ZY, Liu YX. Signal transduction of stem cell factor in promoting early follicle development. Mol Cell Endocrinol. 2005;229:3–10. doi: 10.1016/j.mce.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenngott RA, Vermehren M, Ebach K, Sinowatz F. The role of ovarian surface epithelium in folliculogenesis during fetal development of the bovine ovary: a histological and immunohistochemical study. Sex Dev. 2013;7:180–195. doi: 10.1159/000348881. [DOI] [PubMed] [Google Scholar]
- Kerr B, Garcia-Rudaz C, Dorfman M, Paredes A, Ojeda SR. NTRK1 and NTRK2 receptors facilitate follicle assembly and early follicular development in the mouse ovary. Reproduction. 2009;138:131–140. doi: 10.1530/REP-08-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe E, Boerboom D. WNT signaling and the regulation of ovarian steroidogenesis. Front Biosci (Schol Ed) 2011;3:276–285. doi: 10.2741/s151. [DOI] [PubMed] [Google Scholar]
- Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994–999. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Targeting signal transduction for disease therapy. Curr Opin Cell Biol. 1996;8:239–244. doi: 10.1016/s0955-0674(96)80071-8. [DOI] [PubMed] [Google Scholar]
- Li-Ping Z, Da-Lei Z, Jian H, Liang-Quan X, Ai-Xia X, Xiao-Yu D, Dan-Feng T, Yue-Hui Z. Proto-oncogene c-erbB2 initiates rat primordial follicle growth via PKC and MAPK pathways. Reprod Biol Endocrinol. 2010;8:66. doi: 10.1186/1477-7827-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P. Control of mammalian oocyte growth and early follicular development by the oocyte PI3 kinase pathway: new roles for an old timer. Dev Biol. 2006;299:1–11. doi: 10.1016/j.ydbio.2006.07.038. [DOI] [PubMed] [Google Scholar]
- Mahindra A, Anderson KC. Role of interleukin 16 in multiple myeloma pathogenesis: a potential novel therapeutic target? J Natl Cancer Inst. 2012;104:964–965. doi: 10.1093/jnci/djs274. [DOI] [PubMed] [Google Scholar]
- Mathy NL, Scheuer W, Lanzendorfer M, Honold K, Ambrosius D, Norley S, Kurth R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology. 2000;100:63–69. doi: 10.1046/j.1365-2567.2000.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin EA, McIver SC. Awakening the oocyte: controlling primordial follicle development. Reproduction. 2009;137:1–11. doi: 10.1530/REP-08-0118. [DOI] [PubMed] [Google Scholar]
- Mettouchi A. The role of extracellular matrix in vascular branching morphogenesis. Cell Adh Migr. 2012;6:528–534. doi: 10.4161/cam.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina G, Vogt A, Bakan A, Dai W, Queiroz de Oliveira P, Znosko W, Smithgall TE, Bahar I, Lazo JS, Day BW, Tsang M. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat Chem Biol. 2009;5:680–687. doi: 10.1038/nchembio.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohaku K, Hoshino Y,H, Sato E. Incorporation of phosphatase inhibitor in culture prompts growth initiation of isolated non-growing oocytes. PLoS ONE. 2013;8:e77533. doi: 10.1371/journal.pone.0077533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LJ, Marcinkiewicz JL. Tumor necrosis factor alpha enhances oocyte/follicle apoptosis in the neonatal rat ovary. Biol Reprod. 2002;66:450–457. doi: 10.1095/biolreprod66.2.450. [DOI] [PubMed] [Google Scholar]
- Musse AA, Meloty-Kapella L, Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Semin Cell Dev Biol. 2012;23:429–436. doi: 10.1016/j.semcdb.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Dole G, Skinner MK. Neurotrophin NT3 promotes ovarian primordial to primary follicle transition. Reproduction. 2009;138:697–707. doi: 10.1530/REP-09-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Parrott JA, Skinner MK. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol. 2001;175:123–130. doi: 10.1016/s0303-7207(01)00391-4. [DOI] [PubMed] [Google Scholar]
