Abstract
Redirection of adenovirus vectors by engineering the capsid-coding region has shown limited success because proper targeting ligands are generally unknown. To overcome this limitation, we constructed an adenovirus library displaying random peptides on the fiber knob, and its screening led to successful selections of several particular targeted vectors. In the previous library construction method, the full length of an adenoviral genome was generated by a Cre-lox mediated in vitro recombination between a fiber-modified plasmid library and the enzyme-digested adenoviral DNA/terminal protein complex (DNA-TPC) before transfection to the producer cells. In this system, the procedures were complicated and time-consuming, and approximately 30% of the vectors in the library were defective with no displaying peptide. These may hinder further extensive exploration of cancer-targeting vectors. To resolve these problems, in this study, we developed a novel method with the transfection of a fiber-modified plasmid library and a fiberless adenoviral DNA-TPC in Cre-expressing 293 cells. The use of in-cell Cre recombination and fiberless adenovirus greatly simplified the library-making steps. The fiberless adenovirus was useful in suppressing the expansion of unnecessary adenovirus vectors. In addition, the complexity of the library was more than a 104 level in one well in a 6-well dish, which was 10-fold higher than that of the original method. The results demonstrated that this novel method is useful in producing a high quality live adenovirus library, which could facilitate the development of targeted adenovirus vectors for a variety of applications in medicine.
Keywords: Adenovirus, library, Cre recombination, fiberless, targeting vector
Introduction
An adenovirus is a potent gene-delivery vehicle and has frequently been used for designing oncolytic viruses.1,2,3 However, the lack of specificity on infection due to the widespread distribution of the primary cellular receptor of the adenovirus has prevented the achievement of cancer-specific gene transduction after intravenous administration.4,5 Also, many cancer cells lack sufficient receptor expression for realizing therapeutic effect.4,5 Strategies for redirecting the tropism of the adenovirus vector are therefore being developed to enable efficient target gene delivery to specific cell types.1,2,6
Most of the presently used adenovirus vectors are based on serotype 5 (Ad5). Targeting of Ad5 vectors has been achieved by direct genetic modifications of the capsid proteins: targeting ligands can be incorporated into the fiber proteins ablated for native tropism, and these vectors provide an important platform for evaluating the targeting potential of selected peptide ligands.7–10 Lack of cell-type specific ligands for targeted adenovirus vectors impedes application of fiber-modified adenovirus vectors for targeted therapies. Although a phage display library has been used to identify targeting peptide motifs, the incorporation of the peptides selected by phage display into the adenoviral capsid is difficult and has not been successful for few cases,11–14 possibly due to the unwanted conformational change of the virus capsid protein induced by the inserted peptide or the loss of affinity after ligand incorporation.15,16
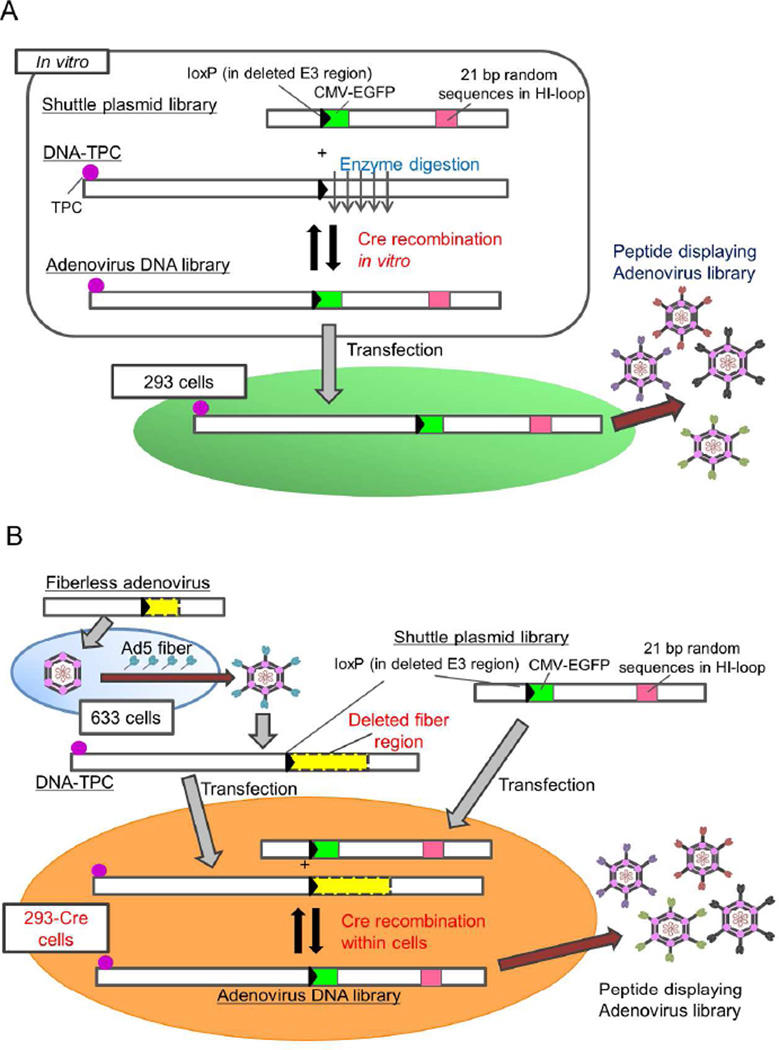
To overcome this limitation, we have developed a system generated by Cre/lox-mediated in vitro recombination between an adenoviral fiber-modified plasmid library and a right end-digested adenoviral DNA-terminal protein complex (DNA-TPC), for producing adenoviral libraries displaying a variety of peptides on the fiber knob (Fig. 1A).16,17 We screened the library on several cancer cell lines in vitro and in vivo with a murine peritoneal dissemination model, and successfully identified the targeted adenoviral vectors with high infectivity.16,18,19
Figure 1.
