Abstract
Objectives
Cognitive-behavioral interventions improve outcomes for many pediatric health conditions, but little is known about which mechanisms mediate these outcomes. The goal of this study was to identify whether changes in targeted process variables from baseline to one week post-treatment mediate improvement in outcomes in a randomized controlled trial of a brief cognitive-behavioral intervention for idiopathic childhood abdominal pain.
Methods
Two-hundred children with persistent functional abdominal pain and their parents were randomly assigned to one of two conditions: a 3-session social learning and cognitive-behavioral treatment (SLCBT) (N=100), or a 3-session educational intervention controlling for time and attention (N=100). Outcomes were assessed at 3, 6 and 12 month follow-ups. The intervention focused on altering parental responses to pain and on increasing adaptive cognitions and coping strategies related to pain in both parents and children.
Results
Multiple mediation analyses were applied to examine the extent to which the effects of the SLCBT condition on child GI symptom severity and pain as reported by children and their parents were mediated by changes in targeted cognitive process variables and parents’ solicitous responses to their child’s pain symptoms. Reductions in parents’ perceived threat regarding their child’s pain mediated reductions in both parent- and child-reported GI symptom severity and pain. Reductions in children’s catastrophic cognitions mediated reductions in child-reported GI symptom severity but no other outcomes. Reductions in parental solicitousness did not mediate outcomes.
Discussion
Results suggest that reductions in reports of children’s pain and GI symptoms following a social learning and cognitive-behavioral intervention were mediated at least in part by decreasing maladaptive parent and child cognitions.
Keywords: Mediation, Cognitive Behavior Therapy, RCT, children, functional abdominal pain
Introduction
Abdominal pain is the most common recurrent pain complaint of childhood [1, 2]. Medical evaluations rarely yield evidence of an organic disease etiology. Instead, the majority of children with persistent complaints of abdominal pain meet criteria for pediatric functional abdominal pain, which is considered to be present when children experience at least three episodes of abdominal pain over a 3-month period that disrupt activities or function, in the absence of physical or laboratory findings that would account for the pain [3]. The disorder is associated with increased psychosocial distress, functional disability, and health care utilization [4-8]. It is also associated with increased emotional distress and decreased quality of life among parents [9].
The role of solicitous responses and cognitions
In the pain research literature, solicitous responses by significant others (responses that are likely to support or reinforce illness behavior, e.g., increased attention and support, expressions of concern, or reduction in demands such as school attendance, chores, or other potentially aversive situations) have been shown to be related to increased pain, activity, and disability [10-16]. In studies focusing on stomach pain in particular, Whitehead and colleagues reported [17, 18] that when IBS (an adult variant of childhood abdominal pain) patients were asked how their parents had responded to their illness complaints during childhood, they were more likely than participants without IBS to recall that their parents provided gifts or special privileges. Our own research has shown that children with parents who were more solicitous in response to their child’s symptoms had significantly higher school absentee levels for gastrointestinal symptoms than children whose parents were less solicitous [19].
Cognitions regarding pain have also emerged as important predictors of pain-related outcomes [20]. For example, research on adults suggests that patients’ catastrophic cognitions are associated with increased pain experience and expression, and increased distress and disability [21-26]. Changes in catastrophizing have also been shown to mediate multidisciplinary pain treatment outcomes [27, 28]. Pain catastrophizing cognitions can be characterized as those that magnify the threat value of a pain stimulus, reflect helplessness in the face of pain, and involve ruminative thinking about pain [20, 29]. In pediatric samples, catastrophizing has been found to be associated with increased pain intensity and avoidance, increased psychological distress, increased depressive symptomatology, increased disability, and decreased quality of life [30-39].
The role of catastrophizing by significant others [40] and parents has received increasing attention in the literature [41-44]. Parental catastrophizing about child pain has been positively associated with child catastrophizing [35], child-reported pain intensity [45], laboratory-demonstrated child pain attentional avoidance [38], and parent-reported child pain behavior [34, 35]; it has also been inversely associated with parent-reported child quality of life [34]. Parental catastrophizing may influence parent behavior as well, specifically, parents’ responses to their children’s pain behaviors. For example, in a questionnaire-based study of 128 mothers and fathers of pediatric chronic pain patients, both maternal and paternal catastrophizing predicted self-reported solicitous responding to their child’s pain [45]. In a laboratory-based study in which children were administered a cold-pressor test, moreover, parents’ catastrophizing about their child’s pain was predictive of the degree to which they reported wanting to stop the procedure [46].
Intervention study
Based on emerging literature regarding the importance of parent and child cognitions about child pain and parent behavioral responses, we developed and tested [47, 48] an intervention which had as the major targets the alteration of parent responses and child and parent cognitions, such as parental perceptions of threat about their child’s pain symptoms. Specifically, the experimental condition (Social Learning/Cognitive Behavior Therapy; SLCBT) provided three intervention sessions focusing on teaching children and their parents cognitive and behavioral strategies to reduce perceived threat and improve coping with the child’s symptoms and also to reduce parents’ solicitous responses to their child’s pain behavior. The comparison condition (Education and Support; ES) was developed to provide a credible alternative condition that would control for therapist and patient time and attention. It included three sessions with the same amount of therapist time as SLCBT, and provided information on the GI system and nutrition. Care was taken to include homework assignments which required similar time and effort as SLCBT assignments. Further detail on participants, inclusion and exclusion criteria, and study procedures can be found in Levy et al. [47]
As reported in the aforementioned article [47], at the end of three treatment sessions spaced one week apart, significantly greater reductions were observed in SLCBT compared to ES for child pain reports, child catastrophizing, and parental perceptions of child pain as a threat. For SLCBT, change in child pain reports was maintained at the 3 and 6 month follow-up assessments with trends for further reductions in child GI symptom severity. At six month follow-up, parents in the SLCBT condition, as compared to parents in the Education Support (ES) control condition, reported greater baseline to follow-up reductions in their child’s pain, in their solicitous responding to their child’s pain reports, and in their perceptions of their child’s pain as a threat. SLCBT parents also reported greater baseline to follow-up increases in their child’s emotion-focused and problem-focused coping. Children in the SLCBT condition, moreover, reported greater baseline to follow-up increases in pain minimization, the ability to distract themselves, and the ability to ignore their pain relative to children in the ES condition. Results at 12 months showed similar patterns [48].
Mechanisms of change
Mediators are intervening variables that are posited to change during or as a result of treatment and that provide a potential explanation of the causal sequences or mechanisms through which treatments affect outcomes [49, 50]. Knowledge about mediators can contribute significantly to our knowledge about mechanisms of intervention efficacy and can be used to determine key targets for future interventions [27, 51, 52]. Researchers have begun to conduct studies that examine the role that potential mediators such as psychological distress, anxiety, visceral sensitivity, cognitions (e.g., self-efficacy to manage symptoms), and expectations for improvement may play in explaining changes following treatments such as cognitive-behavioral therapy (CBT) for functional abdominal pain in adults [53-56]. However, few mediation studies have been done in pediatric pain populations to examine mechanisms of cognitive behavioral interventions. Kashikar-Zuck et al. (2013) [57] reported a mediational analysis examining the role of coping, coping efficacy and catastrophizing as mediators of the effects of a CBT intervention for children 11-18 years old with juvenile fibromyalgia. While significant treatment effects of CBT versus a fibromyalgia education condition were found, the improvements were not mediated by changes in these variables, contrary to the study hypothesis. Wicksell et al. (2011) [58] conducted a study of factors mediating change in a randomized controlled trial of Acceptance and Commitment Therapy compared to multidisciplinary care combined with an antidepressant for adolescents with chronic idiopathic pain. They examined potential mediators of treatment including pain impairment beliefs, pain reactivity, pain intensity, self-efficacy, catastrophizing and kinesiophobia. Pain impairment beliefs and pain reactivity were the only significant mediators of differential outcomes at follow-up. These authors also noted the paucity of studies examining mediation effects in pediatric pain. Sieberg et al. (2011) [59] found that parent protective responses partially mediated the relationship between parent distress and child functional disability in pediatric pain of diverse types, but the study variables were gathered concurrently rather than longitudinally, making it difficult to conduct tests of true mediation. Other studies in pediatric abdominal pain samples have examined effects on coping or cognitions although not in the context of a formal mediation analysis. For example, Wassom et al. (2013) [60] studied a minimal contact CBT intervention (“Gutstrong”) in a small sample of adolescents with functional gastrointestinal disorders. They found that the addition of the intervention to standard medical care resulted in improved pain outcomes and improved adaptive coping compared to standard care alone but did not examine the mediating effects of coping.
Interventions with children that also involve parents introduce different targets for change that may serve as mediators of treatment effects, such as parent cognitions regarding threat and catastrophizing, parental distress, and parental behavioral responses to child pain behavior [61]. While the reports by our group discussed previously, as well as other experimental studies [62, 63] have added support to the position that a cognitive-behavioral and social learning intervention could help reduce the personal and social costs of abdominal pain in children, there has been no research which has specifically identified mediators of these successful interventions.
