Abstract
HCC1/CAPERα is considered to be a novel human tumor-associated antigen, and the tumor-specific immunity of HCC1/CAPERα has been reported in several types of cancer. However, there was very limited evidence indicating its function in tumorigenesis. In the present study, to elucidate the roles and underlying molecular mechanism of HCC1/CAPERα in lung cancer, we examined the expression of HCC1/CAPERα in human non-small cell lung cancer (NSCLC) cell line and NSCLC tissue microarray (TMA). Immunohistochemistry with TMA was performed to detect HCC1/CAPERα expression in NSCLC and adjacent lung tissues. NSCLC cell line constitutively transfected by pcDNA3.1-HCC1/CAPERα, and empty pcDNA3.1 vector were used. These cells were analyzed by Western blot, MTT, immunofluorescence, wound healing assay, and transwell assays. It was found that HCC1/CAPERα was mainly localized in the nucleus of the lung cancer cells and overexpression of HCC1/CAPERα may promote lung cancer cells proliferation and increase cells migration. The frequency of HCC1/CAPERα expression in NSCLC tissues was significantly higher than that in adjacent and normal tissues (P<0.01). Our data suggest that overexpression of HCC1/CAPERα may increase the proliferation and migration of NSCLC cells, and HCC1/CAPERα could be a promising biomarker for lung cancer.
Keywords: Lung cancer, HCC1/CAPERα, Biomarker, Overexpression, Carcinogenesis
Introduction
Cancer has long been recognized as a highly complex disorder that involves not only the interplay of genomic changes but also factors including the immunological, hormonal, environmental, and other disease [1]. There is persuasive evidence that the immune system of the cancer patients have the capability of sensing abnormalities in structure, function, intracellular location, and other alterations of cellular participants in carcinogenesis. Previous studies have demonstrated that cancer sera contain antibodies that react with a unique group of autologous cellular antigens called tumor-associated antigens (TAAs) [2, 3]. These antigens are probably cellular proteins, such as p53, p62, p90, and p16, whose aberrant regulation or overexpression is capable of leading to tumorigenesis [4–8]. Therefore, these cancer-associated anti-TAA antibodies may be regarded as reporters, which can identify aberrant cellular factors involved in the transformation process in cancer [9].
HCC1, a splicing factor that is highly homologous to U2AF65 (splicing factor U2AF 65 kDa subunit), was originally isolated as an autoantigen from a patient with liver cirrhosis who later progressed to hepatocellular carcinoma [10, 11]. It was also known as CAPERα, a protein for transcriptional coactivator of activating protein-1 and estrogen receptors [12]. As a novel nuclear protein, HCC1/CAPERα contains RNA-binding domains and a long region rich in arginine and serine, was previously identified as RNA binding motif protein 39 (RBM39), which might contribute to alternative splicing, an important mechanism to produce mRNA, protein, and functional diversity by modulation of processing of pre-mRNA [13]. HCC1/CAPERα has also been reported to have an important role in modulating the oncogenic activity of Rel/NF-κB [14]. HCC1/CAPERα comprises two alternatively spliced isoforms termed HCC1.3 and HCC1.4. The full-length cDNA clones encoded 530 amino acids termed HCC1.4 and 18-nucleotide (six-amino acid) deletion was another representative clone, named as HCC1.3 [15]. In our recent study, we found that HCC1.4 and HCC1.3 as TAAs had different autoantibody immune responses (data not published yet); however, whether HCC1.4 and HCC1.3 had different function as reporters identifying aberrant cellular mechanisms in tumorigenesis was not sure yet.
Lung cancer has become the most common malignant tumor and the major cause of cancer deaths worldwide [16]. Non-small cell lung cancer (NSCLC) accounts for more than 85 % of all lung cancers when compared with adenocarcinomas, squamous cell, and large cell carcinomas. Approximately 75 % of patients with NSCLC present with advanced stage is un-resectable or metastatic [17]. HCC1/CAPERα has been described to be overexpressed in NSCLC and other cancers [18, 19]. In the current study, we aimed to investigate the roles of HCC1.4 and HCC1.3 in NSCLC cells growth and migration.
Materials and methods
Cell lines and cell extracts
Six different tumor cell lines (lung cancer (H460, H1299), hepatocellular carcinomar (HepG2, SUN449), breast cancer (SKBR3), gastric cancer (AGS)) were obtained from the tumor cell bank of our laboratory and cultured following the specific protocol for each cell line. Cells grown in monolayers were solubilized in sample buffer containing protease inhibitors after sonication. Solubilized lysates were briefly denatured before electrophoresis on sodium dodecyl sulfate-polyacrylamide gels.
