Abstract
Background
The nitroxyl (HNO) donor, Angeli’s salt (AS), exerts positive inotropic, lusitropic, and vasodilator effects in vivo that are cyclic AMP-independent. Its clinical utility is limited by chemical instability and co-generation of nitrite that itself has vascular effects. Here we report on effects of a novel, stable, pure HNO donor (CXl-1020) in isolated myoctyes, and intact hearts in experimental models and in patients with heart failure (HF).
Methods and Results
CXL-1020 converts solely to HNO and inactive CXL-1051 with a t1/2 of 2 minutes. In adult mouse ventricular-myocytes, it dose-dependently increased sarcomere shortening by 75–210% (50–500 µM), with a ~30% rise in the peak Ca2+ transient only at higher doses. Neither protein-kinase-A or soluble guanylate-cyclase inhibition altered this contractile response. Unlike isoproterenol, CXL-1020 was equally effective in myocytes from normal or failing hearts. In anesthetized dogs with coronary microembolization-induced HF, CXL-1020 reduced LV end-diastolic pressure and myocardial oxygen-consumption while increasing ejection fraction from 27 to 40% and maximal ventricular power index by 42% (both p<0.05). In conscious dogs with tachypacing-induced HF, CXL-1020 increased contractility assessed by end-systolic elastance, and provided veno-arterial dilation. Heart rate was minimally altered. In patients with systolic HF, CXL-1020 reduced both left and right heart filling pressures and systemic vascular resistance, while increasing cardiac and stroke volume index. Heart rate was unchanged, and arterial pressure declined modestly.
Conclusions
These data show the functional efficacy of a novel pure HNO donor to enhance myocardial function, and show first-in-man evidence for potential utility in heart failure.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifiers: NCT01096043, NCT01092325.
Keywords: nitroxyl, cardiomyopathy, contractility, myocyte, pharmacology, human, canine
Patients with acute decompensated heart failure (ADHF) present a complex and often life threatening clinical syndrome. New therapeutic advances remain scant and patients are at major risk for recurrent hospitalizations and suffer a high mortality rate1–3. The initial thrust of therapy focuses on decongestion and hemodynamic stabilization, with removal of excess fluid by diuresis or ultrafiltration4, and use of arterial and venous dilators to reduce preload and afterload3, 5. In a substantial number of patients, these approaches prove insufficient or cannot be adequately employed due to renal dysfunction and hypotension. In such individuals, inotropes are often considered6 though this avenue has been historically limited by difficulties in separating therapeutic benefit from unwanted toxicity. The most commonly used agents are dobutamine or milrinone, but both confer adverse myocardial effects, including tachycardia and arrhythmia linked to cAMP-dependent signaling7, and can worsen long-term outcomes8, 9. Several new strategies are being pursued, including omecamtiv mercarbil, an activator of myosin ATPase10, 11, and istaroxime that is thought to impact calcium handling12. Safe and effective therapies that enhance LV function and also aid in decongestion, remain lacking.
Nitroxyl (HNO) is a reactive-nitrogen species that while related to nitric oxide (NO), displays many unique biochemical and pharmacological features13, 14. HNO improves myocardial function by direct positive cAMP-independent lusitropic and inotropic effects, and by combined venous and arterial dilation15–19. HNO targets selective cysteine residues (negatively charged, or thiolates) resulting in covalent bonding and/or formation of a reversible disulfide. In myocytes, HNO enhances sarcoplasmic reticular calcium uptake and release via cysteine modifications on SERCA2a19, 20, phospholamban21, 22 and the ryanodine receptor19, and also improves myofilament calcium sensitivity15, 23. HNO does not alter L-type calcium channel current nor total SR calcium load16. Unlike its myocyte effects, vasodilation from HNO has been attributed to soluble guanylate cyclase activation24, 25 though other pathways remain possible. Importantly, the effects of HNO on the heart are a) independent of cAMP or cGMP; b) similar in normal and failing myocardium; c) minimally impacted by β-adrenergic receptor blockade; and d) are additive with agents stimulating cAMP/PKA pathways (e.g. beta-receptor agonists), unlike NO 17.
The compendium of pharmacological effects of HNO donors has suggested potential for treating both congestion and hemodynamic insufficiency in ADHF. However, major limitations in available HNO donors have impeded progress in the field. Virtually all prior studies have utilized the inorganic compound Angeli’s salt (AS, Na2N2O3)26 that is chemically unstable and thus unsuitable for clinical use. AS also co-generates nitrite which itself has potent vascular effects27. To circumvent these limitations, we developed a novel Piloty’s acid cogener, CXL-1020, which non-enzymatically decomposes to produce pure HNO and an inactive organic by-product (CXL-1051). We tested the impact of CXL-1020 on isolated myocyte function and calcium transients, determined its dose-dependent efficacy in vivo in two canine models of cardiac failure, and performed the first clinical study of an HNO donor testing proof-of-concept for patients with decompensated HF. The results support the potential utility of HNO donors as a novel HF treatment.
Methods
Pharmacology of CLX-1020
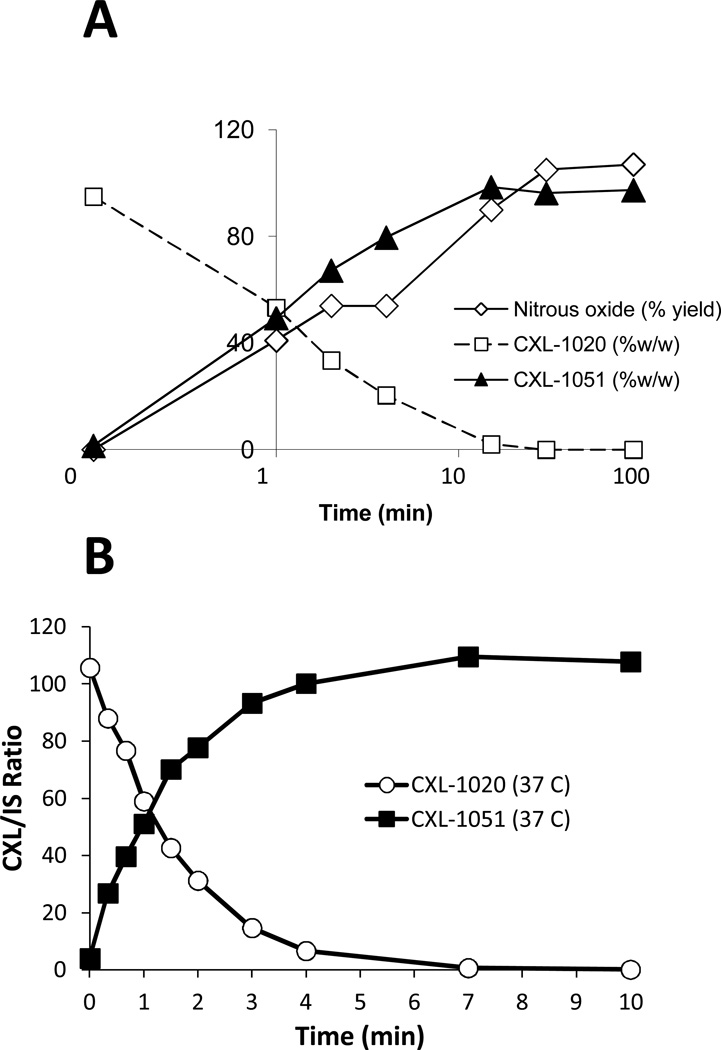
CXL-1020 (Cardioxyl Pharmaceuticals, NC) was synthesized as a pure HNO donor that chemically decomposes to HNO and an organic byproduct (CXL-1051). CXL-1051 has no cardiovascular pharmacological activity, and is not metabolized in vivo but rather excreted unchanged in the urine. In PBS buffer, the decay half-times of CXL-1020 and generation of HNO and CXL-1051 measured by reverse phase HPLC were 1.9, 1.5, and 2.1 minutes, respectively (Figure 1A, conditions for HPLC analysis provided in Supplemental Table 1, quantitation shown in Supplemental Figure 1). At high concentrations, HNO rapidly dimerizes in aqueous solution to HON-NOH which decomposes to nitrous oxide (N2O) and water. Thus, in the test tube, HNO generation is measurable by quantifying N2O by gas chromatography headspace analysis. The disappearance of CXL-1020 and appearance of N2O and CXL-1051 were highly correlated (Figure 1A), and importantly 100% degradation of CXL-1020 yielded 100% appearance of N2O and CXL-1051, confirming CXL-1020 did not generate other NO-species such as NO or nitrite. Quantitative conversion of CXL-1020 to CXL-1051 was also documented in EDTA-treated whole human blood (Figure 1B), with t1/2 for loss of CXL-1020 and formation of CXL-1051 being 2 min. CXL-1020 is stable (>95%) in aqueous solution at pH < 4.5 for at least 24 hrs, and soluble to ~1 mg/mL in H2O for injection, 5% dextrose, and 0.9% saline, and 100 µM Citrate pH 4.0. Higher concentrations (up to 30 mg/mL) were achieved by formulation with a β-cyclodextran.
