Abstract
Objectives
This study sought to determine feasibility and prognostic performance of stress cardiac magnetic resonance (CMR) in obese patients (body mass index [BMI] ≥30 kg/m2).
Background
Current stress imaging methods remain limited in obese patients. Given the impact of the obesity epidemic on cardiovascular disease, alternative methods to effectively risk stratify obese patients are needed.
Methods
Consecutive patients with a BMI ≥30 kg/m2 referred for vasodilating stress CMR were followed for major adverse cardiovascular events (MACE), defined as cardiac death or nonfatal myocardial infarction. Univariable and multivariable Cox regressions for MACE were performed to determine the prognostic association of inducible ischemia or late gadolinium enhancement (LGE) by CMR beyond traditional clinical risk indexes.
Results
Of 285 obese patients, 272 (95%) completed the CMR protocol, and among these, 255 (94%) achieved diagnostic imaging quality. Mean BMI was 35.4 ± 4.8 kg/m2, with a maximum weight of 200 kg. Reasons for failure to complete CMR included claustrophobia (n = 4), intolerance to stress agent (n = 4), poor gating (n = 4), and declining participation (n = 1). Sedation was required in 19 patients (7%; 2 patients with intravenous sedation). Sixteen patients required scanning by a 70-cm-bore system (6%). Patients without inducible ischemia or LGE experienced a substantially lower annual rate of MACE (0.3% vs. 6.3% for those with ischemia and 6.7% for those with ischemia and LGE). Median follow-up of the cohort was 2.1 years. In a multivariable stepwise Cox regression including clinical characteristics and CMR indexes, inducible ischemia (hazard ratio 7.5; 95% confidence interval: 2.0 to 28.0; p = 0.002) remained independently associated with MACE. When patients with early coronary revascularization (within 90 days of CMR) were censored on the day of revascularization, both presence of inducible ischemia and ischemia extent per segment maintained a strong association with MACE.
Conclusions
Stress CMR is feasible and effective in prognosticating obese patients, with a very low negative event rate in patients without ischemia or infarction.
Keywords: cardiac magnetic resonance, obesity, stress testing
Obesity (body mass index [BMI] ≥30 kg/m2) affects more than 1 in 3 Americans (1), with an increased risk of coronary artery disease (CAD) independent of traditional coronary risk factors (2,3). Given the explosion in obesity and obesity-related cardiovascular and metabolic disease, the ability to risk stratify obese patients for adverse cardiovascular events is paramount. Despite their widespread use in a general referral population, both stress echocardiography and nuclear perfusion have important technical challenges in obese patients. Stress echocardiography is limited by operator dependence of acquisition and poor acoustic windows as a result of body habitus, and its impact on long-term prognosis remains unclear. As the mainstay of current clinical practice, single-photon emission computed tomography (SPECT) nuclear perfusion imaging may be hampered by attenuation artifacts and reduced signal-to-noise ratio, limiting diagnostic accuracy in obese patients (4–9). The prognostic impact of a negative nuclear scan may be associated with BMI. In one study, a normal perfusion scan was associated with a nearly 1.7% risk of death at 1 year (9). Although positron emission tomography (PET) overcomes many of these challenges, as a result of superior diagnostic and prognostic performance in obese patients (10), ionizing radiation, radiotracer availability, and cost pose important limitations to its widespread adoption.
Stress cardiac magnetic resonance (CMR) can assess ventricular function, stress and rest perfusion, and viability within a single examination (11). Relative to alternative techniques, stress CMR has high spatial and temporal resolution and is not limited by acoustic windows or image acquisition. However, concerns over feasibility in obese patients because of claustrophobia and safety monitoring have limited the adoption of stress CMR in this important, burgeoning population. To address these fundamental concerns, we sought to determine the feasibility of stress CMR in obese patients and the prognostic impact of stress CMR results on cardiovascular events.
Methods
Study population
We identified patients with a BMI ≥30 kg/m2 from consecutive patients ≥18 years of age referred to Brigham and Women's Hospital between December 2001 and August 2011 for the assessment of suspected myocardial ischemia. Exclusion criteria consisted of known hypersensitivity to gadolinium-based contrast agents, glomerular filtration rate <30 ml/min/1.73 m2, pregnancy, weight >250 kg (because of table limitations), acute myocardial infarction (MI), hemodynamic instability, presence of metallic hazards, and history of asthma or bronchospastic disease. Patients with atrial fibrillation at the time of CMR were included in the study. Clinical data were collected by medical history and clinical examination on the day of stress CMR by a trained physician using standardized criteria. Our institutional review board approved follow-up of clinical events, and all participating patients gave written informed consent for stress CMR.