Plasmids and transfection
pET-HCC1.3 and pET-HCC1.4 containing the human full-length HCC1.3 and HCC1.4 cDNA were kind gifts from Dr. Eng. M. Tan’s lab at The Scripps Research Institute (La Jolla, CA). The gene fragments of HCC1.3 and HCC1.4 were amplified with the below primers: forward primer: 5′-GGGGATCCAAAATGGCAGACGATATTGA-3′ (BamHI site) and reverse primer: 5′-GCTCTAGATCATCGTCTACTTGGA-3′ (XbaI site) using pET-HCC1.3 and pET-HCC1.4 as the templates, respectively. The polymerase chain reaction products containing the full-length coding sequence of HCC1.4 were purified and ligated into the corresponding sites of pcDNA3.1 (Invitrogen, Carlsbad, CA) to create the plasmid expressing HCC1.4. The plasmid expressing HCC1.3 was prepared in a similar way. The plasmids expressing HCC1.3 or HCC1.4 were named pcDNA3.1-HCC1.3 and pcDNA3.1-HCC1.4, which were confirmed by sequencing.
H1299 cells were transfected with pcDNA3.1-HCC1.3, pcDNA3.1-HCC1.4, and pcDNA3.1 vectors respectively, using Lipofectamine™ LTX (Invitrogen, Carlsbad, CA) according to manufacturer’s protocol. The stable cell lines H1299-HCC1.3, H1299-HCC1.4, and their counterparts, H1299-pcDNA3.1 were established following selection with 1 mg/ml G418 and maintained with 0.5 mg/ml G418. The stable expression of HCC1.3 and HCC1.4 proteins were confirmed by Western blot as described below.
Western blotting and indirect immunofluorescence (IIF) assay
Western blotting was performed by using our previously described method [20]. Antibody against HCC1/CAPERα was purchased from Abcam (Cambridge, MA), antibody against β-actin from Santa Cruz (Santa Cruz, CA). Immunoreactive protein bands were visualized by the ECL kit (Amersham, Arlington Heights, IL) according to the manufacturer’s instructions.
IIF assay was performed by using a previously described method [21]. In brief, after transfected with pcDNA3.1-HCC1.3, pcDNA3.1-HCC1.4, and pcDNA3.1vector, respectively, H1299 cells cultured in an eight-well chamber culture slide (BD-Falcon, Bedford, MA) were fixed with 100 % pre-cold methanol for 5 min at −20 °C, and then permeabilized in 100 % pre-cold acetone for 3 min at −20 °C. Cell samples were incubated with polyclonal HCC1/CAPERα antibody with 1:40 for 1 h at room temperature, followed by incubation with anti-human IgG FITC conjugated secondary antibody (Invitrogen, Carlsbad, CA) for 1 h at room temperature at a 1:200 dilution. Confocal fluorescence images were acquired with a laser scanning microscope (LSM 700, Zeiss, New York, NY), using a×20 objective and processed with ZEN 2009 software (Zeiss, CA).
Cell proliferation assay
Cells proliferation was detected using MTT (Promega, Madison, WI). Briefly, H1299 cells were harvested, counted, and seeded into 96-well microliter plates (Thermo Scientific, Waltham, MA) at a density of 5×103 cell/ml (200 μl cell suspensions) and incubated for 24, 48, and 72 h, respectively. After indicated time points, the cell proliferation was determined by the MTT assay as described according to the manufacturer’s instructions.
Cell migration assay
Wound healing assay was performed as described previously [22]. Transwell assay were performed using 24-well permeable supports with 8-μm pore (Corning, NY). After 24 h incubation, cells that had migrated into the lower surface of the filter were stained with 0.05 % crystal violet or fluorescent dye Calcein AM (Corning) according to the manufacturer’s instructions. The fluorescence was read by fluoroskan ascent microplate fluorometer (Thermo Scientific).
Immunohistochemistry (IHC) with tissue microarray (TMA)
NSCLC TMA slides with normal tissue control (LC10012, 50 cases/100 cores, including clinical stages and pathology grades) were purchased (US Biomax, Inc., Rockville, MD) and used to detect the expression of the HCC1/CAPERα. IHC assay was performed according to previously described methods [21, 23]. The slides were then observed under light microscopy. All IHC results were read blindly by two independent researchers. A four-level scoring system (−, negative; +, low expression level; ++, moderate expression level; +++, high expression level) was used to evaluate the staining intensity.