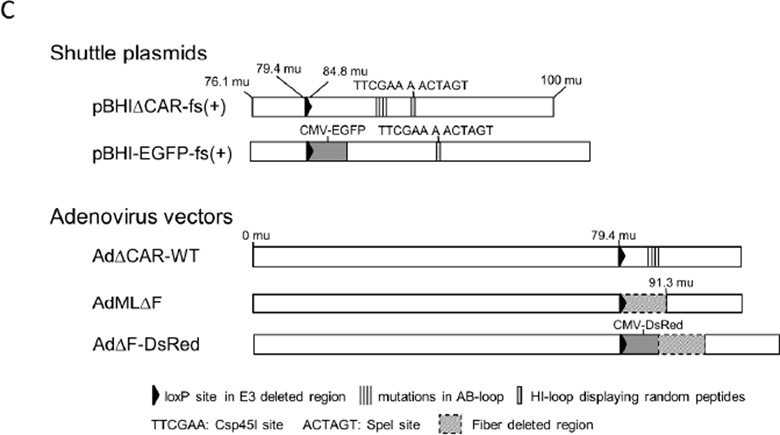
Methods to construct adenovirus libraries displaying random peptides on the fiber knob. A) An original library construction method. DNA-TPC was prepared from a CAR binding-ablated adenovirus. The fiber-modified shuttle plasmid library was recombined with equal moles of the enzymatically digested DNA-TPC by Cre recombinase in vitro to produce a full-length adenovirus genomic DNA library. Then, to generate an adenovirus library, recombined adenoviral DNA was transfected into 293 cells. B) A novel adenovirus library construction method. DNA-TPC was prepared from a fiberless adenovirus, which was propagated in 633 cells. The fiber-modified shuttle plasmid library was co-transfected with equal moles of DNA-TPC in 293-Cre cells. C) Schematic presentation of adenovirus vectors and fiber-modified shuttle plasmids. A single copy of a loxP sequence substitutes for the E3 gene (76.1–84.8 mu). The AdΔCAR-WT, AdMLΔF and AdΔF-DsRed have a wild-type E1 gene, and four point mutations were inserted in the AB-loop of fiber knob in the AdΔCAR-WT, and the fiber region is deleted in AdMLΔF and AdΔF-DsRed. In the pBHIΔCAR-fs(+), four point mutations were inserted in the AB-loop.
Although the screening was effective in finding tumor-specific adenovirus vectors, two points in the library construction method had to be addressed before moving on to the wider screening of targeted adenovirus vectors in various cancer cell lines and cancer animal models: 1) the original library making-procedures such as the preparation of recombined full length of adenovirus DNA-TPC were complicated and time-consuming; 2) although an adenovirus library was expanded three times with re-infection with crude viral lysate (CVL) in 293 cells, approximately 30% of vectors contained in the expanded library (4th seed) were defective with no displaying peptide, which was not much different from the first seed of library. These problems may hinder the construction of a large-scale adenovirus library and the extensive exploration of cancer-targeting vectors. In this study, we successfully established a novel method to construct an adenovirus library more efficiently by using DNA-TPC of a fiberless adenovirus and an in-cell Cre recombination (Fig. 1B). The results showed that this novel method is useful in constructing a high quality live adenovirus library.
Materials and methods
Cell lines
A human embryonic kidney cell line (293) was obtained from American Tissue Culture Collection (ATCC; Rockville, MD), and was cultured in Dulbecco’s modified eagle’s medium (Wako Pure Chemical Industries, Ltd., Osaka, Japan) with 10% fetal bovine serum (FBS). The 633 cells, a derivative of A549 cells expressing E1, E2A, and Ad5 fiber, were provided by Dr. Glen Nemerow (The Scripps Research Institute, La Jolla, CA) and were maintained as reported before.20 The Cre-expressing 293 cells were generated by retrovirus-mediated transduction of Cre recombinase cDNA, and designated as 293-Cre cells.