Cardiac magnetic resonance
Patients were scanned with a 60-cm–bore, 1.5-T magnet (Signa CV/i, GE Medical Systems, Milwaukee, Wisconsin) with an 8-element phased-array coil before 2006 and with a 3.0-T scanner (60-cm–bore Magnetom Trio [TIM system], Siemens, Erlangen, Germany) with a 16-element phased array coil after 2006. With the availability of a wide-bore scanner (70-cm bore diameter, 3.0-T Magnetom Verio, Siemens) in 2007, this scanner was used when requested by the referral clinician, significant patient history of claustrophobia from pre-CMR screening, or failure to perform CMR at either 60-cm–bore scanners. Oral sedation (diazepam 0.5 to 1.0 mg) was given 15 to 20 min before CMR examination where necessary. Intravenous conscious sedation was offered if oral sedation failed to control claustrophobic symptoms (both via short-acting benzodiazepine, intravenous or oral; or fentanyl, intravenous). The stress CMR protocol was optimized for obese patients via: 1) lateral placement of 1 of the electrocardiographic leads to improve gating signal; 2) increasing field of view with real-time adjustment to prevent wrap artifact; and 3) dynamic gradient echo shimming around cardiac structures before cine steady-state free precession imaging. All patients were instructed to refrain from caffeine for 24 h and to fast for at least 6 h before the CMR examination. Our CMR protocol included localizers, cine function, stress and rest perfusion, and late gadolinium enhancement (LGE) imaging. A 12-lead electrocardiogram was performed both before and following CMR examination. Cine images were acquired in both short-axis (contiguous 8-mm slices) and long-axis (4-chamber, 2-chamber, and 3-chamber) views of the left ventricle with steady-state free precession cine imaging (repetition time 3.4 ms; echo time 1.2 ms; matrix 256 × 256; field of view 34 to 40 cm).
Vasodilator stress was achieved using either intravenous adenosine (Adenoscan, Astellas Pharma US, Deerfield, Illinois) (n = 149 [58%]) infused at a rate of 140 μg/kg/min over 6 min or regadenoson (Lexiscan, Astellas Pharma US) (n = 106 [42%]) as an intravenous bolus of 0.4 mg, hand-injected over 10 s. Myocardial perfusion images for both stress and rest were acquired with a saturation-prepared, single-shot spoiled gradient echo sequence (repetition time 6 ms; echo time 2.3 ms; slice thickness 8 mm) during bolus injection of 0.1 mmol/kg intravenous gadolinium (Magnevist, Bayer HealthCare, Wayne, New Jersey) at 4 to 5 ml/s, followed by a saline flush. Myocardial perfusion images were acquired in 3 short-axis (basal, mid, and apical) and 1 long-axis (4-chamber) view (for acquisitions on the Siemens scanners). LGE was performed using a standard inversion recovery gradient echo sequence in short- and long-axis slices 10 to 15 min after rest perfusion imaging (with total gadolinium dose 0.20 mmol/kg, with appropriate TI time to null normal myocardium). Vital signs, electrocardiography, and respiration were monitored contin uously throughout the CMR scan. Blood pressure was measured with noninvasive sphygmomanometry before and following administration of the vasodilator agent. All images were acquired at end-expiration with electrocardiographic gating (with provisional pulse gating for electrocardiographic gating failure).
CMR analysis
Perfusion images were interpreted by 2 experienced observers (R.Y.K. and B.H.) blinded to clinical and follow-up data. CMR images were analyzed by standard post-processing techniques on an offline workstation (Mass Clinical 7.4, Medis, Leiden, the Netherlands). Ventricular volumes, function, and mass were quantified by previously published techniques (12). Cine wall motion was assessed in a 17-segment American Heart Association model, and perfusion imaging was assessed in a 16-segment model (13). Inducible ischemia was defined as any stress perfusion defect that: 1) remained dark and persisted for at least 3 phases beyond peak contrast enhancement; 2) was at least 1 pixel in thickness; and 3) followed a coronary distribution. These criteria are similar to multicenter published studies (14,15). Inducible ischemia was defined as the presence of any segmental subendocardial stress perfusion defect in the absence of LGE in the same segment. Therefore, segments with LGE and matching stress perfusion defects were not counted as “inducible ischemia.” LGE was evaluated qualitatively (presence or absence) and quantitatively using a signal intensity threshold of ≥2 SD above remote normal myocardium, as previously published (12).
Clinical outcomes
Patient follow-up was obtained through review of the electronic medical record, followed by a mailed standard questionnaire or scripted telephone interview by trained study personnel (when medical records were not available). Mortality was confirmed by the Social Security Death Index. Our primary outcome was a composite of cardiac death or nonfatal MI, defined as major adverse cardiac events (MACE). Our secondary outcome was a composite of all-cause mortality or nonfatal MI. Nonfatal MI was defined as a new clinical presentation of chest pain or dyspnea with an elevation of cardiac biomarkers (>99th percentile of the upper limits of normal) in a temporal pattern consistent with an acute MI (16).
Statistics
Parametric continuous data were expressed as means with SD, whereas nonparametrically distributed continuous data were presented as medians with interquartile range (IQR). Categorical variables were expressed as counts with percentages. Comparison between groups was performed with a 2-sample Student t test for continuous, normal data, and Wilcoxon rank sum test for continuous, non-normal data. The Fisher exact test was used to compare stratified categorical data. MACE-free survival stratified by the presence or absence of inducible ischemia was estimated by the Kaplan-Meier method and compared using a log-rank test. Univariable Cox regression models were used to estimate unadjusted hazard of MACE for clinical and CMR characteristics. The independent prognostic value of inducible ischemia for MACE was evaluated using a multivariable Cox model constructed by inclusion of significant covariates on univariable screen in a stepwise selection algorithm (p < 0.05 for model entry and retention). All clinical covariates and CMR indexes (left ventricular and right ventricular function, mass, volumes, wall motion, and perfusion results) were included as potential covariates in the multivariable model. We assessed for the presence of collinearity amongst covariates in the final multivariable model. The proportional hazards assumption for the multivariable model was evaluated quantitatively by including time-dependent covariates in the final multivariable Cox model. All statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, North Carolina). A 2-tailed p < 0.05 was considered statistically significant.
