Abstract
The association of the amyloid-β (Aβ) peptide with cellular membranes is hypothesized to be the underlying phenomenon of neurotoxicity in Alzheimer’s disease. Misfolding of proteins and peptides, as is the case with Aβ, follows a progression from a monomeric state, through intermediates, ending at long, unbranched amyloid fibers. This tutorial review offers a perspective into the association of toxic Aβ structures with membrane as well as details into membrane-associated mechanisms of toxicity.
1.0 Introduction
Amyloid deposits characterize more than twenty different clinical syndromes, each of which is associated with a distinct amyloid-forming protein. Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and Huntington’s diseases, in addition to localized diseases such as Type II Diabetes, are associated with the aggregation and misfolding of amyloidogenic peptides/proteins. Of these amyloid-related diseases, Alzheimer’s disease (AD) is the most known, affecting 5.2 million people in the United States and more than 24 million people worldwide.1 AD is clinically characterized by the presence of intracellular neurofibrillary tangles and extracellular senile plaques.2 The plaques consist of insoluble amyloid deposits composed primarily of aggregates of amyloid-beta (Aβ) in their fibril form. The Aβ peptide is produced through proteolytic cleavage of the amyloid precursor protein (APP) by the β- and γ-secretases.3 The predominant Aβ species formed are the Aβ1–40 and Aβ1–42 peptides (consisting of 40 and 42 residues, respectively); with the Aβ1–42 variant being an indicator of a progressive AD state.4 The accumulation of Aβ1–40 and Aβ1–42 into long, unbranched fibrils is a hallmark of the disease, as is the loss of neurons due to cell death in parallel with the Aβ aggregation process.2, 5 Amyloid fibrils of Aβ1–40 and Aβ1–42 form a parallel, in-register cross β-sheet structure that binds to fibril-specific dyes such as congo red and thioflavin-T.6 Prior to fibril formation, Aβ forms a myriad of structures in the monomeric and oligomeric states, all of which result in similar fibril structures.3 Neurotoxicity by Aβ is convoluted as it is unknown which misfolded species causes cell death, in addition to the mechanism by which a particular misfolded state causes toxicity. This data has been correlated with brain samples from patients with and without AD, and, to this day, the Aβ fibrils define and confirm the diagnosis of AD patients’ post-mortem.4
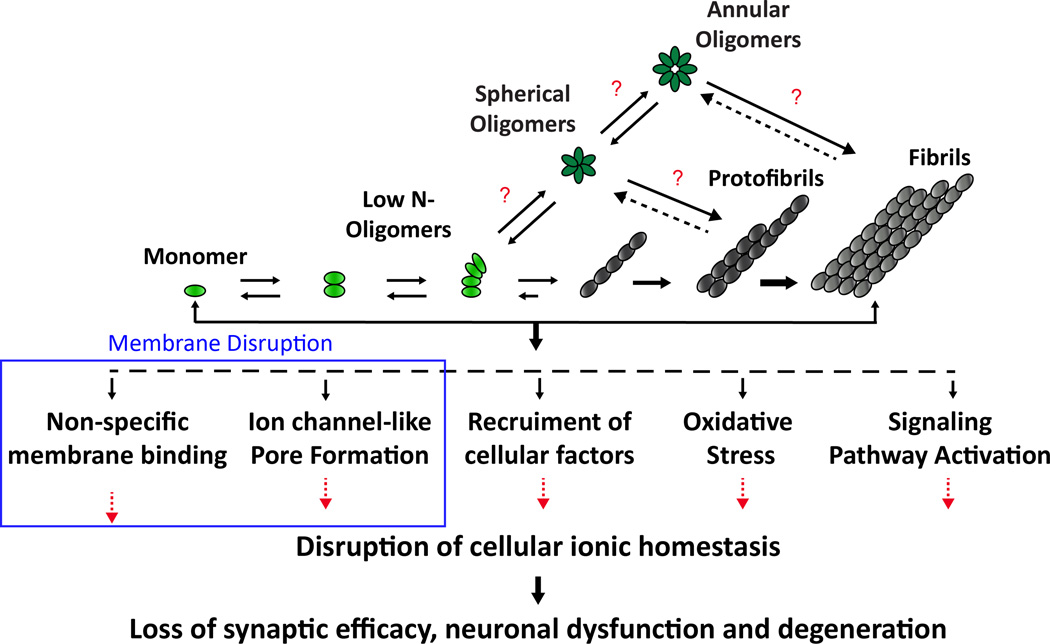
Early AD research pointed to Aβ fibrils specifically as the neurotoxic agent leading to cellular death, memory loss, and other AD characteristics.7 Over the last two decades, further investigation has suggested that the fully-matured fibrils are no longer considered to be the main toxic agent; rather, oligomeric, prefibrillar species of the Aβ peptide have been shown to be most damaging to neuronal cells.3, 5, 8–10 There are several hypotheses regarding the mode of Aβ toxicity and they include the generation of reactive oxygen species, interaction with cell receptors, interactions with metals and direct disruption of cellular membranes (Fig. 1).5 One widely accepted theory, called the amyloid hypothesis, deals with the notion that misfolded intermediate states are responsible for cell death.3 Therefore, it is important to establish the relationship between misfolded entities, their structural properties, and how they confer toxicity.
Fig. 1. The amyloid hypothesis for Alzheimer’s disease.
The aggregation of amyloid-β is highly diverse and poorly understood. Mounting evidence points to oligomers as being the most toxic agent in Alzheimer’s disease; however, intermediate structures are transient and heterogeneous. Additionally, the mechanism by which Aβ can be neurotoxic has not been fully elucidated. One prevailing hypothesis suggests that Aβ can be toxic through a membrane disruption mechanism.
