Abstract
Aims
Pudendal afferent fibers can be excited using electrical stimulation to evoke reflex bladder activity. While this approach shows promise for restoring bladder function, stimulation of desired pathways and integration of afferent signals for sensory feedback remains challenging. At sacral dorsal root ganglia (DRG), the convergence of pelvic and pudendal afferent fibers provides a unique location for access to lower urinary tract neurons. Our goal in this study was to demonstrate the potential of microstimulation in sacral DRG for evoking reflex bladder responses.
Methods
Penetrating microelectrode arrays were inserted in the left S1 and S2 DRG of six anesthetized adult male cats. While the bladder volume was held at a level below the leak volume, single and multiple channel stimulation was performed using various stimulation patterns.
Results
Reflex bladder excitation was observed in five cats, for stimulation in either S1 or S2 DRG at 1 Hz and 30–33 Hz with a pulse amplitude of 10–50 µA. Bladder relaxation was observed during a few trials. Adjacent electrodes frequently elicited very different responses.
Conclusions
These results demonstrate the potential of low-current microstimulation to recruit reflexive bladder responses. An approach such as this could be integrated with DRG recordings of bladder afferents to provide a closed-loop bladder neuroprosthesis.
MeSH Keywords: afferent pathways, cats, electric stimulation, pudendal nerve, rehabilitation, spinal ganglia, urinary bladder
1. Introduction
Sensory fibers originating in the lower urinary tract drive spinal reflexes that contribute to the control of bladder continence and micturition (1). Electrical stimulation of the pudendal nerve, which provides afferent innervation to the urethra as well as afferent and efferent innervation to the external urethral sphincter (EUS) and other pelvic structures, can activate many of these spinal reflexes (1,2). However, extraneural stimulation of the pudendal nerve (e.g. with nerve cuff electrodes) typically recruits efferent fibers to the EUS and spinal reflexes with opposing functions. This is an undesirable effect when the stimulation objective is to empty the bladder, which is a frequently investigated yet elusive goal for people with spinal cord injury (2).
Activation of these opposing functions may be avoided if appropriate populations of afferent fibers within individual pudendal nerve branches or fascicles can be selectively targeted. At locations far from the spinal cord, individual branches of the pudendal nerve can be stimulated to activate different reflex functions (3), although the surgical approach is challenging. A recent nerve cuff model suggested that closer to the spinal cord individual pudendal nerve fascicles may be selectively activated with a multiple contact nerve cuff (4). Penetrating microelectrodes inserted into peripheral nerves can access small groups of axons within fascicles (5), yielding higher selectivity. Recently, arrays of penetrating microelectrodes were inserted in canine pudendal nerves to obtain access to afferent pathways for reflex bladder control (6). This work demonstrated the potential of a pudendal nerve stimulator that can target small sets of afferent fibers for reflex control of micturition and continence.
In addition to control of bladder function, an effective neurostimulator will also require sensory information about the state of the bladder. Pudendal nerve afferents only provide indirect information about the state of the bladder (7). Mechanosensitive afferents from the bladder wall travelling in the pelvic nerve provide direct information about bladder activity, but accessing human pelvic nerves is very challenging (2). Pudendal and pelvic nerve fibers converge at spinal nerves that enter the sacral spinal cord and the cell bodies of afferent fibers are packed together in dorsal root ganglia (DRG). DRG are a unique anatomical structure in which access to afferent-only fibers from multiple peripheral nerves is possible (8). Also, due to their proximity to the spinal column, DRG are more isolated from mechanical forces than peripheral nerves.
Recently we demonstrated recordings of individual afferent fibers from the pelvic and pudendal nerves with penetrating microelectrodes in feline sacral DRG (9). We hypothesize that microstimulation of some of these same sacral DRG fibers can lead to selective excitation of multiple afferent pudendal nerve pathways ultimately enabling reflex bladder control. In this pilot study we show that single and multiple channel stimulation can recruit spinal circuits for bladder excitation and relaxation. While we demonstrate that DRG microstimulation may be a potential approach to restoring bladder function, more studies are needed. Ultimately, we envision a closed-loop bladder neuroprosthesis which utilizes selective DRG recording and stimulation for effective control.
2. Methods
Six spinally intact, adult male cats were used in this study. All procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Each animal was initially sedated with ketamine (10–15 mg/kg) before continuous anesthesia with isoflurane (1.5–2.5%) during surgical steps. Subsequent anesthesia maintenance varied. In the first experiment, after completion of all surgical steps, the animal was transitioned to α-chloralose (70 mg/kg initial dose; 20 mg/kg maintenance doses) augmented with low levels of isoflurane (0.1–0.7%). The second animal underwent precollicular decerebration after bilateral ligation of the carotid arteries, was removed from anesthesia and the absence of consciousness was verified. The remaining experiments were conducted solely under α-chloralose after completion of all surgical steps (70 mg/kg initial dose; 20 mg/kg maintenance doses, augmented with buprenorphine 0.01 mg/kg every 12 hr). Although isoflurane suppresses bladder reflexes (10,11), we did not observe a difference in the stimulation-evoked bladder responses between the first or second experiments and the remainder. On average, 31.1 hours elapsed between sedation and the start of testing described here (range 22–40 hours), due to surgical procedures and testing for other experimental objectives. After all testing was completed, as described below, each cat was euthanized with an intravenous dose of potassium chloride (10 mL of 2 mEq/mL) while deeply anesthetized.