Shuttle plasmids and recombinant adenovirus DNA
The fiber-modified adenoviral shuttle plasmids pBHIΔCAR-fs(+) and pBHI-EGFP-fs(+) include a 76.1–100 map unit (mu) of the adenoviral genome with a single loxP site at the E3 region deleted (79.4–84.8 mu)(Fig. 1C).16 These plasmids contain Csp45I and SpeI restriction sites in the HI-loop to clone DNA sequences coding random peptides.16 The pBHIΔCAR-fs(+) includes 4 point mutations in the AB-loop of the fiber knob that reduces CAR binding.16 The pBHI-EGFP-fs(+) contains a cytomegalovirus immediate early enhancer/promoter (CMV promoter), the enhanced green fluorescent protein (EGFP) gene and a SV40 poly(A) signal in place of the E3 region.
The fiberless adenoviruses (AdMLJF and AdJF-DsRed) have a wild-type E1 gene, a single loxP site replacing the E3 gene, and a deletion of its fiber region (79.4– 91.3 mu)(Fig. 1C).21 The AdJF-DsRed contains a CMV promoter, the red fluorescent protein from Discosoma (DsRed) gene and a SV40 poly(A) signal at the E3 region deleted. These viruses were propagated in 633 cells for pseudotyping with the type 5 adenoviral fiber. The CAR-binding ablated adenovirus (AdΔCAR-WT) has a wild-type E1 gene and 4 point mutations in the AB-loop of the fiber knob that reduces CAR binding. The DNA-TPC from AdMLJF was prepared through a buoyant CsCl density gradient with 4 M guanidine hydrochloride.17
The plasmid plox-DsRed-lox-EGFP contains a loxP-DsRed-loxP cassette and an EGFP cDNA downstream of the CMV promoter. This plasmid expresses the DsRed gene in the absence of Cre recombinase, whereas it expresses the EGFP gene due to the deletion of the DsRed gene after Cre-mediated recombination.
Construction of shuttle plasmid libraries
Fiber-modified shuttle plasmid libraries were constructed in the same method we have described before.16 Briefly, the degenerate oligonucleotide 5’-AACGGTACACAGGAAACAGGAGACACAACTTTCGAA(NNK)7ACTAGTCCA AGTGCATACTCTATGTCATTTTCATGG-3’ (N=A, T, G or C, K=G or T) served as a template for PCR with the primers 5’-GAAACAGGAGACACAACTTTCGAA-3’ and 5’-CATAGAGTATGCACTTGGACTAGT-3’. The PCR product was ligated into the HI-loop portion of the adenovirus shuttle plasmid pBHIΔCAR-fs(+) and pBHI-EGFP-fs(+), and then transfected into Max Efficiency electrocompetent cells (Life Technologies Corp., Carlsbad, CA) by electroporation. The plasmid libraries constructed from pBHIΔCAR-fs(+) and pBHI-EGFP-fs(+) were designated as pBHI-lib and pBHI-EGFP-lib, respectively. Both plasmid libraries contained 2×107 clones, excluding insertless and unsuitable clones. The complexity of the plasmid libraries was estimated by the number of clones growing from a representative aliquot of the transformed bacteria on agar plates containing ampicillin. DNA sequencing of 50 clones verified that different random sequences are encoded in each clone (data not shown).
Novel method to construct random peptides displaying adenovirus libraries
The basic difference between the original and novel methods was that in the original method the full length of the adenovirus genome DNA was constructed in vitro and the DNA was transfected into the producer cells (Fig. 1A), whereas in the novel method the full length of the adenovirus genome DNA was constructed within the producer cells (Fig. 1B). Specifically, in the novel library construction method, the fiber-modified shuttle plasmid libraries were linearized at the right end by PacI and co-transfected with equal moles of DNA-TPC isolated from AdMLΔF in 293-Cre cells. The 0.5 µg of shuttle plasmid and 1.5 µg of DNA-TPC were transfected by the lipofection method (Lipofectamine 2000 Reagent; Life Technologies Corp.) for one well in a 6-well dish. The first generation of the adenovirus library was harvested 2–3 days after the appearance of the CPE in the cells.
PCR and sequencing of adenovirus library
To analyze the inserted sequences in the fiber knob of the adenovirus library, DNA was extracted from the CVL that served as a template for a PCR with the primers containing upstream and downstream sequences of the HI-loop: 5’-GAAACAGGAGACACAACTTTCGAA-3’ and 5’-CATAGAGTATGCACTTGGACTAGT-3’. PCR products were cloned into the pBHI-fs(+) plasmid. Randomly assigned clones were sequenced using the primer 5’- GGAGATCTTACTGAAGGCACAGCC-3’.