Statistics
All data are represented as means±standard deviation (SD). Statistical analysis was performed using an unpaired two-tailed t test or χ2 test in SPSS13.0. Two significant levels (P<0.05 or P<0.01) were used.
Results
Expression of HCC1/CAPERα in different cancer cell lines
The expression of HCC1/CAPERα in six cancer cell lines was analyzed by Western blotting. As shown in Fig. 1a, based on the expression level of internal control β-actin, cancer cell lines such as HepG2, SUN449, and SKBR3 exhibited a strong reactivity to anti-HCC1/CAPERα. H460, H1299, and AGS cell lines exhibited weak reactivity.
Fig. 1.
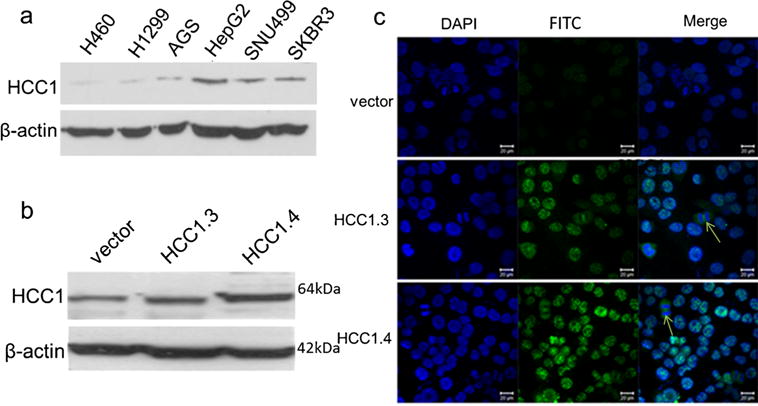
Expression of HCC1/CAPERα in tumor cells. a Expression of HCC1/CAPERα in six tumor cell lines were analyzed by Western blotting. The polyclonal anti-HCC1/CAPERα antibody was used as a probe. H460 and H1299: lung cancer, AGS: gastric cancer, HepG2 and SUN449: liver cancer, SKBR3: breast cancer. β-Actin was used as loading control. b, c Expression of HCC1/CAPERα in H1299 cells were analyzed by Western blotting (b) and immunofluorescence staining (c). H1299 cells were transfected with empty vector pcDNA3.1 (vector), pcDNA3.1-HCC1.3 (HCC1.3), or pcDNA3.1-HCC1.4 (HCC1.4). It showed that HCC1/CAPERα was overexpressed in H1299-HCC1.4 cells. HCC1/CAPERα demonstrated stronger nuclear immunofluorescence staining pattern in H1299 cells transfected with pcDNA3.1-HCC1.4 and also found expressed in the perinuclear cytoplasm of dividing H1299 cells (c). The arrows indicate the diving cells
Differential expression of HCC1.3 and HCC1.4 d in NSCLC cells
The stable HCC1.3 and HCC1.4 overexpression cell lines were distinguished, and their protein expression was confirmed by Western blotting and the intracellular localization by indirect immunofluorescence assay (Fig. 1b–c). As shown in Fig. 1b, the expression of HCC1.3 and HCC1.4 in cells was increased though not very higher after transfected with pcDNA3.1-HCC1.3 and pcDNA3.1-HCC1.4. As shown in Fig. 1c, HCC1/CAPERα had stronger nuclear staining pattern in H1299 cells transfected with pcDNA3.1-HCC1.4 and pcDNA3.1-HCC1.3, while the fluorescent staining was significantly reduced in the cells transfected with empty vector. The observation was consistent with previously reported that HCC1/CAPERα protein was mainly expressed in the nucleus of HeLa cells [15].
Overexpression of HCC1.4 affects cell proliferation and migration
With MTT assay, we observed that the difference of H1299 cells growth transfected with pcDNA3.1-HCC1.3, pcDNA3.1-HCC1.4, and pcDNA3.1 (+) vector. As shown in Fig. 2, H1299-HCC1.4 cells had much higher proliferation activity than H1299-HCC1.3 and H1299-vector. The cell proliferation of H1299-HCC1.4 was significantly promoted compared with H1299-vector cells. Thus, HCC1.4 overexpression resulted in the enhancement of cell proliferation.