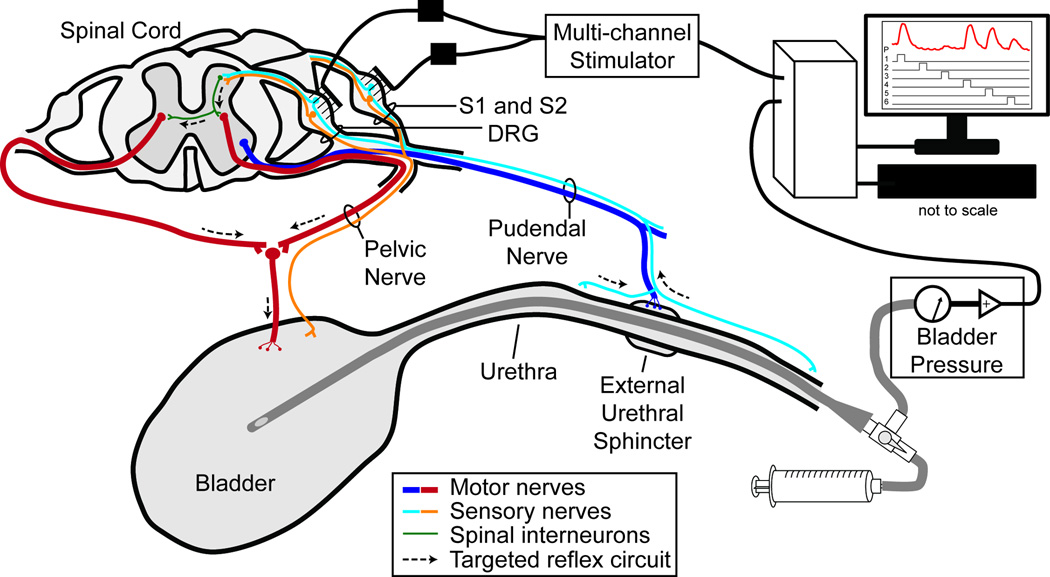
A tracheotomy was performed and the animal was connected to a ventilator. Vitals were monitored and maintained within normal physiological ranges. An intravenous line was inserted in one or both cephalic veins for fluid and drug infusion. A polypropylene catheter (3.5 Fr, Sovereign) was inserted in the urethra to the bladder for controlling the bladder volume and measuring the bladder pressure (Figure 1). A stop cock on the end of the urethral catheter was used to choose between monitoring the pressure or controlling the volume. A disposable pressure transducer (DTX Plus DT-4812, Beckton Dickinson) and transducer amplifier (TA-100, CWE) were used to record the bladder pressure. Bladder volume control was performed with a refillable syringe connected to the other output of the stop cock.
Figure 1.
Experimental setup and simplified lower urinary tract neural innervation diagram. Microelectrode arrays (Blackrock) were inserted in the S1 and S2 DRG. Multiple and single channel cathode-leading, charge-balanced biphasic stimulation pulses were generated by a neural stimulator (TDT). The bladder pressure was monitored with a catheter inserted through the urethra to the bladder and connected to a pressure transducer (Beckton Dickinson). The goal of DRG microstimulation was to target afferent pudendal nerve fibers that contribute to spinal circuits controlling reflex bladder excitation and relaxation.
After exposing the spinal laminae by retracting the paraspinal muscles overlying the lumbosacral vertebrae, a laminectomy was performed to expose the S1 and S2 DRG on the left side. The cat was positioned in a custom-built frame with the torso and pelvis suspended. Penetrating microelectrode arrays (90 channels: 4x10 and 5x10 ICS-96 MultiPort split planar arrays, ~2000 µm2 surface area, 1 mm shaft length, 0.4 mm interelectrode spacing, Blackrock Microsystems) were inserted in the S1 and S2 DRG of each cat with a pneumatic inserter (Blackrock). The wire bundles were secured to the dura with 8-0 silk suture. A bone screw in the iliac crest was used as a stimulus return electrode. The electrode tips were coated with sputtered iridium oxide in the first five cats with average impedances (at 1 kHz) of 46.9 kΩ (5.3 kΩ standard deviation) for the first two and 51.1 kΩ (7.0 kΩ) for the next three cats. In the sixth cat the electrode tips were coated in platinum and had average impedances of 227.3 kΩ (91.2 kΩ).