Results
Feasibility of stress CMR in obese patients
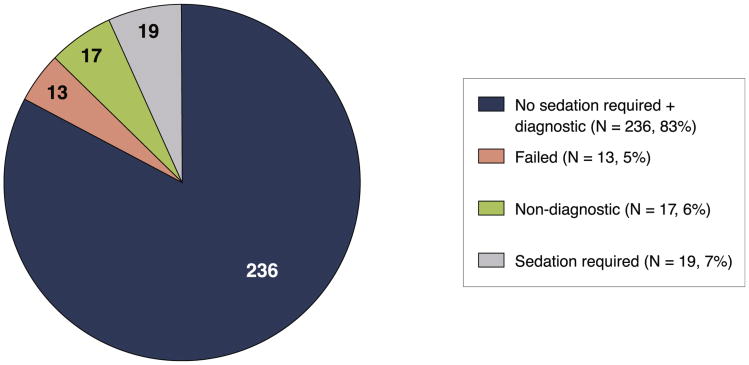
A total of 285 patients fulfilled the inclusion criteria for the study. Thirteen patients (4.6%) were unable to complete the stress CMR study (4 patients were claustrophobic, 4 patients were intolerant of the stress agent, 4 patients had poor gating, and 1 patient declined participation) (Fig. 1). Another 17 patients (6.0%) had nondiagnostic imaging quality, with the remaining 255 patients comprising the study cohort. One hundred thirty-one patients were scanned on the 1.5-T scanner before August 2006. Nineteen (6.7%) patients required sedation for the CMR scan, among them 2 patients requiring intravenous conscious sedation (because oral agents were not effective). Sixteen (5.6%) required scanning using a wide-bore scanner, among them 4 patients requiring oral sedation and 1 requiring intravenous sedation. f the overall population, 24 (8%) had atrial fibrillation, and 3 of 24 had nondiagnostic perfusion studies and were excluded from the study.
Figure 1. Feasibility of CMR in Obese Patients.
The pie chart illustrates the feasibility of performing stress perfusion cardiac magnetic resonance (CMR) in the retrospective cohort of obese patients. A total of 285 patients were referred for CMR, 13 (4.6%) of whom failed largely as the result of claustrophobia, intolerance to vasodilator agent, and poor electrocardiographic gating. Of the remaining 272 patients, 255 (94%) were found by 2 experienced CMR observers to have images of diagnostic quality. Sedation to perform CMR was required in 19 (6.7%) patients.
Baseline characteristics
Patient and CMR characteristics for the entire study cohort and stratified by inducible ischemia are shown in Tables 1 and 2, respectively. Primary indications for CMR included suspicion of cardiac ischemia in 80% and evaluation of suspected cardiac dyspnea in 20%. The overall study cohort (mean age 56 years, 40% female) had a mean BMI of 35.4 ± 4.8 kg/m2. The maximum weight successfully scanned was 200 kg (440 lbs). The majority of patients were in sinus rhythm (92%), whereas 21 patients were in atrial fibrillation during stress CMR. Patients with no evidence of inducible ischemia by CMR had a higher BMI (35.8 ± 5.3 kg/m2 vs. 34.4 ± 3.4 kg/m2, p < 0.05), and lower prevalence of history of heart failure (14% vs. 27%, p < 0.05), symptomatic angina (44% vs. 22%, p < 0.001), prior MI (7% vs. 36%, p < 0.0001), or prior coronary artery bypass surgery (2% vs. 16%, p < 0.001).
Table 1. Patient Characteristics for All Patients and Patients With and Without Inducible Ischemia.
| Inducible Ischemia − (n = 180) | Inducible Ischemia + (n = 75) | p Value | |
|---|---|---|---|
| Age, yrs, median (IQR) | 54 (15) | 61 (15) | <0.0001 |
|
| |||
| Male | 78 (43) | 23 (31) | 0.07 |
| Body mass index, kg/m2, median (IQR) | 34 (7) | 34 (5) | <0.05 |
|
| |||
| Past history | |||
| Diabetes mellitus | 51 (28) | 31 (41) | 0.06 |
| Hypertension | 115 (64) | 56 (75) | 0.14 |
| Current or previous smoking | 36 (20) | 22 (29) | 0.10 |
| Hypercholesterolemia | 95 (53) | 55 (73) | <0.01 |
| Prior myocardial infarction | 12 (7) | 27 (36) | <0.0001 |
| Prior PCI | 21 (12) | 25 (33) | <0.001 |
| Prior CABG surgery | 3 (2) | 12 (16) | <0.0001 |
| Prior congestive heart failure | 26 (14) | 20 (27) | <0.05 |
| Symptomatic angina | 40 (22) | 33 (44) | <0.001 |
|
| |||
| Medications on presentation | |||
| Beta-blocker | 92 (51) | 53 (71) | <0.01 |
| Diuretic therapy | 48 (27) | 32 (43) | <0.05 |
| Aspirin | 82 (46) | 57 (76) | <0.0001 |
| ACE inhibitor | 94 (52) | 48 (64) | 0.10 |
| Statin | 83 (46) | 57 (76) | <0.0001 |
| Calcium channel blocker | 37 (21) | 11 (15) | 0.30 |
| Nitroglycerin | 14 (8) | 21 (28) | <0.0001 |
|
| |||
| Physical examination and electrocardiographic features | |||
| Systolic BP, mm Hg, median (IQR) | 135 (22) | 129 (31) | 0.01 |
| Diastolic BP, mm Hg, median (IQR) | 76 (17) | 70 (18) | 0.05 |
| Heart rate, beats/min, median (IQR) | 68 (20) | 71 (17) | 0.36 |
| Presence of pathologic Q wave | 13 (7) | 17 (23) | <0.01 |
| Left bundle branch block | 12 (7) | 4 (5) | 0.78 |
| Left ventricular hypertrophy | 14 (8) | 5 (7) | 1.0 |
| Nonsinus rhythm | 13 (7) | 8 (11) | 0.46 |
| Resting ST-segment deviation | 15 (8) | 21 (28) | <0.001 |
| T-wave inversion | 31 (17) | 22 (29) | 0.06 |
Values are median (IQR) or n (%).