Fig. 2.
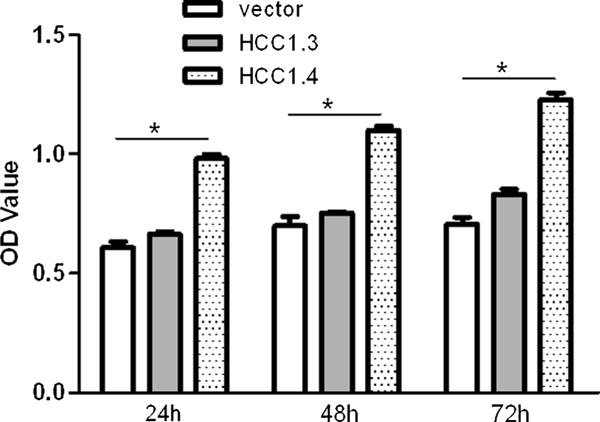
Proliferative activity of H1299 cells. MTT assay was used to estimate the proliferation of H1299 cells after transfected at different time points. OD: Optical density. Data shown are means±SD of at least three independent experiments. It showed that there was higher proliferation activity in H1299-HCC1.4 cells. Asterisk: designated statistical significant, P<0.05
As shown in Fig. 3b, after 24 h incubation with cell cultures, cells that overexpressed HCC1.4 nearly formed 100 % confluent monolayer, while the free surface area of the scratch was still observed for H1299-vector and H1299-HCC1.3 cells in wound healing assay. The transwell assay revealed the different migration capacity of H1299 transfectants (Fig. 3a, c). The H1299-HCC1.4 cells had the significantly higher level of migration activity compared with H1299-vector cells (Fig. 3c). Similar results can also be observed when transfected cells were stained with crystal violet (Fig. 3a).
Fig. 3.
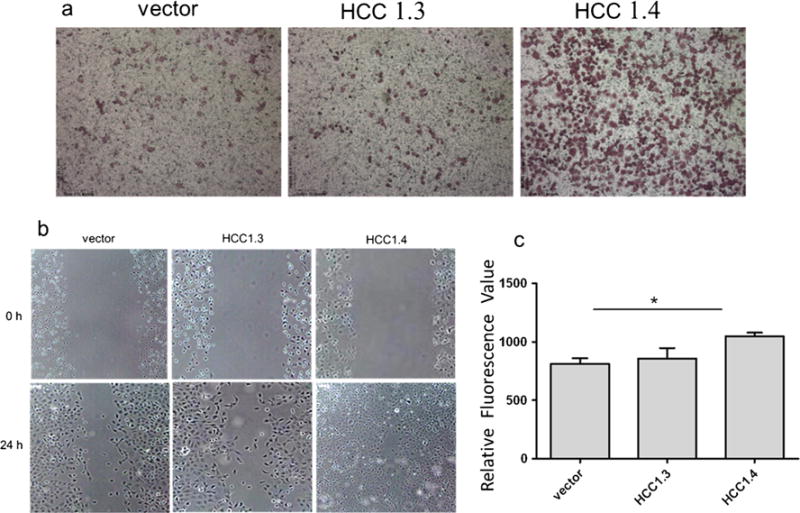
Migration capability of H1299 cells. After being transfected, H1299 cells were subjected to transwell migration assay (a, c) and wound healing assay (b). a The representative fields on membrane. Migrated cells on the bottom membrane of transwell inserts were stained with crystal violet (magnification, ×10). b The transfected H1299 cells at once after applying stripes as control (0 h) and observed again after 24 h. The H1299-HCC1.4 cells nearly formed 100 % confluent monolayer after 24 h incubation. c Migrated cells were stained with fluorescence dye: calcein AM. The fluorescence data of migrated cells were collected by fluorescence plate reader. Data shown were means±SD of at least three independent experiments. It showed that the H1299-HCC1.4 cells had the significantly higher level of migration activity compared with H1299-vector cells. Asterisk statistically different to vector, P<0.05
HCC1/CAPERα is over-expressed in NSCLC tissues
The express of HCC1/CAPERα in NSCLC tissues and adjacent normal lung tissues was examined by immunohistochemistry with TMA slides. The TMA slide containing 45 cases of carcinoma, 45 cases of adjacent tissue, and five normal tissue specimens were commercially available for this study. As shown in Table 1, 43 of 45 (95.6 %) NSCLC tissues were stained positive with monoclonal anti-HCC1/CAPERα antibody, and 18 of the 50 (36 %) adjacent and normal lung tissues were positively stained. The frequency of HCC1/CAPERα expression in lung cancer tissues was significantly higher than that in adjacent and normal lung tissues (P<0.01). Figure 4 indicated the positive reaction of lung cancer tissues with grades I, II, and III, respectively, while the normal and adjacent tissue had negative staining. Analysis on the frequency of positive HCC1/CAPERα staining in lung cancer tissues with different clinical stages had revealed no significant correlation between HCC1/CAPERα expression and tumor stage, gender, and age due to the limited number of tissue specimens in this study.