The bladder pressure was sampled at 100 Hz using a biopotential processor (RZ2, TDT). In the first two experiments, two 16-channel MS16 stimulators were used (Tucker Davis Technologies) and in the last four experiments, a 32-channel IZ2 stimulator (Tucker Davis Technologies) was used. A custom-designed LabVIEW (National Instruments) software interface allowed for control over stimulation parameters and stored the stimulation timings and bladder pressure on a computer for offline analysis.
The bladder was filled (1–2 mL/min) until distension evoked contractions were observed and/or leakage occurred. In the case of leakage, 1–5 mL of saline were removed from the bladder. Electrical stimulation was applied through the microelectrodes using one of three approaches. In multiple channel stimulation, simultaneous stimulation was delivered through 2–32 channels across one or both electrode arrays for 10–60 seconds. In randomized single-channel stimulation, 5 second stimulus trains were delivered through one electrode at a time across 10–32 channels on one or both arrays. There was a 5–10 second delay between stimulus trains. Based on successful responses observed during randomized single-channel stimulation, individual electrode channels were selected for longer-duration stimulus periods (10–60 seconds) in some experiments. Delays between longer duration stimulation trials were at least 30 seconds in length. All stimulation pulses were applied as cathode-leading, constant current, biphasic square pulses. The cathode-leading phase was 200 µs and the anode-trailing phase was 400 µs with half the amplitude of the cathodic phase.
Stimulation frequency was selected within the range of 1–33 Hz, typically either 1 Hz or 30–33 Hz. This range has been shown to include stimulus patterns that excite (12) and inhibit (13) reflex bladder responses. In one experiment, burst-patterned stimuli were used, with six 200-Hz pulses repeated every second as in (12). In the first five experiments, stimulation amplitudes of 5–50 µA were used, which are higher than threshold current levels for activating proprioceptive afferents in lumbar DRG (1–5 µA) (14). In this preliminary work, we did not attempt to find thresholds to evoke responses. In experiment 6, the stimulation amplitude was capped at 10 µA to account for the lower charge injection capabilities of platinum (15).
For each stimulation period, the evoked bladder pressure (PEV) was determined by subtracting the baseline pressure (3-second average before stimulation) from the maximum recorded pressure. Stimulation trials were identified as excitatory if the stimulation-evoked change in bladder pressure was rising or maintained during the stimulation period. A stimulation trial that yielded a transient pressure increase that returned to baseline before the stimulation ceased was not identified as excitatory.
3. Results
Microstimulation in S1 and S2 DRG for reflex bladder control was attempted in six acute cat experiments, designated as 1–6. See Table I for a summary of the number of trials performed per experiment. Stimulation-evoked bladder contractions were observed in five experiments: 1, 2, 4, 5 and 6. Overfilling of the bladder occurred in experiment 3 which we believe led to an unresponsive bladder.
Table I.
Summary of single and multiple channel stimulation trials performed.
| Multiple Channel | Single Channel | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| # Trials |
#Ch. | Excit. Trials |
Inhibit. Trials |
# Trials |
Excitatory Ch. | Inhibit. Ch. |
Max PEV (cm H2O) |
||
| S1 | S2 | ||||||||
| 1 | 17 | 28–32 | 9 (S1, S2, S1+S2) |
0 | 2 | 0/2 | 0/0 | 0 | 16.5 (M) 1.6 (S) |
| 2 | 40 | 2–32 | 29 (S1, S2, S1+S2) |