+ = with; − = without; ACE = angiotensin-converting enzyme; BP = blood pressure; CABG = coronary artery bypass surgery CMR = cardiac magnetic resonance; IQR = interquartile range; LGE = late gadolinium enhancement; LV = left ventricle; PCI = percutaneous coronary intervention; RV = right ventricle.
Table 2. CMR Characteristics for All Patients and Patients With and Without Inducible Ischemia.
| Inducible Ischemia − (n = 180) | Inducible Ischemia + (n = 75) | p Value | |
|---|---|---|---|
| RV ejection fraction, %, median (IQR) | 54 (12) | 55 (12) | 0.64 |
| RV end-diastolic volume index, ml/m2 | 68 (20) | 64 (14) | <0.05 |
| RV end-systolic volume index, ml/m2, median (IQR) | 31 (13) | 28 (10) | <0.05 |
| LV ejection fraction, %, median (IQR) | 63 (14) | 57 (22) | <0.001 |
| LV end-diastolic volume index, ml/m2, median (IQR) | 79 (27) | 81 (37) | 0.27 |
| LV end-systolic volume index, ml/m2, median (IQR) | 29 (20) | 34 (39) | <0.05 |
| LV mass index, g/m2, median (IQR) | 58 (24) | 58 (24) | 0.69 |
| Resting wall motion abnormality | 26 (14) | 35 (47) | <.0001 |
| Presence of LGE, % | 38 (21) | 50 (67) | <.0001 |
| LGE mass, g, median (IQR)* | 10.4 (17.7) | 10.3 (15.4) | 0.18 |
| Number of segments with inducible ischemia | 0.0 ± 0.0 | 4.8 ± 4.2 | <0.0001 |
Values are median (interquartile range), n (%), or mean ± SD.
In patients with LGE presence consistent with infarction.
Abbreviations as in Table 1.
The overall study cohort had a median left ventricular ejection fraction (LVEF) of 62% (IQR: 16%), left ventricular mass index of 58 g/m2 (IQR: 24 g/m2), and right ventricular ejection fraction of 55% (IQR: 12%). Patients with no evidence of inducible ischemia on CMR had a significantly greater LVEF (63% vs. 57%, p < 0.001), lower left ventricular end-systolic volume index (29 ml/m2 vs. 34 ml/m2, p < 0.05), and less frequent resting wall motion abnormalities (14% vs. 47%, p < 0.0001). In those patients with evidence of infarction by LGE imaging, infarct size was not different with respect to the presence or absence of inducible ischemia.
Clinical outcomes
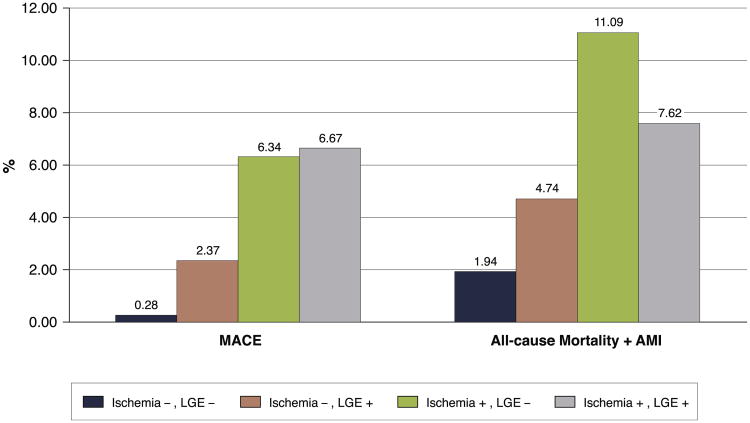
Median follow-up was 2.1 (IQR: 2.8 years) years, with no loss to follow-up in the patients studied. In the entire cohort, there were 19 deaths of any cause (7 cardiovascular [37%]). In addition, 7 patients experienced nonfatal MI during study follow-up. For the whole-study cohort, annualized event rates were 2.2% for MACE, 4.0% for a composite of all-cause mortality and MI, and 3.1% for all-cause mortality alone. The annualized event rates for MACE and composite of all-cause mortality and MI by presence of inducible ischemia and LGE are shown in Figure 2. Patients with no evidence of inducible ischemia or infarction by LGE had a very low annualized rate of MACE (0.3%). Patients with LGE without inducible ischemia experienced a higher annualized rate of MACE (2.4%), which was significantly lower than those patients with presence of inducible ischemia, but no evidence of LGE (2.4% vs. 6.3%, p < 0.0001). The annualized rate of MACE for patients with both inducible ischemia and LGE was highest, at 6.7%.