Therefore, the goal of the present study was to explore an important area not addressed in prior study reports: evaluation of the extent to which changes in children’s symptom severity reported in Levy et al. [47, 48] were mediated by changes in parent or child cognitions and parental solicitous responses. As reported in Levy et al. (2010) [47], mean adjusted change in parental threat perceptions at post-treatment for individuals in the SLCBT group was −0.45 (SE = 0.05). This represents a large effect size change (0.76 standard deviation units) from the value of parental threat at baseline (2.07 [SD =0.59]). Similarly, mean adjusted change from pre- to post-treatment for child catastrophizing was −0.51 (SE = 0.08); this translates to a moderate effect size change (0.59 standard deviations) from the baseline value of 1.63 (SD = 0.86). Finally, mean adjusted change from pre- to post-treatment for parental solicitousness was −0.55 (SE = 0.05) which translates to a large effect size change (1.0 standard deviations) from the baseline value of 1.14 (SD = 0.53). We hypothesized that reductions in a) child catastrophizing, b) parental perceptions regarding the threat of their child’s pain, and c) parental solicitousness in response to child pain behaviors, assessed at baseline and 1 week post-treatment, would mediate child pain and GI symptom severity outcomes assessed at 3, 6 and 12 months post-treatment. Figure 1 illustrates the conceptual model to be tested.
Figure 1.
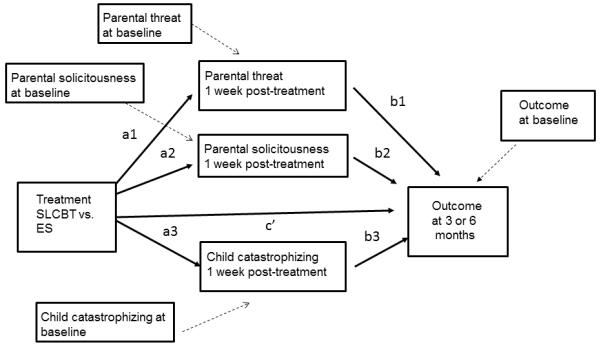
Multiple mediation model
Materials and Methods
Participants and procedure
This study was registered with ClinicalTrials.gov as study number NCT00494260. The original study was a prospective, randomized, controlled trial with blinded assessment of outcome [please see Levy et al.47, 48]. The two intervention conditions consisted of three in-person sessions spaced approximately one week apart (median days between sessions = 7.00; mean number of days from session 1 to session 3 = 19.06, SD = 7.60). All interventionists were trained therapists with a Master’s degree in psychology or social work, or higher. Participants completed assessments at baseline (one week prior to treatment and prior to knowledge of treatment condition assignment) and at one week, three months, six months, and twelve months post-treatment. Children completed assessments via telephone with nurse assessors who were blind to the treatment assignment of the children. Participants included 200 FAP parent /child dyads with children aged 7-17 who were recruited from a pediatric gastroenterology clinic.
Measures
Baseline characteristics
Demographic characteristics were assessed by parent-report: parent age, gender, race, ethnicity, marital status and education; and child age, gender, race and ethnicity.
Outcome variables
Child abdominal pain was assessed using the Faces Pain Scale-Revised (FPS-R) [64], a single-item measure of current pain intensity. Respondents view a row of 6 line-drawn faces depicting various levels of pain, ranging from no pain on the far left to very much pain on the far right. Children were instructed to choose the face that shows “how much they hurt right now”. Options are scored as 0 (no pain) to 10 (very much pain). We administered the same measure to parents with instruction to rate their child’s current level of pain. Validity was demonstrated by a positive correlation between child ratings on the FPS-R and a visual analogue scale in a sample of 76 children age 5-12 undergoing voluntary ear piercing [64].
Child GI symptom severity was assessed using the Children’s Somatization Inventory (CSI) [(CSI65, 66, 67]. This is a measure of children’s nonspecific somatic symptoms such as headaches, back pain, and sore muscles [67, 68]. Each of 35 items is rated with respect to bothersomeness during the past week, using a 0-4 (not at all to a whole lot) scale. We focus here on the GI symptom subscale comprised of 7 items: nausea or upset stomach, constipation, loose bowel movements or diarrhea, stomachaches, vomiting, feeling bloated or gassy, and food making you sick. Children rate the items with respect to their own symptoms and parents rate the items with respect to their child’s symptoms. Internal consistencies (Cronbach’s coefficient alphas) based on the present sample were 0.75 for children and 0.75 for parents. This is commensurate with reports in the literature using the child-report version: 0.73 based on a sample of 101 youth with medically unexplained abdominal pain [69] and 0.80 based on a sample of 188 children with FAP and 61 well controls [70]. With respect to validity, the subscale has been shown to discriminate between persons with and without functional gastrointestinal disorders [70]. It is also sensitive to experimental manipulation [69].
Process variables (mediators)
Parental perceptions regarding the threat of their child’s pain were assessed using the Pain Beliefs Questionnaire (PBQ; Walker et al., 2005) [71]. The parent-report version of the PBQ is a 32-item measure designed to assess parents’ beliefs about various aspects of their children’s abdominal pain [72]. We focus here on the Primary Appraisal or perceived pain threat subscale. Twenty items assess the perceived duration, frequency and seriousness of the child’s abdominal pain condition, as well as the intensity and duration of individual pain episodes. Items such as, “My child’s stomachaches hurt worse than anything” and “My child’s stomachaches mean that he/she is very sick” are rated on a 0-4 (not at all true to very true) scale. Walker and colleagues [72] reported an alpha reliability of 0.83; the value based on the present sample was 0.84. With respect to validity, the subscale has been shown to discriminate between FAP children with differing pain coping profiles [72]. For example, parents of children characterized as “high pain dysfunctional” and “high pain adaptive” (via cluster analysis) reported greater perceived threat regarding their child’s abdominal pain as compared to parents of children characterized as “low pain adaptive” [72].
Child catastrophizing was measured using the Catastrophizing subscale of the Pain Response Inventory. The PRI is a 60-item questionnaire designed to assess children’s responses to pain [73]. It includes 13 subscales representing specific coping strategies such as problem solving, seeking social support, and stoicism. The Catastrophizing subscale contains five items such as, “When you have a stomachache, how often do you … think to yourself that something might be really wrong with you?” and “…think to yourself that you might be really sick?” Ratings are made on a 0-4 (never to always) scale. The developers demonstrated internal consistency using samples of school children and children with abdominal pain; alpha reliabilities were 0.77 and 0.84, respectively [73]. They also demonstrated moderate 6-month test-retest reliability (0.46) and convergent validity through positive associations between catastrophizing and continued pain [73]. Alpha reliability based on the present sample was 0.82.
Parental solicitousness was measured using the Protect subscale of the Adult Responses to Children’s Symptoms [74]. Sample items include “When your child has a stomachache or abdominal pain, how often do you… tell your child that s/he does not have to finish all of his/her homework” and “…tell others in the family not to bother your child or to be especially nice to him/her?” Ratings are made on a 0-4 (never to always) scale. The developers reported an internal consistency value of 0.86 [74]. Cronbach’s coefficient alpha for the present sample was 0.84. Walker and colleagues [75] demonstrated validity of the protect subscale in a sample of 67 mothers. Protect scores correlated positively (r = 0.61, p < 0.001) with subsequent reports of protective responses to their children’s abdominal pain (a diary version of the scale). Medical record data, moreover, revealed greater gastrointestinal-related health care utilization and health care costs among children with mothers scoring high versus low in protectiveness [75].
Mediation analysis
To examine the extent to which the effects of SLCBT on outcomes (child GI symptom severity and pain) were mediated by targeted process variables (parental perceptions regarding the threat of their child’s pain, child catastrophic cognitions, and parental solicitous responses), we applied a multiple mediation model using simultaneous linear regression equations via structural equation modeling [49, 76]. Multiple (compared to a series of single) mediation models assess mediation simultaneously, controlling for the other mediator effects in the model, and produce less biased estimates [76]. To satisfy criteria for causal inferences, we specified prospective, time-lagged models. As can be seen in Figure 1, we tested whether the effect of treatment (SLCBT versus ES) on primary outcomes assessed at 3, 6 or 12 months was mediated by changes in cognitive process variables and solicitousness from baseline assessment to 1 week post-treatment. To focus the analysis on change, we controlled for baseline values of the mediators and the outcome variables by including them as covariates in the model. Figure 1 depicts the simultaneous mediation model comprised by the following: the effect of treatment on the mediators assessed 1 week post- treatment (a1, a2, a3); the effect of the mediators on outcomes assessed at 3, 6 or 12 months (b1, b2, b3); the direct effect of treatment on outcomes assessed at 3, 6 or 12 months (c’); indirect effects of treatment on outcomes assessed at 3, 6 or 12 months, the mediated effects (a1*b1, a2*b2, a3*b3); and the effect of the baseline covariates (represented by variables connected by dashed lines). AMOS 18.0 [77] was employed to conduct full information likelihood estimation and to produce 90 and 95% confidence intervals for parameter estimates based on the normal distribution. The standard error of the indirect effect was calculated as [78].