2(S2) | 58 | 14/26 | 2/32 | 0 | 32.3 (M) 26.7 (S) |
| 3 | 22 | 28–32 | 0 | 0 | 120 | 0/32 | 0/28 | 0 | -- |
| 4 | 6 | 14–16 | 0 | 0 | 223 | 18/32 | 8/40 | 3 S1 9 S1 |
13.3 (M) 30.4 (S) |
| 5 | 10 | 4–30 | 0 | 0 | 138 | 11/48 | 0/0 | 0 | 11.4 (M) 15.9 (S) |
| 6 | 8 | 28 | 4 (S2, S1+S2) |
1(S2) | 185 | 0/34 | 4/40 | 0 | 17.5 (M) 12.8 (S) |
Ch. - Channel; Excit. - Excitatory; Inhibit. - Inhibitory; (M) - across Multiple Channel trials; (S) - across Single Channel trials
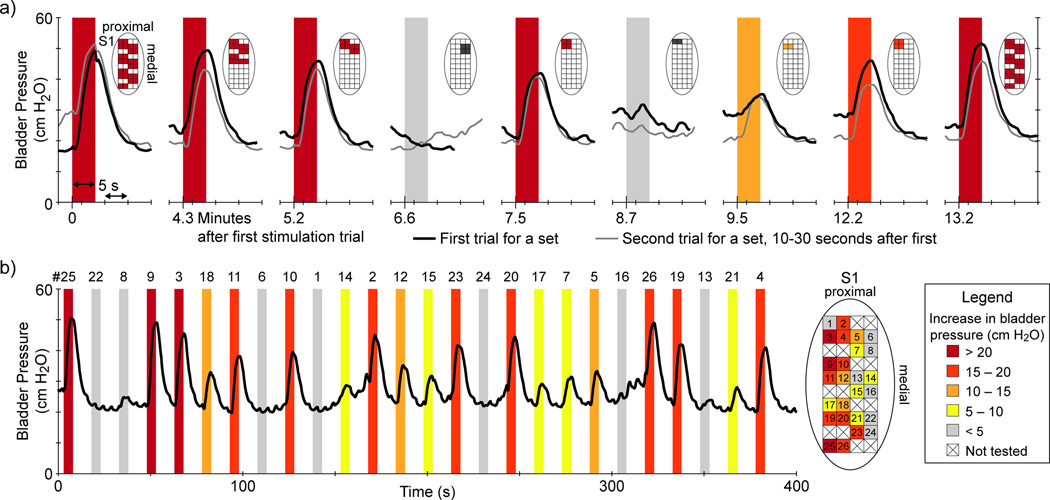
In the first two experiments, the primary focus was on performing multiple channel stimulation trials. In experiment 1, reflex bladder excitation was observed for multiple channel stimulation within either DRG using 33 Hz stimulation at 10–25 µA (mean PEV of 13.1 cm H2O for 4 excitatory trials of 10–30 seconds in duration). In experiment 2, sequential stimulus trials were performed in which the number of simultaneously active electrode channels was varied to evaluate combinations that evoked bladder responses. In this sequence we were able to identify a small subset of the total number of electrodes stimulated that were the primary driver of evoked bladder activity (Figure 2a). In these trials, stimulation with 26 electrodes spanning the S1 DRG led to large bladder contractions, as did 2–14 of the channels at the same stimulation amplitude. The success of these few electrode trials suggested the potential for single channel stimulation, which we evaluated further in experiment 2 and subsequent experiments. The average (standard deviation) PEV for all excitatory multiple channel stimulation trials was 16.2 (8.5) cm H2O.
Figure 2.
DRG-evoked bladder excitation. a) Multiple channel stimulation sequence. Pairs of sequential S1 DRG stimulus trials (first in black, second in grey) for different electrode channel combinations (indicated in diagram above each trial) are given from experiment 2. Although 14- and 28-channel stimulation evoked robust bladder contractions, the majority of the response could be elicited by only 2–4 electrodes. These results motivated subsequent trials evaluating single channel stimulation. All stimulus patterns here were 30 Hz at 10 µA for 5 seconds in duration. b) Single-channel stimulation in S1 DRG during experiment 2. Stimulus parameters were 50 µA at 30 Hz for 5 seconds duration. Eleven of twenty-six channels led to a PEV of at least 15 cm H2O. There was no clear somatotopy, although there was a greater concentration of responsive channels on the lateral side of the array. Note that the excitatory channels labeled here as #3 and #4 were always part of the multiple channel group that evoked bladder activity in (a). For each trial, the PEV range for each stimulus trial is indicated by the color shading and the legend at lower right.
We observed reflex bladder excitation with single channel stimulation in each of experiments 2, 4, 5, and 6. Eighty-nine single channel stimulation trials, across fifty-seven different electrodes, were excitatory.. Stimulus patterns that evoked contractions were 1 Hz and 30–33 Hz patterns at 10, 20, 30 and 50 µA. Higher current levels (30, 50 µA) evoked bladder contractions more frequently (29.8% of all single channel trials at these amplitudes) than lower current levels (10, 20 µA; 5.5%), according to Fisher's exact test (p < 0.001). Figure 2b shows a randomized single channel stimulation sequence, in which a PEV of at least 10 cm H2O was evoked on over half of the electrodes (14/26) in the sequence. In similar sequences across these experiments, the percentage of channel stimulation trials during a randomized single-channel sequence that evoked a bladder response ranged from 6–14% up to 41–54%.