Figure 1. Pharmacological decomposition of CXL-1020.
A) Decomposition of CXL-1020 in aqueous solution into HNO (measured by nitrous oxide) and CXL-1051. Conversion is rapid, and virtually complete by 15 minutes, with stoichiometry confirming pure generation of HNO and CLX-1051 in equal parts. B) Decomposition of CXL-1020 in human whole blood shows similar rapid pharmacokinetics. IS: internal standard, see supplemental methods.
In vitro myocyte studies
Adult left ventricular cardiomyocytes were isolated from male 3–6 month old C57Bl/6 mice (Jackson Laboratory, ME) with either normal or failing hearts (latter induced by 9-wks transverse aortic constriction)28. Details are provided in supplemental methods. Cells were studied at room temperature, superfused in Tyrode's solution, and stimulated at 0.5 Hz. Sarcomere shortening and twitch kinetics were measured by inverted fluorescence microscopy (Ellipse TE2000, Nikon, Inc) using Fourier-image analysis (MyoCam, IonOptix, MA). Cells were pre-incubated with Fura-2/AM (Molecular Probes, 3 μM for 10 min, de-esterification 20 minutes) to measure whole cell Ca2+ transients. Cells were then exposed to CXL-1020 (50–500 µM), prepared from a 100 mM stock solution in 100% DMSO (final concentration of DMSO of 0.05–0.5%).
In-vivo canine studies
Two canine models of cardiac failure were studied. All studies followed procedures approved by the respective institutional Animal Care and Use Committee of the Johns Hopkins Medical Institutions, or Henry Ford Hospital.
Group A: dogs had ischemic cardiomyopathy generated by serial coronary microembolization29 (supplemental methods). An initial dose-finding study (n=3, CXL-1020 3–100 µg/kg/min × 40 min) identified two doses (n=6, 3 or 10 µg/kg/min × 4 hours) for subsequent hemodynamic analysis. CXL-1020 was mixed with 7% Captisol® in sterile water at pH=4 (vehicle), the latter then used as vehicle control. Data were obtained under general anesthesia induced by i.v. hydromorphone (0.22 mg/kg) and diazepam (0.17 mg/kg), and maintained with 1–2% isofluorane. Cardiac function was assessed by micromanometer arterial and left ventricular pressures, right heart catheterization pressures, contrast ventriculography (LV volumes and echoDoppler cardiography, as previously reported29. LV peak power index (PmaxI) was estimated by (peak aortic flow velocity × peak systolic pressure)/EDV2 (30). Diastolic function was assessed by deceleration time of mitral inflow velocity (DT) and ratio of early to late filling time integrals (Ei/Ai). Myocardial oxygen consumption was assessed at baseline and after 4 hours as previously described31 (details in supplemental methods). At the highest dose, blood samples were obtained to determine Nt-pro BNP, pro atrial natriuretic peptide (proANP), and troponin-I (TnI) by ELISA assay per manufacture’s instructions (supplemental methods).
A separate group of animals (n=6) were subjected to programmed ventricular stimulation after receiving 5 µg/kg/min CXL-1020 × 40 min or vehicle. Each study was terminated when extra-stimuli provoked sustained monomorphic ventricular tachycardia (VT) for >30 seconds or ventricular fibrillation (VF).
In Group B dogs, heart failure was induced by 3-week tachypacing. Conscious dogs were chronically instrumented to obtain LV pressure-volume relations, including an LV micromanometer (P22; Konigsberg Instruments, Pasadena, CA), right atrial and descending aortic catheters, 3-pairs of orthogonal endocardial sonomicrometers to assess LV volume, and inferior vena caval cuff occluder17. Epicardial pacing leads sutured to the LV free wall were connected to an implanted pacemaker (Spectrax, Medtronics, Minneapolis). Data were recorded in conscious animals both at initial baseline when the heart was normal, and following induction of HF. Pressure-volume relations were obtained and used to assess end-systolic elastance (Ees) and preload-recruitable stroke measures of contractile function, and steady state data used to assess chamber volumes and pressures and isovolumic relaxation time constant32. Baseline data were obtained during vehicle infusion, and then CXL-1020+vehicle was administered at doses ranging 3–100 µg/kg/min. Data were digitally recorded (200 Hz) at each dose after reaching a steady-state response (~10 minutes).
Human Heart Failure Studies
A prior Phase 1–2a pilot study in patients with stable heart failure (Clinicaltrials.gov NCT01092325) identified 4 hour exposure to CXL-1020 at 1–30 µg/kg/min as safe and potentially active.23 The present study (Clinicaltrials.gov NCT010960430) examined the hemodynamic effects and safety of CXL-1020 at doses of 1–20 µg/kg/min in patients hospitalized for hemodynamic assessment of HF prior to transplantation or for treatment of decompensated HF requiring hemodynamic monitoring. IRB approval was obtained at each institution involved with the trial, pursuant to federal guidelines, and all subjects provided informed consent. Measurements were obtained within 72 hours of hospitalization. Inclusion criteria required a mean CI ≤ 2.5L/min and a mean PCWP or PAD > 20 mmHg at baseline, based on 3 consecutive CI and PCWP measurements within 10% agreement and measured in the hour preceding drug administration. Baseline diuretic and oral vasodilator therapy was withheld for at least 3 hours prior to baseline recordings, and no parenteral hemodynamically-active agents were allowed within 12 hours of baseline measurements. Patients with a HR <50 or ≥ 100 BPM, a systolic blood pressure of <100 or >150 mmHg or a diastolic BP of >95 mmHg at baseline prior to randomization were excluded. Also excluded were patients with atrial fibrillation without adequate rate control, or with evidence of clinically significant non-sustained VT (10 beats or at a rate >120 bpm) in the preceding 12 hours. CXL-1020 was administered intravenously using a placebo-controlled (4:1 active-to-placebo randomization ratio) 6 hour forced titration design, with up-titration at 2 hour intervals. An overall dose range of 1 – 20 µg/kg/min was studied using over-lapping dose ranges in 2 cohorts (Cohort 1 = 1, 3 and 10 µg/kg/min; Cohort 2 = 3, 10 and 20 µg/kg/min).
