Abstract
Mammalian flavin-containing monooxygenase (FMO) is active towards many drugs with a heteroatom having the properties of a soft nucleophile. Thiocarbamides and thiones are S-oxygenated to the sulfenic acid which can either react with glutathione and initiate a redox-cycle or be oxygenated a second time to the unstable sulfinic acid. In this study, we utilized LC-MS/MS to demonstrate that the oxygenation by hFMO of the thioureas under test terminated at the sulfenic acid. With thiones, hFMO catalyzed the second reaction and the sulfinic acid rapidly lost sulfite to form the corresponding imidazole. Thioureas are often pulmonary toxicants in mammals and, as previously reported by our laboratory, are excellent substrates for hFMO2. This isoform is expressed at high levels in the lung of most mammals, including non-human primates. Genotyping to date indicates that individuals of African (up to 49%) or Hispanic (2–7%) ancestry have at least one allele for functional hFMO2 in lung, but not Caucasians nor Asians. In this study the major metabolite formed by hFMO2 with thioureas from Allergan, Inc. was the sulfenic acid that reacted with glutathione. The majority of thiones were poor substrates for hFMO3, the major form in adult human liver. However, hFMO1, the major isoform expressed in infant and neonatal liver and adult kidney and intestine, readily S-oxygenated thiones under test, with Kms ranging from 7–160 μM and turnover numbers of 30–40 min−1. The product formed was identified by LC-MS/MS as the imidazole. The activities of the mouse and human FMO1 and FMO3 orthologs were in good agreement with the exception of some thiones for which activity was much greater with hFMO1 than mFMO1.
Keywords: Flavin-containing monooxygenase, thioureas, thiones, drug development
Introduction
The flavin-containing monooxygenases (FMO) oxygenate numerous endobiotics and xenobiotics, including therapeutic drugs (Cashman, 1995; 2003; Cashman et al., 1995; Krueger and Williams, 2005; Phillips and Shephard, 2008; Ziegler, 2002). FMO, present in the endoplasmic reticulum of cells in numerous tissues, utilizes NADPH to produce a 4a-hydroperoxyflavin intermediate that oxygenates substrates in a two-electron reaction (Ziegler, 1988). Soft nucleophilic heteroatoms typically make the best substrates, but other factors, including size and charge, also determine if the compound will be a substrate (Cashman, 1995; Ziegler, 1988). In most cases, oxygenation by FMO results in a metabolite less toxic than the substrate, but there are some exceptions including two classes of chemicals, found in a variety of industrial chemicals and therapeutic drugs, thiocarbamides and thiones. The initial oxygenation of the sulfur produces the electrophilic sulfenic acid that is capable of reacting with nucleophiles, including glutathione (Decker and Doerge, 1991; Decker et al., 1992; Henderson et al., 2004; Kim and Ziegler, 2000; Poulsen et al., 1979; Russ and Waring, 1991; Vannelli et al., 2002). A number of thioureas have been demonstrated to initiate a redox cycle in which reduced glutathione and NADPH are depleted, resulting in oxidative stress and tissue injury (Krieter et al., 1984; Neal and Halpert, 1982; Smith and Crespi, 2002). If the sulfenic acid is long-lived enough and is also a good substrate for FMO, it is oxygenated to the sulfinic acid. Sulfinic acids are more reactive that the sulfenic acid metabolite and can damage the cell directly or spontaneously decompose to other products (Ji et al., 2007; Onderwater et al., 1999; 2004). Second-line anti-tuberculosis drugs such as ethionamide (thioamide) and thiacetazone (thiourea) are substrates for mammalian FMOs as well as the Mycobacterium tuberculosis equivalent, EtaA, (Francois et al., 2009; Henderson et al., 2008; Qian and Ortiz de Montellano, 2006). In this case, S-oxygenation represents bioactivation of a pro-drug (Dover et al., 2007; Nishida and Ortiz de Montellano, 2011; Qian and Ortiz de Montellano, 2006; Vannelli et al., 2002).
Our laboratory, and others, has demonstrated that numerous thionsulfur compounds are substrates for mammalian FMOs producing toxicity. FMOs are a super-family of enzymes. In all the mammals examined to date, there exists a single member in each family. In humans, there are five FMO genes expressed, FMOs 1–5 (Hernandez et al., 2004). Each FMO is expressed under developmental- and hormonal control, but, unlike the major monooxygenase system, the cytochrome P450s (CYPs), has not been shown to be responsive to inducers or inhibitors, other than competitive inhibition by other substrates (Janmohamed et al., 2004; Shehin-Johnson et al., 1995; Ziegler, 1988; 2002). The majority of drug metabolism is carried out by three FMOs, FMOs 1, 2 and 3. In humans, FMO1 is the major form in fetal liver, but its expression is repressed shortly after parturition and FMO3 expression is turned on so that FMO3 is the major form in adult liver (Hines, 2006; Hines and McCarver, 2002; Koukouritaki et al., 2002). FMO1 is the major FMO in adult human kidney and intestine (Hines, 2006; Koukouritaki et al., 2002; Yeung et al., 2000). This pattern differs from most laboratory animals in that FMO1 is the major hepatic FMO; one exception is the mouse where there is a gender-specific expression of FMO3 in female liver (Janmohamed et al., 2004; Siddens et al., 2008). A number of mutations in FMO3 result in the heritable disease known as trimethylaminuria, in which patients have reduced capacity to N-oxygenate trimethylamine resulting in excretion of high levels of this noxious odorant in urine and sweat (Phillips and Shephard, 2008; Yeung et al., 2007; Zhang et al., 2003). Trimethylaminuria patients also exhibit altered metabolism of some drugs, suggesting expression of these FMO3 allelic variants could play a role in therapeutic efficacy and/or toxicity of certain drugs (Cashman et al., 2003; Shimizu et al., 2007).