Figure 2. Annualized Rate of MACE and Composite of All-Cause Mortality and MI Stratified by Inducible Ischemia and Presence of LGE.
Annual event rates for the entire study cohort (N = 255) of major adverse cardiac events (MACE) (cardiovascular [CV] death and myocardial infarction [MI]) and composite of all-cause mortality are shown stratified by presence of inducible ischemia and late gadolinium enhancement (LGE) by stress perfusion cardiac magnetic resonance.
Prognostic value of stress CMR in obese patients
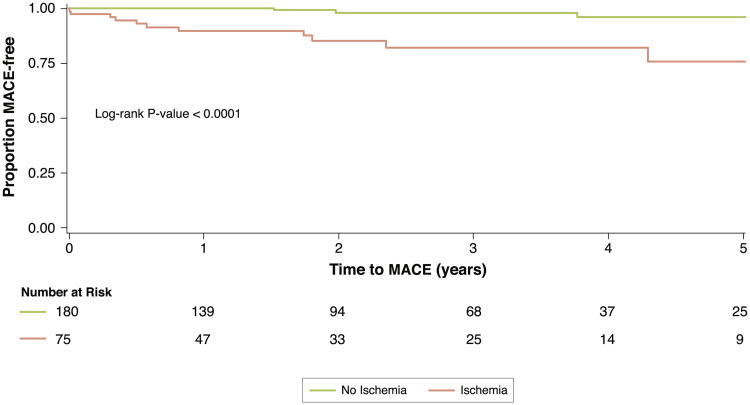
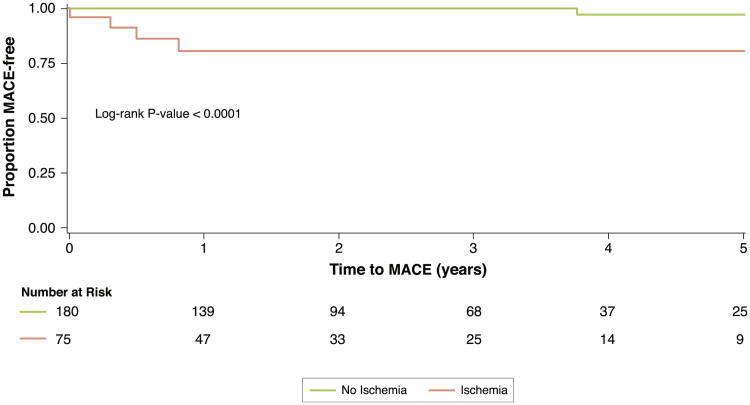
Univariable analysis of patient and CMR characteristics for association with MACE, and all-cause mortality/nonfatal MI are displayed in Table 3. Age, history of diabetes, prior MI, LVEF, presence of resting wall motion abnormalities, LGE, and inducible ischemia were all significantly associated with MACE in univariable Cox models. Kaplan-Meier event-free survival curves for MACE (primary outcome) or all-cause mortality/nonfatal MI (secondary outcome) stratified by inducible ischemia are shown in Figure 3. Obese patients with inducible ischemia had a significantly lower event-free survival for both primary and secondary outcomes as compared with patients without inducible ischemia (p < 0.0001 and p < 0.005, respectively). This relationship held when patients with LGE were excluded (Fig. 4). In a multivariable stepwise Cox regression including clinical characteristics and CMR indexes, inducible ischemia (hazard ratio [HR]: 7.5; 95% confidence interval [CI]: 2.0 to 28.0; p = 0.002) and resting ST abnormalities (HR: 3.2; 95% CI: 1.1 to 9.5; p = 0.04) remained independently associated with MACE. None of the time-dependent covariates included in the final multivariable Cox model were significant, suggesting that the proportionality assumption was not violated.
Table 3. Univariable Analysis of Clinical and CMR Characteristics for Prediction of Adverse Events.
