Background: Whether suppression of glucose production by metformin is through AMPK-dependent inhibition of gluconeogenic gene expression remains controversial.
Results: Metformin inhibits gluconeogenic gene expression in hepatocytes.
Conclusion: Low metformin concentrations found in the portal vein suppress glucose production via AMPK-dependent mechanism.
Significance: The hyperglucagonemia of diabetes mellitus decreases metformin suppression of glucose production through a PKA-mediated phosphorylation of the AMPK α subunit at Ser-485/497.
Keywords: AMP-activated Kinase (AMPK), Gluconeogenesis, Hepatocyte, Metabolism, Protein Kinase A (PKA), Gluconeogenic Gene, Metformin
Abstract
Metformin is a first-line antidiabetic agent taken by 150 million people across the world every year, yet its mechanism remains only partially understood and controversial. It was proposed that suppression of glucose production in hepatocytes by metformin is AMPK-independent; however, unachievably high concentrations of metformin were employed in these studies. In the current study, we find that metformin, via an AMP-activated protein kinase (AMPK)-dependent mechanism, suppresses glucose production and gluconeogenic gene expression in primary hepatocytes at concentrations found in the portal vein of animals (60–80 μm). Metformin also inhibits gluconeogenic gene expression in the liver of mice administered orally with metformin. Furthermore, the cAMP-PKA pathway negatively regulates AMPK activity through phosphorylation at Ser-485/497 on the α subunit, which in turn reduces net phosphorylation at Thr-172. Because diabetic patients often have hyperglucagonemia, AMPKα phosphorylation at Ser-485/497 is a therapeutic target to improve metformin efficacy.
Introduction
Diabetes is the fastest growing chronic disease worldwide. The prime goal in the treatment of patients with diabetes is to control their high blood glucose levels as close to the normal range as possible so as to reduce the occurrence of complications. Metformin, a derivative of guanidine, is currently the most widely prescribed oral antidiabetic agent. Guanidine compounds are the active ingredients in French lilac (Galega officinalis), a plant that has been used as a folk medicine in the treatment of diabetes for several centuries. The biguanide class of drugs (two fused guanidine rings) includes metformin and phenformin and has been used to treat diabetes since the 1950s. In the 1970s, phenformin was withdrawn from the market due to the emergence of lactic acidosis as a serious side effect (1). Metformin was first used in Europe and was approved by the Food and Drug Administration (FDA) for the treatment of diabetes in the United States in 1994. Now, over 150 million people worldwide take this medication. This drug has also received attention because many studies have shown a reduction in cancer incidence in patients with diabetes mellitus treated with metformin (2–5).
After oral administration, the plasma concentration of metformin peaks within 1 h in humans and in animals (6–8), achieving a plasma concentration of 10–40 μm in humans following a therapeutic dose (7, 9). Because metformin is absorbed from the gastrointestinal tract and delivered directly to the liver through the portal vein, the plasma metformin concentration in the portal vein is somewhat higher (40–80 μm) (8). Many studies have demonstrated that the major effect of metformin is to suppress glucose production in the liver (10), reducing hepatic glucose production (HGP)3 by over 60% in a euglycemic clamp study (11). In 2001, Zhou et al. (12) reported that metformin is able to increase the net phosphorylation of the AMPK catalytic α subunit at Thr-172, a crucial phosphorylation site in the activation of AMPK, and activate AMPK activity in primary hepatocytes; also, hepatic knock-out of LKB1 (liver kinase B1), the upstream kinase for AMPKα phosphorylation at Thr-172, abolishes metformin suppression of hepatic glucose production (13). Furthermore, activation of AMPK results in the phosphorylation of CRTC2 (CREB-regulated transcription coactivator 2) and CBP (CREB-binding protein), which then inhibit gluconeogenic gene expression (14, 15).
