Abstract
Background
An intensive, prospective, open-label pharmacokinetic (PK) study in a subset of HIV-infected mothers and their uninfected infants enrolled in the Breastfeeding, Antiretroviral, and Nutrition study was performed to describe drug exposure and antiviral response.
Methods
Women using Combivir®[zidovudine (ZDV)+ lamivudine (3TC)]+Aluvia®[lopinavir/ritonavir(LPV/RTV)] were enrolled. Breast milk (BM) and mother and infant plasma (MP, IP) samples were obtained over 6hrs after observed dosing at 6, 12, or 24wks post-partum for drug concentrations and HIV RNA.
Results
30 mother/infant pairs (10 each at 6, 12,and 24wks post-partum) were enrolled. Relative to MP, BM concentrations of ZDV and 3TC were 35% and 21% higher, while LPV and RTV were 80% lower. Only 3TC was detected in IP with concentrations 96% and 98% lower than MP and BM, respectively. Concentrations in all matrices were similar at 6-24wks. The majority (98.3%) of BM concentrations were >HIVwt IC50, with one having detectable virus. There was no association between PK parameters and MP or BM HIV RNA.
Conclusions
ZDV and 3TC concentrated in BM while LPV and RTV did not, possibly due to protein binding and drug transporter affinity. Undetectable to low ARV concentrations in IP suggests prevention of transmission while breast feeding may be due to ARV effects on systemic or BM HIV RNA in the mother. Low IP 3TC exposure may predispose an infected infant to HIV resistance, necessitating testing and treating infants early.
Introduction
Breastfeeding by HIV infected mothers places the infant at risk of transmission; however, formula feeding increases the risk of infant morbidity and mortality during the first 6 months post-partum [1,2]. UNAIDS has reported that antiretroviral use in mothers is much lower in the breastfeeding period (49%), as compared to pregnancy and delivery (62%) [3]. This is especially concerning since up to 40% of mother-to-child transmission to infants occurs during breastfeeding [3]. The results of several large randomized trials and observational studies have demonstrated substantially reduced transmission by either providing pre-exposure prophylaxis to the uninfected breastfeeding baby with antiretrovirals (ARVs), or by providing combination ARV therapy (ART) to the breastfeeding mother [1,2, 4-14]. With these interventions, the World Health Organization (WHO) has proposed to decrease MTCT to 5% and ensure 90% of breastfeeding infant-mother pairs receive antiretroviral therapy or prophylaxis by 2015.
The BAN (Breastfeeding, Antiretrovirals, and Nutrition) Study showed a decrease of HIV transmission during 28 weeks of breastfeeding with either daily nevirapine given to breastfeeding infants (74% protective efficacy) or combination ART given to the mother (53% protective efficacy). Based on the results of BAN and other studies, the WHO now recommends the use of triple ART to the mother and 4-6 weeks of peripartum daily nevirapine or zidovudine to the infant [15].
The efficacy of combination ART given to the breastfeeding mother is likely due to a combination of suppressed HIV replication in both blood and breast milk. Previous studies of ARV exposure in maternal plasma and breast milk [16-21] have shown differing penetration of antiretroviral drugs into the breast milk. This has potential advantages and disadvantages. ARVs that concentrate in breast milk may have the advantage of directly suppressing viral replication in the breast and/or transferring a large enough drug dose to the breastfeeding infant to provide a local protective effect, but may have a disadvantage of ARV-induced side effects in the infant. ARVs that enter breast milk may cause selective pressure on replicating HIV in the breast, resulting in the development of resistance.
The extent of ARV penetration into breast milk, and into the breast feeding infant, is currently only estimated by sparse sampling investigations at single time points. Drug exposure over a dosing interval for breastfeeding infants and their mothers taking antiretroviral drugs is unknown. Limited but promising data on the systemic and local effects of ARV exposure on HIV RNA in breastfeeding women has been described in one study [21]. This investigation evaluated HIV RNA and multiple dose pharmacokinetics (PK) of zidovudine, lamivudine, lopinavir, and ritonavir in maternal plasma (MP), breast milk (BM), and infant plasma (IP) over 6 hours in 30 HIV-infected mothers and breastfeeding HIV-uninfected infants participating in the BAN Study at 6, 12, and 24 weeks post-partum.
Methods
Study Design
This was a prospective, non-blinded, steady-state intensive PK and HIV RNA investigation in a subset of mother/infant pairs enrolled in the BAN study.
Study Population
A convenience sample of 30 mother-infant pairs was recruited from the 849 pairs participating in the BAN study that had been randomized to the maternal ARV arm. All mothers were HIV infected with CD4+ T cell counts > 200 cells/mm3 and all infants were HIV uninfected. Each mother was given a single dose of nevirapine (sdNVP) prior to delivery. Additionally, all mothers initiated zidovudine (ZDV)+lamivudine (3TC) (1 combination tablet = ZDV 300mg + 3TC 150mg; Combivir®) and 2 tablets of lopinavir (LPV)+ ritonavir (RTV) (1 combination tablet = LPV 200mg + RTV 50mg; Aluvia®) twice daily just after delivery. Therapy was prescribed until the cessation of breastfeeding for a maximum of 28 weeks.