To evaluate the ratio of defective adenovirus displaying no peptide in the library, 100 ng of total DNA isolated from CVL was analyzed by the PCR method with the following primers. The forward primer F1 was set up upstream at loxP site: 5’-AACGTACGAGTGCGTCACCGGCCG-3’. The reverse primer R1 was set up in the CMV promoter: 5’-GGAAATCCCCGTGAGTCAAAC-3’ to detect the adenovirus library, and the reverse primer R2 was set up downstream of the deleted fiber region: GAGGATGTGGCAAATATTTC-3’ to detect the fiberless adenovirus. The band intensity was analyzed using scanning densitometry (LAS-3000 imaging system; Fujifilm Life Science, Tokyo, Japan).
Results
Production of an adenovirus library with DNA-TPC prepared from a fiberless adenovirus
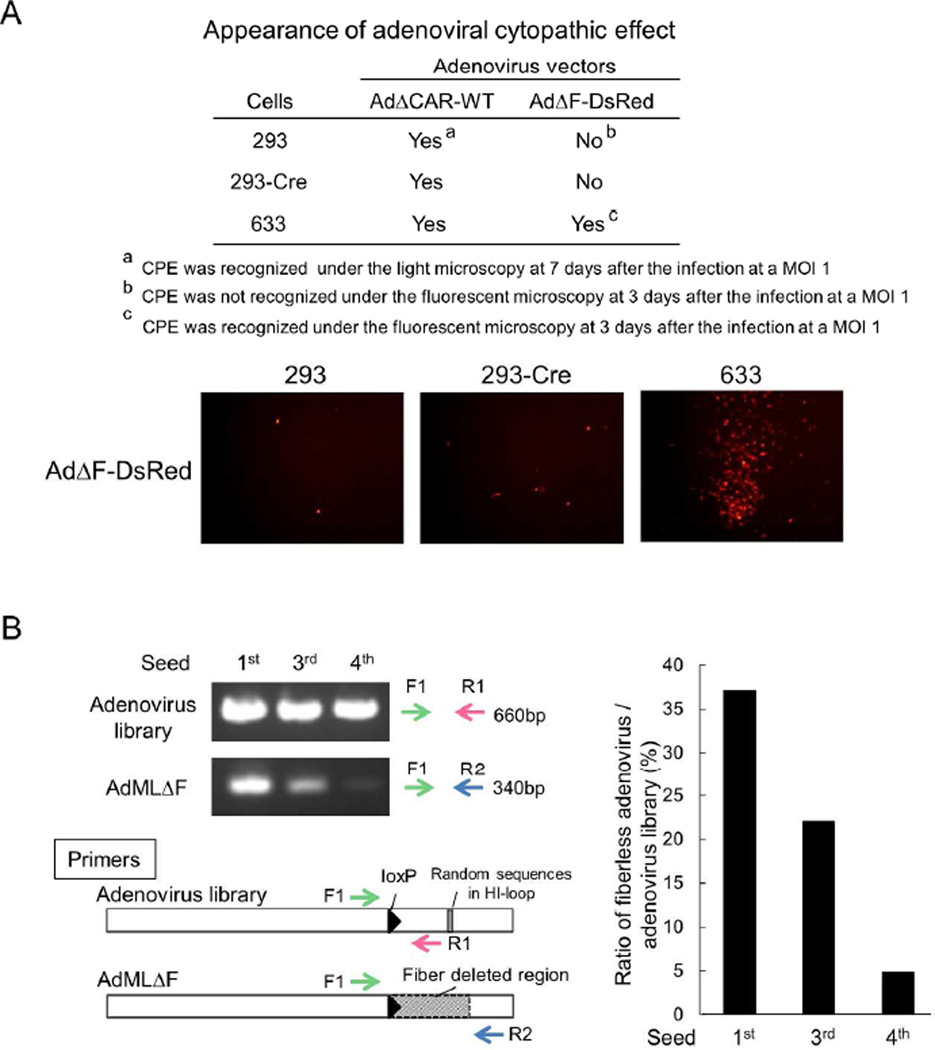
The use of the adenoviral DNA-TPC is crucial to efficiently generate an adenovirus library because the 55KDa terminal protein covalently linked to its 5’ end greatly enhances the efficiency of adenoviral production.22 In this study, we employed the DNA-TPC isolated from a fiberless adenovirus vector to suppress the expansion of unnecessary adenovirus vectors.21 In order to confirm that the expansion of fiberless adenovirus is severely suppressed in 293 cells,23 293, 293-Cre and 633 cells were infected with AdΔCAR-WT and a fiberless adenovirus vector pseudotyped with Ad5 fiber (AdΔF-DsRed) at a MOI (multiplicity of infection) of 1. The DsRed+ CPE was recognized in 633 cells but not in 293 and 293-Cre cells, whereas the infection of AdΔCAR-WT showed CPE in 293, 293-Cre and 633 cells (Fig. 2A), indicating that the expansion of a fiberless adenovirus required the fiber-transcomplementation. We then constructed a peptide-displaying adenovirus library by the transfection of the recombined DNA between a fiber-modified plasmid library (pBHI-EGFP-lib) and DNA-TPC (AdMLΔF) in the producer 293 cells.