Statistical Analysis
For myocyte studies, data were analyzed using paired responses for a given set of cells that were exposed to a particular dose of test drug. As these differences were often not normally distributed, we tested the null-hypothesis (%change=0) using a Wilcoxan sign-rank test. For intact canine studies Group A: within group comparisons were made using repeated measures analysis of variance (ANOVA) with alpha set at 0.05. If significance was attained, then pairwise comparisons were made using the Student-Newman-Kuels test with p<0.05 considered significant. For Group B: parameters were assessed by repeated measures analysis of covariance, with a Tukey test for multiple comparisons, and normal versus failing data compared by non-parametric (Wilcoxan) test. For clinical studies, change from baseline of a hemodynamic parameter measured at a given time point following drug infusion (or placebo) was determined, and these differences were then compared by t-test to to determine if drug-response differed from placebo (placebo-corrected response). Data are reported as the mean ± SEM.
Results
CXL-1020 improves myocyte systolic and diastolic function
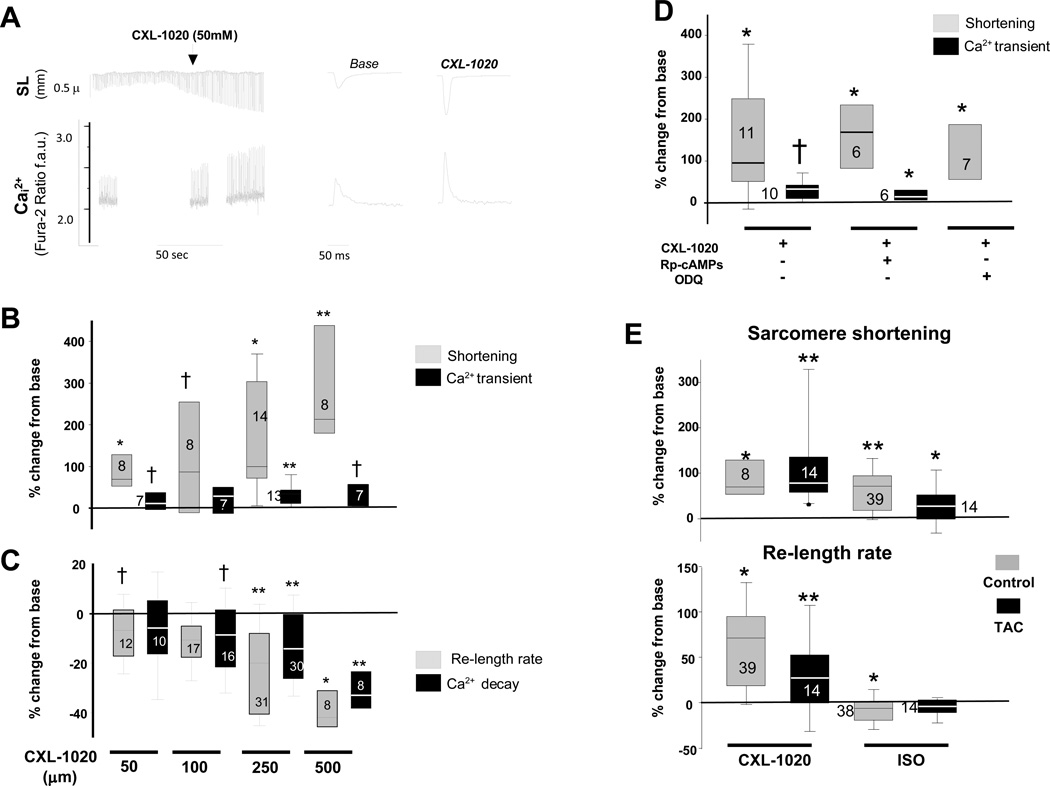
Figure 2A shows example sarcomere length and Ca2+ tracings before and after exposure to CXL-1020 (50 μM). Sarcomere shortening rose substantially in a dose-dependent manner, reaching ~210% over baseline at 500 µM. Peak Ca2+ transients rose more modestly (+30% at the highest dose), displaying little change at lower doses (Figure 2B). Diastolic Ca2+ was little altered (≤3%) at any of the doses (data not shown). Systolic functional changes were accompanied by a faster rate of sarcomere re-lengthening and shortening of the Ca2+ transient decay (Figure 2C). The decomposition product CXL-1051 had no direct impact on the cells (Supplemental Figure 2A).
Figure 2. Influence of CXL-1020 on isolated cardiac myocytes from normal and failing heart.
A) (Left) Isolated myocyte sarcomere length (SL, upper tracing) and calcium transients (Ca2+, lower tracing) after exposure to CLX-1020. There is a marked rise in sarcomere shortening (SS) and a rise in the peak Ca2+ transient, as well as acceleration of the time for re-lengthening and calcium decline. B) Box-plots show percent change in SS and peak Ca2+ transient relative to baseline with incremental CXL-1020 dose. Only one dose was tested per myocyte; the sample size at each dose is provided in the plots. C) Percent reduction in sarcomere relengthening and calcium decay time. D) Percent change in SS and peak Ca2+ transient following CXL-1020 with or without co-inhibition of PKA (Rp-cAMPs) or soluble guanylate cyclase (ODQ). E) Percent change in SS and re-lengthening rate in myocytes isolated from control or failing hearts that are then exposed to either isoproterenol (ISO, 2.5 nM) or CXL-1020 (50 µM). † - p<0.05; * p<0.01; ** p≤0.001 versus respective (pre-CXL1020, or ISO) baseline, by Wilcoxan Sign-rank Test.
To test if functional effects of CXL-1020 required protein kinase A or cGMP dependent signaling, cells were co-incubated with either Rp-cAMPs (PKA inhibitor, 100 µM × 30 min) or 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 µM×30 min) to block soluble guanylate cyclase (Figure 2D). Neither intervention impacted CXL-1020 modulation of sarcomere shortening. Rp-cAMPs or ODQ incubation alone had no effects on basal cell shortening at the concentrations studied (% shortening 3.4±0.3% with Rp-cAMPs, 3.4±0.4% with ODQ, both p>0.22 versus 3.0±0.19% for non –treated cells). As first demonstrated with AS 18, CXL-1020 mediated contractility was also redox sensitive, being suppressed by pre-incubating cells with n-acetyl cysteine (5 mM) (Supplemental Figure 2B).
We next tested whether CXL-1020 influences myocyte contractility in cells from failing hearts. In contrast to the blunted response to the beta-adrenergic agonist isoproterenol (2.5 nM) in failing cells, CXL-1020 induced changes similar to those in normal cells (Figure 2E). In controls, whole cell Ca2+ transients rose (+21±9% and +21±5%) and declined faster (−13±5% and −11±2%) similarly with stimulation by ISO or CXL-1020 (each p<0.05 vs baseline). However, in failing cells, the ISO Ca2+ response was 1/3rd that with CXL-1020, and while the decay rate of the Ca2+-transient was unaltered by ISO it shortened −15±5% with CXL-1020. These data support independence of CXL-1020 effects from cAMP/PKA-dependent inotropy or lusitropy that are blunted in failing myocytes.