1.1 Structurally dissimilar Aβ oligomers give rise to varied neuronal toxicity
While the toxicity of Aβ is under constant debate, the amyloid hypothesis has been supported by bodies of work suggesting that amyloid oligomers are the toxic amyloid species.11 The toxicity of soluble amyloid oligomers is not only relevant to AD, but to other amyloid diseases as well such as Parkinson’s disease and Type-II Diabetes where α-synuclein and human islet amyloid polypeptide (hIAPP) are indicated as the aggregative proteins, respectively.5, 12 In the case of Aβ, it is known that amyloid oligomers are toxic in vitro,13 with further evidence identifying oligomers as the culprit for in vivo toxicity.14 The problem herein arises due to the fact that oligomers ranging in size and structural morphology have exhibited cytotoxicity.15
A wealth of structural data exists for the relatively benign fibrils of Aβ;6 however, intermediate structures such as structured monomers and oligomers have remained relatively unexplored, largely due to their transient nature. In this regard, the inability to crystallize and/or trap a pure (single state) sample has presented much of the difficulty in performing structural studies of oligomeric species. Relatively low-resolution methods, such as AFM, hydrogen-deuterium exchange mass spectrometry, and circular dichroism (CD) have shown the wide size distribution of oligomers and variance in secondary structure. To further complicate matters, increasing the concentration to levels which are more amenable to atomic-resolution structural experiments can affect the aggregation time, decreasing the longevity of the oligomeric sample. Consequently, the sensitivity of aggregation results from a multitude of factors generating heterogeneous samples and hampering structural studies by techniques like nuclear magnetic resonance (NMR) and X-ray crystallography.
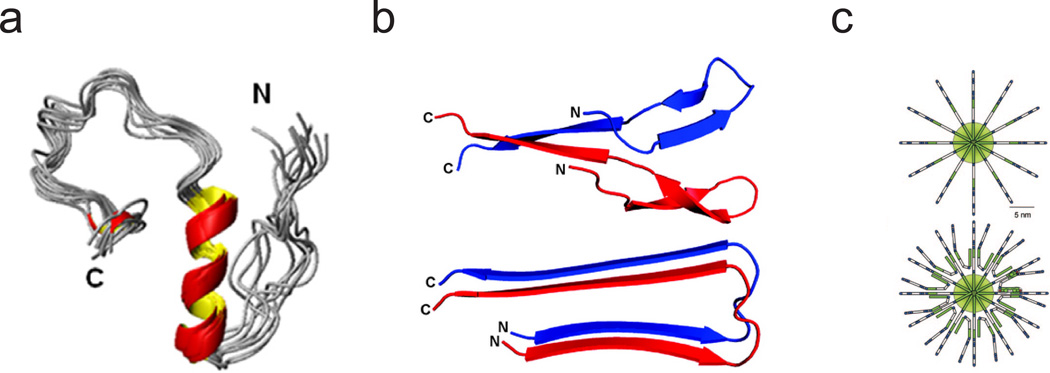
The larger oligomers of Aβ have been mostly described as spherical, although studies have shown that circular, annular oligomers are producible in vitro.16, 17 One interesting aspect of Aβ oligomers and amyloid oligomers in general, is their ability to bind a common antibody regardless of structure. This ability to bind the A11 antibody indicates that amyloid oligomers across diseases and protein sequences share a common structure.13 This common β-sheet-containing structure is fascinating, especially given structural evidence that Aβ1–40 forms a 310 helix in solution (Fig. 2a), meaning a helix-to-β-sheet transition may occur.18 That being said, secondary structure-dependent toxicity has been the source of some debate. While most of the oligomers studied to date have contained β-sheet secondary structure, for relatively small oligomers of Aβ1–42, it was determined that hydrophobic exposure and not the presence of β-sheets are required for toxicity. The study also found that aggregate number is also not necessarily linked to toxicity; two similar sized globular oligomers were characterized and the more-hydrophobic exposed oligomer was toxic.19
Fig. 2. Various structures of Aβ in solution and detergents characterized by NMR.
(a) Aβ1–40 as a partially folded structure in the presence of 50 mM NaCl, with residues 13 to 23 forming a 310 helix.18 (b) Solution NMR structure of 0.05% SDS-stabilized pre-globulomer of Aβ1–42 (top) compared with the basic fold of the fibrils of Aβ1–42 (bottom). Adapted with permission from Yu et al.21 Copyright 2009 American Chemical Society. (c) Structural schematic of β-balls formed at low pH in the absence (top) and presence (bottom) of DSS. These structures both show a pinwheel, or micelle-like, arrangement of monomers. Reprinted with permission from Laurents et al. 2005. Copyright 2005 Journal of Biological Chemistry.20
Some structural studies have utilized detergents or small molecules to stabilize both large20 and small21 oligomeric states with some success while others have utilized the freeze-trapping of purified oligomers.9 Very large oligomers formed at low pH, described as amyloid “β-balls” and having a molecular weight of 764 kDa, have been studied at low-resolution. These very large assemblies can also be stabilized by DSS (4,4-dimethyl-4-silapentane-1-sulfonic acid), and are hypothesized to have a pin-wheel-like structure, with monomers protruding radially outward (Fig. 2c).20 In the case of freeze-trapped large oligomers of Aβ1–40, structural studies by solid state NMR have identified fibril-like contacts.9 These large spherical oligomers contain primarily β-sheet secondary structure and are toxic to cultured neurons. Smaller Aβ1–42 oligomers, thought to be pentamers, show a more loose arrangement with turns and C-termini contained in the center of the oligomer.10 Polymerization of these “disk-shaped” oligomers into fibrils is thought to occur by association and lateral extension of parallel β-strands into fibrils. These pentamers are also toxic to cultured mouse neurons, even in the absence of β-sheet secondary structure. The collective theme associated with the three aforementioned structures is the idea of a pinwheel, or micelle-like, structure which may confirm the notion that oligomers of various size and sequence share a common structural element.