FMO2 is the major pulmonary isoform in most mammals, including non-human primates (Dolphin et al., 1998; Krueger et al., 2001; 2002a). An interesting genetic polymorphism exists in humans. Caucasians and Asians carry a C→T transition mutation (Dolphin et al., 1998; Furnes et al., 2003; Krueger et al., 2002b; 2004b; 2005; Whetstine et al., 2000) resulting in a premature TAG stop codon 64 amino acids from the C-terminal (g.23,238C>T; dbsSNP#rs6661174; FMO2*2). This truncated FMO2 (FMO2.2) is catalytically inactive and cannot be detected in human lung tissue by antibodies recognizing the truncated protein, presumably due to incorrect folding and rapid degradation. Approximately 27% of African-American individuals and up to 49% of individuals from Sub-Saharan Africa (Dolphin et al., 1998; Furnes et al., 2003; Krueger et al., 2002a; Veermah et al., 2008; Whetstine et al., 2000) as well as 2–7% (depending on origin) of Hispanics (Krueger et al., 2005; 2004) possess at least one FMO2*1 allele, coding for the full-length enzymatically active, protein. Although not yet associated with susceptibility to any lung disease, FMO2 expression may play a role in the efficacy and/or toxicity of therapeutic drugs for which lung is a target or a portal of entry.
Expressed hFMO2.1 has high activity toward a series of thiocarbamides synthesized by Allergan, Inc. We have characterized the kinetics of hFMO2.1 toward these thioureas and with a representative of the series, identified the metabolite by LC-MS/MS as the sulfenic acid. No evidence was found for further oxygenation to the sulfinic acid. Addition of glutathione to the reaction resulted in concentration-dependent disappearance of the sulfenic acid. Expressed hFMO1, hFMO3, mFMO1 and mFMO3 were tested with a series of thiones. These compounds were substrates for hFMO1 and to a lesser degree mFMO1 but were generally not oxygenated by hFMO3 or mFMO3. As seen previously by Decker et al. (1992), the major product was identified as the imidazole, indicating that hFMO1 effectively catalyzes the oxygenation of the sulfenic acid to the unstable sulfinic acid which rapidly decomposes to sulfite and the imidazole.
Methods
Chemicals
The compounds under study were provided by Allergan Pharmaceuticals (Irvine, CA). Other reagents and chemicals employed were obtained from Sigma/Aldrich (St. Louis, MO) and were of analytical grade.
Protein Expression and Determination of Kinetics
Human FMOs1, 2 and 3 and mouse Fmo1 and 3 were expressed in Sf9 insect cells utilizing baculovirus as previously described (Henderson et al. 2004; 2008). The kinetics of substrate-dependent oxidation of NADPH, in the presence of Sf9 microsomes containing 50 μg of protein, was determined by loss of absorbance at 340 nm utilizing a Cary 300 dual-beam spectrophotometer. The incubation, containing FMO in 100 mM Tricine, pH 9.5 with 1 mM EDTA and 0.1 mM NADPH, was pre-incubated at 37°C for 3 min prior to the addition of substrate (1–200 μM in ethanol (final concentration of 0.1%)). The reactions were run for 4 min and performed with two different batches of FMO-expressing Sf9 microsomes. The Km and Vmax values were calculated from both Lineweaver-Burk and Eadie-Hofstee plots. For the experiments involving glutathione, concentrations of 0–10 mM glutathione were utilized.
HPLC Conditions
The reaction conditions were the same as used in the substrate-dependent NADPH oxidation with the exception that 50 μg of Sf9 microsomes expressing human or mouse FMOs was utilized, a concentration of 1 mM NADPH employed and substrate (20–500 μM) was added in ethanol or DMSO in a final incubation volume of 100 μl. The reactions were run at 37°C (with shaking), following a 3 min preincubation, for 15 min. The reactions were terminated by the addition of 100 μl methanol and transferred to ice. The incubation was centrifuged at 10,000 × g for 30 minutes at 4°C and an aliquot utilized for analysis by HPLC and/or LC/MS/MS. HPLC was performed with a Waters 2690 equipped with a Model 996 diode array detector. The column utilized was a Waters Nova-Pak C18 4 μm with dimensions of 3.9 × 150 mm. The solvent system utilized was acetonitrile as the organic phase and 10 mM ammonium formate, pH 3.2, as the aqueous phase. The solvent gradient used to elute parent compound and metabolites was 20% acetonitrile for 8 min followed by a linear gradient to 35% acetonitrile over 3 min. The acetonitrile concentration was held at 35% for 3 min and then returned to initial conditions over 3 min. The flow rate was 0.8 ml/min and the column temperature was 35°C. We allowed 5 min between injections for the column to re-equilibrate.
LC/MS/MS
The analysis was performed with a Shimadzu (Shimadzu Scientific Instruments, Columbia, MD) Prominence CBM-20 LC with autosampler connected to a Perkin-Elmer (Applied Biosystems, Foster City, CA) Sciex API 365 mass spectrometer in positive ion mode using TurboIon spray. The runs were performed in both the MS and MS/MS modes. The column, solvents and gradient were as described above only the run was performed at room temperature at a flow rate of 0.5 ml/min and the split ratio was 2:1. The conditions used for multiple reaction monitoring and Q1 full scan modes was as described in Henderson et al. (2008).
Results
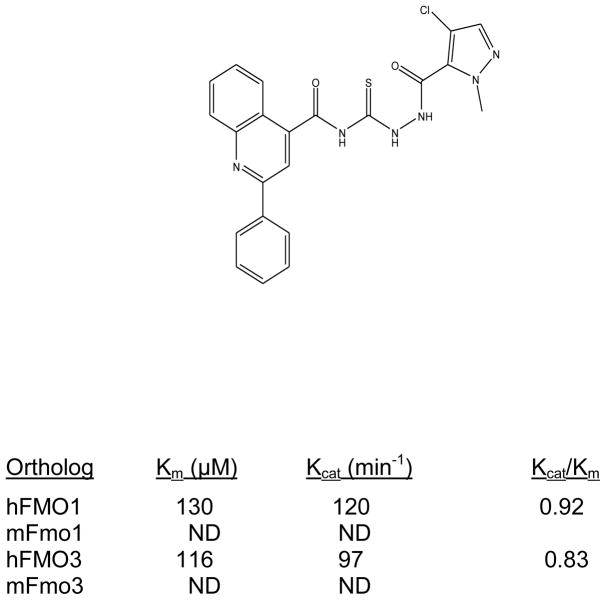
Thioureas have been demonstrated to be effective pulmonary toxicants and some of the Allergan compounds produced pulmonary edema in mice (data not shown). In order to assess the likelihood that FMO2 could bioactivate thioureas in humans expressing the active enzyme (FMO2.1) we determined the kinetics of S-oxygenation with expressed human FMO2.1 by substrate-dependent NADPH oxidation. The thioureas (AGN 1–7) were substrates with Kms varying from 29–165 μM (Table 1). As expected, urea (AGN 9) or imidazolone (AGN 10) derivatives, were not substrates for hFMO2.1 (Table 1). hFMO2.1 is also capable of S-oxygenation of thiones of similar size (AGN 8, Table 1). The size of the halogens (Fl, Cl, Br), meta and/or ortho to the thiourea did not appear to strikingly alter the turnover with hFMO2.1. The lowest specificity constant and highest Km was observed with AGN 5 with chlorines in both positions. The more bulky thiourea, AGN 11 (Figure 1), was oxygenated by both human FMO1 and FMO3 (although the Kms were both > 100 μM); however, the mouse orthologs, Fmo1 and Fmo3 did not demonstrate appreciable activity with this thiourea perhaps indicating a more restricted substrate access channel.