In vivo effects of CXL-1020 in canines with ischemic cardiomyopathy (Group A)
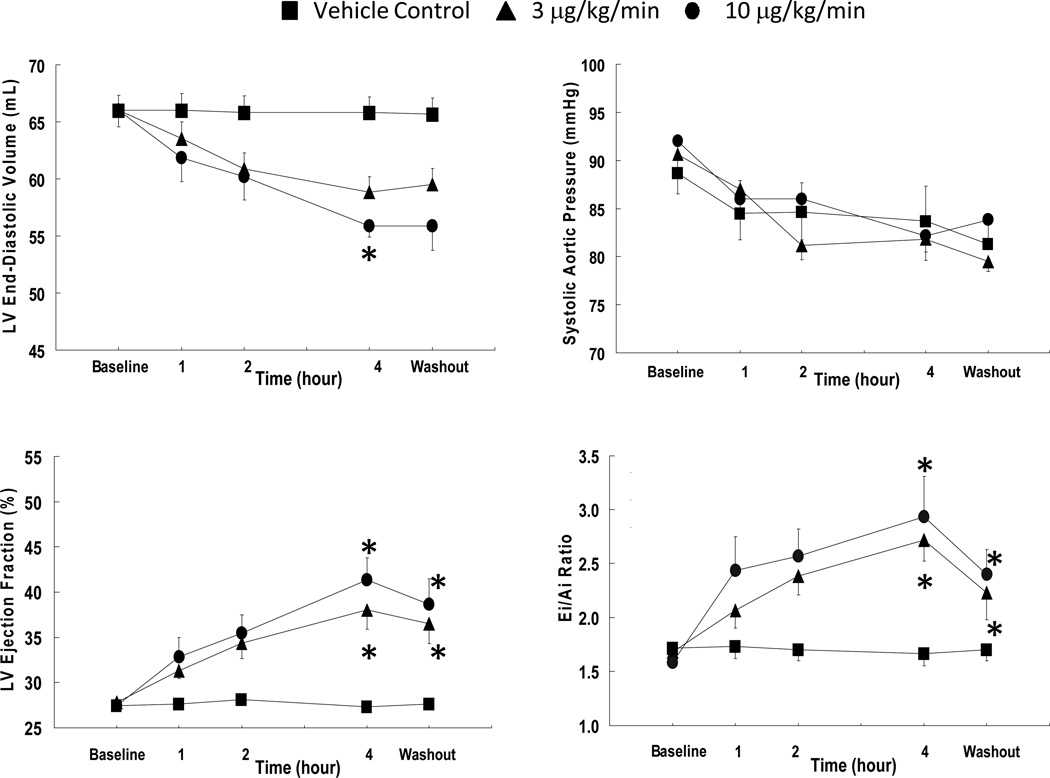
We next assessed integrative cardiovascular effects of a 4-hour CXL-1020 infusion at 3 or 10 µg/kg/min (doses derived from preliminary dose-ranging study). Compared with vehicle control, LV end-diastolic volumes declined by ~15% and ejection fraction rose from 27 to 40% (p<0.05) at the higher CXL-1020 dose (Figure 3A). Arterial blood pressures declined slightly over the course of the procedure. The fell 8–9 mmHg more (diastolic and mean) at 3 µg/kg/min but were unaltered from control at higher doses (Table). End-systolic volume, end-diastolic pressure, and systemic vascular resistance, all declined. Heart rate was unchanged, and systolic contractility indexed by maximal power index30 rose 42±2% (p<0.001) (Table). Early diastolic function reflected by Ei/Ai filling ratio (Figure 3A) and E-wave deceleration time improved, and CXL-1020 also reduced plasma NT-pro BNP and pro-ANP (Table). Plasma troponin I was unchanged.
Figure 3. Influence of CXL-1020 in anesthetized dogs over 4-hour infusion.
A) Left ventricular and systemic hemodynamics at either 3 or 10 µg/kg/min CXL-1020, with 1 hour of washout (n=6). Ei/Ai= ratio of early to late (atrial) mitral inflow. * P<0.05 versus vehicle control by multiple comparisons test (Student-Newman-Keuls) following repeated measures ANOVA.
Table.
Hemodynamic variables and plasma biomarkers during 4-hr infusion of CXL-1020 in dogs with ischemic cardiomyopathy
| Vehicle Control (n=6) | 3 µg/kg/min | 10 µg/kg/min | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 hours | Washout | Baseline | 4 hours | Washout | Baseline | 4 hours | Washout | |
| Heart Rate (bpm) | 75±4 | 69±1 | 71±3 | 74±2 | 75±5 | 65±1 | 74±1 | 77±2 | 75±5 |
| Systolic AoP (mmHg) | 89±3 | 84±4* | 81±2 | 91±2 | 82±1 | 80±1 | 92±6 | 82±3 | 84±2 |
| Diastolic AoP (mmHg) | 62±3 | 55±3 | 57±4 | 64±2 | 55±2* | 53±1* | 64±4 | 50±1* | 53±1 |
| Mean AoP (mmHg) | 75±3 | 71±4 | 68±3 | 76±2 | 68±2* | 66±1* | 77±5 | 63±2* | 65±2* |
| LVEDP (mmHg) | 13.7±0.8 | 15.3±0.7 | 12.3±1.0 | 14.5±0.7 | 13.3±0.3 | 13.8±0.7 | 13.8±0.3 | 10.8±0.9* | 11.7±1.5 |
| LVESV (mL) | 47.8±1.0 | 47.8±1.0 | 47.7±1.1 | 47.7±1.1 | 36.5±2.1* | 37.8±2.4* | 48.0±1.2 | 32.8±1.9* | 34.3±2.5* |
| LV EDV (ml) | 66.0±1.3 | 65.8.0±1.3 | 65.7±1.4 | 66.0±1.3 | 58.8±2.1* | 59.5±2.2* | 66.0±1.5 | 55.8±0.9* | 55.8±2.1* |
| LV FAS (%) | 25.0±0.4 | 25.3±0.7 | 25.2±0.7 | 25.5±0.3 | 35.8±2.1* | 33.5±2.3* | 24.8±0.3 | 38.8±2.2* | 35.3±2.8* |
| Stroke Volume (mL) | 18.2±0.3 | 18.0±0.4 | 18.0±0.4 | 18.3±0.3 | 22.3±1.1* | 21.7±1.0* | 18.0±0.4 | 23.0±1.3* | 21.5±1.5* |
| SVR (dynes.sec.cm5) | 4476±328 | 4640±405 | 4283±200 | 4502±142 | 3335±247* | 3793±234* | 4642±236 | 2904±138* | 3342±311* |
| Deceleration Time (msec) | 93.2±2.5 | 96.3±3.0 | 96.7±3.6 | 94.8±3.8 | 119.8±5.3* | 103.3±2.3* | 97.3±4.0 | 128.0±7.5* | 121.3±6.9* |
| Peak Power Index (mmHg/sec*mL)*100 | 184±12 | 171±10 | 169±9 | 192±11 | 245±19* | 212±14 | 180±14 | 262±16* | 250±20* |
| MVO2 (µmol/min) | --- | --- | --- | --- | --- | --- | 102.5±6.3 | 71.5±14.5* | --- |
| nt-proBNP (fmols/mL) | 165±12 | 164±13 | --- | 164±15 | 133±11* | --- | 166±13 | 93±16* | --- |
| pro-ANP (pmols/mL) | 0.56±0.06 | 0.57±0.06 | --- | 0.57±0.07 | 0.45±0.05* | --- | 0.57±0.06 | 0.31±0.05* | --- |
| TnI (ng/mL) | 0.53±0.03 | 0.56±0.03 | --- | 0.56±0.03 | 0.36±0.03* | --- | 0.58±0.02 | 0.28±0.2† | --- |
AoP=aortic pressure; LV=left ventricular; EDP=end-diastolic pressure; ESV=end-systolic volume; EDV=end-diastolic volume; FAS=fractional area of shortening; SVR=systemic vascular resistance,. MVO2=myocardial oxygen consumption. nt-pro BNP=n-terminal pro-brain natriuretic peptide; pro ANP=pro atrial natriuretic peptide, TnI – troponin I.