Smaller oligomers, coined as pre-globulomers and globulomers, of Aβ1–42 described by Yu et al show similar molecular contacts to amyloid fibers of Aβ (Fig. 2b).21 The pre-globulomers (16 kDa) and globulomers (64 kDa) were stabilized by 0.05% sodium dodecyl sulphate (SDS) and studied by solution NMR. As with larger freeze trapped oligomers of Aβ1–40, the identification of these fibril-like structures provides a basis for the conversion of oligomers to fibrils, but do not directly explain the ability of Aβ to permeabilize cell membranes.
Overall, high-resolution reports of fibril-like contacts for spherical oligomers of Aβ1–40 have shown parallel β-sheets9 while oligomers of Aβ1–42 demonstrated a lack of β-sheet secondary structure,10 highlighting the important difference observed between the structure and toxicity of these Aβ alloforms. Others have pointed to oligomers containing anti-parallel β-sheets for the formation of pores and thus, the permeabilization of cell membranes.22
Annular oligomers, or annular protofibrils, are 8–20 nm in diameter and contain, as with spherical oligomers, a high β-sheet secondary structure.16 Despite the β-sheet content, circular oligomers are quite different from their spherical counterparts. It has been shown that these annular oligomers may share a common structure with β-barrel pores, such as α-hemolysin, through the binding of an anti-annular protofibril antibody to assembled α-hemolysin.16 Despite having a preformed pore-like structure, these circular oligomers do not permeabilize membranes, but rather convert to spherical oligomers in the presence of lipids. Regardless of their inability to permeabilize membranes, annular protofibrils point to the possibility of a β-barrel pore as the Aβ pore structure.
The relationship between structure and cellular toxicity has been of primary focus in recent amyloid research. Given the number of reportedly different oligomeric Aβ species, relating these parameters could provide intuition into how we might go about preventing unfavorable folds from occurring. Despite the propensity of Aβ to form fibril-like intermediates early and late in its aggregation pathway, existing evidence suggests that the fold of toxic oligomers may differ considerably from that of the Aβ fibril.15, 23 As a result of the variance in secondary structure between different oligomer preparations, Aβ toxicity need not derive from a ‘single’ toxic species. In a recent study, Lashuel and co-workers argued that Aβ toxicity results from an “ongoing polymerization process” by demonstrating that a heterogeneous mixture of monomers and protofibrils were prone to heightened aggregation and, in turn, cytotoxicity.24 Neurotoxicity occurring by such a mechanism would only present greater difficulties in seeking therapeutic measures for alleviating AD symptoms, and attributes greater stress to understanding misfolding pathways as opposed to identifying individual toxic structures.
While it is important to understand the structural link between intermediates and fibrils, their ultimate mode of action should be considered. Therefore, it is of utmost importance to gain structural and functional information on intermediates at their site of action, namely the membrane. Solid state NMR has proven to be an effective tool for the characterization of Aβ oligomer structures in the absence of the membrane. This technique has been a cornerstone of lipid biophysics as well, allowing researchers to effectively determine the integrity and dynamics of the lipid bilayer. With solid state NMR having been recently used to solve the structures and dynamics of several membrane proteins25 and membrane disrupting antimicrobial peptides,26 it stands to reason that this technique will be on the forefront of structural determination of Aβ in the membrane environment.
2.0 The role of membrane composition and disruption in AD pathogenesis
A number of studies have identified various possible mechanisms for cytotoxicity mediated by Aβ, and a prominent focus in AD pathology has centered on Aβ’s ability to disrupt membranes.5 In order to be cytotoxic via membrane disruption, Aβ aggregates must interact with the cellular surface by either a receptor or the lipid membrane. The mechanism of Aβ-membrane interactions has not been fully elucidated; however, its interaction with the membrane is likely to affect the structure and properties of any type of aggregate. Dysregulation of ionic homeostasis, particularly of Ca2+, has been a consistent pathology in AD; thus, understanding the molecular mechanics by which Aβ induces ionic flux has become crucial to AD pathology.27 The cellular membrane in live cells is exceedingly complex, involving many variables that are difficult to isolate and control. Consequently, studying structural transitions of Aβ in the presence of membranes (with varying composition) and the ionic flux across a cellular membrane that results due to specific Aβ-membrane interactions has been extremely challenging.