The use of two confidence intervals requires some explanation, particularly the inclusion of a more liberal 90% confidence interval. Recent discussion of mediation analyses following randomized controlled trials suggests that these may be best thought of as tools for hypothesis generation rather than traditional hypothesis testing, and are undertaken to foster stronger hypotheses to be tested in future studies [50]. Furthermore, Type II error (the probability of missing a significant effect) is a concern in conducting mediation analysis within a moderately powered clinical trial (< 400 participants). As such, it is recommended that standard p values not be used as the criterion by which to determine mediator effects [50, 79, 80]. Following these recommendations [50], we do not report standard tests of significance. Instead, we have included unstandardized beta coefficients, standard errors, and 90% and 95% confidence intervals for the mediated (indirect) effect, the direct effect of treatment on outcome and the mediators, and the effect of the mediators on the outcome. Choosing an alpha of .10 (critical t-1.65) to calculate confidence intervals of estimated parameters reduces chances of type II error. However, recognizing that most researchers are accustomed to viewing 95% confidence intervals, we have included these as well. In both cases, a confidence interval that includes zero suggests no mediation effect is present.
In addition, we calculated effect size as another way of measuring the strength of mediation. Specifically we calculated Hedges’ g, which is considered a more accurate effect size measure than Cohen’s d because it adjusts for sample sizes [81]. Hedges’ g reflects the impact of the intervention on the outcome through the mediator in the scale of standard deviation units. As a rule of thumb, an effect size of g = .80 is considered large (explaining 14% of the variance), .50 medium (6% variance explained), and .20 small (1% variance explained) [82].
We also conducted analyses using multi-informant outcome data. These analyses included data from both parent and child and were conducted to address the fact that shared variance due to the same informant (parent or child) might account for some associations in the analyses based on only a single informant. This approach models outcomes based on both parent and child report of similar constructs (e.g., child pain) as a latent variable in a mediation model using structural equation modeling and treats discrepancies between multiple informants as measurement error; see Figure 2. (For further details on this approach, see Cui, Durtschi, Donnellan, Lorenz, & Conger, 2010 [83]). This approach essentially pools parent and child perspectives into a latent variable as an alternative to modeling the child and parent outcomes separately. Again, we calculated 90 and 95% confidence intervals and effect sizes for all parameter estimates.
Figure 2.
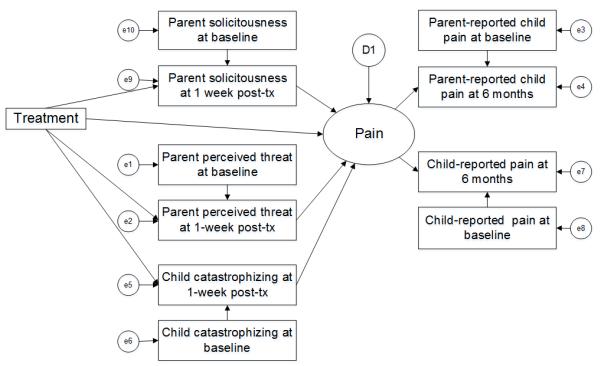
Mediation model specifying the outcome as a latent variable comprised of multiple informant data
Moderators
Participant characteristics may influence the magnitude of treatment effects on outcomes [84]. We examined the potential moderating effects of parent education, parent and child age, gender, and race, and parent and child baseline anxiety (using the Brief Symptom Inventory [85] and the Multidimensional Anxiety Scale for Children [86]) on treatment outcomes. No moderators were found to be significant. As a sensitivity analysis we ran models with age and sex included but, as expected, this did not change the results of the mediation analyses so ultimately these variables were not included in the mediation models.
Results
Sample characteristics
Participants included 200 children with a mean age of 11 years, 73% female, 89% Caucasian, and 4% Hispanic. Parents were on average 44 years old, 94% female, 93% Caucasian, 3% Hispanic, and 93% college-educated [consistent with other research 87]. The SLCBT and ES conditions did not differ significantly on any demographic characteristics as reported in the original publication [47]. Table 1 presents baseline characteristics of our key parent and child-reported variables.
Table 1.
Baseline characteristics of key variables
| M (SD) | |
|---|---|
| Parent-reported variable | |
| Child current pain (FPS-R) | 1.73 (2.07) |
| Child gastrointestinal symptom severity (CSI) | 1.18 (0.70) |
| Parent perception regarding threat of child pain (PBQ) | 2.03 (0.56) |
| Child-reported variable | |
| Child current pain (FPS-R) | 2.04 (2.18) |
| Child gastrointestinal symptom severity (CSI) | 1.19 (0.69) |
| Child catastrophizing (PRI) | 1.59 (0.86) |
Note. FPS-R = Faces Pain Scale-Revised; CSI = Children’s Somatization Inventory; PBQ = Pain Beliefs Questionnaire; PRI = Pain Response Inventory.
Mediation analyses
Tables 2, 3 and 4 present the parameter estimates for the paths that are critical for evaluating the hypothesized mediation. To ease interpretation of mediation effects in the tables, Hedges’ g was calculated for the indirect (a*b) effect and was bolded if the 90% confidence intervals excluded zero, indicating mediation.
Table 2.
Mediation of treatment effects on parent-reported child GI symptom severity and pain at 3, 6 and 12 months by changes from baseline to 1 week post-treatment in parental threat, parental solicitousness, and child catastrophizing
| a | c’ | b | a*b | Effect size of the indirect effect (a*b) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent-reported outcome |
Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI | Hedges’ g |
| Severity at 3 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
0.01 (0.06) |
−0.09, 0.11 |
−0.11, 0.13 |
0.32 (0.09) |
0.17, 0.47 |
0.14, 0.50 |
−0.06 (0.02) |
−0.09, −0.03 |
−0.09, −0.02 |
−0.43 |
| Parent solicitousness |
−0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
0.01 (0.06) |
−0.09, 0.11 |
−0.11, 0.13 |
0.09 (0.10) |
−0.08, 0.26 |
−0.11, 0.29 |
−0.02 (0.02) |
−0.05, 0.02 |
−0.06, 0.02 |
−0.13 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.01 (0.06) |
−0.09, 0.11 |
−0.11, 0.13 |
0.08 (0.07) |
−0.04, 0.20 |
−0.06, 0.22 |
−0.01 (0.01) |
−0.02, 0.01 |
−0.03, 0.01 |
−0.15 |
| Severity at 6 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
−0.01 (0.05) |
−0.09, 0.07 |
−0.11, 0.09 |
0.30 (0.07) |
0.18, 0.42 |
0.16, 0.44 |
−0.05 (0.02) |
−0.08, −0.03 |
−0.08, −0.02 |
−0.49 |
| Parent solicitousness |
−0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.01 (0.05) |
−0.09, 0.07 |
−0.11, 0.09 |
0.002 (0.09) |
−0.15, 0.15 |
−0.17, 0.18 |
−0.0004 (0.02) |
−0.03, 0.03 |
−0.04, 0.04 |
−0.003 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.01 (0.05) |
−0.09, 0.07 |
−0.11, 0.09 |
−0.01 (0.06) |
−0.11, 0.09 |
−0.13, 0.11 |
0.001 (0.01) |
−0.01, 0.01 |
−0.01, 0.02 |
0.02 |
| Severity at 12 months | |||||||||||||
| Parent threat | −0.17 (0.03) |
−0.22, −0.12 |
−0.23, −0.11 |
−0.001 (0.06) |
−0.10, 0.10 |
−0.12, 0.12 |
0.33 (0.09) |
0.18, 0.48 |
0.15, 0.51 |
−0.06 (0.02) |
−0.09, −0.03 |
−0.09, −0.02 |
−0.44 |
| Parent solicitousness |
−0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.001 (0.06) |
−0.10, 0.10 |
−0.12, 0.12 |
0.14 (0.11) |
−0.04, 0.32 |
−0.08, 0.36 |
−0.03 (0.02) |
−0.07, 0.01 |
−0.08, 0.02 |
−0.18 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.001 (0.06) |
−0.10, 0.10 |
−0.12, 0.12 |
0.03 (0.07) |
−0.09, 0.15 |
−0.11, 0.17 |
−0.004 (0.01) |
−0.02, 0.01 |
−0.02, 0.01 |
−0.06 |
| Pain at 3 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
−0.02 (0.19) |
−0.33, 0.29 |
−0.39, 0.35 |
1.26 (0.27) |
0.82, 1.71 |
0.73, 1.79 |
−0.23 (0.06) |
−0.33, −0.13 |
−0.35, −0.11 |
−0.52 |
| Parent solicitousness |
−0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.02 (0.19) |
−0.33, 0.29 |
−0.39, 0.35 |
0.34 (0.32) |
−0.19, 0.87 |
−0.29, 0.97 |
−0.07 (0.07) |
−0.18, 0.04 |
−0.20, 0.06 |
−0.15 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.02 (0.19) |
−0.33, 0.29 |
−0.39, 0.35 |
0.37 (0.21) |
0.02, 0.72 |
−0.04, 0.78 |
−0.04 (0.03) |
−0.10, 0.007 |
−0.11, 0.02 |
−0.20 |
| Pain at 6 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
−0.15 (0.20) |
−0.48, 0.18 |
−0.54, 0.24 |
0.88 (0.29) |
0.40, 1.36 |
0.31, 1.45 |
−0.16 (0.06) |
−0.25, −0.06 |
−0.27, −0.04 |
−0.38 |
| Parent solicitousness |
−0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.15 (0.20) |
−0.48, 0.18 |
−0.54, 0.24 |
−0.27 (0.34) |
−0.83, 0.29 |
−0.94, 0.40 |
0.06 (0.07) |
−0.06, 0.18 |
−0.08, 0.20 |
0.11 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.15 (0.20) |
−0.48, 0.18 |
−0.54, 0.24 |
0.03 (0.22) |
−0.33, 0.39 |
−0.40, 0.46 |
−0.004 (0.03) |
−0.05, 0.04 |
−0.06, 0.05 |
−0.02 |
| Pain at 12 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
0.24 (0.18) |
−0.06, 0.54 |
−0.11, 0.59 |
0.87 (0.25) |
0.46, 1.28 |
0.38, 1.36 |
−0.16 (0.05) |
−0.24, −0.07 |
−0.26, −0.06 |
−0.43 |
| Parent solicitousness |
−0.20 (0.03) |
−0.25, −0.15 |
−0.26, −0.14 |
0.24 (0.18) | −0.06, 0.54 |
−0.11, 0.59 |
0.32 (0.30) |
−0.18, 0.82 |
−0.27, 0.91 |
−0.06 (0.06) |
−0.16, 0.04 |
−0.18, 0.06 |
−0.15 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.24 (0.18) | −0.06, 0.54 |
−0.11, 0.59 |
0.25 (0.20) |
−0.08, 0.58 |
−0.14, 0.64 |
−0.03 (0.03) |
−0.07, 0.01 |
−0.08, 0.02 |
−0.16 |
Note.