Table 1.
Relationship between HCC1/CAPERα expression and clinicopathologial factors in human lung cancer
| Characteristic | No. of patients | HCC1 positive |
|---|---|---|
| Adjacent+normal | 50 | 18 (36%) |
| Malignant tumor | 45 | 43 (95.6%)* |
| Pathology grade | ||
| I | 18 | 17 (94.4%) |
| II | 16 | 15 (93.8%) |
| III+IV | 11 | 11 (100%) |
| Age (years) | ||
| <60 | 62 | 27 (43.5%) |
| ≥60 | 33 | 16 (48.5%) |
| Gender | ||
| Male | 64 | 30 (46.9%) |
| Female | 31 | 14 (45.1%) |
P<0.01, the P value of lung cancer tissues to adjacent and normal tissues
Fig. 4.
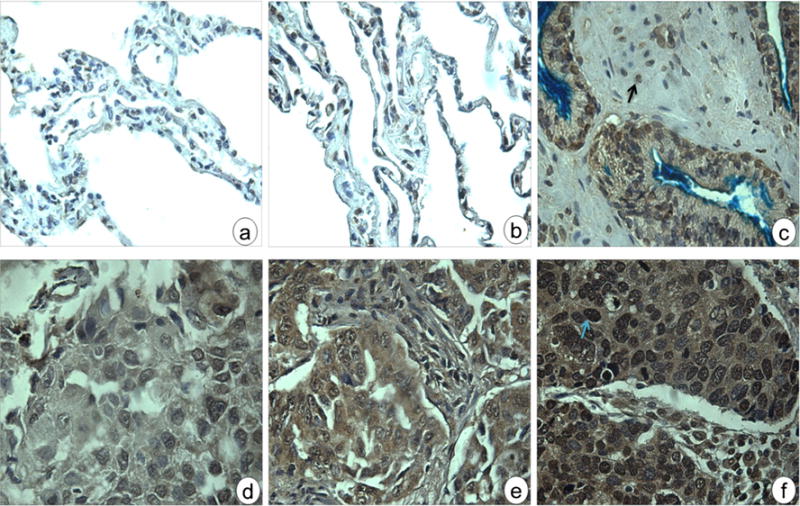
Expression of HCC1/CAPERα in normal, adjacent lung, and NSCLC tissues by IHC. Tissue microarray slide was stained with polyclonal anti-HCC1/CAPERα antibody to detect the expression of HCC1/CAPERα. One representative normal lung tissue (a) and a representative adjacent lung tissue (b) were negatively stained; c one positive control prostate cancer tissue; d, e, and f representative NSCLC grades I, II, and III tissues were positively stained (magnification, ×400). The NSCLC tissue with higher grade was intensively staining. The arrows indicate positive nuclear staining
Discussion
Identification of the intracellular antigens that involved in the immune response of cancer patients can provide vital information regarding the molecular mechanisms participating in the malignant transformation process. The possible mechanisms underlying the production of the autoantibodies may be related to the alteration of molecular structure or location of autoantigens [24, 25]. Splicing, the removal of introns from mRNA precursors, is an essential step in eukaryotic gene expression [26]. HCC1/CAPERα, as a splicing factor, regulates both steroid hormone receptor-mediated transcription and alternative splicing, which are functionally coupled to modulate gene expression [13]. In our previous study, we have also found that HCC1/CAPERα can induce immune responses in lung cancer patients (data not published yet). The six-amino acid deletion in HCC1.3 was located in the third RNA recognition motif (RRM) domain [12, 15]. The RRMs consisted of ribonucleoprotein consensus sequence (RNP-CS), which had been shown to be responsible for both RNA and single-stranded DNA binding specificity [27–30], and mediate protein–protein interaction [31–33]. In another study from our lab, both HCC1.3 and HCC1.4 have been demonstrated as tumor-associated antigens which can induce humoral immune responses in hepatocellular carcinoma patients (data were not published yet). The study has also shown that the deletion of amino acid of intracellular antigen may reduce its affinity for the autoimmune immune response to cellular components. The fact that the deletion of six-amino acid in HCC1.3 results in the loss of the RNP-like motif in the third RNP-CS domain may have some functional consequences, suggesting that HCC1.3 and HCC1.4 were indistinguishable in their binding and transcriptional coactivation properties [12]. HCC1/CAPERα can also interact with both subunits of U2AF heterodimer and accumulate distinct complexes of splicing factors within the nucleus [34], but the function of HCC1/CAPERα in tumorigenesis has not previous reported yet.