2.1 Ganglioside-containing membranes influence structural changes during Aβ aggregation
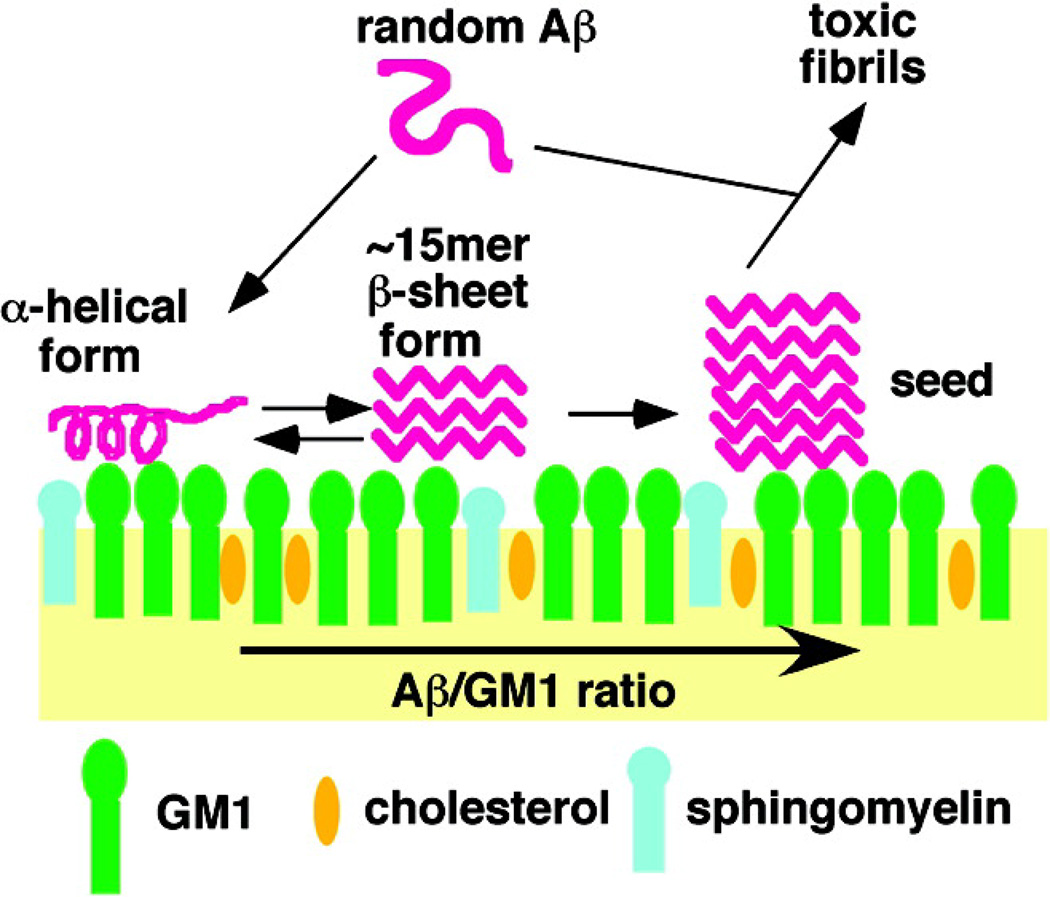
Although obtaining structural models of Aβ oligomers is paramount to understanding the pathology of AD, the presence of membranes has been shown to strongly influence the Aβ aggregation pathway. While a number of studies have reported on the Aβ-membrane interaction, a great body of evidence points to gangliosides playing a significant role in altering the aggregation pathway of Aβ.28, 29 Gangliosides are glycospingolipids that contain a sialic acid headgroup moiety and play a substantial role in cell signaling and memory function. Early work identified Aβ fibrils tightly bound to monosialoganglioside (GM1), generating a conformationally distinct Aβ species.30 Since this work, a series of in vitro and in vivo studies demonstrated GM1 strongly influences the secondary structure of Aβ in the early and late stages of aggregation.31 Initial biophysical characterization demonstrated the pivotal role the sialic acid played in inducing such structural transitions.32 Work along this direction from the Matsuzaki group showed structural changes of Aβ upon interaction with GM1 was dependent upon the Aβ:GM1 ratio—for a low Aβ:GM1 ratio Aβ assumes an α-helix conformation, while at a high Aβ:GM1 ratio fibril formation ensues (Fig. 3).29, 33 A great body of evidence from the Matsuzaki group has demonstrated Aβ amyloidogenicity can be altered in the presence of GM1-containing membranes, with this effect being amplified by introducing Aβ to a lipid raft-like membrane mimetic. Cell viability assays showed that co-incubation of Aβ1–40 with GM1 in a lipid raft-like environment generated toxic amyloid species. Furthermore, concurrent measurements of the accumulation of Aβ and oligomer formation at the cell membrane were visualized with TIRF microscopy, revealing membrane-mediated aggregation affects fibril morphology.34
Fig. 3. Model of GM1 ganglioside-clusters leading to the formation of toxic Aβ species.
Studies have found that low Aβ:GM1 ratios yield an α-helical Aβ structure, while an increasing Aβ:GM1 ratio produces fibrils. Reprinted with permission from Ikeda et al. 2011. Copyright 2011 American Chemical Society.33
The enhanced binding of Aβ to gangliosides is largely dependent upon the sialic acid of the headgroup moiety. Yet, McLaurin and Chakrabartty demonstrated that neither the ceramide nor the sialic acid alone could induce a partial helical structure; rather, the structural transition brought about by gangliosides is dependent upon the association of the sialic acid with the carbohydrate backbone.32 Recently, an NMR study came to a similar conclusion using the paramagnetic relaxation enhancement (PRE) effect to identify Aβ binding to GM1 at the sugar-ceramide junction.35 In a related study, Williamson et al employed the use of chemical shift perturbations of uniformly 15N-labeled Aβ1–40 upon titration of GM1 and asialo-GM1 micelles as monitored by HSQC (heteronuclear single-quantum coherence) and revealed: i) the presence of the sialic acid in the ganglioside headgroup enhanced Aβ-GM1 binding, ii) Aβ binding to GM1 was N-terminally driven, and iii) Aβ association is localized to His13 and Leu17.36 In accordance with these data, a separate NMR study using 1H-15N TROSY and TROSY-based saturation transfer experiments, it was found that Aβ assumes a partial helical structure with an “up and down topological mode” when bound to GM1 micelles.37 A structure was not determined by this study from Utsumi et al; however, it was found through backbone chemical shifts that the regions of Aβ bound to GM1 micelles assumes discontinuous α-helices between residues His14-Val24 and Ile31-Val26 (other regions of the peptide being unstructured).