Figure 2.
Reduction of a fiberless adenoviral genome in the library. A) Appearance of CPE in 633 cells infected with AdΔF-DsRed. The 293, 293-Cre and 633 cells were infected with AdΔCAR-WT and AdΔF-DsRed at a MOI of 1. The appearance of DsRed+ CPE was examined under fluorescent microscopy at day 3 after the infection in AdΔF-DsRed-infected cells, while CPE was examined in 293 and 293-Cre cells under light microscopy at day 7 after the infection in AdΔCAR-WT-infected cells (upper panel). DsRed+ cells in 293, 293-Cre and 633 cells 3 days after the infection of AdΔF-DsRed (lower panel). B) The reduction of a fiberless adenoviral genome during the expansion of the library. The 293 cells were infected with the 1st generation of the adenovirus library generated by the original method. Four days later, the cells were harvested and the 293 cells were re-infected with CVL. This re-infection was repeated three times. DNA was extracted from each CVL and subjected to PCR analysis for the detection of an adenovirus library and AdMLΔF (left panel). The intensity of the PCR band of AdMLΔF was analyzed by densitometry and was compared with that of the adenovirus library in 1st, 3rd and 4th seeds (right panel).
Reduction of a fiberless adenovirus genome in the library
To examine whether the use of DNA-TPC prepared from AdMLΔF is able to reduce the defective adenovirus displaying no peptide in the process of library expansion, the CVL of the adenovirus library was subjected to three more rounds of re-infection in 293 cells. During the sequential re-infections of CVL, the PCR band of the fiberless adenovirus genome was obviously reduced (Fig. 2B left). The densitometry showed that the amount of fiberless adenovirus was decreased until less than 5% in the 4th round of library seed (Fig. 2B right).
Generation of Cre-expressing 293 cells
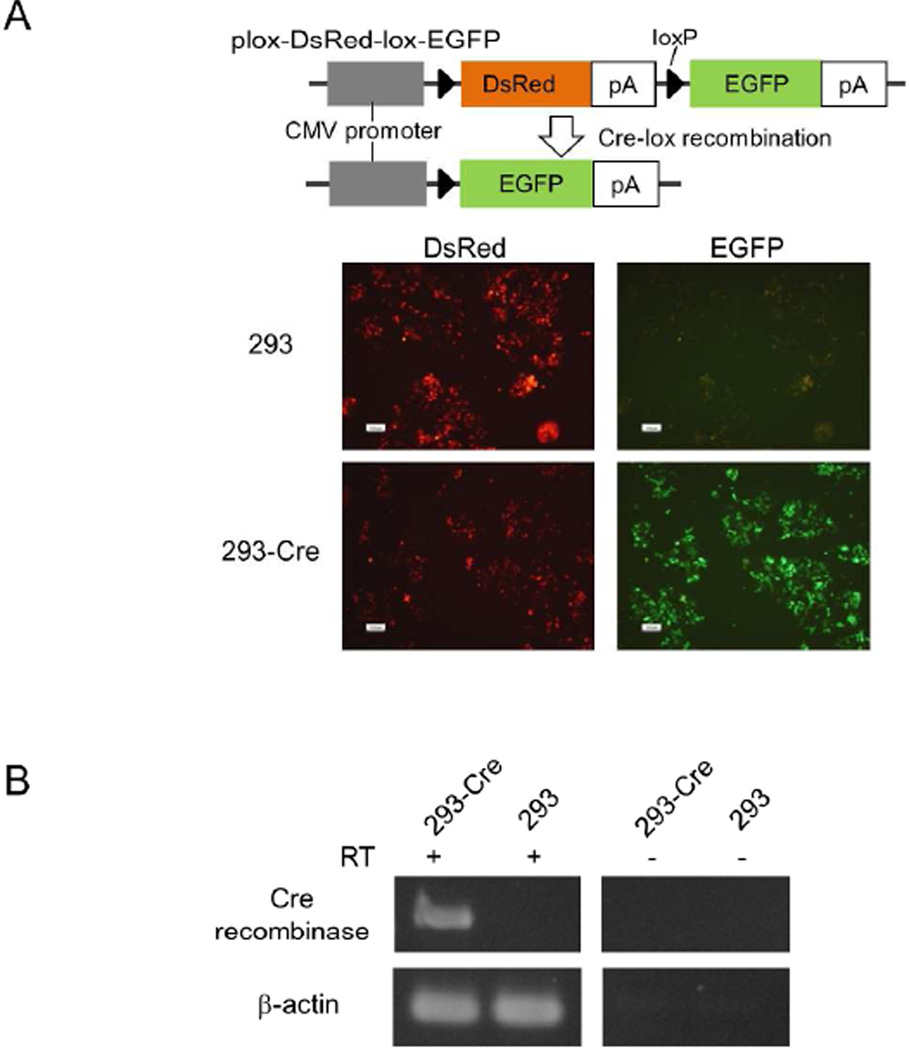
To simplify the steps to generate an adenovirus library, we made Cre-expressing 293 cells. The cDNA of Cre recombinase was transduced in 293 cells by a retrovirus vector, and 5 colonies were picked up after G418 selection. To select the clone with higher activity of Cre recombinase, 5 clones were transfected with a plasmid plox-DsRed-lox-EGFP, in which the Cre-lox reaction results in the EGFP expression (Fig. 3A upper panel). In all 5 clones, the plasmid transfection showed many EGFP+ cells, indicating that Cre recombinase recognized the loxP sites of the plasmid and recombined the DNA within the cells, whereas the plasmid showed the expression of DsRed but not the EGFP gene in the parental 293 cells. We selected a 293-Cre clone (293-Cre2) among the 5 clones, one that showed the highest number of EGFP+ cells after the plasmid transfection (Fig. 3A lower panel). The expression of Cre mRNA in the selected clone was confirmed by the reverse transcription (RT)-PCR method (Fig. 3B).