Table 1.
S-Oxygenation by hFMO2 of thioureas with varying o- and m-halogen substituents.
| Compound | Km (μM) | Kcat (min−1) | kcat/Km |
|---|---|---|---|
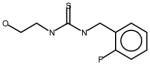 AGN1 |
55 | 36 | 0.65 |
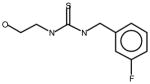 AGN2 |
74 | 42 | 0.57 |
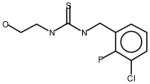 AGN3 |
29 | 23 | 0.79 |
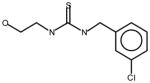 AGN4 |
31 | 35 | 1.13 |
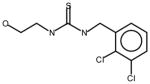 AGN5 |
165 | 38 | 0.23 |
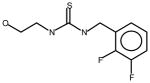 AGN6 |
51 | 34 | 0.67 |
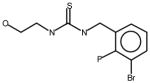 AGN7 |
51 | 33 | 0.65 |
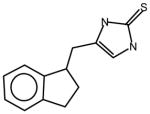 AGN8 |
36 | 25 A thione derivative |
0.69 |
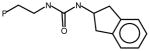 AGN9 |
No Activity – urea derivative | ||
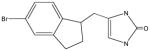 AGN10 |
No activity – imidazolone derivative | ||
Figure 1. S-Oxygenation of AGN 11 by hFMO1 and hFMO3 Compared to the Mouse Orthologs mFmo1 and mFmo3.
Activity of human, hFMO1 and hFMO3, as well as mouse, mFmo1 and mFmo3, toward AGN 11, a bulky thiourea. Kinetic constants were determined using Lineweaver-Burk and Eadie-Hofstee plots taking the average from two Sf9 insect cell microsomal preparations. ND indicates that the activity was too low to determine kinetic constants.
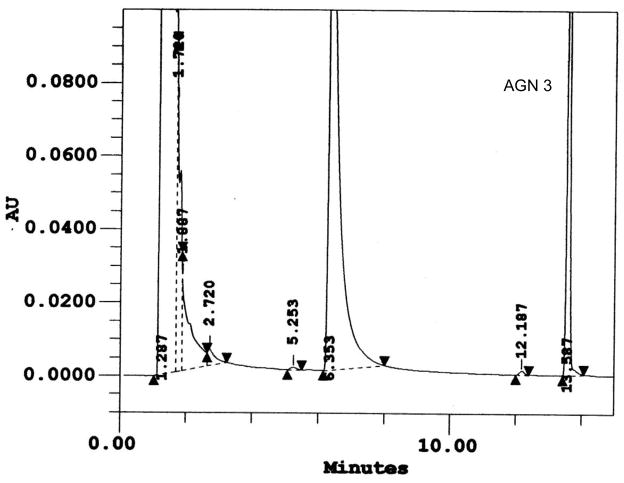
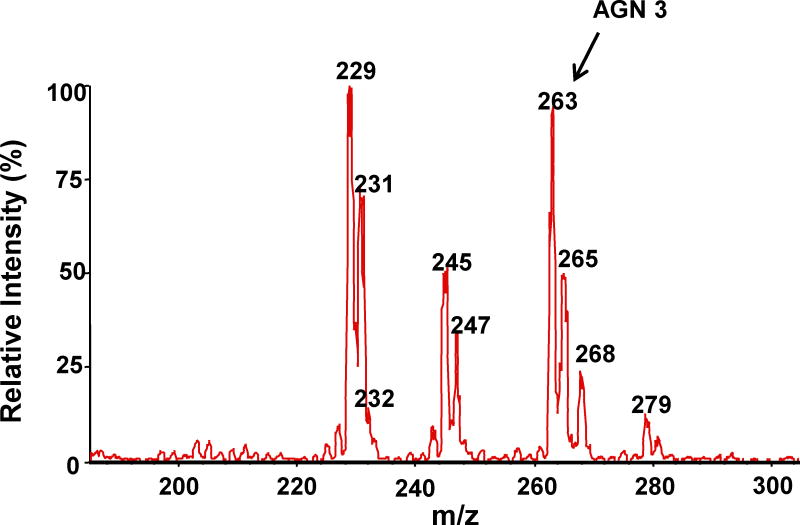
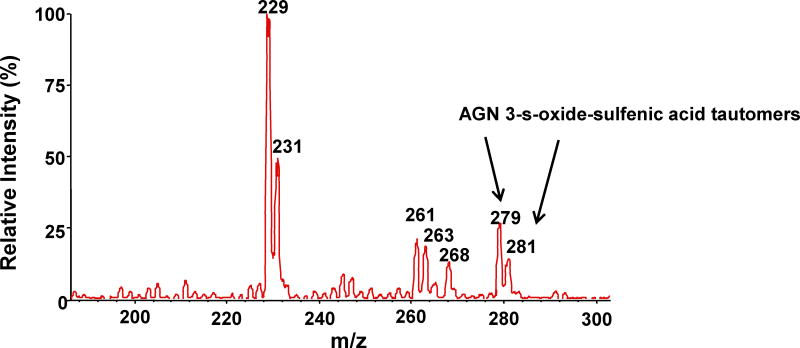
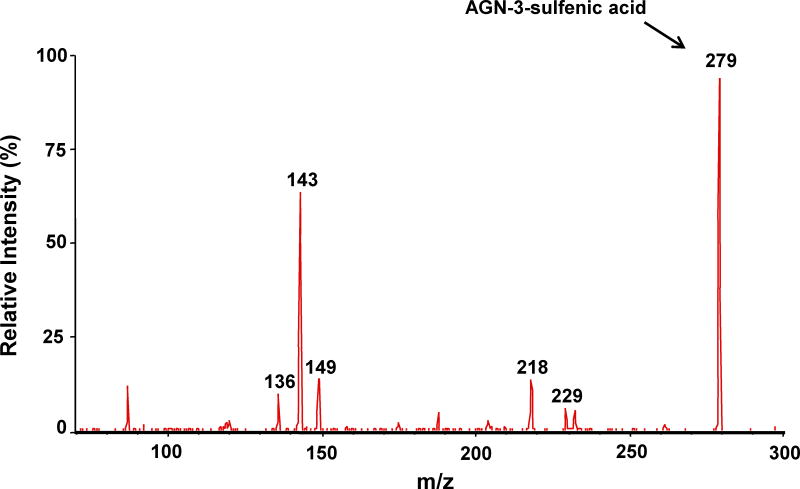
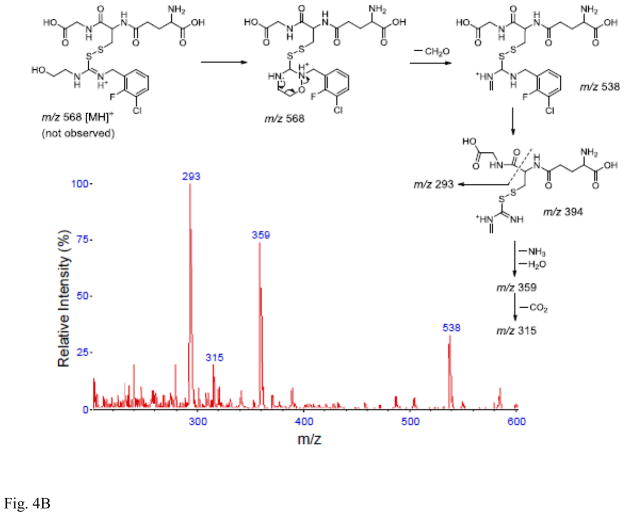
Following incubation of expressed hFMO2.1 with AGN 3, a single polar metabolite was detected by HPLC (Figure 2). We performed LC-MS/MS to identify the major metabolite that was determined to be the S-oxide-sulfenic acid tautomer (Figure 3). Addition of reduced glutathione to the incubation resulted in disappearance of the sulfenic acid (Figure 4A) and in formation of a glutathione adduct, which appeared as a fragment ion [MH-CH2O]+ at m/z 538 (Figure 4B). No sulfenic acid was detected in incubations of hFMO2.1. Significant GSH-dependent reduction in sulfenic acid yield and presence of AGN 3 was observed even when the GSH was added 15 min after initiation of the incubation, suggesting that AGN 3 sulfenic acid undergoes both glutathione adduction and redox-cycling (Figure 5). These results document that expression of FMO2 in human lung is very capable of bioactivation of this series of thioureas to sulfenic acids.