The studies from Williamson et al and Utsumi et al seem to generate conflicting results of Aβ binding to gangliosides being N- or C-terminally driven. Yet, one can imagine a multi-step process involving initial electrostatic interactions with the N-terminus and hydrophobic interactions driving binding and/or insertion of the C-terminus into the GM1-containing membrane. The early biophysical characterization of the Aβ-GM1 interaction demonstrating the subtle dependence on the peptide:lipid ratio was corroborated by the more recent NMR studies. Collectively, these data provide a better understanding for the production of structurally dissimilar Aβ aggregation states and heterogeneity in the aggregation pathway; the central dogma of the amyloid problem. It is well known that Aβ membrane binding is amplified by the presence of negatively charged lipid headgroups. Nonetheless, answering questions as to what makes for favorable binding to gangliosides and how gangliosides generate toxic amyloid species remain inadequate in the broader scope of AD pathology. While there is still much to be learned about the Aβ-ganglioside interaction, recent progress has been made in understanding how gangliosides might play a role in the mechanism of Aβ toxicity.
2.2 Gangliosides mediate a two-step mechanism of amyloid-β membrane disruption
Studying the interactions between Aβ and cellular membranes has proven to be of great importance, yet more insight must be gained by understanding the mechanism by which these oligomers can be toxic to cells. Aβ generates a multifactorial response in neurons, making the study of Aβ’s cytotoxicity difficult to pinpoint. While the exact mechanism of Aβ neuronal toxicity remains elusive, one of the long-standing pathologies is believed to arise from increased levels of cytosolic Ca2+ resulting from plasma membrane disruption.38 A number of in vitro studies over the last two decades demonstrated that ionic dyshomestasis can be caused by Aβ’s ability to form ion channel-like pores: an annular oligomer structure that allows ions to permeate the membrane through a pore of a hydrophilic interior and hydrophobic exterior.27 The resulting unregulated influx of Ca2+ is a result of the general properties of Aβ pores being cation selective and capable of being blockaded by Zn2+, suggesting these pores adopt a specific structure.38–40 Yet, there is little information on the structure adopted by Aβ upon its insertion to the membrane. Studies have suggested that pore structures contain a β-sheet rich conformation which is consistent with the current available structural data of Aβ; particularly the common U-shaped motif found in amyloid structures.41 The concept of an explicit pore structure is further supported from a study by Capone et al, which demonstrated Aβ ion channels could be modulated by non-natural amino acid substitutions and emphasized the necessity of secondary structure for such channels to form.42 In a related study from Lal and colleagues, Aβ with an all D-amino acid sequence retains strikingly similar features known for the all L-amino acids Aβ, demonstrating that chirality had no bearing on Aβ channel-like activity.43 The results using the all D-amino acids Aβ peptide are quite intriguing; however, in vivo toxicity measurements would provide a more definitive answer as to whether stereospecific, non-stereospecific, or both mechanisms are involved in membrane perturbations. Nevertheless, both neurons and mitochondria are highly sensitive to perturbations in ionic strength, and a small perturbation in intracellular calcium levels caused by unregulated Aβ channel activity can trigger an apoptotic cascade.
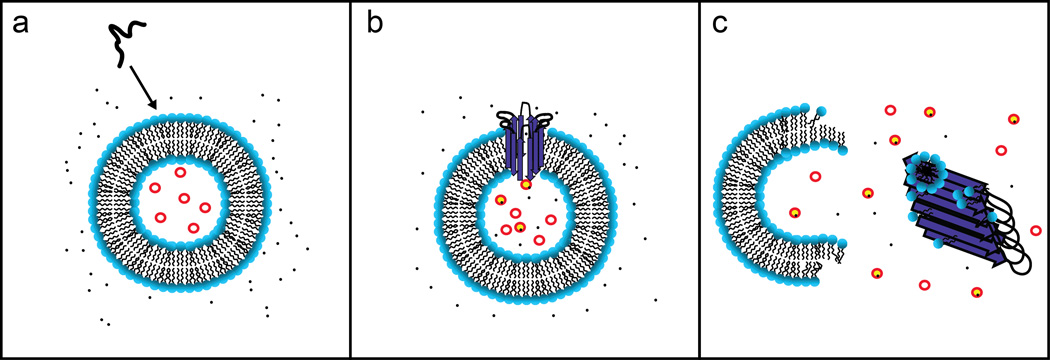
The amyloid channel hypothesis provides much insight into the membrane disruption mechanism by Aβ and other amyloid peptides; however, the mechanism by which Aβ stimulates ionic dyshomeostasis has not been fully elucidated. A multivariate analysis of Aβ cytotoxicity found a negative correlation with both the particular cross-β sheet structure of amyloid fibers as well as the overall β-sheet content, contrary to the predicted models of Aβ pore structures.44 Moreover, spherical aggregates of Aβ have repeatedly shown to exhibit cytotoxicity,45, 46 and it is difficult to fathom how such structural polymorphs could form an Aβ ion channel. Recently, Sciacca et al demonstrated that membrane disruption by Aβ involves a two-step mechanism: (i) Aβ oligomers bind to the membrane to form ion permeable pores and (ii) the process of Aβ fibrillization causes membrane fragmentation via a detergent-like mechanism (Fig. 4).47 The first phase of this proposed two-step mechanism (Fig. 4b) shares many of the qualities observed for the previously described Aβ channel structures; namely, cation selectively and the ability to be blocked by Zn2+. The second step was found to be correlated with the formation of Aβ fibrils as revealed by leakage of the dye 6-carboxyfluorescein from large unilamellar vesicles (LUVs) and time course ThT measurements. Further examination of the fiber-dependent form of membrane disruption by 31P NMR and a lipid sedimentation assay led to the conclusion that this mechanism acted in a detergent-like manner. Unlike the Aβ pores, the second phase of membrane disruption displayed neither charge nor size selectively, lending support to the loss of the integrity of the membrane (Fig. 4c). Interestingly, completion of fibril polymerization halted progression of further membrane fragmentation, and, perhaps more intriguing, this phase of membrane disruption was entirely dependent upon gangliosides being part of the membrane composition. Given the overwhelming evidence for the effect of ganglioside-containing membranes on Aβ aggregation, the finding of a relationship between aggregation and membrane disruption is a step forward in bridging ideals for further elucidation of a mechanism. In a similar train of thought, fiber-dependent membrane disruption can be correlated with the results previously discussed from Lashuel that ongoing polymerization was a key factor in cytotoxicity.