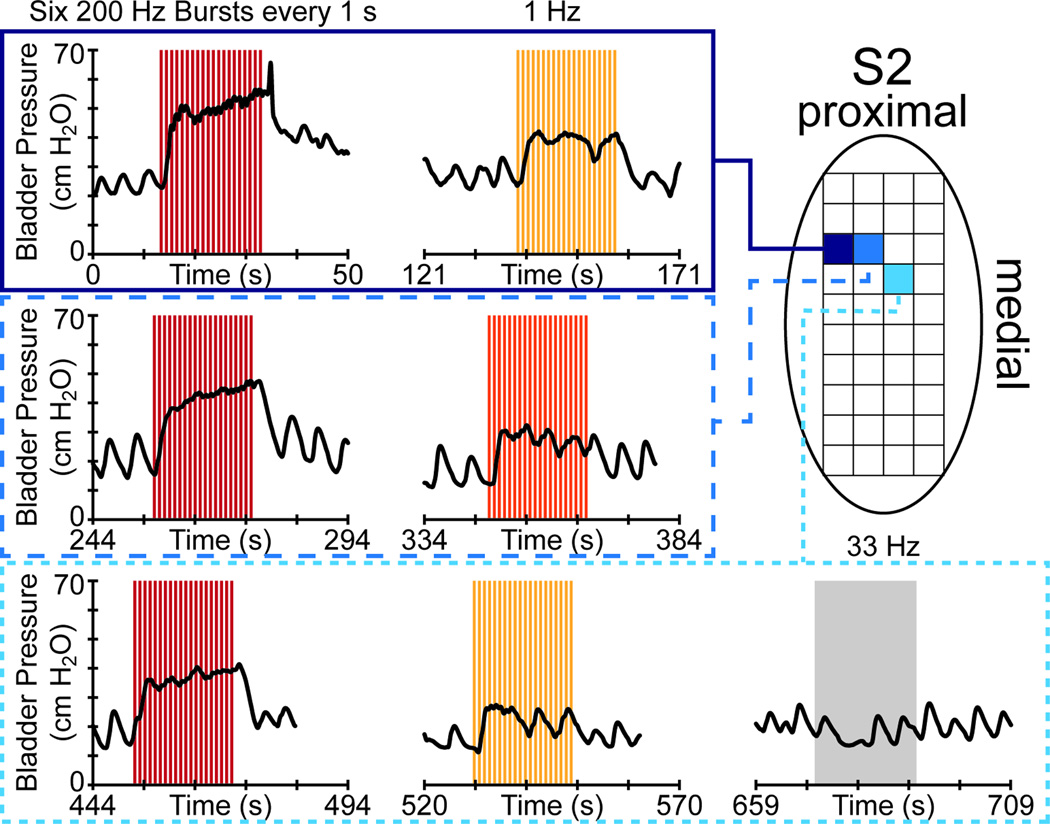
We attempted longer duration single channel stimulation in only a few trials. In experiment 4 after a randomized single channel stimulation sequence, several electrodes were selected for further parameter evaluation. Figure 3 shows some of these trials, in which 20-second duration stimuli evoked sustained bladder contractions. In these trials, a low frequency (1 Hz) bursting stimulus pattern led to greater evoked contractions (mean PEV of 27.1 cm H2O) than non-bursting 1 Hz stimulus patterns (mean PEV of 15.0 cm H2O). In experiment 5, ten-second 30 Hz single channel stimulation trials excited the bladder at 30 and 50 µA on three different electrodes (9 trials), but not at 10 or 20 µA (7 trials), with a 20.5% greater average PEV evoked at higher amplitudes. The average (standard deviation) PEV for all excitatory single channel stimulation trials was 12.5 (5.6) cm H2O. As 88% of these trials were shorter than 5 s, we expect that a greater quantity of longer duration trials would have yielded higher pooled results.
Figure 3.
Stimulation-evoked bladder excitation sustained for twenty seconds in a single DRG (S2) during experiment 4. The stimulation pattern is given at the top of each column of trials, with each row reflecting stimulation on a single electrode. In these trials, a bursting stimulus pattern led to larger contractions than 1 Hz or 33 Hz patterns. The stimulation amplitude was fixed at 30 µA in each trial. The colors for each stimulation trial period reflect the PEV, based on the Figure 2 legend.
Across experiments, bladder excitation was seen for stimulation on both the S1 and the S2 DRG (Table I). In experiment 2 (83% of excitatory multiple and single channel stimulation trials on one root; e.g. Figure 2) and experiment 5 (100%) the majority of responsive trials occurred for S1 stimulation. In experiments 1 (80% of excitatory stimulation trials on a single root) and 6 (100%), the majority of responsive excitatory trials occurred for S2 stimulation. Excitatory trials were more evenly split in experiment 4, with 55% of excitatory trials occurring on S1 and 45% on S2 (e.g. Figure 3). As the range of stimulation parameters was limited in the evaluations performed here, it is possible that stimulation at either DRG level can evoke bladder contractions if the appropriate stimulation pattern, amplitude and location(s) are determined.