| MACE | All-Cause Mortality + MI | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| LR Chi-Square | Hazard Ratio (95% CI) | p Value | LR Chi-Square | Hazard Ratio (95% CI) | p Value | |
| Age | 4.58 | 1.05 (1–1.11) | <0.05 | 6.31 | 1.05 (1.01–1.08) | 0.012 |
|
| ||||||
| Female | 0.02 | 1.08 (0.37–3.12) | 0.89 | 0.07 | 0.90 (0.41–1.98) | 0.79 |
|
| ||||||
| Body mass index | 0.82 | 0.94 (0.81–1.08) | 0.36 | 1.24 | 0.94 (0.85–1.05) | 0.27 |
|
| ||||||
| Hypertension | 2.89 | 5.84 (0.76–44.66) | 0.09 | 0.21 | 1.23 (0.52–2.92) | 0.65 |
|
| ||||||
| Diabetes mellitus | 6.79 | 4.68 (1.47–14.95) | <0.01 | 1.47 | 1.61 (0.75–3.49) | 0.23 |
|
| ||||||
| Hypercholesterolemia | 1.38 | 2.15 (0.6–7.71) | 0.24 | 2.30 | 0.55 (0.25–1.20) | 0.13 |
|
| ||||||
| Smoking | 0.56 | 1.56 (0.49–4.97) | 0.46 | 4.83 | 2.43 (1.10–5.36) | 0.03 |
|
| ||||||
| Diamond Forrester pre-test probability of CAD | 2.97 | 1.02 (1–1.04) | 0.09 | 4.20 | 1.02 (1.00–1.03) | 0.04 |
|
| ||||||
| Prior MI | 14.20 | 7.68 (2.66–22.19) | <0.001 | 7.89 | 3.23 (1.42–7.31) | 0.005 |
|
| ||||||
| Prior PCI | 8.36 | 4.7 (1.65–13.41) | <0.01 | 1.90 | 1.85 (0.77–4.43) | 0.17 |
|
| ||||||
| Prior CABG | 0.94 | 2.1 (0.47–9.4) | 0.33 | 0.78 | 1.72 (0.51–5.77) | 0.38 |
|
| ||||||
| LBBB | 3.75 | 3.54 (0.99–12.75) | 0.05 | 0.88 | 1.78 (0.53–5.97) | 0.35 |
|
| ||||||
| Presence of Q waves | 3.76 | 3.15 (0.99–10.06) | 0.05 | 1.80 | 1.96 (0.73–5.22) | 0.18 |
|
| ||||||
| ST-segment changes at baseline | 9.91 | 5.59 (1.91–16.3) | <0.01 | 5.45 | 2.85 (1.18–6.87) | 0.02 |
|
| ||||||
| LV ejection fraction | 4.35 | 0.97 (0.94–1) | <0.05 | 2.06 | 0.98 (0.96–1.01) | 0.15 |
|
| ||||||
| LV mass index | 7.43 | 1.03 (1.01–1.05) | <0.01 | 2.19 | 1.01 (1.00–1.03) | 0.14 |
|
| ||||||
| LVESVI | 5.47 | 1.02 (1–1.03) | <0.05 | 2.30 | 1.01 (1.00–1.02) | 0.13 |
|
| ||||||
| Resting WMA | 10.65 | 7.11 (2.19–23.11) | <0.01 | 7.17 | 2.92 (1.33–6.41) | 0.007 |
|
| ||||||
| Any stress perfusion defect | 9.84 | 25.93 (3.39–198.24) | <0.01 | 10.29 | 3.76 (1.67–8.43) | 0.001 |
|
| ||||||
| Revascularization within 60 days of CMR | 4.93 | 3.46 (1.16–10.34) | <0.05 | 0.67 | 1.50 (0.57–3.99) | 0.41 |
|
| ||||||
| LGE | 4.61 | 6.56 (1.18–36.5) | <0.05 | 0.87 | 1.61 (0.59–4.38) | 0.35 |
|
| ||||||
| RVEF | 3.54 | 0.95 (0.9–1) | 0.06 | 1.49 | 0.97 (0.93–1.02) | 0.22 |
|
| ||||||
| Presence of inducible ischemia | 12.28 | 9.81 (2.74–35.19) | <0.001 | 10.665 | 3.66 (1.68–7.97) | 0.001 |
CAD = coronary artery disease; CI = confidence interval; LBBB = left bundle branch block; LR chi-square = chi-square likelihood ratio; LVESVI = left ventricular end-systolic volume index; MACE = major adverse cardiac events; MI = myocardial infarction; RVEF = right ventricular ejection fraction; WMA = wall motion abnormality; other abbreviations as in Table 1.
Figure 3. Kaplan-Meier Survival Curves Stratified by Presence of Inducible Ischemia.
Kaplan-Meier survival curves for MACE (CV death and MI) stratified by presence of inducible ischemia by stress perfusion CMR. Abbreviations as in Figures 1 and 2.
Figure 4. Kaplan-Meier Survival Curves for Patients With No LGE, Stratified by Presence of Inducible Ischemia.
Kaplan-Meier survival curves of patients with no evidence of prior MI determined by any presence of subendocardial LGE on CMR for MACE (CV death and MI), stratified by presence of inducible ischemia. Abbreviations as in Figures 1 and 2.
Censoring of patients who underwent early coronary revascularization
Of the 255 patients in the study, 32 patients underwent early coronary revascularization (within 90 days of stress CMR). In the 223 patients remaining in the cohort after exclusion of these patients, there were 9 MACE events (5 nonfatal MI and 4 cardiac deaths). When these patients were censored on the day of the early revascularization, both presence of inducible ischemia and ischemia extent per segment remained highly associated with MACE (HR: 9.81; 95% CI: 2.74 to 35.19; p = 0.0005 and HR: 1.15; 95% CI: 1.07 to 1.23; p = 0.0001, respectively).
Discussion
In this cohort of 285 obese patients clinically referred for stress CMR, we found that stress CMR produced diagnostic quality imaging in more than 89% of patients. Despite lingering concerns over claustrophobia, only 7% of obese patients required sedation to achieve a diagnostic CMR. CMR inducible ischemia was a strong, independent predictor of MACE in multivariable models, with lack of inducible ischemia or LGE associated with a low annual MACE rate of 0.3%. Collectively, these results suggest that stress CMR is highly feasible and has excellent prognostic utility in obese patients.