= change in the mediator from baseline to post−treatment due to SLCBT treatment compared to ES;
= direct effect of change in the mediator from baseline to post−treatment on change in the outcome;
= direct effect of treatment on the outcome variable;
=indirect (mediated) effect of treatment on the outcome. To facilitate interpretation of the table, Hedges’ g for the a*b effect was bolded if the .90 confidence intervals (CI) excluded zero, suggesting that mediation is present. Hedges’ g values can be interpreted approximately as follows: .80 large, .50 medium and .20 small.
Table 3.
Mediation of treatment effects on child-reported GI symptom severity and pain at 3, 6 and 12 months by changes from baseline to 1 week post-treatment in parental threat, parental solicitousness, and child catastrophizing
| a | c’ | b | a*b | Effect size of the indirect effect (a*b) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Child-reported outcome | Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI |
Estimate (SE) |
90 CI | 95 CI |
Hedges’ g |
| Severity at 3 months | |||||||||||||
| Parent threat | −0.17 (0.03) |
−0.22, −0.12 |
−0.23, −0.11 |
0.02 (0.05) |
−0.06, 0.10 |
−0.08, 0.12 |
0.06 (0.07) |
−0.06, 0.18 |
−0.08, 0.20 |
−0.01 (0.01) |
−0.03, 0.01 |
−0.03, 0.01 |
−0.12 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
0.02 (0.05) |
−0.06, 0.10 |
−0.08, 0.12 |
−0.01 (0.08) |
−0.14, 0.12 |
−0.17, 0.15 |
0.002 (0.02) |
−0.03, 0.03 |
−0.03, 0.03 |
0.02 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.02 (0.05) |
−0.06, 0.10 |
−0.08, 0.12 |
0.14 (0.05) |
0.06, 0.22 |
0.04, 0.24 |
−0.02 (0.01) |
−0.03, −0.002 |
−0.03, −0.001 |
−0.26 |
| Severity at 6 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
−0.003 (0.05) |
−0.09, 0.08 |
−0.10, 0.10 |
0.13 (0.07) |
0.02, 0.25 |
−0.01, 0.27 |
−0.02 (0.01) |
−0.05, −0.002 |
−0.05, 0.002 |
−0.25 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.003 (0.05) |
−0.09, 0.08 |
−0.10, 0.10 |
−0.08 (0.08) |
−0.21, 0.05 |
−0.24, 0.08 |
0.02 (0.02) |
−0.01, 0.04 |
−0.02, 0.05 |
0.14 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.003 (0.05) |
−0.09, 0.08 |
−0.10, 0.10 |
0.18 (0.05) |
0.10, 0.26 |
0.08, 0.28 |
−0.02 (0.01) |
−0.04, −0.004 |
−0.04, 0.0004 |
−0.28 |
| Severity at 12 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
−0.11 (0.06) |
−0.21, −0.01 |
−0.23, 0.01 |
0.05 (0.09) |
−0.10, 0.20 |
−0.13, 0.23 |
−0.01 (0.02) |
−0.04, 0.02 |
−0.04, 0.02 |
−0.08 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.11 (0.06) |
−0.21, −0.01 |
−0.23, 0.01 |
−0.08 (0.10) |
−0.25, 0.09 |
−0.28, 0.12 |
0.02 (0.02) |
−0.02, 0.05 |
−0.02, 0.06 |
0.11 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.11 (0.06) |
−0.21, −0.01 |
−0.23, 0.01 |
0.12 (0.07) |
0.004, 0.24 |
−0.02, 0.26 |
−0.01 (0.01) |
−0.03, 0.003 |
−0.03, 0.006 |
−0.20 |
| Pain at 3 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
0.03 (0.13) |
−0.18, 0.24 |
−0.22, 0.28 |
0.59 (0.19) |
0.28, 0.90 |
0.22, 0.96 |
−0.11 (0.04) |
−0.17, −0.04 |
−0.18, −0.03 |
−0.39 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
0.03 (0.13) |
−0.18, 0.24 |
−0.22, 0.28 |
−0.16 (0.23) |
−0.54, 0.22 |
−0.61, 0.29 |
0.03 (0.05) |
−0.05, 0.11 |
−0.06, 0.13 |
0.10 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.03 (0.13) |
−0.18, 0.24 |
−0.22, 0.28 |
0.24 (0.15) |
−0.01, 0.49 |
−0.05, 0.53 |
−0.03 (0.02) |
−0.06, 0.007 |
−0.07, 0.01 |
−0.19 |
| Pain at 6 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
0.06 (0.12) |
−0.14, 0.26 |
−0.18, 0.30 |
0.37 (0.17) |
0.09, 0.65 |
0.04, 0.70 |
−0.07 (0.03) |
−0.12, −0.01 |
−0.13, −0.003 |
−0.29 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
0.06 (0.12) |
−0.14, 0.26 |
−0.18, 0.30 |
−0.37 (0.20) |
−0.70, −0.04 |
−0.76, 0.02 |
0.08 (0.04) |
0.006, 0.15 |
−0.01, 0.16 |
0.25 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.06 (0.12) |
−0.14, 0.26 |
−0.18, 0.30 |
0.26 (0.13) |
0.05, 0.47 |
0.005, 0.52 |
−0.03 (0.02) |
−0.06, 0.002 |
−0.07, 0.009 |
−0.22 |
| Pain at 12 months | |||||||||||||
| Parent threat | −0.17 (0.03) |
−0.22, −0.12 |
−0.23, −0.11 |
0.09 (0.13) |
−0.12, 0.30 |
−0.16, 0.34 |
0.28 (0.19) |
−0.03, 0.59 |
−0.09, 0.65 |
−0.05 (0.03) |
−0.10, 0.007 |
−0.11, 0.02 |
−0.20 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
0.09 (0.13) |
−0.12, 0.30 |
−0.16, 0.34 |
−0.10 (0.23) |
−0.48, 0.28 |
−0.55, 0.35 |
0.02 (0.05) |
−0.06, 0.10 |
−0.07, 0.12 |
0.06 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.09 (0.13) |
−0.12, 0.30 |
−0.16, 0.34 |
0.16 (0.15) |
−0.09, 0.41 |
−0.13, 0.45 |
−0.02 (0.02) |
−0.05, 0.01 |
−0.06, 0.02 |
−0.14 |
Note.
= change in the mediator from baseline to post−treatment due to SLCBT treatment compared to ES;
= direct effect of change in the mediator from baseline to post−treatment on change in the outcome;
= direct effect of treatment on the outcome variable;
=indirect (mediated) effect of treatment on the outcome. To facilitate interpretation of the table, Hedges’ g for the a*b effect was bolded if the .90 confidence intervals (CI) excluded zero, suggesting that mediation is present. Hedges’ g values can be interpreted approximately as follows: .80 large, .50 medium and .20 small.
Table 4.