Study Procedure
The protocol was approved by the Institutional Review Boards of the US Centers for Disease Control and Prevention, the University of North Carolina at Chapel Hill, and the National Health Sciences Research Committee of Malawi [UNC IRB 03-MED-184]. Each mother was approached and agreed to participate at either 6, 12, or 24 weeks post partum. Mothers recorded the date and time of ARV administration for 3 days prior to their scheduled PK visit. As overnight visits could not be accommodated at the site in Malawi, an abbreviated 6-hour PK visit followed a morning dose. Subjects were instructed to report to the UNC Project building in the morning prior to their dose and 10-11 hours after their previous evening dose. An intravenous catheter was placed and normal saline was administered to keep the line patent. Prior to the mother's morning dose of ARVs (t=0hr), 5mL of whole blood and 10mL of breast milk from the mothers, and 3mL of infant whole blood were collected. Breast milk was collected from the right breast unless clinically contraindicated at which time it was collected from both breasts and labeled as right or left breast. One tablet of Combivir® and 2 tablets of Aluvia® were administered and plasma (MP and IP) and breast milk samples were obtained at 2, 4, and 6 hours after these witnessed doses. Infants were allowed to breastfeed as often as needed and mothers were allowed to eat after the t=0 and 6hr blood draws. Breakfast and lunch selections were similar for all subjects, consisting of bun and tea for breakfast and nsima and chicken for lunch (local foods in Malawi).
As a control comparison for HIV RNA in breast milk, 10 mothers randomized to the infant nevirapine arm (i.e., mothers were not on ART) while enrolled in the BAN study were selected. Daily nevirapine was only given to their infants. Breast milk samples from these women were also taken at 6, 12, and 24 weeks post-partum for evaluation. In addition, historical control data from a Western population of both HIV infected patients and health volunteers including men and women for each antiretroviral were included for a qualitative comparison.
Drug Assays from Blood and Breast Milk
All whole blood samples were collected in K3 containing Vacutainer tubes (Fisher Scientific, Hampton, New Hampshire, USA) and centrifuged at 2600rpm at 4°C for 10 minutes. Plasma was removed and aliquoted to 2mL cryovials and stored at -70°C until shipped to the UNC Center for AIDS Research Clinical Pharmacology and Analytical Chemistry lab for analysis. Drug concentrations for ZDV, 3TC, LPV, and RTV in plasma and breast milk were analyzed using a validated HPLC-MS/MS method [22]. Briefly, drugs were extracted from 50-100uL of plasma and 200μL of whole breast milk. A solid-phase extraction procedure (BOND ELUT-C18, Harbor City, California, USA) was used with a 90:10 methanol:water solution as the eluent. Eluted samples were dried at 40°C under a gentle steam of nitrogen and reconstituted in 100μL of mobile phase before using a LEAP HTC Pal thermostated autosampler to inject the samples onto an Applied Biosystems API 4000 triple quadruple mass spectrometer. Antiretrovirals were separated using an Aquasil C18 column (Thermo-Electron, San Jose, CA). Analyst 1.3.1 software was used for data collection. The concentration range for all ARVs was 10-10,000 ng/mL, with an intra-and inter-day precision and accuracy falling within 15%. The laboratory participates in two external proficiency testing programs twice annually: the Division of AIDS Clinical Pharmacology Quality Assurance Program, and the Dutch Association for Quality Assessment in TDM and Clinical Toxicology (KKGT) International and Interlaboratory QC Program [23, 24].
HIV RNA Assays from Blood and Breast Milk
HIV RNA was measured in 0.6ml plasma or whole breast milk using the Abbott RealTime HIV-1 assay. Breast milk was pre-treated by adding 0.21ml Promega RNA Lysis Buffer and 0.06ml Proteinase K (Abbott) to 0.6ml whole breast milk, then incubating at 53°C for 20 min. The breast milk mixture was extracted the same as for plasma.
Statistical Analysis
Non-compartmental methods were employed to calculate pharmacokinetic parameters using WinNonlin Pro 4.0.1 (Pharsight Corp, Mountain View, California, USA). AUC0-6hr (area under the concentration time curve from 0 to 6 hrs post dose) was calculated using the linear up/log down trapezoidal rule. Since several of the plasma C0hr,ss (steady state concentrations just prior to the dose) (4.8% from ZDV, 0.4% from 3TC, 0.8% from LPV and 1.5% from RTV) were undetectable, likely due to the variability of the length of time that elapsed from the previous dose to sampling, Cavg (average concentration over the sampling time) was used in the statistical analyses in lieu of the trough concentration. The median (range) elapsed time from previous dose to sampling was 14.9 (13-17) hrs, which were all outside the range of the dosing interval. Cavg was calculated by dividing the AUC by the time interval over which the AUC was calculated. ARV adherence rates were determined based on diary cards, and self-reported adherence assessments taken at 1, 4, 8, 21, and 28 weeks. Median and interquartile range (IQR) were calculated for all demographic data, PK parameters, and PK parameter ratios. Associations of drug concentrations between compartments and over time were analyzed using linear regression [SAS Version 9.2].
HIV RNA was quantified as copies/mL, assessed as a continuous variable and categorized as detectable (MP viral load (VL) ≥ 40; BM VL ≥ 56) or undetectable (MP VL < 40; BM < 56). HIV RNA concentrations that were detected but below the lower limit of quantification were imputed to 50% of the lower limit of quantification (MP VL = 20; BM VL = 28). PK parameters were assessed as continuous variables and were dichotomized using the following cut-points: median, geometric mean, 25th percentile, and 75th percentile.