Figure 2. HPLC of the Major Metabolite Following Incubation of AGN 3 (see Table 1) with hFMO2.
Incubation of hFMO2 with the thiourea AGN 3 in the presence of NADPH produced a single polar metabolite (peak at 6.4 minutes). The parent compound eluted at 13.6 minutes under these conditions.
Figure 3.
LC-MS/MS of the polar metabolite of AGN 3. A. MS scan of the parent AGN 3 thiourea (m/z 263); there appears to be a small amount of contamination with formamidine sulfenic acid; B. MS scan of the major polar metabolite (m/z 279 & 281) identified as the S-oxide-sulfenic acid tautomer; C. MS/MS scan of the AGN 3 sulfenic acid metabolite (m/z 279).
Figure 4.
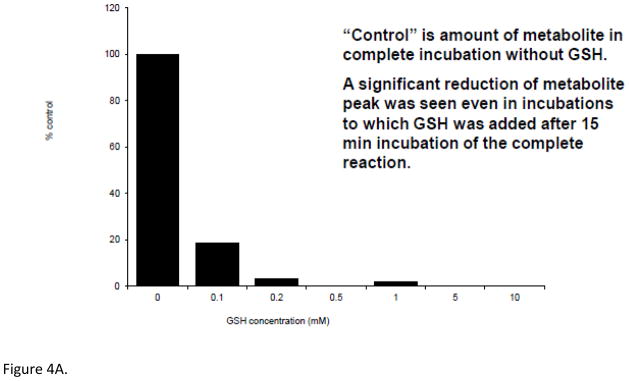
Addition of glutathione eliminates the sulfenic acid metabolite. A. Concentration-dependent yield of the AGN 3 sulfenic acid in the presence of glutathione. Metabolite was reduced even when GSH was added 15 min after incubation began. The percent metabolite on the y axis is compared to the yield in the absence of glutathione. B. Mass spectrum and proposed mass fragmentation pathwy of the glutathione adduct of AGN 3.
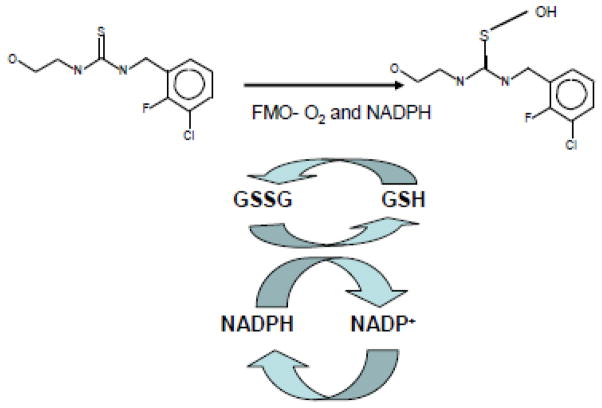
Figure 5. Diagram of Redox-Cycling Generated by S-Oxygenation of Thiourea AGN 3 (.
Diagram of the proposed redox cycle that occurs with AGN 3 in the presence of glutathione.
In order to test the activity toward thiones of the major FMOs expressed in liver, kidney and intestine, we examined the metabolism of a series of Allergan, Inc. thiones utilizing expressed hFMO1 and hFMO3. We also thought it important to examine the expressed mouse orthologs to determine the suitability of this species as a model for FMO-dependent drug metabolism. hFMO1 S-oxygenated these thiones with Kms ranging from 7–161 μM (Table 2). In each example, the Km with the hFMO1 was lower than with mFMO1. With the majority of the thiones, the Km of the mFMO1 was so high as to be undeterminable. The thiones were poor substrates for both human FMO3 (Table 2) and mouse Fmo3 (data not shown). A Km could not be calculated with hFMO3 or with mFmo3 for any of the thiones. At concentrations of 200 μM, a Kcat an order of magnitude slower than with FMO1 was detected (data not shown).