Mediation of treatment effects on multiple informant (parent/child) reported child GI symptom severity and pain at 3, 6 and 12 months by changes from baseline to 1 week post-treatment in parental threat, parental solicitousness, and child catastrophizing
| a | c’ | b | a*b | Effect size of the indirect effect (a*b) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multiple informant outcome |
Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI | Estimate (SE) |
90 CI | 95 CI | Hedges’ g |
| Severity at 3 months | |||||||||||||
| Parent threat | −0.17 (0.03) |
−0.22, −0.12 |
−0.23, −0.11 |
0.01 (0.06) |
−0.09, 0.11 |
−0.11, 0.13 |
0.29 (0.09) |
0.14, 0.44 |
0.11, 0.47 |
−0.05 (0.02) |
−0.08, −0.02 |
−0.08, −0.02 |
−0.40 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
0.01 (0.06) |
−0.09, 0.11 |
−0.11, 0.13 |
0.10 (0.10) |
−0.07, 0.27 |
−0.10, 0.30 |
−0.02 (0.02) |
−0.06, 0.01 |
−0.06, 0.02 |
−0.14 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.01 (0.06) |
−0.09, 0.11 |
−0.11, 0.13 |
0.14 (0.07) |
0.03, 0.26 |
0.003, 0.28 |
−0.02 (0.01) |
−0.03, 0.001 |
−0.04, 0.01 |
−0.22 |
| Severity at 6 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
−0.01 (0.04) |
−0.08, 0.06 |
−0.09, 0.07 |
0.25 (0.07) |
0.13, 0.37 |
0.11, 0.39 |
−0.05 (0.01) |
−0.07, −0.02 |
−0.07, −0.02 |
−0.43 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.01 (0.04) |
−0.08, 0.06 |
−0.09, 0.07 |
−0.03 (0.08) |
−0.16, 0.10 |
−0.19, 0.13 |
0.01 (0.02) |
−0.02, 0.03 |
−0.03, 0.04 |
0.05 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.01 (0.04) |
−0.08, 0.06 |
−0.09, 0.07 |
0.08 (0.05) |
−0.003, 0.16 |
−0.02, 0.18 |
−0.01 (0.01) |
−0.02, 0.002 |
−0.02, 0.005 |
−0.19 |
| Severity at 12 months | |||||||||||||
| Parent threat | −0.17 (0.03) |
−0.22, −0.12 |
−0.23, −0.11 |
−0.02 (0.06) |
−0.12, 0.08 |
−0.14, 0.10 |
0.32 (0.09) |
0.17, 0.47 |
0.14, 0.50 |
−0.05 (0.02) |
−0.08, −0.02 |
−0.09, −0.02 |
−0.43 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.02 (0.06) |
−0.12, 0.08 |
−0.14, 0.10 |
0.12 (0.11) |
−0.06, 0.30 |
−0.10, 0.34 |
−0.03 (0.02) |
−0.06, 0.01 |
−0.07, 0.02 |
−0.15 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.02 (0.06) |
−0.12, 0.08 |
−0.14, 0.10 |
0.06 (0.07) |
−0.06, 0.18 |
−0.08, 0.20 |
−0.01 (0.01) |
−0.02, 0.01 |
−0.02, 0.01 |
−0.11 |
| Pain at 3 months | |||||||||||||
| Parent threat | −0.17 (0.03) |
−0.22, −0.12 |
−0.23, −0.11 |
−0.01 (0.16) |
−0.27, 0.25 |
−0.32, 0.30 |
1.30 (0.26) |
0.87, 1.73 |
0.79, 1.81 |
−0.22 (0.06) |
−0.32, −0.12 |
−0.34, −0.11 |
−0.53 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.01 (0.16) |
−0.27, 0.25 |
−0.32, 0.30 |
0.14 (0.28) |
−0.32, 0.60 |
−0.41, 0.69 |
−0.03 (0.06) |
−0.13, 0.07 |
−0.14, 0.09 |
−0.07 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.01 (0.16) |
−0.27, 0.25 |
−0.32, 0.30 |
0.42 (0.18) |
0.12, 0.72 |
0.07, 0.77 |
−0.05 (0.03) |
−0.10, −0.01 |
−0.11, 0.009 |
−0.24 |
| Pain at 6 months | |||||||||||||
| Parent threat | −0.18 (0.03) |
−0.23, −0.13 |
−0.24, −0.12 |
−0.01 (0.14) |
−0.24, 0.22 |
−0.28, 0.26 |
0.64 (0.26) |
0.21, 1.07 |
0.13, 1.15 |
−0.12 (0.05) |
−0.20, −0.03 |
−0.21, −0.02 |
−0.32 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
−0.01 (0.14) |
−0.24, 0.22 |
−0.28, 0.26 |
−0.37 (0.26) |
−0.80, 0.06 |
−0.88, 0.14 |
0.08 (0.06) |
−0.01, 0.17 |
−0.03, 0.19 |
−0.20 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
−0.01 (0.14) |
−0.24, 0.22 |
−0.28, 0.26 |
0.26 (0.17) |
−0.02, 0.54 |
−0.07, 0.59 |
−0.03 (0.02) |
−0.07, 0.01 |
−0.08, 0.02 |
−0.18 |
| Pain at 12 months | |||||||||||||
| Parent threat | −0.17 (0.03) |
−0.22, −0.12 |
−0.23, −0.11 |
0.20 (0.17) |
−0.08, 0.48 |
−0.13, 0.53 |
0.78 (0.24) |
0.38, 1.18 |
0.31, 1.25 |
−0.13 (0.05) |
−0.21, −0.05 |
−0.22, −0.04 |
−0.40 |
| Parent solicitousness | −0.21 (0.03) |
−0.26, −0.16 |
−0.27, −0.15 |
0.20 (0.17) |
−0.08, 0.48 |
−0.13, 0.53 |
0.23 (0.29) |
−0.25, 0.71 |
−0.34, 0.80 |
−0.05 (0.06) |
−0.15, 0.05 |
−0.17, 0.07 |
−0.11 |
| Child CAT | −0.12 (0.05) |
−0.20, −0.04 |
−0.22, −0.02 |
0.20 (0.17) |
−0.08, 0.48 |
−0.13, 0.53 |
0.28 (0.19) |
−0.03, 0.59 |
−0.09, 0.65 |
−0.03 (0.03) |
−0.08, 0.01 |
−0.09, 0.02 |
−0.18 |
Note.
= change in the mediator from baseline to post-treatment due to SLCBT treatment compared to ES;
= direct effect of change in the mediator from baseline to post-treatment on change in the outcome;
= direct effect of treatment on the outcome variable;
=indirect (mediated) effect of treatment on the outcome. To facilitate interpretation of the table, Hedges’ g for the a*b effect was bolded if the .90 confidence intervals (CI) excluded zero, suggesting that mediation is present. Hedges’ g values can be interpreted approximately as follows: .80 large, .50 medium and .20 small.
Parent-reported outcomes
Results of analyses examining the mediating effects of changes in parental pain threat, parental solicitousness, and child catastrophizing measured from baseline to 1 week post-treatment on parent-reported outcomes measured at 3, 6 and 12 months are displayed in Table 2. As can be seen in the table, reductions in parents’ perceptions regarding the threat of their child’s pain showed moderate-sized mediation effects (g = −0.38 to −0.52) on parent-reported outcomes of child pain and symptoms. Changes in parental solicitousness and child catastrophizing, however, did not mediate changes in these parent-reported outcomes.
Child-reported outcomes
Results of analyses examining the mediating effects of changes measured from baseline to 1 week post-treatment in parental pain threat, parental solicitousness, and child catastrophizing on child-reported outcomes measured at 3, 6 and 12 months are displayed in Table 3. Reductions in parents’ perceptions regarding the threat of their child’s pain mediated reductions in child-reported GI symptom severity at 6 months (g = −0.25, a small effect) and in child-reported pain at 3 and 6 months (g = −0.39 and −0.29, respectively, moderate to small-sized effects). Reductions in children’s catastrophic thinking mediated small effect-size reductions in child-reported GI symptom severity at 3 (g = −0.26) and 6 (g = −.28) months. Reductions in child catastrophic thinking did not mediate treatment effects on child-reported pain. Changes in parental solicitousness did not mediate any child outcomes.
Multi-informant latent variable outcomes
Results of analyses examining the mediating effects of changes measured from baseline to 1 week post-treatment in parental pain threat, parental solicitousness, and child catastrophizing on both parent- and child-reported outcomes measured at 3, 6 and 12 months are displayed in Table 4. Reductions in parents’ perceptions regarding the threat of their child’s pain mediated reductions in GI symptom severity at 3, 6 and 12 months (g = −0.40, −0.43 and −0.43, respectively, moderate-sized effects) and in pain at 3, 6 and 12 months (g = −0.53, −0.32, and −0.40, respectively). Reductions in child catastrophic thinking mediated reductions in pain at 3 months (g = −0.24). Reductions in parental solicitousness did not mediate treatment effects on GI symptom severity and pain.
Discussion
A primary aim of this study was to determine whether cognitive process variables and parental solicitousness mediated change in parent and child reports of child pain and symptoms following a very brief social learning/cognitive behavior therapy intervention for children with functional abdominal pain. Parental beliefs about the threat posed by their child’s illness emerged as a significant mediator of the treatment effects of the intervention. Similarly, child catastrophizing also appeared to mediate changes in child-reported GI symptom severity, although these effects were in general smaller. These findings lend support to the cognitive-behavioral model tested in this study and suggest that targeting change in parent and child beliefs should be an important goal of programs aimed at improving child pain-related outcomes. The current results are thus consistent with a recent Cochrane review suggesting parental involvement in behavioral interventions increased effectiveness [61].