In the present study, we have examined the HCC1/CAPERα protein level in NSCLC by immunohistochemistry. As expected, low levels of HCC1/CAPERα protein were observed in adjacent and normal lung tissues which showed negative staining for anti-HCC1/CAPERα (Fig. 4a–b) compared with a prostate adenocarcinoma specimen (Fig. 4c) and lung cancer tissues (Fig. 4d–f). Moreover, the staining occurred in both the cytoplasm and nuclei of these cells. Collectively, our results showed that HCC1/CAPERα have significant higher immunogenicity in NSCLC tissues compared with adjacent and normal tissues (P<0.01), suggesting that HCC1/CAPERα may play an important role in human lung carcinogenesis.
To further investigate the function of HCC1.3 and HCC1.4 in lung cancer tumorigenesis, NSCLC H1299 cells were transfected to demonstrate the overexpression of HCC1.3 or HCC1.4 in vitro. Since poly- or monoclonal antibody against HCC1.3 was not commercially available, we used polyclonal antibody against HCC1/CAPERα to detect the expression of HCC1.3 or HCC1.4 in Western blot and immunofluorescence assay. It was found that HCC1/CAPERα mainly expressed in the nucleus of H1299 cells, also expressed in the peri-nuclear region or cytoplasm of dividing cells. It is of great importance to explore the localization of proteins at the subcellular level, as it leads to a better understanding of protein function, interaction networks, and cellular signaling pathways [35]. This special localization might be correlated to the function of HCC1/CAPERα protein as a splicing factor. Our finding indicated the novel distribution of HCC1/CAPERα protein, which might contribute to its roles and function in lung cancer tumorigenesis. We also showed that overexpression of HCC1.4 significantly increased the proliferation of H1299 cells compared with HCC1.3 and empty vector. Therefore, it may suggest that HCC1.4, the full length of CAPERα, not HCC1.3, play an important role in lung cancer cell proliferation. Moreover, when the trans-membraned cells in transwell assay were stained with crystal violet, similar results can be observed. The data indicated that cells overexpressing HCC1.4 showed the highest level of migratory ability compared with cells transfected with either empty vector or HCC1.3. The results may provide new evidence that HCC1.3 and HCC1.4 have differential expression in lung cancer cells and their distinct roles in regulation of tumor cell biology. It is consistent with that SR-related proteins may not only be involved in pre-mRNA processing but have a more complex array of functions in mammalian cells [36].
In summary, the present findings in this study indicate that the full length of HCC1.4, or CAPERα may play an important role in lung cancer tumorigenesis. These data provide further information to explain why HCC1.3 and HCC1.4 can induce different autoimmune responses in patients with cancers. This preliminary study also suggests that HCC1.4/CAPERα may be a potential diagnostic target for lung cancer.
Acknowledgments
Authors thank Dr. Eng M. Tan (The Scripps Research Institute, La Jolla, CA) for his support to this study. This work was supported by grant (SC1CA166016) from the National Institutes of Health (NIH). We would also like to thank the Border Biomedical Research Center (BBRC) Core facilities at The University of Texas at El Paso (UTEP) for their help, which were funded by NIH grant (5G12MD007592).
Footnotes
Conflicts of interest None
Contributor Information
Yurong Chai, Department of Biological Sciences, The University of Texas at El Paso, 500 W. University Ave., El Paso, TX 79968, USA, Department of Histology and Embryology, College of Basic Medicine, Zhengzhou University, Zhengzhou, Henan 45001, China.
Xinxin Liu, Department of Biological Sciences, The University of Texas at El Paso, 500 W. University Ave., El Paso, TX 79968, USA.