Table 2.
S-oxygenation of thiones by human and mouse FMOs.
| Compound | hFMO1 | mFMO1 | hFMO3 | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Km (μM) | kcat (min−1) | kcat/Km | Km (μM) | kcat (min−1) | kcat/Km | Km | |
 AGN12 |
139 | 49 | 0.35 | 159 | 47 | 0.35 | ND |
|
AGN13 |
40 | 40 | 1.00 | 40 | 48 | 1.20 | ND |
|
AGN14 |
161 | 40 | 0.24 | ND | 23 @ 200 μM | -- | ND |
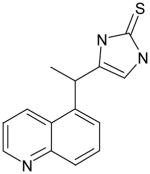 AGN15 |
111 | 33 | 0.30 | ND | 28 @ 200 μM | -- | ND |
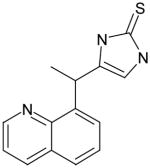 AGN-16 |
7 | 38 | 5.43 | ND | 34 @ 200 μM | -- | ND |
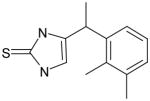 AGN-17 |
31 | 41 | 1.32 | ND | 40 @ 200 μM | -- | ND |
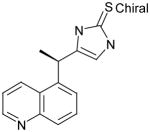 AGN18 |
60 | 30 | 0.50 | ND | 27 @ 200 μM | -- | ND |
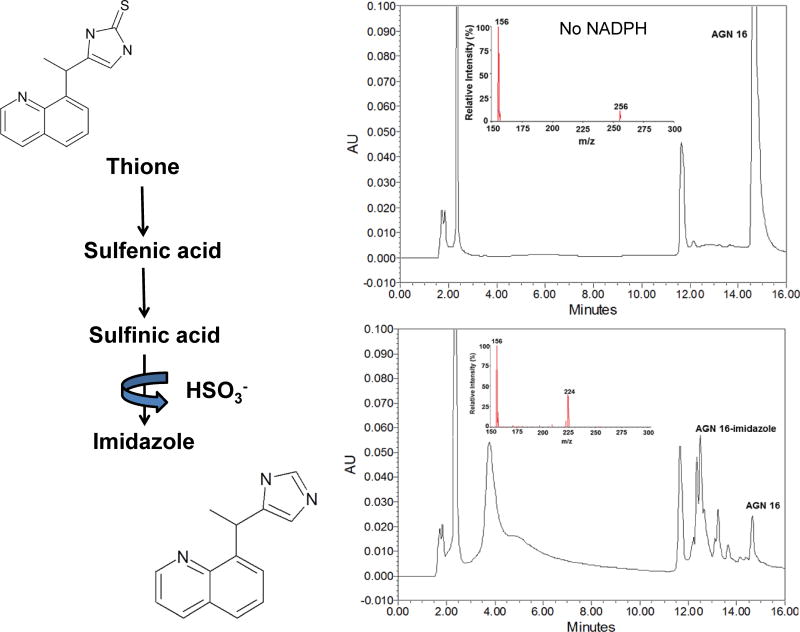
The products formed following incubation of one of the thiones (AGN 16) were determined by LC-MS/MS. The major metabolite from HPLC gave an MS peak at minus 32 mass units from the original thione, consistent with formation of an imidazole (Figure 6). The imidazole is postulated to be formed through a two-step oxygenation to the sulfenic and sulfinic acids with subsequent decomposition, release of the sulfur as sulfite and formation of the imidazole (Figure 6). This pathway has been previously described for benzimidazoline-2-thiones by Decker et al. (1992).
Figure 6.
HPLC-MS Analysis of hFMO1 Conversion of the Thione AGN 16 to the Imidazole
LC-MS/MS analysis of hFMO1 conversion of the thione AGN 16 to the imidazole. HPLC chromatogram of AGN 16 (top right panel) with corresponding MS spectra (m/z 256), following incubation with expressed hFMO1. Addition of NADPH (bottom right panel) converts most of the parent compound (14.8 min peak) to slightly more polar compounds (12.5 min peak; MS spectra m/z 224), consistent with the loss of S.
Discussion
Mammalian FMOs typically display high activity toward oxygenation of the sulfur in thioureas and thiocarbamides (Decker and Doerge, 1991; Decker et al., 1992; Henderson et al., 2004; Kim and Ziegler, 2000; Krieter et al., 1984; Neal and Halpert, 1982; Poulsen et al., 1979; Russ and Waring, 1991; Smith and Crespi, 2002; Vannelli et al., 2002). Previous work has identified the initial metabolite as the sulfenic acid. Sulfenic acids either react with cellular nucleophiles, such as glutathione, or can be oxygenated a second time to the reactive and unstable sulfinic acid. In our previous studies with expressed hFMO2 typically a single oxygenation would occur and the sulfenic acid would be eliminated in a dose-dependent manner by the addition of glutathione, even after 15 min of reaction (Henderson et al., 2004; 2008). Thiourea, phenylthiourea, ethylenethiourea and α-naphthylthiourea had Kms of 27, 29, 14 and 42 μM, respectively and kcats of 51, 24, 48 and 33 min−1, respectively (Henderson et al., 2004). Rabbit FMO2 displayed Kms for these thioureas in the same range (21–82 μM) as the human enzyme (Henderson et al., 2004; Nagata et al., 1990). Diphenylthiourea is not a substrate for rabbit or human FMO2, apparently because the larger cross sectional area prevents the sulfur from access to the FAD hydroperoxide active site of the enzyme; FMO2 has the most restrictive active site channel for substrates (Nagata et al., 1990). The activity of hFMO2.1 toward the Allergan thioureas displays similar kinetics (Kms of 29–165 μM and kcat/Kms of 0.23–1.13) to that previously seen with hFMO2.1 and thiourea, ethylenethiourea, phenylthiourea and α-naphthylthiourea (kcat/Km of 0.8–3.4, Henderson et al., 2004). These data reinforce the hypothesis that the major determinant of oxygenation of thioureas by FMO2 is size and the nucleophilic character of the sulfur heteroatom (Poulsen et al., 1979; Ziegler, 1988). With the AGN thioureas tested here, the size and electron-withdrawing potential of the ortho- and meta-substituted halogens did not significantly impact activity of hFMO2.1 towards S-oxygenation in any consistent way. Onderwater et al. (2006) had shown, utilizing a series of N-substituted N′-(4-imidazole-ethyl)thioureas, that the Km of hFMO1 (but not FMO3) was reduced with the electron withdrawing capacity of the N-p-phenyl substituents.