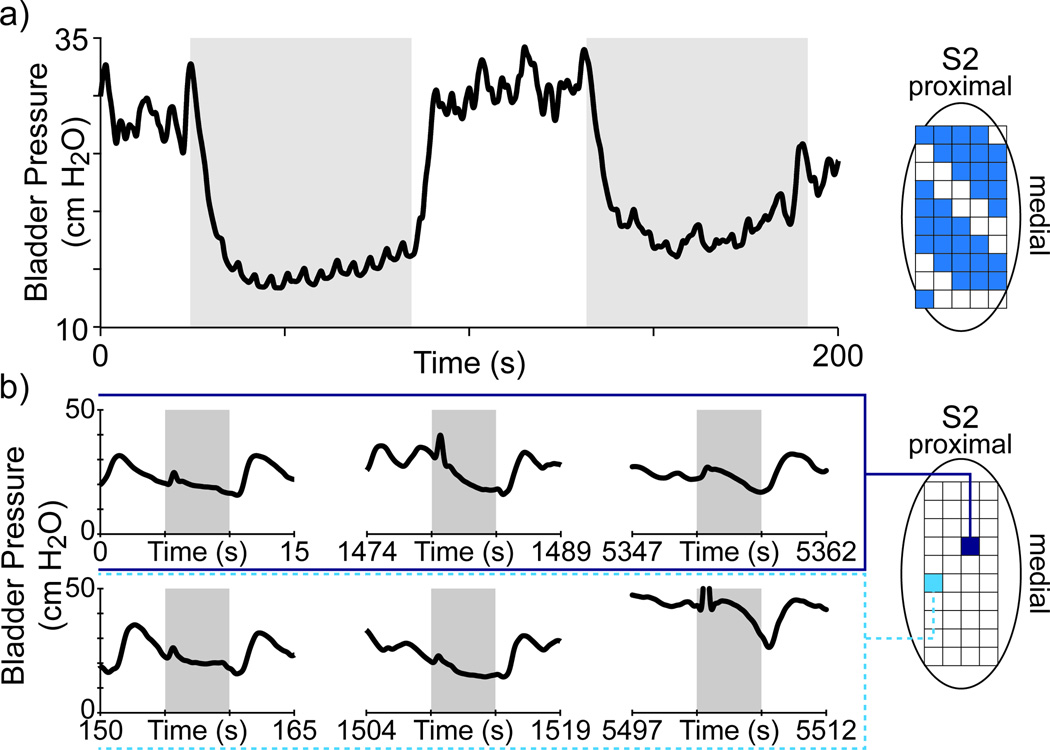
In several DRG stimulation trials we observed an inhibition or relaxation of the bladder. These observations were limited primarily because we typically stayed below bladder pressures at which there was leakage and we only performed a few stimulation trials using stimulation frequencies usually reported as inhibitory (5–10 Hz). During multiple channel stimulation trials, two 5 Hz trials were performed while the bladder pressure was high (Figure 4a). The resulting decrease in the bladder pressure clearly corresponded to the stimulation period. In twenty trials in experiment 4, the bladder pressure during brief 33 Hz stimulation periods appeared to decrease. Figure 4b shows several of these trials, where the baseline bladder pressure was fluctuating prior to stimulation but stabilized or decreasing during stimulation. These decreases occurred both on electrodes for which other stimulation patterns were excitatory (e.g. lower row in Figure 3) and on electrodes which only showed this response (e.g. lower row in Figure 4).
Figure 4.
Example of stimulus-evoked bladder relaxation. a) Multiple channel stimulation at 5 Hz, 10 µA in experiment 2 led to decreases in the bladder pressure. b) Some single-channel stimulation trials evoked bladder relaxation. Here, replicates of 33Hz, 30 µA stimulation on two channels in experiment 4 showed bladder relaxation.
4. Discussion
Microstimulation in sacral dorsal root ganglia is a novel approach for recruiting spinal circuits that control reflex micturition and continence. The results presented here indicate the feasibility of this approach for evoking bladder responses with multiple microelectrode channels (Table I, Figures 2, 4) and individual electrode channels (Table I, Figures 2, 3, 4). Excitatory responses were seen for stimulus amplitudes of 10 to 50 µA, using both lower (around 1 Hz) and higher (30–33 Hz) frequency stimulus patterns (Figures 2b, 3). Relaxation of the bladder was also observed in a few trials, for multiple channel and single channel stimulation (Table I, Figure 4).
The reflexive bladder responses evoked here are similar to responses reported for direct stimulation of the pudendal nerve (12) and its branches (3), with contractions evoked at a level (Figures 2, 3) that can lead to functional bladder emptying (16,17). Typical pudendal nerve stimulation with nerve cuffs (4,12) or relatively large electrodes placed nearby (16) require hundreds to thousands of microamps to evoke a response. Our approach of inserting microelectrodes into DRG allowed for the use of lower current levels, on the order of tens of microamps. This low current level is only occasionally successful at evoking bladder activity with peripheral nerve cuffs (3). It is possible that stimulation amplitudes less than 10 µA may be sufficient to recruit bladder responses, as thresholds for recruiting type I and type II myelinated afferent fibers with lumbar DRG microstimulation has been reported below 5 µA (14). Further study is warranted to evaluate thresholds for activating sacral DRG fibers, as pudendal nerve myelinated afferents are smaller type II and type III fibers (18). Although we did not conclusively demonstrate selective stimulation of peripheral nerve sources to the DRG, these results show that low current stimulation can yield selective recruitment of fibers in close proximity to individual microelectrodes. For example, many excitatory microelectrodes in Figure 2b had adjacent microelectrodes which evoked a very different response, suggesting that fibers activated by stimulation at each location were different.