p<0.05 versus Baseline
In vivo effects of CXL-1020 in conscious canines using PV loop analysis (Group B)
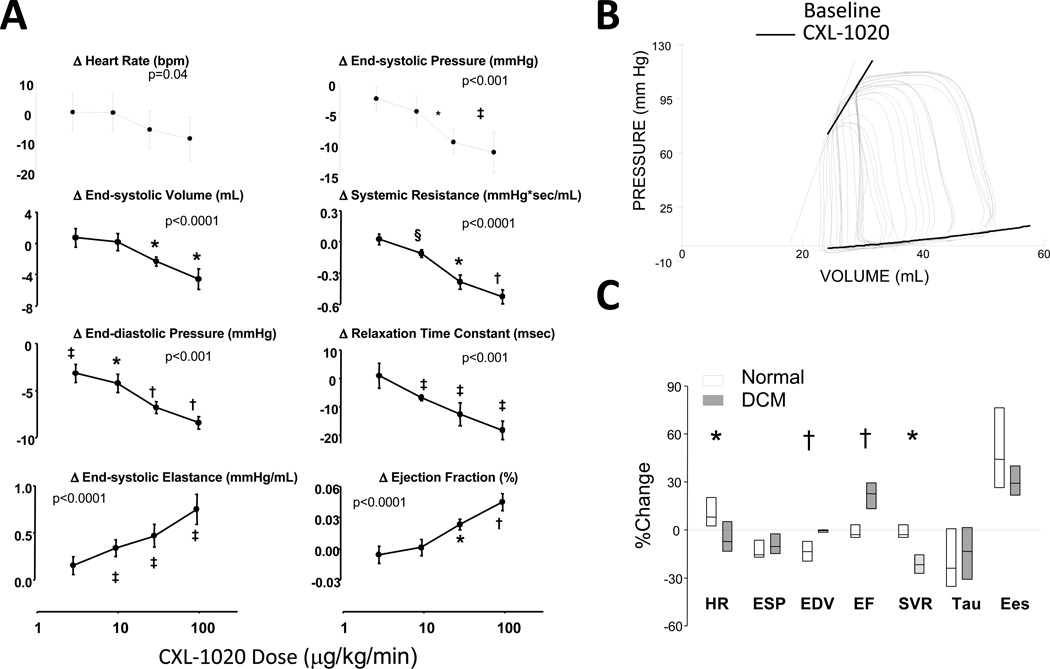
To more directly test if CXL-1020 enhanced in vivo contractility, pressure-volume analysis was performed in conscious dogs before and after the induction of heart failure due to tachypacing. HF was more severe in Group B than Group A animals (Supplemental Table 2). In failing hearts, CXL-1020 (3–100 µg/kg/min) lowered end-systolic pressure and volume, and end-diastolic pressure (declined nearly 30%) in a dose-dependent manner, whereas HR was little altered (Figure 4A). Systemic vascular resistance and time-constant of relaxation both declined whereas contractile function indexed by load-insensitive end-systolic elastance (Ees) increased. Figure 4B displays example PV relations before and after CXL-1020, showing a rise in Ees.
Figure 4. Influence of CXL-1020 in conscious heart failure dogs.
A) Absolute change in hemodynamic parameters in Group 2 (conscious) heart failure dogs as a function of increasing CXL-1020 dose. P-values in each plot are for a repeated measures analysis of co-variance (with drug dose as the continuous variable, n=5). Post-hoc multiple comparisons test for dose response versus baseline: * - p<0.005; † - p<0.001; ‡ - p≤0.01; § - p=0.02. B) Example pressure volume loops at baseline and after CXL-1020 infusion, showing an increase in the slope of the end-systolic pressure volume relationship (solid line is control, dashed after CXL-1020). C) Box-plot for percent change in hemodynamic parameters before and after 100 mg/kg/min CXL-1020 in normal dogs and the same dogs after inducing heart failure. * - p<0.05, † p<0.01 between groups (n=5 per group, Kruskal-Wallis used to test for effect of heart failure on the response).
Many of these responses were also observed when CXL-1020 was administered to the dogs in the control state prior to inducing HF (Supplemental Table 3), however there were some differences. At the maximal CXL-1020 dose, there was a greater percent decline in preload volume (EDV) yet less reduction of systemic vascular resistance in the normal versus HF state (Figure 4C), with ejection fraction consequently rising more in dogs with HF. A similar disparity has been observed with AS17, 18. Heart rate tended to rise in controls likely reflecting a baro-reflex response, but declined slightly in HF dogs. The percent change in end-systolic elastance was similar in both conditions.
Electrophysiology and CXL-1020
Neither canine model revealed electrophysiological instability in association with CXL-1020 infusion. There were no changes in QTc interval (Supplemental Figure 3). In animals subjected to programmed ventricular stimulation, CXL-1020 did not alter the threshold for inducing ventricular tachycardia or fibrillation, nor impede cardioversion when either were induced (Supplemental Table 4).
Hemodynamic effects of CXL-1020 in patients with decompensated heart failure
The demographics, co-morbidities, and medications of the 31 study patients are provided in Supplemental Table 5. Subject age, gender, HF etiology, and function class were similar between the treatment groups. Baseline hemodynamics (Supplemental Table 6) demonstrated severe systolic heart failure with a depressed cardiac index (1.9–2.2 L/m/m2), and elevated right and left heart filling pressures (15–17, 25–30 mmHg, respectively). CXL-1020 was infused at rates of 1–20 µg/kg/min in a placebo-controlled, forced titration protocol in which 2 patient groups received overlapping dose ranges (low dose group (N=12) = 1, 3 and 10 µg/kg/min; high dose group (N=12) = 3, 10 and 20 µg/kg/min) and matching placebo (N=7).