Liping Dai, Department of Biological Sciences, The University of Texas at El Paso, 500 W. University Ave., El Paso, TX 79968, USA.
Yang Li, Department of Biological Sciences, The University of Texas at El Paso, 500 W. University Ave., El Paso, TX 79968, USA.
Mei Liu, Department of Biological Sciences, The University of Texas at El Paso, 500 W. University Ave., El Paso, TX 79968, USA.
Jian-Ying Zhang, Email: jzhang@utep.edu, Department of Biological Sciences, The University of Texas at El Paso, 500 W. University Ave., El Paso, TX 79968, USA.
References
- 1.Tan HT, Lee YH, Chung MC. Cancer proteomics. Mass Spectrom Rev. 2012;31:583–605. doi: 10.1002/mas.20356. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM, Zhang J. Autoantibodies to tumor-associated antigens: reporters from the immune system. Immunol Rev. 2008;222:328–40. doi: 10.1111/j.1600-065X.2008.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JY, Tan EM. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev Mol Diagn. 2010;10:321–8. doi: 10.1586/erm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soussi T. P53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–88. [PubMed] [Google Scholar]
- 5.Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY. Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Onco Rep. 2006;16:1105–10. [PubMed] [Google Scholar]
- 6.Zhang JY, Chan EK, Peng XX, Lu M, Wang X, Mueller F, et al. Autoimmune responses to mRNA binding proteins p62 and koc in diverse malignancies. Clin Immunol. 2001;100:149–56. doi: 10.1006/clim.2001.5048. [DOI] [PubMed] [Google Scholar]
- 7.Zhang JY, Chan EK, Peng XX, Tan EM. A novel cytoplasmic protein with RNA-binding motifs is an autoantigen in human hepatocellular carcinoma. J Exp Med. 1999;189:1101–10. doi: 10.1084/jem.189.7.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soo Hoo L, Zhang JY, Chan EK. Cloning and characterization of a novel 90 kda ‘companion’ auto-antigen of p62 overexpressed in cancer. Oncogene. 2002;21:5006–15. doi: 10.1038/sj.onc.1205625. [DOI] [PubMed] [Google Scholar]
- 9.Tan EM. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J Clin Invest. 2001;108:1411–5. doi: 10.1172/JCI14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valcarcel J, Gaur RK, Singh R, Green MR. Interaction of u2af65 rs region with pre-mrna branch point and promotion of base pairing with u2 snRNA [corrected] Science. 1996;273:1706–9. doi: 10.1126/science.273.5282.1706. [DOI] [PubMed] [Google Scholar]
- 11.Rappsilber J, Ryder U, Lamond AI, Mann M. Large-scale proteomic analysis of the human spliceosome. Genome Res. 2002;12:1231–45. doi: 10.1101/gr.473902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung DJ, Na SY, Na DS, Lee JW. Molecular cloning and characterization of caper, a novel coactivator of activating protein-1 and estrogen receptors. J Biol Chem. 2002;277:1229–34. doi: 10.1074/jbc.M110417200. [DOI] [PubMed] [Google Scholar]
- 13.Dowhan DH, Hong EP, Auboeuf D, Dennis AP, Wilson MM, Berget SM, et al. Steroid hormone receptor coactivation and alternative RNA splicing by u2af65-related proteins caperalpha and caperbeta. Mol Cell. 2005;17:429–39. doi: 10.1016/j.molcel.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Dutta J, Fan G, Gelinas C. Caperalpha is a novel rel-tad-interacting factor that inhibits lymphocyte transformation by the potent rel/nf-kappab oncoprotein v-rel. J Virol. 2008;82:10792–802. doi: 10.1128/JVI.00903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai H, Chan EK, Kiyosawa K, Fu XD, Tan EM. Novel nuclear autoantigen with splicing factor motifs identified with antibody from hepatocellular carcinoma. J Clin Invest. 1993;92:2419–26. doi: 10.1172/JCI116848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 17.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 18.Bangur CS, Switzer A, Fan L, Marton MJ, Meyer MR, Wang T. Identification of genes over-expressed in small cell lung carcinoma using suppression subtractive hybridization and cDNA microarray expression analysis. Oncogene. 2002;21:3814–25. doi: 10.1038/sj.onc.1205480. [DOI] [PubMed] [Google Scholar]