Microstimulation in the DRG to recruit spinal reflexes is similar to recent work which used microelectrode arrays placed in the pudendal nerve to selectively activate sensory pathways to control the bladder and motor pathways to close the sphincter (6). That study excited bladder reflexes with individual microelectrode channels, however the typical stimulation levels were 3–10 times larger than used here. Unlike a pudendal nerve interface, which has access to direct motor sphincter recruitment, closure of the sphincter with DRG-based electrodes must target reflex pathways that were not evaluated here. A DRG-based interface allows for direct access to sensory fibers from the bladder in the pelvic nerve (9), which is not possible with a pudendal nerve interface.
DRG microstimulation with bursts of pulses can lead to greater evoked bladder contractions than stimulus patterns without bursting (Figure 3), as has been previously shown with stimulation on the pudendal nerve (12) and in the urethra (16,19). These results demonstrate the potential for further optimization of an afferent stimulation approach. It is possible that bursting stimulation patterns lead to greater recruitment of spinal interneurons or pelvic pre-ganglionic neurons, although the exact mechanisms are unknown (12).
One potential limitation of this study is that direct excitation of the ventral roots was not unequivocally ruled-out. Activation of the ventral roots, either by placement of electrode tips directly in the ventral root, or by current spread from the DRG to the ventral root could lead to excitation of pelvic pre-ganglionic axons travelling to the bladder. However, we believe that this is unlikely based on several lines of evidence. First, stimulation-evoked relaxation of bladder is only possible through a reflex pathway at the sacral level since the hypogastric nerve, which originates in thoracolumbar roots, mediates sympathetic evoked bladder relaxation. Secondly, the electrode shank lengths were 1 mm and in most cases the insertion was incomplete. This depth is shorter than the diameter of feline S1 and S2 DRG (unpublished data in lab). In each of these experiments afferent recordings were obtained from many channels distributed across both microelectrode arrays, as in (9), providing further support for their placement in DRG. Finally, the small stimulation amplitudes used in this study are unlikely to have led to significant current spread that would have recruited bladder efferents in the ventral root.
A further limitation of this study is that changes in urethral outlet resistance were not recorded. Low outlet resistance resulting from relaxation of the external urethral sphincter is a requirement for effective voiding. We expect that future studies will be able to demonstrate that increases in bladder pressure can be achieved using DRG microstimulation without unwanted excitation of the urethral sphincter. Previous electrical stimulation approaches for voiding have been complicated by concomitant excitation of the urethral sphincter due to activation of the sphincter muscle or its innervating axons from direct current spread, or by poor specificity in activating reflex pathways (16). DRG microstimulation enables highly localized activation of neural pathways and is significantly different from previous approaches in that current intensities are typically very low, which limits current spread to unwanted targets and activation of undesired reflex pathways.
There are several key opportunities for further examination of microstimulation in sacral DRG to control the bladder. Threshold stimulation currents can be thoroughly evaluated, as previously done at the lumbar level (14), as well as stimulation in one or more DRG on one or both sides of the spinal cord. Cell bodies within DRG are, in general, packed around the perimeter close beneath the perineurium (20). Planar penetrating electrode arrays, as used here, may not provide an optimal approach to targeting DRG neurons. The use of variable-length microelectrodes (5,6) or nonpenetrating electrodes placed in close contact to DRG (21) may improve access to individual neurons. Continued customization of electrodes for DRG applications may allow for a percutaneous insertion that does not require a laminectomy, as used by the Spinal Modulation device to relieve pain via DRG stimulation (22). As the ultimate goal of this work is the development of a neuroprosthesis for controlling the bladder, longterm behaving animal studies and integration of stimulation with DRG recordings (9) for closed-loop control are important steps.
5. Conclusions
This is the first demonstration of reflex bladder responses evoked by microstimulation in sacral dorsal root ganglia. This approach may be useful for obtaining highly selective control over the bladder and lower urinary tract as part of a bladder neuroprosthesis. A microstimulation interface with sacral DRG may be coupled with neural recordings from the same electrode to provide closed-loop control of the bladder. Further experiments are necessary to evaluate long-term efficacy and microelectrodes custom-designed for DRG anatomy.
Acknowledgments
The authors thank members of the Rehabilitation Neural Engineering Lab for their assistance during experiments. In particular we acknowledge Jim Hokanson for his work developing the stimulation control interface. This work was supported in part by NIH NIBIB grant R01EB007748 (DJW) and NIH NINDS grant F32NS074565 (TMB). Additional support was provided by the Telemedicine and Advanced Technology Research Center (TATRC) of U.S. Army Medical Research and Material Command Agreements W81XWH-07-1-0716 (DJW) and W81XWH-10-C-0208 (DJW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, NIBIB, NINDS or TATRC.