Figure 3.
Making of Cre recombinase-expressing 293 cells. A) Functional assay of Cre activity. In the plox-DsRed-lox-EGFP plasmid-transfected cells, Cre-lox reaction deletes the DsRed gene and expresses the EGFP gene. B) Expression of Cre recombinase mRNA in a selected 293-Cre clone. Total RNA was isolated from 293-Cre2 cells and subjected to RT-PCR analysis.
Efficiency of adenovirus production by the novel method
To examine whether the peptide displaying adenovirus library can be constructed by the novel method with the fiberless adenoviral genome DNA and in-cell Cre recombination, 293-Cre2 cells were co-transfected with a DNA-TPC of AdMLΔF and a pBHI-EGFP-lib in a 6-well dish. The adenoviral CPE appeared, and the cells were harvested 10 days after the transfection. The re-infection of the first seed CVL showed many EGFP+ cells in the 293 cells, indicating that the adenovirus library was efficiently generated by the novel method.
To estimate how many different peptides were displayed on these adenoviruses, we set up dilution experiments with two shuttle plasmid libraries: pBHI-EGFP-lib was mixed with pBHI-lib at various ratios (1:102, 1:103, 1:104, 1:105) and then the mixture was co-transfected with the fiberless adenoviral DNA-TPC in 293-Cre2 cells. The CPE was recognized in the wells 10–12 days after the transfection, and to confirm the virus production, the 293 cells were infected with 15% of the CVL. EGFP-expressing adenoviruses, which were derived from pBHI-EGFP-lib, were detected at up to 1×104 dilution level. The result showed that at least 1×104 kinds of peptides on the fiber knob were displayed in the adenovirus library produced by the novel method per one well in the 6-well dish, whereas in the original method the production of EGFP-expressing adenovirus was recognized at 1×103 dilution but not at the 1×104 level, indicating that the complexity generated by the novel method was 10-fold higher than that of the adenovirus library by the original method (Table). The DNA sequences of 50 randomly selected clones of PCR products from the library generated by the novel method verified that each clone contained a different peptide in the HI-loop of the adenovirus library, and that the frequencies of amino acids was comparable to those in the plasmid library, indicating that a wide variety of peptides were displayed on the fiber knob of the adenovirus library evenly representing original random library sequences (data not shown).
Table.
Efficiency of EGFP-expressing adenovirus production
| pBHI-EGFP-lib/pBHI-liba |
||||
|---|---|---|---|---|
| method | 1/102 | 1/103 | 1/104 | 1/105 |
| Originalb | Yesd | Yes | Noe | No |
| Newc | Yes | Yes | Yes | No |
Various ratios of pBHI-EGFP-lib and pBHI-lib were mixed. EGFP-expressing adenovirus was only produced from pBHI-EGFP-lib.
A shuttle plasmid and DNA-TPC were recombined in vitro by Cre-lox reaction and the recombined DNA was transfected in 293 cells.
A shuttle plasmid and DNA-TPC were transfected in 293-Cre2 cells.
EGFP-expressing cells were detected after the infection of CVL.
EGFP-expressing cells were not detected after the infection of CVL.
Discussion
It is attractive to explore targeted vectors with an adenovirus library on the various cells or other materials for the development of next generation vectors. In fact, we have reported that in vitro and in vivo screenings successfully identified tumor-specific adenovirus vectors, which display unique sequences on their fiber knob.16,18,19 However, in the original library construction method, the procedures were time-consuming and a part of library viruses were defective with no displaying peptide. To apply this library technology for screenings in wide variety of cancer materials as a next step, we established a novel method to construct, simply and efficiently, an adenovirus library with a higher quality by using the DNA-TPC of a fiberless adenovirus and Cre-expressing 293 cells.