Descriptive statistics were computed for PK parameters from each antiretroviral drug and viral load type (maternal plasma and breast milk). Viral load and PK parameters were compared with adherence data. Percent adherence was assessed as a continuous variable, and dichotomized by having either reported“all ARV doses taken prior to visit” at week 1, 4, 8, 21, and 28 (100%) or “some doses missed” prior to at least one of the aforementioned weeks (<100%). Weeks were chosen based on available adherence assessments conducted in the BAN study. Fisher's exact tests were used to test for an association between detectable versus undetectable MP HIV RNA and BM HIV RNA.
Differences in breast milk HIV RNA by study arm (maternal ARV versus infant NVP) were assessed using Fisher's exact tests and generalized estimating equations with an exchangeable correlation matrix. For the maternal arm single time points for HIV RNA were available; however, multiple time points were utilized for the infant NVP arm. For the purposes of modeling, HIV RNA was log10 transformed when used as a continuous outcome. Unadjusted linear regression, exact logistic regression, and Fisher's exact tests were used to look at the association between PK parameters and viral load.
Results
Thirty mother-infant pairs were enrolled for PK evaluation (10 at each of 6, 12, and 24 weeks postpartum). The median (IQR) for characteristics of the mother/infants pairs included: maternal age=25 (23,29) yrs; maternal weight=53 (51,59) kg; maternal CD4+ T cell count at screening (≤ 30 weeks gestation) =431(345,576) cells/mm3; HIV RNA at screening =5201 (1384,22461) copies/mL; infant birth weight=3 (2.7,3.4) kg; and 17 infants were male. There were no demographic differences between the women at the three time points postpartum. All women were 100% adherent based on diary cards for three days prior to the study visit. Infants were fed a median of 6 times per study visit with a range of 2 to 20 minutes at each feeding. PK parameters are presented in Table 1 and graphically represented in Figure 1.
Table 1.
Median (IQR) Antiretroviral PK Parameters (N=samples with detectable concentrations/total samples)
| ZDV | 3TC | LPV | RTV | |||||
|---|---|---|---|---|---|---|---|---|
| Cavg (ug/mL) | AUC0-6hr (hr*ug/mL) | Cavg (ug/mL) | AUC0-6hr (hr*ug/mL) | Cavg (ug/mL) | AUC0-6hr (hr*ug/mL) | Cavg (ug/mL) | AUC0-6hr (hr*ug/mL) | |
| Mothers' plasma | 0.143 (0.107,0.192) N=105/115 | 0.879 (0.686,1.205) | 0.717 (0.588, 0.945) N=113/115 | 4.36 (3.47, 5.67) | 7.79 (4.51, 10.13) N=110/115 | 46.38 (30.25, 56.89) | 0.364 (0.280, 0.489) N=111/115 | 2.30 (1.76, 2.98) |
| Breast Milk | 0.2 (0.119,0.263) N=98/121 | 1.201 (0.781,1.576) | 0.944 (0.682, 1.112) N=107/121 | 5.70 (4.29, 6.70) | 1.43 (0.705, 2.07) N=117/121 | 8.74 (46.91, 12.56) | 0.079 (0.047, 0.112) N=112/121 | 0.490 (0.293, 0.678) |
| Infant Plasma | ND N=0/115 | ND | 0.018 (0.010, 0.028) N=107/115 | 0.121 (0.074, 0.150) | ND N=0/115 | ND | ND N=0/115 | ND |
| BM/MP | 1.35 (1.02, 1.63) | 1.21 (1.06, 1.57) | 0.194 (0.136, 0.267) | 0.195 (0.159, 0.243) | ||||
| IP/BM | NA | 0.02 (0.013, 0.027) | NA | NA | ||||
| IP/MP | NA | 0.025 (0.017, 0.035) | NA | NA | ||||
ND = not detected; NA= not applicable; BM/MP=ratio of all BM and MP concentrations; IP/BM=ratio of all IP and BM concentrations; IP/MP=ratio of all IP and MP concentrations
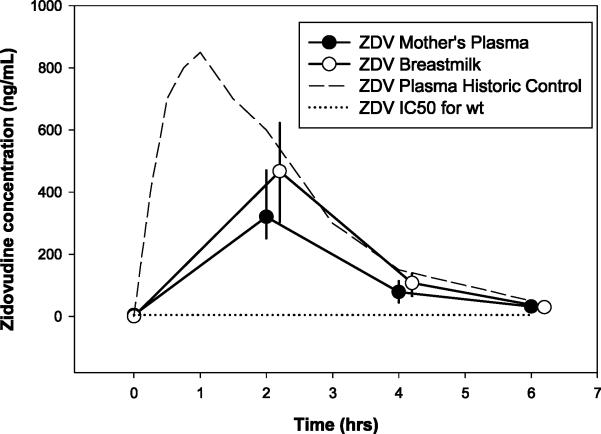
ZDV concentration-time profiles are shown in Figure 1a. Thirty percent (10/30) of ZDV maternal plasma concentrations at t=0 were below the limit of detection. Of all the detectable concentrations from all time points, exposures in BM were 35% higher than in MP. There was no detectable ZDV in infants. PK parameters for ZDV did not differ over the 6, 12, and 24 week time periods (p=0.78 and 0.68 for MP and BM, respectively). Of the detectable MP and BM ZDV concentrations, only 4.8% (5/105) and 0.8% (1/118) were below the HIVwt IC50, respectively (5ng/mL). ZDV AUC and Cavg for mothers’ plasma were similar to historic control data in 24 healthy Western volunteers [25]. Statistical comparisons were not made since the original dataset was not available.