The data also demonstrate that hFMO2.1 is capable of S-oxygenation of a wide range of thioureas and readily forms the sulfenic acid. The sulfenic acid is then capable of establishing a redox cycle, consuming glutathione and NADPH, resulting in oxidative stress and toxicity in the lung. Dr. Paul Ortiz de Montellano’s laboratory and others have shown that 2-ethylthioisonicotinamide (ethionamide), a second line anti-tuberculosis drug, is oxygenated by human FMO (Francosis et al., 2009; Henderson et al., 2008; Nishida and Ortiz de Montellano, 2011; Qian and Ortiz de Montellano, 2006; Vannelli et al., 2002). We recently have found that, unlike the thioureas, hFMO2.1 does carry out the second S-oxygenation to the sulfinic acid but at a slower rate (Henderson et al., 2008). This finding is important, given that ethionamide is a prodrug and is bioactivated through the sulfinic acid by a similar monooxygenase found in Mycobacterium tuberculosis (Dover et al., 2007; Hannoulle et al., 2006). Our laboratory has recently found mice, null for Fmo2 expression, exhibit markedly lower ratios of ethionamide sulfoxide/ethionamide in plasma (Palmer et al., 2012) and pulmonary lavage fluid (Singh et al., unpublished) following dosing with this anti-tuberculosis prodrug. Taken together, these data suggest that the genetic polymorphism in expression of hFMO2 may have a number of consequences with respect to the toxicity and/or therapeutic efficacy of drugs containing a thiocarbamide or related structural moiety in the lung.
The thiones examined in the study were typically good substrates for hFMO1. mFMO1 appears to have a more restricted substrate channel and only slight activity was observed with mFmo1 and thiones at concentrations of ≥ 200 μM. These thiones were poor substrates for hFMO3 and mFmo3. Thiones, capable of being S-oxygenated by human FMO, are also being used as therapeutic agents against disease such as tuberculosis (Qian and Ortiz de Montellano, 2006). It is interesting to note that with hFMO1 the orientation of the quinoline nitrogen relative to the dihydro-imidazole-3-thione results in a 10-fold or greater difference in the kcat/Km (compare AGN 16 with AGN 15 and AGN 18 in Table 2).
In summary, drugs containing the thiocarbamide or thione structure are S-oxygenated by multiple FMOs. FMO2 S-oxygenates thioureas to the sulfenic acid which may result in pulmonary oxidative stress and toxicity. Only humans (a percentage of individuals of African or Hispanic descent) expressing the full-length FMO2.1 enzyme would be expected to be susceptible. Thiones are substrates for hFMO1, expressed in fetal liver and adult intestine and kidney. Mice are unusual among mammals in that mFmo1 is expressed at appreciable levels in lung (Janmohamed et al., 2004; Siddens et al., 2008) and therefore studies of FMO-dependent toxicity of these compounds in this model must be interpreted in that light.
Thiones appear to be poor substrates for hFMO3, making it unlikely that this enzyme would be responsible for any hepatotoxicity associated with these drugs. As previously demonstrated by Decker et al. (1992), thiones are S-oxygenated by FMO first to the sulfenic acid, then the sulfinic acid which rapidly and spontaneous decomposes to the imidazole with release of HSO3−. In the presence of GSH, the imidazole is not formed as, with thioureas, GSH binds to the intermediates. FMO1 metabolism and bioactivation in intestine could impact oral bioavailability and in kidney result in nephrotoxicity. Our results reinforce the need to consider FMO activity in drug development for thiocarbamides and related compounds capable of S-oxygenation to reactive intermediates.
Acknowledgments
The authors thank Jonathan VanDyke for technical assistance. This research was conducted with the assistance of the Mass Spectrometry Core of the Environmental Health Sciences Center (ES000210) at Oregon State University. The work was funded by PHS grant HL038650 (DEW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cashman JR. Structure and catalytic properties of the mammalian flavin-containing monooxygenase. Chem Res Toxicol. 1995;8:165–181. doi: 10.1021/tx00044a001. [DOI] [PubMed] [Google Scholar]
- Cashman JR. The role of flavin-containing monooxygenases in drug metabolism and development. Curr Opin Drug Discov Devel. 2003;6:486–493. [PubMed] [Google Scholar]
- Cashman JR, Camp K, Fakharzadeh SS, Fennessey PV, Hines RN, Mamer OA, Mitchell SC, Nguyen GP, Schlenk D, Smith RL, Tjoa SS, Williams DE, Yannicelli S. Biochemical and clinical aspects of the human flavin-containing monooxygenase form 3 (FMO3) related to trimethylaminuria. Curr Drug Metab. 2003;4:151–170. doi: 10.2174/1389200033489505. [DOI] [PubMed] [Google Scholar]
- Cashman JR, Park SB, Berkman CE, Cashman LE. Role of hepatic flavin-containing monooxygenase in drug and chemical metabolism in adult humans. Chem-Biol Interact. 1995;96:33–46. doi: 10.1016/0009-2797(94)03581-r. [DOI] [PubMed] [Google Scholar]
- Decker CJ, Doerge DR. Rat hepatic microsomal metabolism of ethylenethiourea. Contributions of the flavin-containing monooxygenase and cytochrome P-450 isozymes. Chem Res Toxicol. 1991;4:482–489. doi: 10.1021/tx00022a013. [DOI] [PubMed] [Google Scholar]
- Decker CJ, Doerge DR, Cashman JR. Metabolism of benzimidazoline-2-thiones by rat hepatic microsomes and hog liver flavin-containing monooxygenase. Chem Res Toxicol. 1992;5:726–733. doi: 10.1021/tx00029a021. [DOI] [PubMed] [Google Scholar]