The decrease in parent threat cognitions in treatment is consistent with the intervention targets, which explicitly educated parents and children on chronic pain, including the fact that recurrent abdominal pain is not typically a sign of tissue damage or pathophysiology (i.e., hurt does not equal harm), and that their physicians had cleared the child medically to participate in the study. The intervention also targeted catastrophizing thought patterns (which include perceptions of threat, but also include appraisals of inability to cope with a stressor). During the intervention, child participants were taught how to replace such thoughts with more adaptive coping thoughts and to use coping skills such as relaxation. Decreases in catastrophizing reported by children mediated (albeit to a small extent) reported changes in their self-reported pain.
In general, change in parent threat perception was a more consistent and powerful mediator of outcomes than was change in child catastrophizing. Of note is that changes in parent perceptions of the threat posed by the child’s pain mediated changes in both parent and child-reported GI symptoms, as well as child-reported pain, suggesting that change in parental cognition may affect not only parent report of child GI symptoms but possibly also child self-report. It could be hypothesized that parents may communicate less concern or sense of threat when child symptoms occur after the SLCBT treatment, lessening child concern or sense of threat. Another possibility is that when sense of threat is lowered, parent and child attention is more easily diverted to non-symptom concerns and activities. Further research is needed to confirm these findings and examine alternative explanations. These findings, if replicated, also suggest that interventions to decrease parental concern and sense of threat may be an effective and efficient way of reducing symptom perception in both parents and children.
Our findings are also consistent with studies that have provided evidence that changing beliefs and cognitions about pain are at least in part responsible for changes in outcomes. For example, studies by Burns Kubilis et al. (2003) [27] and Smeets et al. (2006) [28] found changes in catastrophizing to be a significant mediator of multidisciplinary pain treatment effects. Turner, Holtzman and Mancl (2011) [88] found that pre- to post-treatment changes in pain beliefs, catastrophizing and self-efficacy related to pain predicted outcomes at one year post-treatment. However, these studies used adult, not child samples. In one of the few pediatric pain studies to examine mediation processes in treatment, Wicksell et al. (2011) [58] found that pain impairment beliefs and reactivity but not self-efficacy or catastrophizing, significantly mediated outcomes of treatment for adolescents with chronic idiopathic pain. Further studies examining not only catastrophizing but other potential child mediators such as coping, relaxation and self-efficacy, and potential parent mediators such as beliefs, catastrophizing, distress and responses to pain behaviors will be important in furthering understanding of treatment processes in pediatric pain. In addition, it would be useful to determine if professionals other than trained mental health therapists (e.g., nurses) could implement these strategies, and if strategies such as booster sessions or a longer intervention period would increase the intervention effect on outcomes.
Despite significant reductions in parental solicitousness from baseline to post-treatment, we did not find that these changes mediated reductions in the outcomes of pain and GI symptoms as reported by parents and children. One possible explanation for this finding is that these outcomes may not be those most influenced by solicitous responses. Behavioral theory would posit that if solicitous responses by parents reinforce pain behaviors and disability, reductions in solicitousness should mediate improvement in those outcomes, but may not have a similar impact on subjective pain ratings or symptom reports. We did not find a significant treatment effect for disability in the RCT [47] and thus could not assess mediation for this outcome. We also did not obtain a direct observational measure of child pain behavior, which might be more sensitive to changes in solicitousness. Direct observational measures such as those used in studies of patients and their spouses [89] may provide the ability to capture these in future research. Another possibility is that solicitous responses not captured by the ARCS (and thus not measured in the current study), such as changes in nonverbal communications of concern via body language or voice tone, may be important aspects of solicitousness that could mediate outcomes. The intervention stressed replacing solicitous behavior with other responses such as engaging the child in adaptive coping or other activities, but we did not assess the degree to which parents implemented these alternative responses and the extent to which the use of these strategies may have affected outcomes is unknown. It may also be the case that changing parent beliefs about the threat of the child’s pain and child catastrophizing have direct effects on parent perceptions of child well-being and children’s own perceptions of pain and disability in addition to any indirect effects through reductions in solicitousness. Finally, another possible explanation may be the frequency and timing of the assessment of the process and outcome measures. It is possible that changes in solicitousness may have mediated changes in outcomes earlier in the intervention, following the first or second intervention sessions, but as we do not have assessments of solicitousness during the intervention, we may have failed to capture its potential influence. [90].
Limitations of the study should be noted in interpreting the findings. The data are primarily self-reported, although the use of assessments from both parents and children helps to mitigate this to some extent, especially given that some parallels in the findings from both were found. The effect sizes for mediation are moderate to small, and it is possible that other non-assessed mechanisms or factors may have been responsible for findings or may have been more powerful mediators. Although we used a time-lagged mediation model, the mediator was only assessed at baseline and at one-week post-treatment, but not during treatment, and outcomes were assessed at 3, 6 and 12 months post treatment. Expanded measurement of potential mediators during the process of treatment could potentially provide more detail on mediational processes [91, 92].
The literature in this area may be enhanced by the use of more sophisticated designs such as cross lagged panel analyses which allow researchers to more precisely determine timing of changes in mediators and how these may be associated with changes in outcomes longitudinally. In addition, incorporation of moderator effects, baseline predictor variables and nonspecific treatment variables (such as treatment expectancies and therapeutic relationship quality) may allow more comprehensive examination of the process of change in pediatric pain treatments.
In conclusion, these findings support the importance of including a focus on changing parent and child beliefs in the cognitive-behavioral treatment of chronic functional abdominal pain in children. Research examining mechanisms of treatment is critical to progress in testing not only whether but why treatments work, so that the theoretical basis of treatments can be refined and efficient interventions can be developed and delivered [51, 93]. Further research, with study designs that include direct observations of behavior, expanded measurement of possible moderator and nonspecific treatment variables, as well as expanded measurement points of mediators, could potentially provide more detail on the process of change in cognitive-behavioral treatment of pediatric abdominal pain.
Acknowledgments
Source of Funding: Funded by NIH grant R01HD36069 awarded to Dr. Levy.
Footnotes
Conflicts of Interest
There are no conflicts of interest to report.