Figure 1a.
Median (IQR) Zidovudine Concentation vs Time Profile
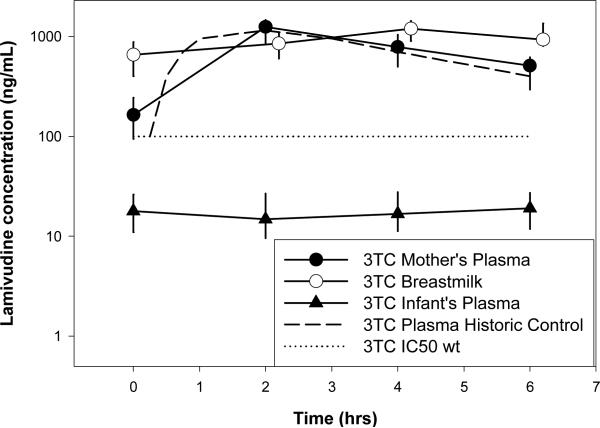
3TC concentration-time profiles are shown in Figure 1b. Only 6% (2/30) of MP 3TC concentrations at t=0 were undetectable. Of all the detectable 3TC concentrations for all time points, exposure in BM was 21% higher than in MP. 3TC concentrations were detectable in 93% (107/115) of infant plasma samples, which was 2% and 3.7% of BM and MP, respectively. PK parameters for 3TC did not differ across post-partum weeks (p=0.33 and 0.31 for MP and BM, respectively). Of the detectable MP, BM, and IP concentrations, 5.3%, 3.3%, and 83% were below the HIVwt IC50 for wild type virus, respectively (100ng/mL). No infant had concentrations consistently above the IC50 over the 6 hr sampling interval. Concentrations in mothers’ plasma were similar to historic controls [25].
Figure 1b.
Median (IQR) Lamivudine Concentration vs Time Profile
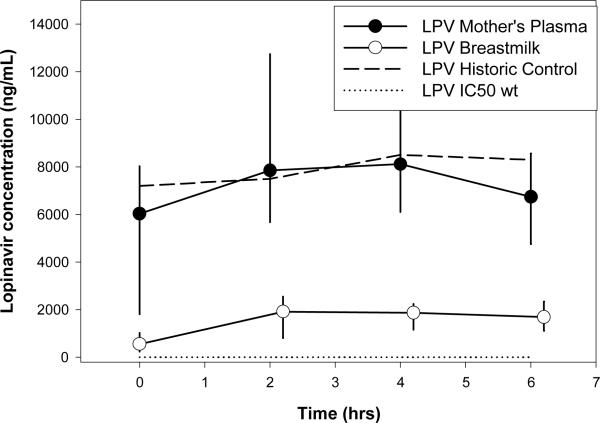
LPV concentration-time profiles are shown in Figure 1c. Sixteen percent (5/30) of MP concentrations and 13% (4/30) BM concentrations obtained at t=0h were undetectable. Lopinavir AUC and Cavg in BM were 80% lower than in MP. There were no detectable LPV concentrations in infants’ plasma. PK parameters for LPV were not different across time post-partum (p=0.99 and 0.56 for MP and BM, respectively). Of the detectable MP and BM concentrations, 1.8% and 0% were below the HIVwt IC50, respectively (2ng/mL). Concentrations in the maternal plasma were similar to historic controls [26].
Figure 1c.
Median (IQR) Lopinavir Concentration vs Time Profile
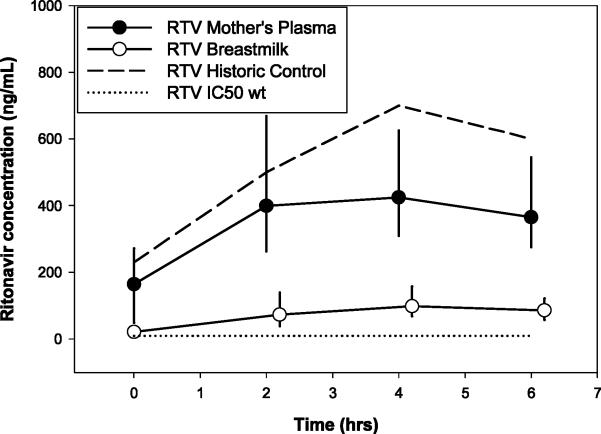
RTV concentration-time profiles are shown in Figure 1d. Thirteen percent (4/30) of MP concentrations and 16% (5/30) BM concentrations obtained at t=0 were undetectable. Exposures in BM were 80% lower than in MP. There were no detectable RTV concentrations in infants’ plasma. A 52% decrease in BM concentrations was noted from 6 to 24 weeks post-partum which could be interpreted as clinically significant; however, was not statistically significant (p=0.21). No changes occurred in the RTV exposure in MP (p=0.79). Of the detectable MP and BM concentrations, 0 and 2.7% were below the HIVwt IC50, respectively (10ng/mL). RTV concentrations in mothers’ plasma were approximately 30% lower than historic controls [27].