- Nilsson E, Rogers N, Skinner MK. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134:209–221. doi: 10.1530/REP-07-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Zhang B, Skinner MK. Gene bionetworks that regulate ovarian primordial follicle assembly. BMC Genomics. 2013;14:496. doi: 10.1186/1471-2164-14-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Detzel C, Skinner MK. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction. 2006a;131:1007–1015. doi: 10.1530/rep.1.00978. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Kezele P, Skinner MK. Leukemia inhibitory factor (LIF) promotes the primordial to primary follicle transition in rat ovaries. Mol Cell Endocrinol. 2002;188:65–73. doi: 10.1016/s0303-7207(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Savenkova MI, Schindler R, Zhang B, Schadt EE, Skinner MK. Gene bionetwork analysis of ovarian primordial follicle development. PLoS ONE. 2010;5:e11637. doi: 10.1371/journal.pone.0011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Schindler R, Savenkova MI, Skinner MK. Inhibitory actions of Anti-Mullerian Hormone (AMH) on ovarian primordial follicle assembly. PLoS ONE. 2011;6:e20087. doi: 10.1371/journal.pone.0020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Bone morphogenetic protein-4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol Reprod. 2003;69:1265–1272. doi: 10.1095/biolreprod.103.018671. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Stanfield J, Skinner MK. Interactions between progesterone and tumor necrosis factor-alpha in the regulation of primordial follicle assembly. Reproduction. 2006b;132:877–886. doi: 10.1530/REP-06-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem O, Buyuk E, Oktay K. Preantral follicle growth is regulated by c-Jun-N-terminal kinase (JNK) pathway. Reprod Sci. 2011;18:269–276. doi: 10.1177/1933719110385709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–4271. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. doi: 10.1172/JCI41350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricken A, Lochhead P, Kontogiannea M, Farookhi R. Wnt signaling in the ovary: identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology. 2002;143:2741–2749. doi: 10.1210/endo.143.7.8908. [DOI] [PubMed] [Google Scholar]
- Serafica MD, Goto T, Trounson AO. Transcripts from a human primordial follicle cDNA library. Hum Reprod. 2005;20:2074–2091. doi: 10.1093/humrep/dei030. [DOI] [PubMed] [Google Scholar]
- Sieber C, Kopf J, Hiepen C, Knaus P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009;20:343–355. doi: 10.1016/j.cytogfr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Sirotkin AV. Growth factors controlling ovarian functions. J Cell Physiol. 2011;226:2222–2225. doi: 10.1002/jcp.22588. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–471. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- Smolikova K, Mlynarcikova A, Scsukova S. Role of interleukins in the regulation of ovarian functions. Endocr Regul. 2012;46:237–253. doi: 10.4149/endo_2012_04_237. [DOI] [PubMed] [Google Scholar]
- Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology. 2002;143:2221–2232. doi: 10.1210/endo.143.6.8845. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Baker K, Han J, Calvo C, Nurmi H, Eichmann AC, Alitalo K. Interactions between VEGFR and Notch signaling pathways in endothelial and neural cells. Cell Mol Life Sci. 2013;70:1779–1792. doi: 10.1007/s00018-013-1312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama T, Yamaguchi TP. Mice lacking Wnt2b are viable and display a postnatal olfactory bulb phenotype. Neurosci Lett. 2012;512:48–52. doi: 10.1016/j.neulet.2012.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houten EL, Themmen AP, Visser JA. Anti-Mullerian hormone (AMH): regulator and marker of ovarian function. Ann Endocrinol (Paris) 2010;71:191–197. doi: 10.1016/j.ando.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Visser JA, Themmen AP. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234:81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Wang J, Roy SK. Growth differentiation factor-9 and stem cell factor promote primordial follicle formation in the hamster: modulation by follicle-stimulating hormone. Biol Reprod. 2004;70:577–585. doi: 10.1095/biolreprod.103.023234. [DOI] [PubMed] [Google Scholar]
- Yang HY, Kim J, Kim SH, Choe CH, Jang YS. Pro-IL-16 is associated with MHC class II-mediated negative regulation of mouse resting B cell activation through MAP kinases, NF-kappaB and Skp2-dependent p27kip regulation. Scand J Immunol. 2013;77:177–186. doi: 10.1111/sji.12026. [DOI] [PubMed] [Google Scholar]