To overcome the problem of time-consuming process in the original method, we tried to improve and simplify the original technology by using in-cell Cre recombination and the fiberless adenovirus system, since Miura et al. recently reported that a high-diversity adenovirus library carrying random sequences in the AB-loop was generated by the infection with a rescue fiberless adenovirus followed by the transfection of a shuttle plasmid in a Cre-expressing fiber-transcomplementing cell line.21 In fact, the employment of Cre-expressing 293 cells and the fiberless virus was much useful to avoid complicated procedures such as the preparation of restriction enzyme-digested DNA-TPC, in vitro Cre recombination between a plasmid library and DNA-TPC and purification of the recombined DNA in the original method. Furthermore, Cre-recombination within the cells appeared to increase the library complexity 10-fold over that of the original method. In the original method, the shuttle plasmid is recombined with DNA-TPC in vitro; however, the recombination efficiency was less than 30%, and future recombination does not occur after the transfection of DNA in the producer cells.17 In the novel method, the transcription and packaging of a virus genome into the virus particles can proceed as soon as the full length of the adenovirus genomic DNA is constructed by Cre-mediated recombination, and the continuous Cre-lox reaction within the 293-Cre cells continues to generate the full length of the adenovirus genome, which is supposed to be the reason of marked enhancement of the adenovirus production efficiency.
Furthermore, in the original method, a part of produced library lacked peptide coding sequences in the HI-loop region. The use of a fiberless adenovirus genome DNA markedly reduced the level of defective adenovirus vectors during the expansion of the adenovirus library (Fig. 2B). Although the infectivity of the CAR-binding ablated adenovirus vector was much lower than that of a wild fiber, it can still infect and expand autonomously in 293 cells, whereas the infectivity of a fiberless adenovirus was significantly reduced and the virus particles were produced only in fiber-transcomplementing producer cell lines such as 633 cells (Fig. 2A). Although the genome of a fiberless adenovirus was obviously decreased in the library, it was not completely eliminated even after a repeated infection of the 293 cells with CVL (Fig. 2B). Probably, since the genome of a fiberless adenovirus can amplify in the cells, the genome might be packaged into the virus capsid with the fiber, that was produced from the adenovirus library, making pseudo-typed virus particles. However, since the fiberless adenovirus is not able to produce a functional fiber, the contamination of a lower amount of a fiberless genome in the library should not hinder the screening process for targeted vectors.
The complexity of the library in the novel method reached at least a 104 level per one well in a 6-well dish. Although the theoretical peptide complexity of 7 amino acids is over 109, our previous examination showed that the total diversity of the live virus library was estimated at a 108 level, because 90% of the peptide insertions into the HI-loop may impede virus production.16 Therefore, 1000×6-well dishes may be necessary to construct the full diversity of an adenovirus library. However, since the complexity of an adenovirus library depends on the number of helper cells transfected with DNA, the simple scaling up of the number and the size of plates is able to increase the library complexity.
At present, delivery tools to efficiently infect and transduce genes in the organs, tissues and cells of interest are awaited in hopes of developing a useful oncolytic virus therapy as well as gene therapy. Oncolytic viruses are designed as a potent approach by post-delivery tumor-selective vector amplification and virus-induced cell lysis.3 However, it is necessary to reduce any undesirable infection of non-target normal tissues, whereas the antitumor effect of an oncolytic adenovirus vector is determined by its capacity to infect tumor cells. Thus, the addition of a tumor-targeting potential to an oncolytic adenovirus is important for enhancing its therapeutic index. A library approach with a replication-competent adenovirus may be highly useful for isolating a targeted oncolytic adenovirus, because the most efficient adenovirus should be selected from the library based on its high infectivity and replication capacity through the process of several rounds of virus amplification and spread through target cells. Hence, as a future work, we will screen this high quality library not only in such various cancer materials as cancer cell lines or murine cancer models but also in human biopsy and surgical specimens. When we confirm the reliability of our novel library technology by successful isolation of various targeted vectors, we will propose to distribute the library as a resource. By screening of the adenovirus library with specific sequences, the most suitable adenovirus vectors will be developed for a variety of applications in medicine.
Acknowledgments
We thank Dr. Glen Nemerow (Scripps Research Institute, La Jolla, CA) for providing us with the 633 cells. This work was supported in part by a grant-in-aid for the 3rd Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan, grants-in-aid for research from the Ministry of Health, Labour and Welfare of Japan, by the National Cancer Center Research and Development Fund (23-A-9) and by R01CA094084, R01CA168448, P50CA101955 (NIH/NCI).