Figure 1d.
Median (IQR) Ritonavir Concentration vs Time Profile
Association Between Breast milk and Mothers’ Plasma
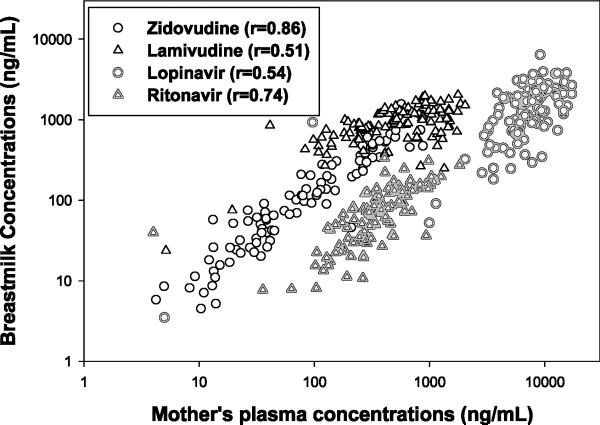
Combining all paired MP-BM concentrations from all time points, breast milk exposures for each drug were correlated with maternal plasma (Figure 2). Pearson correlation coefficients (rho) for ZDV, 3TC, LPV, and RTV were 0.86, 0.51, 0.54, 0.74, respectively (p value <0.0001 for all).
Figure 2.
Correlations Between Breast Milk Concentrations and Mothers’ Plasma Concentrations
Associations of Drug Concentrations, Adherence, and Viral Load
HIV RNA was detected in 34% (10/29) of mothers’ plasma samples with a median (range) of 393 (54-49,708) copies/mL. The median (range) for all mothers’ plasma HIV RNA was 20 (<40 - 49,708) copies/mL. The median of 20 copies/mL was an imputed value and represented those samples with HIV RNA >0 but ≤40 copies/mL. In breast milk, HIV RNA was detected in 3% (1/30) of samples, hence no association was found with plasma (p>0.9). Utilizing logistic regression, there were no significant associations between MP PK parameters and HIV RNA. Estimated odds ratios (95% CI) for the association between drug AUC > the AUC geometric mean and having detectable HIV RNA in MP ranged from 0.73 (0.16-3.39) for 3TC to 1.70 (0.33-8.67) for LPV. However, both MP and BM PK parameters appeared to be higher in patients with undetectable HIV RNA compared to those with detectable HIV RNA.
A large number of undetectable BM viral loads precluded us from detecting an association between BM HIV RNA and antiretroviral exposure. Therefore, we further explored the exposures of each antiretroviral agent relative to their HIVwt IC50 for the ability to predict HIV RNA response. The Cavg concentration in mothers’ plasma and breast milk for all antiretrovirals was > HIVwt IC50, although not all concentrations at all time points were > HIVwt IC50,. Despite this, only 19/29 mothers had MP viral loads < 40 copies/mL.
A total of 25 breast milk viral loads were obtained from 10 mothers in the control arm (infant nevirapine arm) at 6, 12, and 24 weeks post-partum in order to have a comparison of breast milk viral loads within the same study in a similar population. Ninety percent (9/10) of mothers in the control arm had at least one detectable breast milk viral load while only 3% (1/30) in the maternal ARV arm had detectable breast milk viral load confirming the effectiveness in this investigation of women for antiretroviral based suppression of HIV RNA in breast milk.
Self-reported adherence to antiretrovirals did not predict either drug exposure or HIV RNA. Adherence immediately prior to each PK visit was 100% for each subject. A median of five adherence assessments for each mother over the 24-week study visits was utilized to determine overall adherence. Among the 30 women in the maternal ARV arm, twenty-four mothers had 100% overall adherence (optimal adherence group) in the BAN study with the other 6 mothers having 60-80% adherence (sub-optimal adherence group). There were no differences between the median Cavg (or AUC) for any of the antiretrovirals for the optimal vs the suboptimal adherence groups [ZDV=97 vs 121 ng/mL p=0.1; 3TC=626 vs 667 ng/mL p=0.6; LPV 8364 vs 7474 ng/mL p>0.9; RTV 393 vs 360 ng/mL p>0.9, respectively]. Additionally, there was no difference in the number of detectable viral loads in the sub-optimal adherence group vs the optimal adherence group for MP [16% (1/6) vs 39% (9/23); p=0.6] or BM [16% (1/6) vs 0% (0/24); p=0.2], respectively.
Discussion
This investigation supports previous reports of higher ZDV and 3TC exposure in breast milk compared to blood plasma, while LPV and RTV have lower penetration into breast milk[16-18, 20]. The magnitude of ZDV and 3TC exposures with single point concentrations have ranged from 186-557% plasma[16-18, 20], while this intensive PK evaluation found the range of exposure to be less variable at 121-135% when an abbreviated AUC sampling strategy was used. In previous studies including some of our own, single point sampling of LPV and RTV BM exposures have been reported to be 1-32% of plasma, while in this investigation we found them to be 10-39% of plasma with more intensive sampling [16]. Therefore, although qualitative evaluations of breast milk penetration can be made from single-time point studies, we have demonstrated here that more extensive sampling is required to provide accurate, less variable data on the magnitude of drug exposure.