References
- 1.McGrath PA. Pain in children: nature, assessment, and treatment. Guilford; New York: 1990. [Google Scholar]
- 2.Schwille IJD, Giel KE, Zipfel S, Enck P, Ellert U. A community-based survey of abdominal pain prevalence, characteristics, and health care use among children. Clin Gastroenterol Hepatol. 2009;7:1062–1068. doi: 10.1016/j.cgh.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE. Rome III: the functional gastrointestinal disorders. Degnon Associates; McLean, VA: 2006. [Google Scholar]
- 4.Campo JV, Bridge J, Ehmann M, Altman S, Lucas A, Birmaher B, Di Lorenzo C, Iyengar S, Brent DA. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics. 2004;113:817–24. doi: 10.1542/peds.113.4.817. [DOI] [PubMed] [Google Scholar]
- 5.Di Lorenzo C, Colletti RB, Lehmann HP, Boyle JT, Gerson WT, Hyams JS, Squires RH, Jr., Walker LS, Kanda PT, Subcommittee AAP, Pain NCoCA Chronic Abdominal Pain In Children: a Technical Report of the American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:249–61. doi: 10.1097/01.mpg.0000154661.39488.ac. [DOI] [PubMed] [Google Scholar]
- 6.Scharff L. Recurrent abdominal pain in children: a review of psychological factors and treatment. 1997;17:145–166. doi: 10.1016/s0272-7358(96)00001-3. [DOI] [PubMed] [Google Scholar]
- 7.Walker LS, Garber J, Greene JW. Psychosocial correlates of recurrent childhood pain: a comparison of pediatric patients with recurrent abdominal pain, organic illness, and psychiatric disorders. J Abnorm Psychol. 1993;102:248–58. doi: 10.1037//0021-843x.102.2.248. [DOI] [PubMed] [Google Scholar]
- 8.Wendland M, Jackson Y, Stokes LD. Functional disability in paediatric patients with recurrent abdominal pain. Child Care Health Dev. 2010;36:516–523. doi: 10.1111/j.1365-2214.2010.01087.x. [DOI] [PubMed] [Google Scholar]
- 9.Campo JV, Bridge J, Lucas A, Savorelli S, Walker L, Di Lorenzo C, Iyengar S, Brent DA. Physical and emotional health of mothers of youth with functional abdominal pain. Arch Pediatr Adolesc Med. 2007;161:131–7. doi: 10.1001/archpedi.161.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Rosen NO, Bergeron S, Glowacka M, Delisle I, Baxter ML. Harmful or helpful: perceived solicitous and facilitative partner responses are differentially associated with pain and sexual satisfaction in women with provoked vestibulodynia. J Sex Med. 2012;9:2351–2360. doi: 10.1111/j.1743-6109.2012.02851.x. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham JL, Hayes SE, Townsend CO, Laures HJ, Hooten WM. Associations between spousal or significant other solicitous responses and opioid dose in patients with chronic pain. Pain Med. 2012;13:1034–1039. doi: 10.1111/j.1526-4637.2012.01434.x. [DOI] [PubMed] [Google Scholar]
- 12.Romano JM, Turner JA, Jensen MP, Friedman LS, Bulcroft RA, Hops H, Wright SF. Chronic pain patient-spouse behavioral interactions predict patient disability. Pain. 1995;63:1034–1039. doi: 10.1016/0304-3959(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 13.Flor H, Kerns RD, Turk DC. The role of spouse reinforcement, perceived pain, and activity levels of chronic pain patients. J Psychosom Res. 1987;31:251–259. doi: 10.1016/0022-3999(87)90082-1. [DOI] [PubMed] [Google Scholar]
- 14.Flor H, Turk DC, Rudy TE. Relationship of pain impact and significant other reinforcement of pain behaviors: the mediating role of gender, marital status and marital satisfaction. Pain. 1989;38:45–50. doi: 10.1016/0304-3959(89)90071-7. [DOI] [PubMed] [Google Scholar]
- 15.Keefe FJ, Abernethy AP, Campbell C. L. Psychological approaches to understanding and treating disease-related pain. Annu Rev Psychol. 2005;56:601–630. doi: 10.1146/annurev.psych.56.091103.070302. [DOI] [PubMed] [Google Scholar]
- 16.Kerns R, Southwick S, Giller E, Haythornthwaite J, Jacob M, Rosenberg R. The relationship between reports of pain-related social interactions and expressions of pain and affective distress. Behav Ther. 1991;22:101–111. [Google Scholar]
- 17.Whitehead WE, Winget C, Fedoravicius AS, Wooley S, Blackwell B. Learned illness behavior in patients with irritable bowel syndrome and peptic ulcer. 1982;27:202–8. doi: 10.1007/BF01296915. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead WE, Crowell MD, Heller BR, Robinson JC, Schuster MM, Horn S. Modeling and reinforcement of the sick role during childhood predicts adult illness behavior. Psychosomatic medicine. 1994:56. doi: 10.1097/00006842-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Levy RL, Whitehead WE, Walker LS, Von Korff M, Feld AD, Garner M, Christie D. Increased somatic complaints and health-care utilization in children: Effects of parent IBS status and parent response to gastrointestinal symptoms. Am J Gastroenterol. 2004;99:2442–2451. doi: 10.1111/j.1572-0241.2004.40478.x. [DOI] [PubMed] [Google Scholar]
- 20.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing a critical review. Expert Rev Neurother. 2009;9:745–758. doi: 10.1586/ERN.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boothby JL, Thorn BE, Overduin LY, Charles Ward. L. Catastrophizing and perceived partner responses to pain. Pain. 2004;109:500–506. doi: 10.1016/j.pain.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 22.Giardino ND, Jensen MP, Turner JA, Ehde DM, Cardenas DD. Social environment moderates the association between catastrophizing and pain among persons with a spinal cord injury. Pain. 2003;106:19–25. doi: 10.1016/s0304-3959(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 23.Keefe FJ, Lipkus I, Lefebvre JC, Hurwitz H, Clipp E, Smith J, Porter L. The social context of gastrointestinal cancer pain: a preliminary study examining the relation of patient pain catastrophizing to patient perceptions of social support and caregiver stress and negative responses. Pain. 2003;103:151–156. doi: 10.1016/s0304-3959(02)00447-5. [DOI] [PubMed] [Google Scholar]
- 24.Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17:165–72. doi: 10.1097/00002508-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan MJ, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77:253–260. doi: 10.1016/S0304-3959(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Burns JW, Kubilus A, Bruehl S, Harden RN, Lofland K. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? A cross-lagged panel analysis. J Consult Clin Psychol. 2003;71:81–91. doi: 10.1037//0022-006x.71.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. doi: 10.1016/j.jpain.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 30.Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–46. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 31.Eccleston C, Crombez G, Scotford A, Clinch J, Connell H. Adolescent chronic pain: patterns and predictors of emotional distress in adolescents with chronic pain and their parents. Pain. 2004;108:221–9. doi: 10.1016/j.pain.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Langer SL, Romano JM, Levy RL, Walker LS, Whitehead WE. Catastrophizing and parental response to child symptom complaints. Child Health Care. 2009;38:1–16. doi: 10.1080/02739610903038750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Q, Tsao JCI, Myers CD, Kim SC, Zeltzer LK. Coping predictors of children’s laboratory-induced pain tolerance, intensity, and unpleasantness. J Pain. 2007;8:708–717. doi: 10.1016/j.jpain.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Lynch-Jordan AM, Kashikar-Zuck S, Goldschneider KR. Parent perceptions of adolescent pain expression: The adolescent pain behavior questionnaire. Pain. 2010;151:834–842. doi: 10.1016/j.pain.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lynch-Jordan AM, Kashikar-Zuck S, Szabova A, Goldschneider KR. The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior. Clin J Pain. 2013;29:681–688. doi: 10.1097/AJP.0b013e3182757720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vervoort T, Craig KD, Goubert L, Dehoorne J, Joos R, Matthys D, Buysse A, Crombez G. Expressive dimensions of pain catastrophizing: a comparative analysis of school children and children with clinical pain. Pain. 2008;134:59–68. doi: 10.1016/j.pain.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 37.Vervoort T, Goubert L, Eccleston C, Bijttebier P, Crombez G. Catastrophic thinking about pain is independently associated with pain severity, disability, and somatic complaints in school children and children with chronic pain. J Pediatr Psychol. 2006;31:674–683. doi: 10.1093/jpepsy/jsj059. [DOI] [PubMed] [Google Scholar]
- 38.Vervoort T, Trost Z, Van Ryckeghem DM. Children’s selective attention to pain and avoidance behaviour: the role of child and parental catastrophizing about pain. Pain. 2013;154:1979–1988. doi: 10.1016/j.pain.2013.05.052. [DOI] [PubMed] [Google Scholar]
- 39.Warschburger P, Hanig J, Friedt M, Posovszky C, Schier M, Calvano C. Health-related quality of life in children with abdominal pain due to functional or organic gastrointestinal disorders. J Pediatr Psychol. 2013 doi: 10.1093/jpepsy/jst070. [DOI] [PubMed] [Google Scholar]
- 40.Cano A, Johansen A, Leonard MT, Hanawalt J. What are the marital problems of patients with chronic pain? Curr Pain and Headache Rep. 2005;9:96–100. doi: 10.1007/s11916-005-0045-0. [DOI] [PubMed] [Google Scholar]
- 41.Caes L, Vervoort T, Eccleston C, Goubert L. Parents who catastrophize about their child’s pain prioritize attempts to control pain. Pain. 2012;153:1695–1701. doi: 10.1016/j.pain.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Caes L, Vervoort T, Trost Z, Goubert L. Impact of parental catastrophizing and contextual threat on parents’ emotional and behavioral responses to their child’s pain. Pain. 2012;153:687–695. doi: 10.1016/j.pain.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Goubert L, Vervoort T, De Ruddere L, Crombez G. The impact of parental gender, catastrophizing and situational threat upon parental behaviour to child pain: a vignette study. Eur J Pain. 2012;16:1176–1184. doi: 10.1002/j.1532-2149.2012.00116.x. [DOI] [PubMed] [Google Scholar]