References
- 1.Yamamoto M, Curiel DT. Current issues and future directions of oncolytic adenoviruses. Mol Ther. 2010;18:243–250. doi: 10.1038/mt.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pesonen S, Kangasniemi L, Hemminki A. Oncolytic adenoviruses for the treatment of human cancer: focus on translational and clinical data. Mol Pharm. 2011;8:12–28. doi: 10.1021/mp100219n. [DOI] [PubMed] [Google Scholar]
- 3.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, Horwitz MS, Crowell RL, Finberg RW. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 5.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coughlan L, Alba R, Parker AL, Bradshaw AC, McNeish IA, Nicklin SA, Baker AH. Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses. 2010;2:2290–2355. doi: 10.3390/v2102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas JT, Rogers BE, Rosenfeld ME, Michael SI, Feng M, Curiel DT. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. doi: 10.1038/nbt1196-1574. [DOI] [PubMed] [Google Scholar]
- 8.Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT. Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol. 1998;72:1844–1852. doi: 10.1128/jvi.72.3.1844-1852.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida Y, Sadata A, Zhang W, Saito K, Shinoura N, Hamada H. Generation of fiber-mutant recombinant adenoviruses for gene therapy of malignant glioma. Hum Gene Ther. 1998;9:2503–2515. doi: 10.1089/hum.1998.9.17-2503. [DOI] [PubMed] [Google Scholar]
- 11.Nicklin SA, Von Seggern DJ, Work LM, Pek DC, Dominiczak AF, Nemerow GR, Baker AH. Ablating adenovirus type 5 fiber-CAR binding and HI loop insertion of the SIGYPLP peptide generate an endothelial cell-selective adenovirus. Mol Ther. 2001;4:534–542. doi: 10.1006/mthe.2001.0489. [DOI] [PubMed] [Google Scholar]
- 12.Nicklin SA, White SJ, Nicol CG, Von Seggern DJ, Baker AH. In vitro and in vivo characterisation of endothelial cell selective adenoviral vectors. J Gene Med. 2004;6:300–308. doi: 10.1002/jgm.526. [DOI] [PubMed] [Google Scholar]
- 13.Joung I, Harber G, Gerecke KM, Carroll SL, Collawn JF, Engler JA. Improved gene delivery into neuroglial cells using a fiber-modified adenovirus vector. Biochem Biophys Res Commun. 2005;328:1182–1187. doi: 10.1016/j.bbrc.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 14.Nicol CG, Denby L, Lopez-Franco O, Masson R, Halliday CA, Nicklin SA, Kritz A, Work LM, Baker AH. Use of in vivo phage display to engineer novel adenoviruses for targeted delivery to the cardiac vasculature. FEBS Lett. 2009;583:2100–2107. doi: 10.1016/j.febslet.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 15.Muller OJ, Kaul F, Weitzman MD, Pasqualini R, Arap W, Kleinschmidt JA, Trepel M. Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat Biotechnol. 2003;21:1040–1046. doi: 10.1038/nbt856. [DOI] [PubMed] [Google Scholar]
- 16.Miura Y, Yoshida K, Nishimoto T, Hatanaka K, Ohnami S, Asaka M, Douglas JT, Curiel DT, Yoshida T, Aoki K. Direct selection of targeted adenovirus vectors by random peptide display on the fiber knob. Gene Ther. 2007;14:1448–1460. doi: 10.1038/sj.gt.3303007. [DOI] [PubMed] [Google Scholar]
- 17.Hatanaka K, Ohnami S, Yoshida K, Miura Y, Aoyagi K, Sasaki H, Asaka M, Terada M, Yoshida T, Aoki K. A simple and efficient method for constructing an adenoviral cDNA expression library. Mol Ther. 2003;8:158–166. doi: 10.1016/s1525-0016(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 18.Nishimoto T, Yoshida K, Miura Y, Kobayashi A, Hara H, Ohnami S, Kurisu K, Yoshida T, Aoki K. Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Ther. 2009;16:669–680. doi: 10.1038/gt.2009.1. [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto T, Yamamoto Y, Yoshida K, Goto N, Ohnami S, Aoki K. Development of peritoneal tumor-targeting vector by in vivo screening with a random peptide-displaying adenovirus library. PLoS One. 2012;7:e45550. doi: 10.1371/journal.pone.0045550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Seggern DJ, Huang S, Fleck SK, Stevenson SC, Nemerow GR. Adenovirus vector pseudotyping in fiber-expressing cell lines: improved transduction of Epstein-Barr virus-transformed B cells. J Virol. 2000;74:354–362. doi: 10.1128/jvi.74.1.354-362.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura Y, Yamasaki S, Davydova J, Brown E, Aoki K, Vickers S, Yamamoto M. Infectivity-selective oncolytic adenovirus developed by high-throughput screening of adenovirus-formatted library. Mol Ther. 2013;21:139–148. doi: 10.1038/mt.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci U S A. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legrand V, Spehner D, Schlesinger Y, Settelen N, Pavirani A, Mehtali M. Fiberless recombinant adenoviruses: virus maturation and infectivity in the absence of fiber. J Virol. 1999;73:907–919. doi: 10.1128/jvi.73.2.907-919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]