Antiretroviral concentrations are not always detected in the plasma of breastfeeding infants whose mothers are utilizing antiretrovirals. ZDV and protease inhibitors have been inconsistently detected in infant plasma[16-18, 20, 21]. Nevirapine and efavirenz exposures were found to be in four studies 12-60% of mothers’ plasma concentrations [17-20]. Data in this investigation showed only 3TC concentrations detectable in infant plasma at 2% of the concentration in breast milk and at 3.7% of the concentration in mothers’ plasma. This is similar to past reports of 3TC exposure in breastfeeding infants at 1-6% of concentrations seen in mothers’ plasma[16-18, 20].
For the benefit of future studies investigating breast milk concentration of these drugs as a predictor of outcomes, we conclude that BM concentrations are correlated with MP for all 4 ARVs tested (r=0.51-0.86). The relationship for all 4 drugs was linear, and we did not see any saturable pharmacokinetic relationship within the exposures measured, or dichotomous results suggesting altered mechanisms of drug distribution. The finding of nucleoside reverse transcriptase inhibitors concentrating in breast milk and the protease inhibitors achieving very low exposure in breast milk suggests drug specific factors likely play a role. Multiple characteristics contribute to the degree of exposure in breast milk including degree of protein binding, lipo- or hydrophilicity and ionization [28]. Overall the plasma protein binding has been the best predictor of breast milk penetration where the higher the protein binding the less penetration into breast milk[28,29]. In vitro and in vivo evaluations have concluded that large intra- and inter-subject variability exists for breast milk drug concentrations compared to mothers plasma. Previous data has suggested that the infant plasma concentrations serve as predictors of exposure in breast milk; however, data from this investigation would not support this finding as minimal drug exposure is seen in infant plasma [28]. Additionally, drug transporters have been identified in breast tissue, including MRP1, OCT1, and OCT3; however, expression of MRP4 has not been identified [30,31]. Lopinavir and ritonavir are substrates for MRP1 while ZDV and 3TC are substrates for MDR4 [32]. Additionally, 3TC is a substrate of OCT1 and 3. We theorize that the presence of MRP1 is effluxing LPV and RTV away from the breast milk while the absence of MRP4 is allowing the penetration of ZDV and 3TC into breast milk. Additionally, OCT1 and 3 may be facilitating the transport of 3TC into breast milk. This theory would need to be validated to confirm this mechanism.
There was a clear difference in the HIV RNA measured in the BM of mothers on ART and those that were not (97% vs 40% undetectable, respectively). The number of mothers with undetectable HIV RNA in plasma compared to breast milk was also quite different (65% vs 97% undetectable, respectively). In general, BM HIV RNA is 2-3 logs lower than matched plasma and highly correlated [32]. Limited data exist on the degree of breast milk viral load decline secondary to antiretroviral therapy [21]. For mothers taking ART the average HIV RNA in MP was 3.30log vs 0.77log in BM (2.53 log difference), which is consistent with previously reported data [32]. The exact mechanism of HIV transmission through breast milk is somewhat unclear but is believed to involve both cell free and cell associated virus [32]. Inter-subject changes in antiretroviral exposures and viral loads over the 24-week post partum period were evaluated. No significant changes in MP or BM exposure were noted.
Since a large number of breast milk viral loads were undetectable, the correlation of breast milk viral load and concentration was not evaluated. Concentrations in mother's plasma relative to HIVwt IC50 did not seem to predict undetectability of virus in the plasma. The lack of correlation may simply be secondary to the small sample size or to the large interpatient variability typically seen with antiretroviral concentrations. The only other study that has collected information on antiretroviral concentrations in breast milk and viral load did not report whether or not these correlated [21].
Since adherence to study medications obtained from 3-day medication diaries was reported to be 100% for all subjects, this was not used to assess drug exposure or efficacy. Overall median adherence in the BAN study over 24 weeks for these 30 subjects was 60-100% based on self-reported adherence questionnaires at 5 scheduled study visits during the 24-week period. Therefore, this measure was utilized in further comparisons. There did not appear to be an association between adherence and PK parameters, likely due to the small sample size and high rate of overall adherence. It should be noted that self-reported adherence may have overestimated the actual adherence and could be considered a limitation of the study.
In conclusion, antiretroviral penetration into breast milk is higher for ZDV and 3TC and lower for LPV and RTV compared to mothers’ plasma. This is likely due to higher protein binding of lopinavir and ritonavir compared to zidovudine and lamivudine as well as the potential for differences in drug transporter affinities. Additionally, this study has shown that greater variability exists in using single paired PK time points to predict the ARV exposure in breast milk [ZDV,3TC=186-557% vs 121-135% of mother's plasma; LPV,RTV=1-32% vs 10-39% of mother's plasma], and sampling over a dosing interval should occur at the outset of a breast milk PK study to avoid misleading results. However, we did note that a linear relationship could be developed for drugs with this information and sparse plasma sampling utilized in future investigations to estimate breast milk concentrations. Concentrations in mothers’ plasma and breast milk were not markedly different between 6, 12, and 24 weeks post-partum. Therefore, drug exposure can likely be studied at any timeframe from 6 weeks to 6 months post-partum and yield accurate assessments. In these mothers taking combined antiretroviral therapy, only one breast milk HIV RNA was greater than 40 copies/mL. These data, taken with the low to undetectable ARV exposure in infants, suggest that antiretroviral therapy given to mothers during breast feeding is effective at suppressing breast milk viral load which in turn will have an effect on MTCT by rendering the mother less infectious. Low extracellular concentrations in the infant seen in this investigation would not suggest protection of the infant if exposed to virus; however, multiple mechanisms for protection are unknown from this data including infant intracellular antiretroviral concentrations. Low 3TC concentrations in infants may predispose infected infants to HIV resistance via selective drug pressure or resistant virus from the mother could be transmitted to the infant via breastfeeding. Therefore, it is important to test breastfeeding infants early for HIV in mothers who are taking antiretrovirals to limit the possibility of viral resistance.