- Dolphin CT, Beckett DJ, Janmohamed A, Cullingford TE, Smith RL, Shephard EA, Phillips IR. The flavin-containing monooxygenase 2 gene (FMO2) of humans, but not of other primates, encodes a truncated, nonfunctional protein. J Biol Chem. 1998;273:30599–30607. doi: 10.1074/jbc.273.46.30599. [DOI] [PubMed] [Google Scholar]
- Dover LG, Alahari A, Gratraud P, Gomes JM, Bhowruth V, Reynolds RC, Besra GS, Kremer L. EthA, a common activator of thiocarbamide-containing drugs acting on different mycobacterial targets. Antimicrob Agents Chemother. 2007;51:1055–1063. doi: 10.1128/AAC.01063-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois AA, Nishida CR, Ortiz de Montellano PR, Phillips IR, Shephard EA. Human flavin-containing monooxygenase 2.1 catalyzes oxygenation of the antitubercular drugs thiacetazone and ethionamide. Drug Metabol Dispos. 2009;37:178–186. doi: 10.1124/dmd.108.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnes B, Feng J, Sommer SS, Schlenk D. Identification of novel variants of the flavin-containing monooxygenase gene family in African Americans. Drug Metab Disp. 2003;31:187–193. doi: 10.1124/dmd.31.2.187. [DOI] [PubMed] [Google Scholar]
- Hanoulle X, Wieruszeski JM, Rousselot-Pailley P, Landrieu I, Locht C, Lippens G, Baulard AR. Selective intracellular accumulation of the major metabolite issued from the activation of the prodrug ethionamide in mycobacteria. J Antimicrob Chemother. 2006;58:768–772. doi: 10.1093/jac/dkl332. [DOI] [PubMed] [Google Scholar]
- Henderson MC, Krueger SK, Stevens JF, Williams DE. Human flavin-containing monooxygenase form 2 S-oxygenation: Sulfenic acid formation from thioureas and oxidation of glutathione. Chem Res Toxicol. 2004;17:633–640. doi: 10.1021/tx034253s. [DOI] [PubMed] [Google Scholar]
- Henderson MC, Siddens LK, Morré, Krueger SK, Ortiz de Montellano PR, Williams DE. Metabolism of the anti-tuberculosis drug ethionamide by mouse and human FMO1, FMO2 and FMO3 and mouse and human lung microsomes. Toxicol Appl Pharmacol. 2008;233:420–427. doi: 10.1016/j.taap.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez D, Janmohamed A, Chandan P, Phillips IR, Shephard EA. Organization and evolution of the flavin-containing monooxygenase genes of human and mouse: identification of novel gene and pseudogene clusters. Pharmacogenetics. 2004;14:117–130. doi: 10.1097/00008571-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Hines RN. Developmental and tissue-specific expression of human flavin-containing monooxygenase 1 and 3. Expert Opin Drug Metab Toxicol. 2006;2:41–49. doi: 10.1517/17425255.2.1.41. [DOI] [PubMed] [Google Scholar]
- Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300:355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- Janmohamed A, Hernandez D, Phillips IR, Shephard EA. Cell-, tissue-, sex- and developmental stage-specific expression of mouse flavin-containing monooxygenases (Fmos) Biochem Pharmacol. 2004;68:73–83. doi: 10.1016/j.bcp.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Ji T, Ikehata K, Koen YM, Esch SW, Williams TD, Hanzlik RP. Covalent modification of microsomal lipids by thiobenzamide metabolites in vivo. Chem Res Toxicol. 2007;20:701–708. doi: 10.1021/tx600362h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Ziegler DM. Size limits of thiocarbamides accepted as substrates by human flavin-containing monooxygenase 1. Drug Metabol Disp. 2000;28:1003–1006. [PubMed] [Google Scholar]
- Koukouritaki SB, Simpson P, Yeung CK, Rettie AE, Hines RN. Human hepatic flavin-containing monooxygenase 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr Res. 2002;51:236–243. doi: 10.1203/00006450-200202000-00018. [DOI] [PubMed] [Google Scholar]
- Krieter PA, Ziegler DM, Hill KE, Burk RF. Increased biliary GSSG efflux from rat livers perfused with thiocarbamides substrates for the flavin-containing monooxygenase. Molec Pharmacol. 1984;26:122–127. [PubMed] [Google Scholar]
- Krueger SK, Williams DE. Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism. Pharmacol Therap. 2005;106:357–387. doi: 10.1016/j.pharmthera.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger SK, Martin SR, Yueh MF, Pereira CB, Williams DE. Identification of active flavin-containing monooxygenase isoform 2 in human lung and characterization of expressed protein. Drug Metab Dispos. 2002a;30:34–41. doi: 10.1124/dmd.30.1.34. [DOI] [PubMed] [Google Scholar]
- Krueger SK, Siddens LK, Henderson MC, Andreasen EA, Tanguay RL, Pereira CB, Cabacungan ET, Hines RN, Ardlie KG, Williams DE. Haplotype and functional analysis of four flavin-containing monooxygenase isoform 2 (FMO2) polymorphisms in Hispanics. Pharmacogenet Genomics. 2005;15:245–256. doi: 10.1097/01213011-200504000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger SK, Siddens LK, Martin SR, Yu Z, Pereira CB, Cabacungan ET, Hines RN, Ardlie KG, Raucy JL, Williams DE. Differences in FMO2*1 allelic frequency between Hispanics of Puerto Rican and Mexican descent. Drug Metab Disp. 2004;32:1337–1340. doi: 10.1124/dmd.104.001099. [DOI] [PubMed] [Google Scholar]
- Krueger SK, Williams DE, Yueh MF, Martin SR, Hines RN, Raucy JL, Dolphin CT, Shephard EA, Phillips IR. Genetic polymorphisms of flavin-containing monooxygenase (FMO) Drug Metabol Rev. 2002b;34:523–532. doi: 10.1081/dmr-120005653. [DOI] [PubMed] [Google Scholar]
- Krueger SK, Yueh MF, Martin SR, Pereira CB, Williams DE. Characterization of expressed full-length and truncated FMO2 from rhesus monkey. Drug Metab Disp. 2001;29:693–700. [PubMed] [Google Scholar]
- Nagata T, Williams DE, Ziegler DM. Substrate specificities of rabbit lung and porcine liver flavin-containing monooxygenases: differences due to substrate size. Chem Res Toxicol. 1990;3:372–376. doi: 10.1021/tx00016a016. [DOI] [PubMed] [Google Scholar]