Fig. 4. A two-step mechanism of membrane disruption.
Upon addition of Aβ to a membrane solution (a), it is capable of binding to the membrane and forming ion channel-like pores (b). Appearance of Aβ pores is increased by the presence of gangliosides in the membrane composition. Furthermore, gangliosides mediate a second step of membrane disruption, a fiber-dependent step, which acts via a detergent-like mechanism to fragment the lipid bilayer (c).
It should be noted that both the pore and fiber-dependent mechanisms are not strictly characteristic of Aβ. For example, IAPP displays a similar biphasic effect in membrane disruption, and our group demonstrated that the fiber-dependent membrane permeabilization could be prevented by insulin48 and both mechanisms could be modulated by the presence of PE lipids in the membrane composition.49 Furthermore, α-synuclein has been implicated as a membrane disrupting protein by pore formation and membrane fragmentation, in addition to other amyloidogenic proteins.39, 50 While there is an abundance of existing biophysical and biochemical data to support the amyloid hypothesis for Aβ and other amyloids, much more characterization needs to be done; namely, in the area of the therapeutic prevention of membrane disruption and further elucidation in the structure of misfolded intermediate states.
2.3 Preventing membrane disruption by Aβ
Small molecule compounds blocking Aβ channel formation and other forms of membrane disruption have the potential to alleviate Aβ-induced cytotoxicity. Current amyloid inhibitors target Aβ oligomers and fibrils outside the membrane.51, 52 For Aβ structures formed on and in the membrane this can be problematic as the small molecule EGCG has been shown to be less effective at the membrane surface.53 Given Aβ’s reactivity with the membrane, it is greatly important that small inhibitors such as EGCG have equal or superior efficacy at the peptide-membrane interface. Inhibitors of channel formation would therefore seem to be a very attractive target for stopping Aβ cytotoxicity. Such small molecules designed by Arispe and co-workers protected against the neurotoxic effect of Aβ and blocked ion conductance activity in model membranes.54 Unfortunately, the number of known channel inhibitors of Aβ is currently very limited. Since the Aβ channel is formed by the oligomerization of Aβ, aggregation inhibitors are likely to influence Aβ channel formation. However, many aggregation inhibitors are specific to the fibrillar form of Aβ and therefore may not have activity against the specific oligomerization process that creates Aβ channels. Thus, an attractive approach would be to seek a set(s) of molecules to synergistically prevent all possible modes of membrane disruption by screening known inhibitors of Aβ aggregation as inhibitors of Aβ membrane disruption. If pore and oligomer formation are at least partially independent, as our previous data indicates, the elimination of either process alone will be insufficient to stop Aβ toxicity. However, the addition of both pore blockers and aggregation inhibitors simultaneously may eliminate the cellular dysfunction caused by Aβ. Given the common pathology associated with many amyloidogenic proteins, this avenue of research seems promising in light of the amyloid hypothesis.
3.0 Obtaining high-resolution structures of membrane-bound Aβ species
Due to the inability to crystallize stable and homogeneous Aβ preparations on membranes, the use of computational techniques has become the principal mode to model Aβ structure in membranes.27, 41 Yet, the details of Aβ-membrane interactions and the membrane disruption process are not merely of theoretical importance, but have great practical implications for the prevention of membrane damage by amyloidosis. Membrane damage that is primarily mediated by discrete ion channels can be alleviated by channel blockers designed specifically to plug the channels formed by toxic amyloid species. Such small molecules were designed with the intention of complementary binding to distinct Aβ channel structures, thereby reducing cytotoxic effects and strengthening the amyloid channel hypothesis.54 However, prevention of a non-specific mechanism involving membrane fragmentation requires a different approach aimed at blocking the interaction of protofibrillar Aβ with the membrane. To accomplish this it is necessary to have some understanding of the interactions involved in binding of Aβ to the membrane in fibrillar and prefibrillar conformations and some idea of the conformation of the membrane-bound peptide in a given aggregation state. Unfortunately, while many of the mechanistic details of Aβ amyloid formation in solution remain to be determined, experimental evidence of structural polymorphisms involved in amyloid formation on or in a membrane beyond the resolution of CD, ThT fluorescence, and other biophysical measurements are almost completely unknown.