Acknowledgements
We are grateful to the following: BAN Study Team at University of North Carolina Chapel Hill, Centers for Disease Control and Prevention, Atlanta, and UNC Project team in Lilongwe including: Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang’ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, Chifundo Zimba. Finally and most especially, all the women and infants that have agreed to participate in the study.
Footnotes
The authors declare no competing interests
References
- 1.Nduati R, Richardson BA, John G, Mbori-Ngacha D, Mwatha A, Ndinya-Achola J, Bwayo J, Onyango FE, Kreiss J. Effect of breastfeeding on mortality among HIV-1 infected women: a randomised trial. Lancet. 2001;357:1651–1655. doi: 10.1016/S0140-6736(00)04820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, Gilbert PB, Stevens L, Peter T, Kim S, van Widenfelt E, Moffat C, Ndase P, Arimi P, Kebaabetswe P, Mazonde P, Makhema J, Mcintosh K, Novitsky V, Lee TH, Marlink R, Lagakos S, Essex M. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana. A randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS . UNAIDS report on the global AIDS epidemic 2013. WHO; Geneva, Switzerland: 2013. Global Report . available at http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 4.Bedri A, Gudetta B, Isehak A, Kumbi S, Lulseged S, Mengistu Y, Bhore AV, Bhosale R, Varadhrajan V, Gupte N, Sastry J, Suryavanshi N, Tripathy S, Mmiro F, Mubiru M, Onyango C, Taylor A, Musoke P, Nakabiito C, Abashawl A, Adamu R, Antelman G, Bollinger RC, Bright P, Chaudhary MA, Coberly J, Guay L, Fowler MG, Gupta A, Hassen E, Jackson JB, Moulton LH, Nayak U, Omer SB, Propper L, Ram M, Rexroad V, Ruff AJ, Shankar A, Zwerski S. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson D, Chasela C, Hudgens M, King C, Kourtis A, Kayira D. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised, controlled trial. The Lancet. 2012:379. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kesho Bora Study G, de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 7.Kilewo C, Karlsson K, Massawe A, Lyamuya E, Swai A, Mhalu F, Biberfeld G. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. J Acquir Immune Defic Syndr. 2008;48:315–323. doi: 10.1097/QAI.0b013e31816e395c. [DOI] [PubMed] [Google Scholar]
- 8.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, Lipyoga R, Mhalu F, Biberfeld G. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 9.Marazzi CM, Germano P, Liotta G, Guidotti G, Loureiro S, Gomes Ada C, Blazquez MC, Narciso P, Perno CF, Mancinelli S, Altan AD, Nielsen-Saines K, Palombi L. Implementing anti-retroviral triple therapy to prevent HIV mother-to-child transmission: a public health approach in resource-limited settings. Eur J Pediatr. 2007;166:1305–1307. doi: 10.1007/s00431-006-0405-8. [DOI] [PubMed] [Google Scholar]
- 10.Peltier CA, Ndayisaba GF, Lepage P, van Griensven J, Leroy V, Pharm CO, Ndimubanzi PC, Courteille O, Arendt V. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. Aids. 2009;23:2415–2423. doi: 10.1097/QAD.0b013e32832ec20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shapiro RL, Hughes MD, Ogwu A, Kitch D, Lockman S, Moffat C, Makhema J, Moyo S, Thior I, Mcintosh K, van Widenfelt E, Leidner J, Powis K, Asmelash A, Tumbare E, Zwerski S, Sharma U, Handelsman E, Mburu K, Jayeoba O, Moko E, Souda S, Lubega E, Akhtar M, Wester C, Tuomola R, Snowden W, Martinez-Tristani M, Mazhani L, Essex M. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taha TE, Li Q, Hoover DR, Mipando L, Nkanaunena K, Thigpen MC, Taylor A, Kumwenda J, Fowler MG, Mofenson LM, Kumwenda NI. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi trial. J Acquir Immune Defic Syndr. 2011;57:319–325. doi: 10.1097/QAI.0b013e318217877a. [DOI] [PubMed] [Google Scholar]
- 13.Thomas TK, Masaba R, Borkowf CB, Ndivo R, Zeh C, Misore A, Otieno J, Jamieson D, Thigpen MC, Bulterys M, Slutsker L, De Cock KM, Amornkul PN, Greenberg AE, Fowler MG. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding--the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyankandondera J, Luchters S, Hassink E, Pakker N, Mmiro F, Okong P. Simba—Stopping Infection from Mother to Child via Breastfeeding in Africa. Program and abstracts of the 3rd International AIDS Society Conference on HIV Pathogenesis. 2003 [Google Scholar]
- 15.Organization WHO. The use of antiretroviral drugs for treating and preventing HIV infection. WHO; Jun, 2013. [PubMed] [Google Scholar]