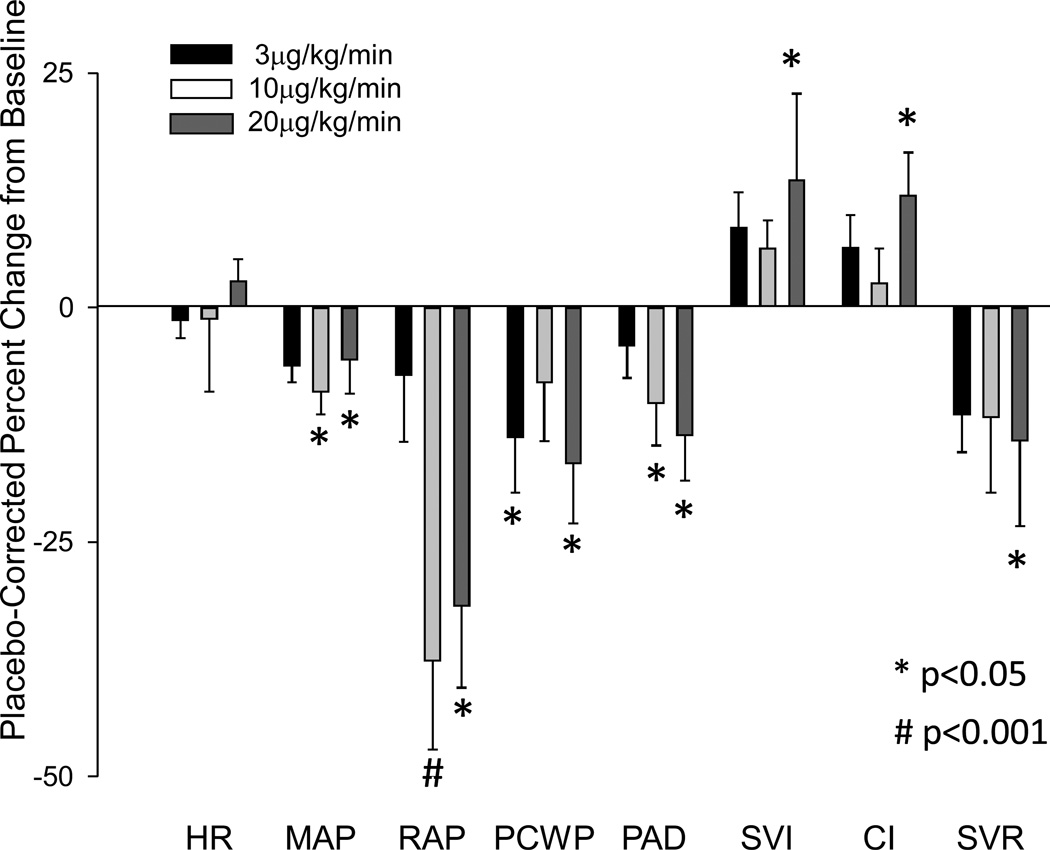
Heart rate was unchanged at all doses of CXL-1020, and there were no statistically significant changes in any hemodynamic measure at 1 or 3 µg/kg/min. Hemodynamic effects of CXL-1020 infusion at 10 and 20 µg/kg/min are shown in Figure 5. At 10 µg/kg/min, pulmonary artery diastolic and mean arterial pressure declined. At 20 µg/kg/min, PCWP also declined, accompanied by increased cardiac index and stroke volume index, whereas the mean arterial pressure change was insignificant. Systemic vascular resistance (SVR) tended to fall modestly at both 10 and 20 µg/kg/min doses, reaching significance at the higher dose, while right atrial pressure (RAP) fell significantly at both doses.
Figure 5. Hemodynamic effects of CXL-1020 in patients with symptomatic heart failure.
Effects of CXL-1020 at 3, 10 or 20 ug/kg/min on heart rate (HR), mean arterial pressure (MAP), right atrial pressure (RAP), pulmonary capillary wedge pressure (PCWP), stroke volume index (SVI), cardiac index (CI) and systemic vascular resistance (SVR) in 12 patients with symptomatic heart failure ( ‘high dose’ titration group described in Methods). Data show the mean percent change ±SEM at each dose minus theresponse observed in the placebo group at the same time point (e.g. i.e. corrected to placebo). * p<0.05 versus baseline, # - p<0.005 versus baseline.
CXL-1020 was also evaluated in several echocardiography cohorts (online supplement) at doses up to 20µg/kg/min. After 6 hours of infusion, MAP was unchanged at any dose, however at the highest dose, HR declined from baseline relative to placebo (p<0.01). There were non-significant trends for decreases in LV end-diastolic and end-systolic volumes, an increase in ejection fraction, and increase in stroke volume (Supplemental Figure 4).
Safety and tolerability of CXL-1020 in patients with heart failure
CXL-1020 was well tolerated with few apparent side effects. Both Adverse and Significant Adverse Events for the study are provided in Supplemental Table 7. Drug treatment was not terminated for adverse experiences in any patient in this study. There were no adverse trends in routine laboratory parameters for hematology, chemistry or urinalysis.
Discussion
We report on a novel pure HNO donor, CXL-1020, and demonstrate direct positive inotropic and lusitropic effects in cardiac myocytes from normal and failing hearts, and positive contractile and lusitropic effects and mild vasodilatory effects in failing canine hearts in vivo. Importantly, we translate these findings for the first time to the clinic, finding CXL-1020 enhances cardiac performance while unloading the left ventricle in patients with decompensated systolic heart failure. CXL-1020 effects were stable over infusion periods ranging from 4–6 hours, and did not alter heart rate or induce arrhythmia. This first cell-to-human evaluation of a pure HNO donor suggests the potential efficacy and utility of this pharmacological approach to improve the function of the failing heart.
Despite being discovered over a century ago26, Angeli’s salt and the chemistry and physiologic role of HNO have only recently received attention. HNO’s chemical cousin, nitric oxide, has been far more studied, and its signaling roles mediated by chemical modification of cysteines (S-nitrosylation)33 and activation of sGC34 is widely appreciated. Nonetheless, the two molecules are chemically and physiologically distinct, and they do not interconvert under normal physiological conditions. While the chemistry of HNO also involves post-translational modifications of selective reduced cysteines (thiolates), those modifications result in either a single modified residue (sulfinamide) or induction of a reversible disulfide between neighboring cysteines13, 21, 23. While NO synthases can produce HNO under conditions of oxidative stress, the endogenous production of HNO remains the subject of considerable speculation and debate. The lack of a bioassay has prevented definitive elucidation of this question. However, there is a growing body of data regarding physiological/pharmacological effects of HNO donors, and this has spawned considerable interest in understanding its biochemistry and potential therapeutic potential.
Paolocci and colleagues originally reported that AS augmented cardiac contractility and relaxation in a cAMP-independent manner18. This study also first suggested a link between HNO and secretion of the neuropeptide calcitonin gene related peptide (CGRP). However, CGRP signaling is coupled to cAMP stimulation, and in subsequent studies, we showed vivo modulation of contractility by CGRP depended on local sympathetic activation rather than a direct effect on cardiomyocytes35. Furthermore, the inotropic response to CGRP was markedly blunted in the failing heart35, consistent with down regulation of sympathetic stimulation responses in this syndrome. Since AS had direct activity on cardiomyocytes and its impact was not blunted in the failing heart17, an alternative to a CGRP mechanism was sought.
More direct mechanistic insights followed the discovery that AS directly improves Ca2+ uptake and release from the SR in a manner independent of cAMP or cGMP generation. This cellular behavior was also very different from that induced by the NO donor, DEA/NO. Importantly, HNO does not alter L-type calcium channel current16 nor augment SR calcium load19, in contrast to agents that generate inotropy by elevating cAMP. Subsequent work revealed a direct impact on enhancing myofilament calcium sensitivity15. More recent studies have begun identifying the molecular targets of HNO that could underlie these myocardial effects. Glutathiolation at cysteine 674 has been proposed to link AS inotropy to enhanced SERCA2a activity, and formation of an internal disulfide in phospholamban by AS was shown to enhance SR-Ca2+ uptake as well21. Recently, Sivakumaran et al.22 showed PLN is required to observe HNO augmentation of both inotropy and Ca2+ transients, and enhance SR Ca2+ uptake and Ca2+-dependent SERCA2a conformational flexibility. This was achieved by stabilizing PLN in an oligomeric disulfide bond-dependent configuration, decreasing the amount of free monomeric (inhibitory) PLN. HNO-induced disulfide links between actin-tropomyosin and myosin heavy chain and myosin light chain have been linked to increased myofilament Ca2+ sensitivity23. Vascular studies have reported vasodilation attributed to activation of soluble guanylate cyclase and/or voltage-gated potassium channels25. However, it remains possible that smooth muscle SR calcium cycling is involved, and the potential role of NO2- co-generated by AS decomposition remains unresolved.