Cardiovascular disease in obese patients represents a unique challenge for clinicians, especially in light of technical limitations inherent to current, widely utilized methods of risk stratification (e.g., nuclear, echocardiographic, treadmill stress testing, or computed tomography angiography). Despite the growing at-risk obese population, current literature examining the prognostic importance of stress echocardiography and nuclear imaging in this population remains limited. Although a negative dobutamine stress echocardiogram carries an annualized rate of cardiac MACE of 1.3% in a general referral population (17), the prognostic performance of dobutamine stress echocardiography in obese patients is not widely known. With regard to nuclear imaging, Elhendy et al. (18) reported on the prognostic importance and feasibility of dobutamine 99mTc-tetrofosmin SPECT imaging without attenuation correction in 267 obese patients (mean BMI 37 kg/m2) with a similar risk profile as our study. These authors demonstrated significant breast or diaphragmatic attenuation in 40 patients (16%) leading to non-diagnostic stress perfusion in 6 (2%). This technical limitation is not experienced with stress perfusion CMR. At a mean follow-up of 5.5 years, 22 cardiac deaths were observed, with an annualized rate of cardiac death and MI of 1% in patients with normal perfusion, significantly higher than observed with stress CMR. Importantly, abnormal nuclear stress perfusion was a strong univariate and multivariate predictor of MACE in this study. Although the authors suggest that attenuation correction may have improved stratification of risk in obese patients, the high annual MACE rate and the frequent presence of imaging artifacts with SPECT methods may more accurately reflect real-world clinical practice. Finally, computed tomography angiography (CTA) has emerged as a useful modality to assess for obstructive CAD, with high diagnostic accuracy (8,19). Indeed, in a study of more than 13,000 patients, BMI was strongly associated with the extent of obstructive CAD at CTA (20). Despite these excellent results, however, CTA requires radiation exposure, and its diagnostic performance may be limited by a low signal-to-noise ratio in the obese population.
The prognostic importance of negative nuclear myocardial perfusion imaging has been shown to be associated with BMI. In a retrospective study of 433 patients with a significantly higher BMI (47.3 kg/m2) with exercise or vasodilator 99mTc SPECT, Duvall et al. (9) reported that despite having only 2% poor-quality scans, patients with normal perfusion had up to a 3.7% risk of death at 2 years, and 1.7% risk of death at 1 year, higher than stress perfusion CMR in our study. Importantly, 82Rb-PET has also shown high diagnostic and prognostic performance in obese patients. In a landmark study of 134 obese patients referred for clinically indicated dipyridamole 82Rb-PET, patients with normal PET perfusion had a 0% cardiac event rate at a mean 3.1-year follow-up (10), significantly better than SPECT imaging. These findings are comparable to our reported results for stress perfusion CMR. In addition to ischemia, however, the improvements in spatial resolution with CMR allow detection of small subendocardial MI that is prognostically important, without ionizing radiation (21).
Our results in an obese population are in keeping with recent large reports in overall referral populations attesting to the prognostic role of stress CMR (22,23), suggesting the generalizability of standard perfusion techniques to obese patients. In a recent large study involving dobutamine stress CMR in 3,138 patients, Kelle et al. (23) describe complementary prognostic information from wall motion abnormalities and LGE, though data on average BMI in this cohort was not provided. These results have been confirmed in a large recent meta-analysis (22). Our work extends the aggregate data in stress CMR by demonstrating its prognostic utility specifically in patients with obesity, in whom imaging methods are limited, with potentially nonspecific results (21). Moreover, we also confirm in an obese population that inducible ischemia and LGE by stress perfusion CMR are robust markers of risk even in those patients without a clinical history of prior infarction, in keeping with prior reports suggesting the prognostic importance of LGE in patients with clinically unrecognized MI (24,25). In addition, as technical developments increasing the field strength possible with open magnetic resonance imaging systems emerge, the possibility of further application of stress perfusion CMR in obese patients may become more generalizable.
Study limitations
The results of our study must be taken in the context of its design. The relatively low event rate in our study reduces the number of clinical and CMR parameters that may be evaluated in multivariable modeling. Despite the low number of events, this event rate and our patient risk profile are comparable to similar studies in the nuclear literature. Even with this low overall rate of MACE, the absence of inducible ischemia and LGE still identified patients with a dramatically low rate of MACE. Over the 10-year study period, both 1.5-and 3.0-T field strengths were used for stress perfusion imaging. It should be noted that the proportion of nondiagnostic scans were similar at 6% between 1.5- and 3.0-T. Therefore, although our imaging protocol was tailored to obese patients, the methods used to optimize cine, perfusion, and LGE imaging were found to be highly transportable across scanner platforms and field strengths, suggesting this was not a significant limitation to generalizability. In addition, given the increasing adoption of 3.0-T scanners worldwide for CMR, it is likely that image quality and standardization will continue to improve. Although we report in the current study that a negative CMR is associated with a very low annualized rate of cardiac events, comparison of prognostic utility of CMR between obese and nonobese patients is not possible on the basis of the current evidence. Finally, although the determination of a threshold extent of inducible ischemia is a clinically important question, the limited number of events in this select population does not allow us to calculate this threshold, although other studies in nuclear and stress CMR have examined this important question (26,27).
Conclusions
Stress CMR is feasible in an obese population, and absence of ischemia or myocardial scar is associated with very low MACE in long-term follow-up. With a growing population of obese patients at significant risk for cardiovascular disease, these results suggest the use of stress CMR to stratify risk in symptomatic obese patients with suspected CAD may be warranted.