Currently, the only membrane bound models of Aβ that exist are high-resolution structures of Aβ monomers bound to detergent micelles and computer simulations of channels or oligomers constructed from fragments of the Aβ fiber.27, 41, 55 The computer simulations of Aβ channels have been invaluable in identifying molecular features of potential Aβ pore structures, yet more structural data is necessary to further refine computational models and to collectively understand all forms of membrane disruption. It is apparent that membranes have a two-fold effect on Aβ: (i) membranes can greatly accelerate the rate of fibrillization and (ii) Aβ can directly disrupt plasma and possibly organelle membranes. The cell membrane therefore contributes to Aβ toxicity as both a site for the accumulation/nucleation of toxic oligomers and as a target for their cytotoxic effect. Consequently, it is imperative to better understand how environmental factors within the cell membrane (such as the lipid composition) contribute to structural polymorphisms of Aβ oligomers. Such data will help us understand both how membrane disruption occurs and possibly if certain cell types are more sensitive to Aβ toxicity due to an altered lipid composition, generating a more toxic conformation.
4.0 Conclusions
The dependence on gangliosides in the membrane disruption process corroborates previous results in that they clearly influence an alternative pathway for Aβ aggregation. Aβ polymerization in the presence of gangliosides generates structurally distinct aggregates; however, specific morphological features of these aggregates have yet to be determined. Yanagisawa et al have demonstrated distinct fibril morphology resulting from GM1-bound Aβ. Following studies of Aβ-ganglioside interactions elucidated a specific pathway for the generation of toxic amyloid structures. Whether distinct toxic species are formed as a result of this interaction or ganglioside clusters trigger Aβ membrane disruption remains to be deciphered. The universal characteristics shared by a variety of amyloids and the great body of biophysical evidence in the literature suggests that such data could be useful for understanding the underlying toxicity of Aβ and other amyloids. While there is much work to be done in this regard, a directed focus should be geared towards obtaining high-resolution structural information on membrane-bound Aβ species to advance the understanding of AD pathogenesis, with the ultimate intention of improved and novel therapeutics to alleviate and/or reverse symptoms.
Key Learning Points.
This review seeks to explain/explore:
The amyloid hypothesis and how it relates to the toxicity of Aβ assemblies.
How the toxicity of Aβ is linked to both AD as well as mechanisms of membrane disruption.
The current structural models of amyloid intermediates.
The influence of the cell membrane on the changes of secondary structure along the misfolding pathway of Aβ.
The differences in the prominent mechanisms of membrane disruption: ion channel-like pores and membrane fragmentation.
References
- 1.Alzheimer's Association: Facts & Figures. Alzheimer’s & Dementia. 2013;9 doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Benilova I, Karran E, De Strooper B. Nat Neurosci. 2012;15:349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 4.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterfield SM, Lashuel HA. Angew Chem Int Ed Engl. 2010;49:5628–5654. doi: 10.1002/anie.200906670. [DOI] [PubMed] [Google Scholar]
- 6.Tycko R. Q Rev Biophys. 2006;39:1–55. doi: 10.1017/S0033583506004173. [DOI] [PubMed] [Google Scholar]
- 7.Ross CA, Poirier MA. Nat Med. 2004;10(Suppl):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- 8.Teplow DB. Alzheimers Res Ther. 2013;5:39. doi: 10.1186/alzrt203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimon S, Shaibat MA, Jones CR, Calero DC, Aizezi B, Ishii Y. Nat Struct Mol Biol. 2007;14:1157–1164. doi: 10.1038/nsmb1345. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed M, Davis J, Aucoin D, Sato T, Ahuja S, Aimoto S, Elliott JI, Van Nostrand WE, Smith SO. Nat Struct Mol Biol. 2010;17:561–567. doi: 10.1038/nsmb.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brender JR, Salamekh S, Ramamoorthy A. Acc Chem Res. 2011 doi: 10.1021/ar200189b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, Glabe CG. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 14.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 15.Ono K, Condron MM, Teplow DB. Proc Natl Acad Sci U S A. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayed R, Pensalfini A, Margol L, Sokolov Y, Sarsoza F, Head E, Hall J, Glabe C. J Biol Chem. 2009;284:4230–4237. doi: 10.1074/jbc.M808591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 18.Vivekanandan S, Brender JR, Lee SY, Ramamoorthy A. Biochem Biophys Res Commun. 2011;411:312–316. doi: 10.1016/j.bbrc.2011.06.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladiwala AR, Litt J, Kane RS, Aucoin DS, Smith SO, Ranjan S, Davis J, Van Nostrand WE, Tessier PM. J Biol Chem. 2012;287:24765–24773. doi: 10.1074/jbc.M111.329763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurents DV, Gorman PM, Guo M, Rico M, Chakrabartty A, Bruix M. Journal of Biological Chemistry. 2005;280:3675–3685. doi: 10.1074/jbc.M409507200. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, Hillen H, Barghorn S, Ebert U, Richardson PL, Miesbauer L, Solomon L, Bartley D, Walter K, Johnson RW, Hajduk PJ, Olejniczak ET. Biochemistry. 2009;48:1870–1877. doi: 10.1021/bi802046n. [DOI] [PubMed] [Google Scholar]