- 44.Goubert L, Vervoort T, Cano A, Crombez G. Catastrophizing about their children’s pain is related to higher parent-child congruency in pain ratings: an experimental investigation. Eur J Pain. 2009;13:196–201. doi: 10.1016/j.ejpain.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Hechler T, Vervoort T, Hamann M, Tietze AL, Vocks S, Goubert L, Hermann C, Wager J, Blankenburg M, Schroeder S, Zernikow B. Parental catastrophizing about their child’s chronic pain: are mothers and fathers different? Eur J Pain. 2011;15:515 e1–9. doi: 10.1016/j.ejpain.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Caes L, Vervoort T, Eccleston C, Vandenhende M, Goubert L. Parental catastrophizing about child’s pain and its relationship with activity restriction: the mediating role of parental distress. Pain. 2011;152:212–222. doi: 10.1016/j.pain.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 47.Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, DuPen MM, Feld AD, Ballard SA, Welsh EM, Jeffery RW, Young M, Coffey MJ, Whitehead WE. Cognitive-behavioral therapy for children with functional abdominal pain and their parents decreases pain and other symptoms. Am J Gastroenterol. 2010;105:946–56. doi: 10.1038/ajg.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy RL, Langer SL, Walker LS, Romano JM, Christie DL, Youssef N, Dupen MM, Ballard SA, Labus JS, Welsh E, Feld LD, Whitehead WE. Twelve-month follow-up of cognitive behavioral therapy for children with functional abdominal pain. JAMA Pediatr. 2013;167:178–184. doi: 10.1001/2013.jamapediatrics.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes AF. Beyond Baron and Kenny: Statistical Mediation Analysis in the New Millenium. Commun Monogr. 2009;76:408–420. [Google Scholar]
- 50.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–83. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 51.Kazdin AE, Nock MK. Delineating mechanisms of change in child and adolescent therapy: methodological issues and research recommendations. J Child Psychol Psychiatry. 2003;44:1116–1129. doi: 10.1111/1469-7610.00195. [DOI] [PubMed] [Google Scholar]
- 52.Morley S, Keefe FJ. Getting a handle on process and change in CBT for chronic pain. Pain. 2007;127:197–198. doi: 10.1016/j.pain.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 53.Lackner JM, Jaccard J, Krasner SS, Katz LA, Gudleski GD, Blanchard EB. How Does Cognitive Behavior Therapy for Irritable Bowel Syndrome Work? A Mediational Analysis of a Randomized Clinical Trial. Gastroenterology. 2007;133:433–444. doi: 10.1053/j.gastro.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones M, Koloski N, Boyce P, Talley NJ. Pathways connecting cognitive behavioral therapy and change in bowel symptoms of IBS. J Psychosom Res. 2011;70:278–285. doi: 10.1016/j.jpsychores.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Lackner JM, Keefer L, Jaccard J, Firth R, Brenner D, Bratten J, Dunlap LJ, Ma C, Byroads M, Group IR. The Irritable Bowel Syndrome Outcome Study (IBSOS): rationale and design of a randomized, placebo-controlled trial with 12 month follow up of self-versus clinician-administered CBT for moderate to severe irritable bowel syndrome. Contemp Clin Trials. 2012;33:1293–310. doi: 10.1016/j.cct.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolitzky-Taylor K, Craske MG, Labus JS, Mayer EA, Naliboff BD. Visceral sensitivity as a mediator of outcome in the treatment of irritable bowel syndrome. Behav Res Ther. 2012;50:647–650. doi: 10.1016/j.brat.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kashikar-Zuck S, Sil S, Lynch-Jordan AM, Ting TV, Peugh J, Schikler KN, Hashkes PJ, Arnold LM, Passo M, Richards-Mauze MM, Powers SW, Lovell DJ. Changes in pain coping, catastrophizing, and coping efficacy after cognitive-behavioral therapy in children and adolescents with juvenile fibromyalgia. J Pain. 2013;14:492–501. doi: 10.1016/j.jpain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wicksell RK, Olsson GL, Hayes SC. Mediators of change in acceptance and commitment therapy for pediatric chronic pain. Pain. 2011;152:2792–2801. doi: 10.1016/j.pain.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Sieberg CB, Williams SE, Simons LE. Do parent protective responses mediate the relation between parent distress and child functional disability among children with chronic pain. J Pediatr Psychol. 2011;36:1043–1051. doi: 10.1093/jpepsy/jsr043. [DOI] [PubMed] [Google Scholar]
- 60.Wassom MC, Schurman JV, Friesen CA, Rapoff MA. A pilot study of “Gutstrong” for adolescents with functional gastrointestinal disorders. Clin Pract Pediatr Psychol. 2013;1:201–213. [Google Scholar]
- 61.Eccleston C, Palermo TM, Fisher E, Law E. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD009660.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Duarte MA, Penna FJ, Andrade EM, Cancela CS, Neto JC, Barbosa TF. Treatment of nonorganic recurrent abdominal pain: cognitive-behavioral family intervention. J Pediatr Gastroenterol Nutr. 2006;43:59–64. doi: 10.1097/01.mpg.0000226373.10871.76. [DOI] [PubMed] [Google Scholar]
- 63.Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol. 1994;62:306–14. doi: 10.1037//0022-006x.62.2.306. [DOI] [PubMed] [Google Scholar]
- 64.Hicks CL, von Baeyer CL, Spafford PA, van Korlaar I, Goodenough B. The Faces Pain Scale - Revised: toward a common metric in pediatric pain measurement. Pain. 2001;93:173–183. doi: 10.1016/S0304-3959(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 65.Garber J, Walker LS, Zeman J. Somatization symptoms in a community sample of children and adolescents: further validation of the Children’s Somatization Inventory. Psychol Assess. 1991;3:588–595. [Google Scholar]
- 66.Walker LS, Beck JE, Garber J, Lambert W. Children’s Somatization Inventory: psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009;34:430–440. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker LS, Garber J, Greene JW. Somatization symptoms in pediatric abdominal pain patients: relation to chronicity of abdominal pain and parent somatization. J Abnorm Child Psychol. 1991;19:379–94. doi: 10.1007/BF00919084. [DOI] [PubMed] [Google Scholar]
- 68.Walker LS, Garber J. Manual for the Children’s Somatization Inventory. Vanderbilt University Medical Center, Department of Pediatrics; Nashville, TN: 2003. [Google Scholar]
- 69.Anderson JL, Acra S, Bruehl S, Walker LS. Relation between clinical symptoms and experimental visceral hypersensitivity in pediatric patients with functional abdominal pain. J Pediatr Gastroenterol Nutr. 2008;47:309–315. doi: 10.1097/MPG.0b013e3181653a6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dengler-Crish CM, Horst SN, Walker LS. Somatic complaints in childhood functional abdominal pain are associated with functional gastrointestinal disorders in adolescence and adulthood. J Pediatr Gastroenterol Nutr. 2011;52:162–165. doi: 10.1097/MPG.0b013e3181ec1d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker LS, Smith CA, Garber J, Claar RL. Testing a model of pain appraisal and coping in children with chronic abdominal pain. Health Psychol. 2005;24:364–374. doi: 10.1037/0278-6133.24.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walker LS, Sherman AL, Bruehl S, Garber J, Smith CA. Functional abdominal pain patient subtypes in childhood predict functional gastrointestinal disorders with chronic pain and psychiatric comorbidities in adolescence and adulthood. Pain. 2012;153:1798–1806. doi: 10.1016/j.pain.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker LS, Smith CA, Garber J, Van Slyke DA. Development and validation of the Pain Response Inventory for Children. Psychol Assess. 1997;9:392. [Google Scholar]
- 74.Van Slyke DA, Walker LS. Mothers’ responses to children’s pain. Clin J Pain. 2006;22:387–391. doi: 10.1097/01.ajp.0000205257.80044.01. [DOI] [PubMed] [Google Scholar]
- 75.Walker LS, Levy RL, Whitehead WE. Validation of a measure of protective parent responses to children’s pain. Clin J Pain. 2006;22:712–716. doi: 10.1097/01.ajp.0000210916.18536.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 77.Wothke W, Arbuckle J. Full-information missing data analysis with Amos. 1996. SPSS White Paper. [Google Scholar]
- 78.Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociol Methodol. American Sociological Association; Washington, DC: 1982. pp. 290–312. [Google Scholar]
- 79.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fairchild AJ, MacKinnon DP. A general model for testing mediation and moderation effects. Prev Sci. 2009;10:87–99. doi: 10.1007/s11121-008-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen WB, McNeal RB. The law of maximum expected potential effect: constraints placed on program effectiveness by mediator relationships. Health Educ Res. 1996;11:501–507. [Google Scholar]
- 82.Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 83.Cui M, Durtschi JA, Donnellan MB, Lorenz FO, Conger RD. Intergenerational transmission of relationship aggression: a prospective longitudinal study. J Fam Psychol. 2010;24:688–697. doi: 10.1037/a0021675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296:1286–1289. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- 85.Derogatis LR. Brief Symptom Inventory (BSI) 18: Administration, scoring, and procedures manual. NCS Pearson; Minneapolis, MN: 2000. [Google Scholar]
- 86.March J, Parker J, Sullivan K, Stallings P, Connors C. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 87.Howell S, Poulton R, Talley NJ. The natural history of childhood abdominal pain and its association with adult irritable bowel syndrome: birth-cohort study. Am J Gastroenterol. 2005;100:2071–2078. doi: 10.1111/j.1572-0241.2005.41753.x. [DOI] [PubMed] [Google Scholar]
- 88.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Romano J, Jensen M, Turner J, Good A, Hops H. Chronic pain patient-partner interactions: Further support for a behavioral model of chronic pain. Behav Ther. 2000;31:415–440. [Google Scholar]
- 90.Deković M, Asscher JJ, Manders WA, Prins PJM, van der Laan P. Within-intervention change: Mediators of intervention effects during multisystemic therapy. Journal of consulting and clinical psychology. 2012;80:574–587. doi: 10.1037/a0028482. [DOI] [PubMed] [Google Scholar]
- 91.Selig JP, Preacher KJ. Mediation models for longitudinal data in developmental research. Res Hum Dev. 2009;6:144–164. [Google Scholar]
- 92.Thorn BE, Burns JW. Common and specific treatment mechanisms in psychosocial pain interventions: The need for a new research agenda. Pain. 2011;152:705–706. doi: 10.1016/j.pain.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Day MA, Thorn BE, Burns JW. The continuing evolution of biopsychosocial interventions for chronic pain. J Cogn Psychother. 2012;26:114–129. [Google Scholar]