- Neal RA, Halpert J. Toxicology of thiono-sulfur compounds. Ann Rev Pharmacol Toxicol. 1982;22:321–339. doi: 10.1146/annurev.pa.22.040182.001541. [DOI] [PubMed] [Google Scholar]
- Nishida CR, Ortiz de Montellano PR. Bioactivation of antituberculosis thioamide and thiourea prodrugs by bacterial and mammalian flavin monooxygenases. Chem-Biol Interact. 2011;192:21–25. doi: 10.1016/j.cbi.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onderwater RCA, Commandeur JNM, Menge WMPB, Vermeulen NPE. Activation of microsomal glutathione S-transferase and inhibition of cytochrome P4501A1 activity as a model system for detection protein alkylation by thiourea-containing compounds in rat liver microsomes. Chem Res Toxicol. 1999;12:396–402. doi: 10.1021/tx980198p. [DOI] [PubMed] [Google Scholar]
- Onderwater RCA, Commandeur JNM, Vermeulen NPE. Comparative cytotoxicity of N′-substituted N′-(4-imidazole-ethyl)thiourea in precision-cut rat liver slices. Toxicol. 2004;197:81–91. doi: 10.1016/j.tox.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Onderwater RCA, Rettie AE, Commandeur JNM, Vermeulen NPE. Bioactivation of N-substituted N′-(4-imidazole-ethyl)thioureas by human FMO1 and FMO3. Xenobiotica. 2006;36:645–657. doi: 10.1080/00498250500354329. [DOI] [PubMed] [Google Scholar]
- Palmer AL, Leykam VL, Larkin A, Krueger SK, Phillips IR, Shepard EA, Williams DE. Metabolism and pharmacokinetics of the anti-tuberculosis drug ethionamide in a flavin-containing monooxygenase null mouse. Pharmaceut. 2012;5:1147–1159. doi: 10.3390/ph5111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips IR, Shephard EA. Flavin-containing monooxygenases: mutations, disease and drug response. Trends Pharmacol Sci. 2008;29:294–301. doi: 10.1016/j.tips.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Poulsen LL, Hyslop RM, Ziegler DM. S-Oxygenation of N-substituted thioureas catalyzed by the pig liver microsomal FAD-containing monooxygenase. Arch Biochem Biophys. 1979;198:78–88. doi: 10.1016/0003-9861(79)90397-7. [DOI] [PubMed] [Google Scholar]
- Qian L, Ortiz de Montellano PR. Oxidative activation of thiacetazone by the Mycobacterium tuberculosis flavin monooxygenase EtaA and human FMO1 and FMO3. Chem Res Toxicol. 2006;19:443–449. doi: 10.1021/tx050328b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ MJ, Waring RH. The effect of methimazole on thioamide bioactivation and toxicity. Toxicol Lett. 1991;58:37–41. doi: 10.1016/0378-4274(91)90188-c. [DOI] [PubMed] [Google Scholar]
- Shehin-Johnson S, Williams DE, Larsen-Su S, Stresser DM, Hines DM. Tissue-specific expression of flavin-containing monooxygenase (FMO) forms 1 and 2 in the rabbit. J Pharmacol Exp Therap. 1995;272:1293–1299. [PubMed] [Google Scholar]
- Shimizu M, Yano H, Nagashima S, Murayama N, Zhang J, Cashman JR, Yamazaki H. Effects of genetic variants of the human flavin-containing monooxygenase 3 on N- and S-oxygenation activities. Drug Metab Dispos. 2007;35:328–330. doi: 10.1124/dmd.106.013094. [DOI] [PubMed] [Google Scholar]
- Siddens LK, Henderson MC, VanDyke JE, Williams DE, Krueger SK. Characterization of mouse flavin-containing monooxygenase transcript levels in lung and liver, and activity of expressed isoforms. Biochem Pharmacol. 2008;75:750–759. doi: 10.1016/j.bcp.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Crespi C. Thiourea toxicity in mouse C3H/10T1/2 cells expressing human flavin-containing monooxygenase 3. Biochem Pharmacol. 2002;63:1941–1948. doi: 10.1016/s0006-2952(02)00978-4. [DOI] [PubMed] [Google Scholar]
- Vannelli TA, Dykman A, Ortiz de Montellano PR. The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J Biol Chem. 2002;277:12824–12829. doi: 10.1074/jbc.M110751200. [DOI] [PubMed] [Google Scholar]
- Veermah KR, Thomas MG, Weale ME, Aeitlyn D, Tarekegn A, Bekele E, Mendell NR, Shephard EA, Bradman N, Phillips IR. The potentially deleterious functional variant flavin-containing monooxygenase 2*1 is at high frequency throughout sub-Saharan Africa. Pharmacogenet Genomics. 2008;18:877–886. doi: 10.1097/FPC.0b013e3283097311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstine JR, Yueh MF, Hopp KA, McCarver DG, Williams DE, Park CS, Kang JH, Cha YN, Dolphin CT, Shephard EA, Phillips IR, Hines RN. Ethnic differences in human flavin-containing monooxygenase 2 (FMO2) polymorphisms: detection of expressed protein in African Americans. Toxicol Appl Pharmacol. 2000;168:216–224. doi: 10.1006/taap.2000.9050. [DOI] [PubMed] [Google Scholar]
- Yeung CK, Adman ET, Rettie AE. Functional characterization of genetic variants of human FMO3 associated with trimethylaminuria. Arch Biochem Biophys. 2007;464:251–259. doi: 10.1016/j.abb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CK, Lang DH, Thummel KE, Rettie AE. Immunoquantitation of FMO1 in human liver, kidney, and intestine. Drug Metab Disp. 2000;28:1107–1111. [PubMed] [Google Scholar]
- Zhang J, Tran Q, Lattard V, Cashman JR. Deleterious mutations in the flavin-containing monooxygenase 3 (FMO3) gene causing trimethylaminuria. Pharmacogenetics. 2003;13:495–500. doi: 10.1097/00008571-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Ziegler DM. Flavin-containing monooxygenases: catalytic mechanism and substrate specificities. Drug Metab Rev. 1988;19:1–32. doi: 10.3109/03602538809049617. [DOI] [PubMed] [Google Scholar]
- Ziegler DM. An overview of the mechanism, substrate specificities and structure of FMOs. Drug Metab Rev. 2002;34:503–511. doi: 10.1081/dmr-120005650. [DOI] [PubMed] [Google Scholar]