- 22.Cerf E, Sarroukh R, Tamamizu-Kato S, Breydo L, Derclaye S, Dufrene YF, Narayanaswami V, Goormaghtigh E, Ruysschaert JM, Raussens V. Biochem J. 2009;421:415–423. doi: 10.1042/BJ20090379. [DOI] [PubMed] [Google Scholar]
- 23.Sarroukh R, Cerf E, Derclaye S, Dufrene YF, Goormaghtigh E, Ruysschaert JM, Raussens V. Cell Mol Life Sci. 2011;68:1429–1438. doi: 10.1007/s00018-010-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jan A, Adolfsson O, Allaman I, Buccarello AL, Magistretti PJ, Pfeifer A, Muhs A, Lashuel HA. J Biol Chem. 2011;286:8585–8596. doi: 10.1074/jbc.M110.172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, Degrado WF, Hong M. Nature. 2010;463:689–692. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramamoorthy A, Xu J. J Phys Chem B. 2013;117:6693–6700. doi: 10.1021/jp4034003. [DOI] [PubMed] [Google Scholar]
- 27.Jang H, Connelly L, Arce FT, Ramachandran S, Lal R, Kagan BL, Nussinov R. Phys Chem Chem Phys. 2013;15:8868–8877. doi: 10.1039/c3cp00017f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaurin J, Chakrabartty A. Eur J Biochem. 1997;245:355–363. doi: 10.1111/j.1432-1033.1997.t01-2-00355.x. [DOI] [PubMed] [Google Scholar]
- 29.Kakio A, Nishimoto S, Yanagisawa K, Kozutsumi Y, Matsuzaki K. Biochemistry. 2002;41:7385–7390. doi: 10.1021/bi0255874. [DOI] [PubMed] [Google Scholar]
- 30.Yanagisawa K, Odaka A, Suzuki N, Ihara Y. Nat Med. 1995;1:1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaki K, Kato K, Yanagisawa K. Biochim Biophys Acta. 2010;1801:868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 32.McLaurin J, Franklin T, Fraser PE, Chakrabartty A. J Biol Chem. 1998;273:4506–4515. doi: 10.1074/jbc.273.8.4506. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda K, Yamaguchi T, Fukunaga S, Hoshino M, Matsuzaki K. Biochemistry. 2011;50:6433–6440. doi: 10.1021/bi200771m. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi M, Matsuzaki K. J Mol Biol. 2007;371:924–933. doi: 10.1016/j.jmb.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Yagi-Utsumi M, Kameda T, Yamaguchi Y, Kato K. FEBS Lett. 2010;584:831–836. doi: 10.1016/j.febslet.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Williamson MP, Suzuki Y, Bourne NT, Asakura T. Biochem J. 2006;397:483–490. doi: 10.1042/BJ20060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utsumi M, Yamaguchi Y, Sasakawa H, Yamamoto N, Yanagisawa K, Kato K. Glycoconj J. 2009;26:999–1006. doi: 10.1007/s10719-008-9216-7. [DOI] [PubMed] [Google Scholar]
- 38.Arispe N, Rojas E, Pollard HB. Proc Natl Acad Sci U S A. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quist A, Doudevski I, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R. Proc Natl Acad Sci U S A. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhee SK, Quist AP, Lal R. J Biol Chem. 1998;273:13379–13382. doi: 10.1074/jbc.273.22.13379. [DOI] [PubMed] [Google Scholar]
- 41.Jang H, Zheng J, Lal R, Nussinov R. Trends Biochem Sci. 2008;33:91–100. doi: 10.1016/j.tibs.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Capone R, Jang H, Kotler SA, Kagan BL, Nussinov R, Lal R. Biochemistry. 2012;51:776–785. doi: 10.1021/bi2017427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capone R, Jang H, Kotler SA, Connelly L, Teran Arce F, Ramachandran S, Kagan BL, Nussinov R, Lal R. J Chem Theory Comput. 2012;8:1143–1152. doi: 10.1021/ct200885r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prangkio P, Yusko EC, Sept D, Yang J, Mayer M. PLoS One. 2012;7:e47261. doi: 10.1371/journal.pone.0047261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solomonov I, Korkotian E, Born B, Feldman Y, Bitler A, Rahimi F, Li H, Bitan G, Sagi I. J Biol Chem. 2012;287:20555–20564. doi: 10.1074/jbc.M112.344036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoshi M, Sato M, Matsumoto S, Noguchi A, Yasutake K, Yoshida N, Sato K. Proc Natl Acad Sci U S A. 2003;100:6370–6375. doi: 10.1073/pnas.1237107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sciacca MF, Kotler SA, Brender JR, Chen J, Lee DK, Ramamoorthy A. Biophys J. 2012;103:702–710. doi: 10.1016/j.bpj.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brender JR, Lee EL, Hartman K, Wong PT, Ramamoorthy A, Steel DG, Gafni A. Biophys J. 2011;100:685–692. doi: 10.1016/j.bpj.2010.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sciacca MF, Brender JR, Lee DK, Ramamoorthy A. Biochemistry. 2012;51:7676–7684. doi: 10.1021/bi3009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sparr E, Engel MF, Sakharov DV, Sprong M, Jacobs J, de Kruijff B, Hoppener JW, Killian JA. FEBS Lett. 2004;577:117–120. doi: 10.1016/j.febslet.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 51.DeToma AS, Salamekh S, Ramamoorthy A, Lim MH. Chem Soc Rev. 2012;41:608–621. doi: 10.1039/c1cs15112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Necula M, Kayed R, Milton S, Glabe CG. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 53.Engel MF, vandenAkker CC, Schleeger M, Velikov KP, Koenderink GH, Bonn M. J Am Chem Soc. 2012;134:14781–14788. doi: 10.1021/ja3031664. [DOI] [PubMed] [Google Scholar]
- 54.Diaz JC, Simakova O, Jacobson KA, Arispe N, Pollard HB. Proc Natl Acad Sci U S A. 2009;106:3348–3353. doi: 10.1073/pnas.0813355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jarvet J, Danielsson J, Damberg P, Oleszczuk M, Graslund A. J Biomol NMR. 2007;39:63–72. doi: 10.1007/s10858-007-9176-4. [DOI] [PubMed] [Google Scholar]