The present study of CXL-1020 addresses a number of prior critical limitations of AS research. First, it isolates the response to HNO alone, excluding potential effects of the nitrite released by AS. Second, it enables studies of sustained exposure (e.g. 4–6 hours), whereas AS instability made such infusion experiments difficult to impossible. The findings with CXL-1020 at both cellular and intact organ levels – in mammalian species ranging from mouse to man – are remarkably compatible with prior data with AS. We observed modest vasodilator effects from HNO that contributed to the integrative functional improvement (e.g. stroke volume, EF) observed. However, using measures that were less load-dependent, such as peak power index or Ees, we showed significant increases in contractile function from CXL-1020. The decline in arterial pressure at high doses indicates a direct vasodilator impact of HNO, though CXL-1020 either did not change heart rate or even resulted in a slight decline at these doses, likely a result of improved contractile performance. As with AS, the onset of hemodynamic effects with CL-1020 were rapid, though recovery was somewhat slower. Lastly, our data show that sustained intravenous administration of a pure HNO donor is not arrhythmogenic. The ischemic HF model (Group A) displays easily inducible malignant ventricular arrhythmias36, and neither rate, rhythm or QT interval were altered by CXL-1020.
Our clinical data for CXL-1020 corroborates the experimental animal data, providing the first-in-man demonstration of the hemodynamic effects of an HNO donor. At the doses and administration studied, CXL-1020 was found to be well tolerated, and a decline in diastolic filling pressures, modest fall in SVR, and rise in cardiac output resulting from increased stroke volume with no change in heart rate, were consistent with inotropic and vasodilator actions observed in dogs, and positive inotropy measured in myocytes.
The clinical study also identified a threshold dose of CXL-1020 for hemodynamic effects at 10–20 µg/kg/min. As this dose range did not induce maximal responses in dogs, higher or more prolonged doses were considered. However, in a subsequent longer duration (12–24 hours, at a dose of 20µg/kg/min) study, CXL-1020 was found to produce an inflammatory irritation at the intravenous insertion site. Based on this, it was not felt a viable candidate for further development as a human therapeutic. However, the results with CXL-1020, AS (and other novel HNO donors) confirm that the hemodynamic effects of HNO are a class phenomenon independent of the donor. Second generation HNO donors have since been developed that abrogate the venous irritation experienced with CXL-1020, while preserving the full spectrum of HNO’s hemodynamic effects in animal models, thus providing a viable option for further investigation of the HNO class.
In conclusion, we show that a novel pure HNO donor enhances cardiac systolic function while reducing arterial and venous tone, and without increasing heart rate. Direct contractile enhancement from CXL-1020 is observed in isolated myocytes and experimental hearts in vivo, and supported by improved systolic function in humans with congestive heart failure. The combination of effects differentiates HNO donors from other classes of inotropes or ino-dilators, and provides a strong rationale for continuing studies to develop donors with optimized pharmacological and clinical efficacy for the treatment of congestive heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
National Heart, Lung, and Blood Institute HL074237-07 (HNS), RO1 HL-091923 (NP), HL089297 and HL077180, and Fondation Leducq (DAK), the Italian Society of Cardiology (SIC) and Servier-International Society for Heart Research-European Section (ISHR-ES) (CGT), and a grant from Cardioxyl Inc. (HNS).
Footnotes
Disclosures
H.N. Sabbah received research grants from and is a consultant to Cardioxyl Pharmaceuticals. W.S. Colucci is a consultant to Cardioxyl Pharmacuticals. D.A. Kass and N Paolocci are co-founders and are consultants to Cardioxyl Pharmacauticals. D. Cowart is an employee and R. Mazhari a prior employee of Cardioxyl Pharmaceuticals. None of the other authors has anything do disclose.
References
- 1.Abraham WT, Fonarow GC, Albert NM, Stough WG, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (optimize-hf) J Am Coll Cardiol. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Oliva F, Mortara A, Cacciatore G, Chinaglia A, Di Lenarda A, Gorini M, Metra M, Senni M, Maggioni AP, Tavazzi L. Acute heart failure patient profiles, management and in-hospital outcome: Results of the italian registry on heart failure outcome. Eur J Heart Fail. 2012;14:1208–1217. doi: 10.1093/eurjhf/hfs117. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg B. Acute decompensated heart failure - treatments and challenges. Circulation Journal. 2012;76:532–543. doi: 10.1253/circj.cj-12-0130. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 5.Majure DT, Teerlink JR. Update on the management of acute decompensated heart failure. Current Treatment Options in Cardiovascular Medicine. 2011;13:570–585. doi: 10.1007/s11936-011-0149-2. [DOI] [PubMed] [Google Scholar]
- 6.Hasenfuss G, Teerlink JR. Cardiac inotropes: Current agents and future directions. Eur Heart J. 2011;32:1838–1845. doi: 10.1093/eurheartj/ehr026. [DOI] [PubMed] [Google Scholar]
- 7.Thackray S, Easthaugh J, Freemantle N, Cleland JG. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure-a meta-regression analysis. Eur.J.Heart Fail. 2002;4:515–529. doi: 10.1016/s1388-9842(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 8.Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O'Connor CM. Heart failure etiology and response to milrinone in decompensated heart failure: Results from the optime-chf study. J Am Coll Cardiol. 2003;41:997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 9.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, Massie BM, O'Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: A randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 10.Cleland JG, Teerlink JR, Senior R, Nifontov EM, Mc Murray JJ, Lang CC, Tsyrlin VA, Greenberg BH, Mayet J, Francis DP, Shaburishvili T, Monaghan M, Saltzberg M, Neyses L, Wasserman SM, Lee JH, Saikali KG, Clarke CP, Goldman JH, Wolff AA, Malik FI. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: A double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 11.Malik FI, Hartman JJ, Elias KA, Morgan BP, Rodriguez H, Brejc K, Anderson RL, Sueoka SH, Lee KH, Finer JT, Sakowicz R, Baliga R, Cox DR, Garard M, Godinez G, Kawas R, Kraynack E, Lenzi D, Lu PP, Muci A, Niu C, Qian X, Pierce DW, Pokrovskii M, Suehiro I, Sylvester S, Tochimoto T, Valdez C, Wang W, Katori T, Kass DA, Shen YT, Vatner SF, Morgans DJ. Cardiac myosin activation: A potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Blair JE, Filippatos GS, Macarie C, Ruzyllo W, Korewicki J, Bubenek-Turconi SI, Ceracchi M, Bianchetti M, Carminati P, Kremastinos D, Valentini G, Sabbah HN. Hemodynamic, echocardiographic, and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: A randomized controlled trial in patients hospitalized with heart failure. J Am Coll Cardiol. 2008;51:2276–2285. doi: 10.1016/j.jacc.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Flores-Santana W, Salmon DJ, Donzelli S, Switzer CH, Basudhar D, Ridnour L, Cheng R, Glynn SA, Paolocci N, Fukuto JM, Miranda KM, Wink DA. The specificity of nitroxyl chemistry is unique among nitrogen oxides in biological systems. Antioxid Redox Signal. 2011;14:1659–1674. doi: 10.1089/ars.2010.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp-Harper BK. Nitroxyl (hno): A novel redox signaling molecule. Antioxid Redox Signal. 2011;14:1609–1613. doi: 10.1089/ars.2011.3937. [DOI] [PubMed] [Google Scholar]