- 16.Corbett A, Martinson F, Rezk N. Lopinavir/ritonavir concentrations in breast milk and breast-feeding infants [abstract no. 947]. 16th Conference on retroviruses and opportunistic infections. 2009 [Google Scholar]
- 17.Corbett A, Martinson F, Rezk N, Kashuba A, Jamieson D, Chasela C, Hyde L, Ahmed Y, Tegha G, Joaki G. 15th Conference on retroviruses and opportunistic infections. Boston, MA: Feb, 2008. Antiretroviral drug concentrations in breast milk and breastfeeding infants. [Poster 648] [Google Scholar]
- 18.Mirochnick M, Thomas T, Capparelli E, Zeh C, Holland D, Masaba R, Odhiambo P, Fowler MG, Weidle PJ, Thigpen MC. Antiretroviral concentrations in breast-feeding infants of mothers receiving highly active antiretroviral therapy. Antimicrob Agents Chemother. 2009;53:1170–1176. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider S, Peltier A, Gras A, Arendt V, Karasi-Omes C, Mujawamariwa A, Ndimubanzi PC, Ndayisaba G, Wennig R. Efavirenz in human breast milk, mothers', and newborns’ plasma. J Acquir Immune Defic Syndr. 2008;48:450–454. doi: 10.1097/QAI.0b013e31817bbc21. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro RL, Holland DT, Capparelli E, Lockman S, Thior I, Wester C, Stevens L, Peter T, Essex M, Connor JD, Mirochnick M. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis. 2005;192:720–727. doi: 10.1086/432483. [DOI] [PubMed] [Google Scholar]
- 21.Palombi L, Pirillo MF, Andreotti M, Liotta G, ERba F, Sagno JB, Maulidi M, Ceffa S, Jere H, Marchei E, Pichini S, Galluzzo CM, Marazzi MC, Vella S, Guiliano M. Antiretroviral prophylaxis for breastfeeding transmission in Malawi: drug concentrations, virological efficacy and safety. Antivir Ther. 2012;17:1511–1519. doi: 10.3851/IMP2315. [DOI] [PubMed] [Google Scholar]
- 22.Rezk NL, White N, Bridges AS, Abdel-Megeed MF, Mohamed TM, Moselhy SS, Kashuba AD. Studies on antiretroviral drug concentrations in breast milk: validation of a liquid chromatography-tandem mass spectrometric method for the determination of 7 anti-human immunodeficiency virus medications. Ther Drug Monit. 2008;30:611–619. doi: 10.1097/FTD.0b013e318186e08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Institute for Allergy and Infectious Diseases Division of AIDS, Clinical Pharmacology Quality Assurance Antiretroviral Proficience Testing Program. available at www.fstrf.org.
- 24.KKGT Association for Quality Assessment in TDM and Clinical Toxicology; A section of the Dutch Foundation for Quality Assessment in Medical Laboratories. available at www.kkgt.nl.
- 25.Moore KH, Shaw S, Laurent AL, Lloyd P, Duncan B, Morris DM, O'Mara MJ, Pakes GE. Lamivudine/zidovudine as a combined formulation tablet: bioequivalence compared with lamivudine and zidovudine administered concurrently and the effect of food on absorption. J Clin Pharmacol. 1999;39:593–605. doi: 10.1177/00912709922008209. [DOI] [PubMed] [Google Scholar]
- 26.Kaletra package insert. Abbott Laboratories; North Chicago, IL: [Google Scholar]
- 27.Veldkamp AI, van Heeswijk RP, Mulder JW, Meenhorst PL, Schreij G, van der Geest S, Lange JM, Beijnen JH, Hoetelmans RM. Steady-state pharmacokinetics of twice-daily dosing of saquinavir plus ritonavir in HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2001;27:344–349. doi: 10.1097/00126334-200108010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Anderson GD. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert Opin Drug Metab Toxicol. Dec. 2006;2(6):947–60. doi: 10.1517/17425255.2.6.947. [DOI] [PubMed] [Google Scholar]
- 29.Riant P, Urien S, Albengres E, Duche JC, Tillement JP. High plasma protein binding as a parameter in the selection of betablockers for lactating women. Biochem Pharmacol. 1986;35:4579–4581. doi: 10.1016/0006-2952(86)90783-5. [DOI] [PubMed] [Google Scholar]
- 30.Olagunju A, Owen A, Cressey TR. Potential effect of pharmacogenetics on maternal, fetal and infant antiretroviral drug exposure during pregnancy and breastfeeding. Pharmacogenomics. 2012;13:1501–1522. doi: 10.2217/pgs.12.138. [DOI] [PubMed] [Google Scholar]
- 31.Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303:487–496. doi: 10.1124/jpet.102.038315. [DOI] [PubMed] [Google Scholar]
- 32.Fiscus SA, Aldrovandi GM. Virologic determinants of breast milk transmission of HIV-1. Adv Exp Med Biol. 2012;743:69–80. doi: 10.1007/978-1-4614-2251-8_5. [DOI] [PubMed] [Google Scholar]