Footnotes
Work performed at: University of Pittsburgh, Pittsburgh, PA, USA
References
- 1.Birder L, De Groat WC, Mills I, Morrison J, Thor K, Drake MJ. Neural control of the lower urinary tract: Peripheral and spinal mechanisms. Neurourol Urodyn. 2010;29:128–139. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaunt RA, Prochazka A. Control of urinary bladder function with devices: successes and failures. Prog Brain Res. 2006;152:163–194. doi: 10.1016/S0079-6123(05)52011-9. [DOI] [PubMed] [Google Scholar]
- 3.Yoo PB, Woock JP, Grill WM. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212(1):218–225. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kent AR, Grill WM. Model-based analysis and design of nerve cuff electrodes for restoring bladder function by selective stimulation of the pudendal nerve. J Neural Eng. 2013;10(3):036010. doi: 10.1088/1741-2560/10/3/036010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann RA. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng. 2004;51(1):146–151. doi: 10.1109/TBME.2003.820321. [DOI] [PubMed] [Google Scholar]
- 6.Wark HAC, Dowden BR, Cartwright PC, Normann RA. Selective activation of the muscles of micturition using intrafascicular stimulation of the pudendal nerve. IEEE J Emerg Sel Topics Circuits Sys. 2011;1(4):631–636. [Google Scholar]
- 7.Wenzel BJ, Boggs JW, Gustafson KJ, Grill WM. Detecting the onset of hyperreflexive bladder contractions from the electrical activity of the pudendal nerve. IEEE Trans Neural Sys Rehab Eng. 2005;13(3):428–435. doi: 10.1109/TNSRE.2005.848355. [DOI] [PubMed] [Google Scholar]
- 8.Aoyagi Y, Pearson KG, Stein RB, Branner A, Normann RA. Capabilities of a penetrating microelectrode array for recording single units in dorsal root ganglia of the cat. J Neurosci Methods. 2003;128:9–20. doi: 10.1016/s0165-0270(03)00143-2. [DOI] [PubMed] [Google Scholar]
- 9.Bruns TM, Gaunt RA, Weber DJ. Multielectrode array recordings of bladder and perineal primary afferent activity from the sacral dorsal root ganglia. J Neural Eng. 2011;8(5):056010. doi: 10.1088/1741-2560/8/5/056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn. 2000;19(1):87–99. doi: 10.1002/(sici)1520-6777(2000)19:1<87::aid-nau9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Smith PP, DeAngelis AM, Kuchel GA. Evidence of central modulation of bladder compliance during filling phase. Neurourol Urodyn. 2012;35:30–35. doi: 10.1002/nau.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruns TM, Bhadra N, Gustafson KJ. Variable patterned pudendal nerve stimuli improves reflex bladder activation. IEEE Trans Neural Sys Rehab Eng. 2008;16(2):140–148. doi: 10.1109/TNSRE.2007.914460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snellings AE, Grill WM. Effects of stimulation site and stimulation parameters on bladder inhibition by electrical nerve stimulation. BJU Int. 2012;110(1):136–143. doi: 10.1111/j.1464-410X.2011.10789.x. [DOI] [PubMed] [Google Scholar]
- 14.Gaunt RA, Hokanson JA, Weber DJ. Microstimulation of primary afferent neurons in the L7 dorsal root ganglia using multielectrode arrays in anesthetized cats: thresholds and recruitment properties. J Neural Eng. 2009;6:055009. doi: 10.1088/1741-2560/6/5/055009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cogan SF. Neural stimulation and recording electrodes. Ann Rev Biomed Eng. 2008;10:275–309. doi: 10.1146/annurev.bioeng.10.061807.160518. [DOI] [PubMed] [Google Scholar]
- 16.Bruns TM, Bhadra N, Gustafson KJ. Bursting stimulation of proximal urethral afferents improves bladder pressures and voiding. J Neural Eng. 2009;6:06606. doi: 10.1088/1741-2560/6/6/066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tai C, Wang J, Wang X, De Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26(4):570–577. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 18.Martin WD, Fletcher TF, Bradley WE. Innervation of feline perineal musculature. Anat Rec. 1974;180(1):15–29. doi: 10.1002/ar.1091800104. [DOI] [PubMed] [Google Scholar]
- 19.Bruns TM, Bhadra N, Gustafson KJ. Intraurethral stimulation for reflex bladder activation depends on stimulation pattern and location. Neurourol Urodyn. 2009;28:561–566. doi: 10.1002/nau.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer PS, Raine CS, Wiśniewski H. Axon diameter and myelin thickness - unusual relationships in dorsal root ganglia. Anat Rec. 1973;176(2):225–243. doi: 10.1002/ar.1091760209. [DOI] [PubMed] [Google Scholar]
- 21.Gaunt RA, Bruns TM, Crammond D, Tomycz N, Moossy JJ, Weber DJ. 33rd Ann Int Conf IEEE EMBS. Boston, MA, USA: IEEE; 2011. Singleand multi-unit activity recorded from the surface of the dorsal root ganglia with non-penetrating electrode arrays; pp. 6713–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation. 2013;16(1):67–72. doi: 10.1111/ner.12013. [DOI] [PubMed] [Google Scholar]