Acknowledgments
Dr. Shah is supported by an American Heart Association Post-Doctoral Fellowship Award (11POST000002) and a training grant from the Heart Failure National Institutes of Health Clinical Research Network (U01-HL084877). Dr. Heydari is supported by a Clinical Fellowship Award from the Alberta Heritage Foundation for Medical Research. Dr. Kwong is supported by National Institutes of Health grant RO1-HL091157. Dr. Kwong receives research support from Astellas Pharma US.
Abbreviations and Acronyms
- BMI
body mass index
- CAD
coronary artery disease
- CTA
computed tomography angiography
- CI
confidence interval
- CMR
cardiac magnetic resonance
- HR
hazardratio
- IQR
interquartile range
- LGE
late gadolinium enhancement
- LVEF
leftventricular ejection fraction
- MACE
major adverse cardiac events
- MI
myocardial infarction
- PET
positron emission tomography
- SPECT
single-photon emissioncomputed tomography
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. for the BMI-CHD Collaboration Investigators. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–8. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 3.Prospective Studies Collaboration. Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DePuey EG., 3rd How to detect and avoid myocardial perfusion SPECT artifacts. J Nucl Med. 1994;35:699–702. [PubMed] [Google Scholar]
- 5.Dunn JP, Huizinga MM, See R, Irani WN. Choice of imaging modality in the assessment of coronary artery disease risk in extreme obesity. Obesity (Silver Spring) 2010;18:1–6. doi: 10.1038/oby.2009.150. [DOI] [PubMed] [Google Scholar]
- 6.Einstein AJ, Weiner SD, Bernheim A, et al. Multiple testing, cumulative radiation dose, and clinical indications in patients undergoing myocardial perfusion imaging. JAMA. 2010;304:2137–44. doi: 10.1001/jama.2010.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson RC, Heller GV, Johnson LL, et al. Value of attenuation correction on ECG-gated SPECT myocardial perfusion imaging related to body mass index. J Nucl Cardiol. 2005;12:195–202. doi: 10.1016/j.nuclcard.2004.12.298. [DOI] [PubMed] [Google Scholar]
- 8.Shaw LJ, Hausleiter J, Achenbach S, et al. Coronary computed tomographic angiography as a gatekeeper to invasive diagnostic and surgical procedures: results from the multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter) registry. J Am Coll Cardiol. 2012;60:2103–14. doi: 10.1016/j.jacc.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 9.Duvall WL, Croft LB, Corriel JS, et al. SPECT myocardial perfusion imaging in morbidly obese patients: image quality, hemodynamic response to pharmacologic stress, and diagnostic and prognostic value. J Nucl Cardiol. 2006;13:202–9. doi: 10.1007/BF02971244. [DOI] [PubMed] [Google Scholar]
- 10.Yoshinaga K, Chow BJ, Williams K, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48:1029–39. doi: 10.1016/j.jacc.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Heydari B, Jerosch-Herold M, Kwong RY. Assessment of myocardial ischemia with cardiovascular magnetic resonance. Prog Cardiovasc Dis. 2011;54:191–203. doi: 10.1016/j.pcad.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–9. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 13.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 14.Schwitter J, Wacker CM, Wilke N, et al. MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-photon emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial. Eur Heart J. 2013;34:775–81. doi: 10.1093/eurheartj/ehs022. [DOI] [PubMed] [Google Scholar]
- 15.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480–9. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–95. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Poldermans D, Fioretti PM, Boersma E, et al. Long-term prognostic value of dobutamine-atropine stress echocardiography in 1737 patients with known or suspected coronary artery disease: a single-center experience. Circulation. 1999;99:757–62. doi: 10.1161/01.cir.99.6.757. [DOI] [PubMed] [Google Scholar]
- 18.Elhendy A, Schinkel AF, van Domburg RT, et al. Prognostic stratification of obese patients by stress 99mtc-tetrofosmin myocardial perfusion imaging. J Nucl Med. 2006;47:1302–6. [PubMed] [Google Scholar]
- 19.Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. New Engl J Med. 2008;359:2324–36. doi: 10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 20.Labounty TM, Gomez MJ, Achenbach S, et al. Body mass index and the prevalence, severity, and risk of coronary artery disease: an international multicentre study of 13,874 patients. Eur Heart Journal Cardiovasc Imaging. 2013;14:456–63. doi: 10.1093/ehjci/jes179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;361:374–9. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:826–38. doi: 10.1016/j.jacc.2013.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelle S, Nagel E, Voss A, et al. A bicenter cardiovascular magnetic resonance prognosis study focusing on dobutamine wall motion and late gadolinium enhancement in 3,138 consecutive patients. J Am Coll Cardiol. 2013;61:2310–2. doi: 10.1016/j.jacc.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 24.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890–6. doi: 10.1001/2012.jama.11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43. doi: 10.1161/CIRCULATIONAHA.105.570648. [DOI] [PubMed] [Google Scholar]
- 26.Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 27.Shah R, Heydari B, Coelho-Filho O, et al. Stress cardiac magnetic resonance imaging provides effective cardiac risk reclassification in patients with known or suspected stable coronary artery disease. Circulation. 2013;128:605–14. doi: 10.1161/CIRCULATIONAHA.113.001430. [DOI] [PMC free article] [PubMed] [Google Scholar]