- 15.Dai T, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD. Nitroxyl increases force development in rat cardiac muscle. J Physiol. 2007;580:951–960. doi: 10.1113/jphysiol.2007.129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohr MJ, Kaludercic N, Tocchetti CG, Dong Gao W, Kass DA, Janssen PM, Paolocci N, Ziolo MT. Nitroxyl enhances myocyte ca2+ transients by exclusively targeting sr ca2+-cycling. Frontiers in bioscience (Elite edition) 2010;2:614–626. doi: 10.2741/e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paolocci N, Katori T, Champion HC, St John ME, Miranda KM, Fukuto JM, Wink DA, Kass DA. Positive inotropic and lusitropic effects of hno/no- in failing hearts: Independence from beta-adrenergic signaling. Proc.Natl.Acad.Sci.U.S.A. 2003;100:5537–5542. doi: 10.1073/pnas.0937302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paolocci N, Saavedra WF, Miranda KM, Martignani C, Isoda T, Hare JM, Espey MG, Fukuto JM, Feelisch M, Wink DA, Kass DA. Nitroxyl anion exerts redox-sensitive positive cardiac inotropy in vivo by calcitonin gene-related peptide signaling. Proc.Natl.Acad.Sci.U.S.A. 2001;98:10463–10468. doi: 10.1073/pnas.181191198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tocchetti CG, Wang W, Froehlich JP, Huke S, Aon MA, Wilson GM, Di Benedetto G, O'Rourke B, Gao WD, Wink DA, Toscano JP, Zaccolo M, Bers DM, Valdivia HH, Cheng H, Kass DA, Paolocci N. Nitroxyl improves cellular heart function by directly enhancing cardiac sarcoplasmic reticulum ca2+ cycling. Circ Res. 2007;100:96–104. doi: 10.1161/01.RES.0000253904.53601.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lancel S, Zhang J, Evangelista A, Trucillo MP, Tong X, Siwik DA, Cohen RA, Colucci WS. Nitroxyl activates serca in cardiac myocytes via glutathiolation of cysteine 674. Circ Res. 2009;104:720–723. doi: 10.1161/CIRCRESAHA.108.188441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Froehlich JP, Mahaney JE, Keceli G, Pavlos CM, Goldstein R, Redwood AJ, Sumbilla C, Lee DI, Tocchetti CG, Kass DA, Paolocci N, Toscano JP. Phospholamban thiols play a central role in activation of the cardiac muscle sarcoplasmic reticulum calcium pump by nitroxyl. Biochemistry. 2008;47:13150–13152. doi: 10.1021/bi801925p. [DOI] [PubMed] [Google Scholar]
- 22.Sivakumaran V, Stanley BA, Tocchetti CG, Ballin JD, Caceres V, Zhou L, Keceli G, Rainer PP, Lee DI, Huke S, Ziolo MT, Kranias EG, Toscano JP, Wilson GM, O'Rourke B, Kass DA, Mahaney JE, Paolocci N. Hno enhances serca2a activity and cardiomyocyte function by promoting redox-dependent phospholamban oligomerization. Antioxid Redox Signal. 2013;19:1185–1197. doi: 10.1089/ars.2012.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, Paolocci N. Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res. 2012;111:1002–1011. doi: 10.1161/CIRCRESAHA.112.270827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrews KL, Irvine JC, Tare M, Apostolopoulos J, Favaloro JL, Triggle CR, Kemp-Harper BK. A role for nitroxyl (hno) as an endothelium-derived relaxing and hyperpolarizing factor in resistance arteries. British Journal of Pharmacology. 2009;157:540–550. doi: 10.1111/j.1476-5381.2009.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irvine JC, Favaloro JL, Kemp-Harper BK. No- activates soluble guanylate cyclase and kv channels to vasodilate resistance arteries. Hypertension. 2003;41:1301–1307. doi: 10.1161/01.HYP.0000072010.54901.DE. [DOI] [PubMed] [Google Scholar]
- 26.Paolocci N, Wink DA. The shy angeli and his elusive creature: The HNO route to vasodilation. Am J Physiol Heart Circ Physiol. 2009;296:H1217–H1220. doi: 10.1152/ajpheart.00243.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda KM, Dutton AS, Ridnour LA, Foreman CA, Ford E, Paolocci N, Katori T, Tocchetti CG, Mancardi D, Thomas DD, Espey MG, Houk KN, Fukuto JM, Wink DA. Mechanism of aerobic decomposition of angeli's salt (sodium trioxodinitrate) at physiological ph. Journal of the American Chemical Society. 2005;127:722–731. doi: 10.1021/ja045480z. [DOI] [PubMed] [Google Scholar]
- 28.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, Lazzarino G, Paolocci N, Gabrielson KL, Wang Y, Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J.Clin.Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabbah HN, Stein PD, Kono T, Gheorghiade M, Levine TB, Jafri S, Hawkins ET, Goldstein S. A canine model of chronic heart failure produced by multiple sequential coronary microembolizations. Am.J.Physiol. 1991;260:H1379–H1384. doi: 10.1152/ajpheart.1991.260.4.H1379. [DOI] [PubMed] [Google Scholar]
- 30.Sharir T, Feldman MD, Haber H, Feldman AM, Marmor A, Becker LC, Kass DA. Ventricular systolic assessment in patients with dilated cardiomyopathy by preload-adjusted maximal power. Validation and noninvasive application. Circulation. 1994;89:2045–2053. doi: 10.1161/01.cir.89.5.2045. [DOI] [PubMed] [Google Scholar]
- 31.Chandler MP, Stanley WC, Morita H, Suzuki G, Roth BA, Blackburn B, Wolff A, Sabbah HN. Short-term treatment with ranolazine improves mechanical efficiency in dogs with chronic heart failure. Circ Res. 2002;91:278–280. doi: 10.1161/01.res.0000031151.21145.59. [DOI] [PubMed] [Google Scholar]
- 32.Senzaki H, Kass DA. Analysis of isovolumic relaxation in failing hearts by monoexponential time constants overestimates lusitropic change and load dependence: Mechanisms and advantages of alternative logistic fit. Circ Heart Fail. 2010;3:268–276. doi: 10.1161/CIRCHEARTFAILURE.109.865592. [DOI] [PubMed] [Google Scholar]
- 33.Haldar SM, Stamler JS. S-nitrosylation: Integrator of cardiovascular performance and oxygen delivery. J Clin Invest. 2013;123:101–110. doi: 10.1172/JCI62854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derbyshire ER, Marletta MA. Structure and regulation of soluble guanylate cyclase. Annual review of biochemistry. 2012;81:533–559. doi: 10.1146/annurev-biochem-050410-100030. [DOI] [PubMed] [Google Scholar]
- 35.Katori T, Hoover DB, Ardell JL, Helm RH, Belardi DF, Tocchetti CG, Forfia PR, Kass DA, Paolocci N. Calcitonin gene-related peptide in vivo positive inotropy is attributable to regional sympatho-stimulation and is blunted in congestive heart failure. Circ Res. 2005;96:234–243. doi: 10.1161/01.RES.0000152969.42117.ca. [DOI] [PubMed] [Google Scholar]
- 36.Sabbah HN, Goldberg AD, Schoels W, Kono T, Webb C, Brachmann J, Goldstein S. Spontaneous and inducible ventricular arrhythmias in a canine model of chronic heart failure: Relation to haemodynamics and sympathoadrenergic activation. Eur Heart J. 1992;13:1562–1572. doi: 10.1093/oxfordjournals.eurheartj.a060102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.