- 19.Mercier I, Casimiro MC, Zhou J, Wang C, Plymire C, Bryant KG, et al. Genetic ablation of caveolin-1 drives estrogen-hypersensitivity and the development of dcis-like mammary lesions. Am J Pathol. 2009;174:1172–90. doi: 10.2353/ajpath.2009.080882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen Y, Zhou Y, Qiu S, Wang K, Liu S, Peng XX, et al. Autoantibodies to tumor-associated antigens combined with abnormal alpha-fetoprotein enhance immunodiagnosis of hepatocellular carcinoma. Cancer Lett. 2010;289:32–9. doi: 10.1016/j.canlet.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shao Q, Ren P, Li Y, Peng B, Dai L, Lei N, et al. Autoantibodies against glucose-regulated protein 78 as serological diagnostic biomarkers in hepatocellular carcinoma. Int J Oncol. 2012;41:1061–7. doi: 10.3892/ijo.2012.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Chai Y, Li J, Ren P, Liu M, Dai L, et al. Autoantibody response to a novel tumor-associated antigen p90/cip2a in breast cancer immunodiagnosis. Tumor Biol. 2014 doi: 10.1007/s13277-013-1350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan EM, Chan EK, Sullivan KF, Rubin RL. Antinuclear antibodies (ANAs): diagnostically specific immune markers and clues toward the understanding of systemic autoimmunity. Clin Immunol Immunopathol. 1988;47:121–41. doi: 10.1016/0090-1229(88)90066-9. [DOI] [PubMed] [Google Scholar]
- 25.Tan EM. Autoantibodies in pathology and cell biology. Cell. 1991;67:841–2. doi: 10.1016/0092-8674(91)90356-4. [DOI] [PubMed] [Google Scholar]
- 26.Lamond AI. The spliceosome. BioEssays. 1993;15:595–603. doi: 10.1002/bies.950150905. [DOI] [PubMed] [Google Scholar]
- 27.Nagai K, Oubridge C, Jessen TH, Li J, Evans PR. Crystal structure of the RNA-binding domain of the u1 small nuclear ribonucleoprotein a. Nature. 1990;348:515–20. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- 28.Query CC, Bentley RC, Keene JD. A common RNA recognition motif identified within a defined u1 RNA binding domain of the 70 k u1 snrnp protein. Cell. 1989;57:89–101. doi: 10.1016/0092-8674(89)90175-x. [DOI] [PubMed] [Google Scholar]
- 29.Shamoo Y, Abdul-Manan N, Williams KR. Multiple RNA binding domains (rbds) just don’t add up. Nucleic Acids Res. 1995;23:725–8. doi: 10.1093/nar/23.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanford JR, Ellis J, Caceres JF. Multiple roles of arginine/serine-rich splicing factors in RNA processing. Biochem Soc Trans. 2005;33:443–6. doi: 10.1042/BST0330443. [DOI] [PubMed] [Google Scholar]
- 31.Wan L, Kim JK, Pollard VW, Dreyfuss G. Mutational definition of RNA-binding and protein–protein interaction domains of heterogeneous nuclear rnp c1. J Biol Chem. 2001;276:7681–8. doi: 10.1074/jbc.M010207200. [DOI] [PubMed] [Google Scholar]
- 32.Scherly D, Dathan NA, Boelens W, van Venrooij WJ, Mattaj IW. The u2b” rnp motif as a site of protein–protein interaction. EMBO J. 1990;9:3675–81. doi: 10.1002/j.1460-2075.1990.tb07579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen H, Green MR. A pathway of sequential arginine–serine-rich domain-splicing signal interactions during mammalian spliceosome assembly. Mol Cell. 2004;16:363–73. doi: 10.1016/j.molcel.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 34.Ellis JD, Lleres D, Denegri M, Lamond AI, Caceres JF. Spatial mapping of splicing factor complexes involved in exon and intron definition. J Cell Biol. 2008;181:921–34. doi: 10.1083/jcb.200710051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stadler C, Rexhepaj E, Singan VR, Murphy RF, Pepperkok R, Uhlen M, et al. Immunofluorescence and fluorescent-protein tagging show high correlation for protein localization in mammalian cells. Nat Methods. 2013;10:315–23. doi: 10.1038/nmeth.2377. [DOI] [PubMed] [Google Scholar]
- 36.Cazalla D, Newton K, Caceres JF. A novel sr-related protein is required for the second step of pre-mRNA splicing. Mol Cell Biol. 2005;25:2969–80. doi: 10.1128/MCB.25.8.2969-2980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