Metformin has also been proposed to inhibit complex 1 of the mitochondrial respiratory chain, leading to an increase in the ADP/ATP ratio (16, 17). The increment in this ratio leads to phosphorylation of AMPKα at Thr-172 and increased AMPK activity (18, 19). In 2010, however, a study showed that metformin inhibits hepatic gluconeogenesis through a mechanism that does not involve suppression of gluconeogenic gene expression (20). Furthermore, metformin suppressed glucose production in primary hepatocytes lacking AMPKα1 and AMPKα2, and the authors proposed that inhibition of mitochondrial complex 1 by metformin results in a decrease in ATP levels together with an increase in the AMP/ATP ratio, which then both directly suppress gluconeogenesis. These results challenge the role of LKB1-AMPK in mediating metformin suppression of hepatic gluconeogenesis (20). However, long term metformin administration did not affect the mitochondrial complex 1 activity both in the liver of mice and in the muscle of humans (21, 22). In studies proposing an AMPK-independent mechanism, the authors employed metformin concentrations (1–2 mm) that were 10–100-fold higher than maximal concentrations achieved in the hepatic portal vein after standard therapeutic dosing, and the high concentration of metformin used in these studies has raised safety concerns.4 In the current study, we tested 1)whether metformin at therapeutic concentrations is able to suppress expression of genes related to gluconeogenesis and 2) whether metformin directly affects AMPKα subunit phosphorylation at Thr-172 to suppress glucose production in hepatocytes.
EXPERIMENTAL PROCEDURES
Plasmids and Adenoviruses
The BLOCK-iT adenoviral RNAi expression system (Invitrogen) was used to construct adenoviral shRNAs for AMPKα1 and AMPKα2, LKB1, and scrambled shRNA as we previously described (23). For the generation of adenoviral expression vectors of WT and mutant AMPKα1, the AMPKα1 gene was cloned into a pENTR2B vector (Invitrogen). AMPKα1S485A and α1S497A were created using site-directed mutagenesis (Stratagene) and transferred into the pAd/CMV/V5-DEST vector (Invitrogen) by recombination to generate expression clones.
Glucose Production Assays
Mouse primary hepatocytes were cultured in William's medium E supplemented with ITS (BD Biosciences) and dexamethasone. After 16 h of planting, primary hepatocytes were subjected to 3 h of serum starvation. Cells were washed twice with PBS, and then the medium was replaced with 1 ml of glucose production medium consisting of glucose-free DMEM supplemented with 20 mm sodium lactate (Sigma) and 2 mm sodium pyruvate (Sigma) or with metformin (Alexis Biochemicals), 0.2 mm Bt-cAMP (Sigma), or 5 nm glucagon (Sigma). In assays with 3 h of metformin pretreatment, metformin was added to FBS-free DMEM medium (5.5 mm glucose) during the serum starvation and added to the glucose production medium. In assays with 24 h of metformin pretreatment, metformin was added into DMEM medium (5.5 mm glucose) plus 10% FBS for 21 h followed by two washes with PBS. After 3 h of serum starvation in DMEM (5.5 mm glucose) supplemented with metformin, cells were washed twice with PBS, and the 1-ml glucose production medium was supplemented with metformin and Bt-cAMP. After a 3–4-h incubation with glucose production medium, both medium and cells were collected. The medium was used to determine glucose concentrations with EnzyChrom glucose assay kit (24).
AMPK Activity Assay
The FLAG-tagged protein and AMPK protein were immunoprecipitated from cell lysates using anti-AMPKα antibody or anti-FLAG M2 monoclonal antibody (Sigma) after incubation overnight at 4 °C. AMPK activity was determined by measuring the incorporation of 32P into the synthetic SAMS peptide (Abcam) as described previously (25). Briefly, immunoprecipitates were incubated at 30 °C for 20 min in the presence of [32P]ATP (1 μCi), AMP (200 μm), and with or without SAMS peptide (200 μm). Aliquots of the reaction mixture were spotted on Whatman p81 phosphocellulose filter papers, which were dropped immediately in 1% phosphoric acid for 20 min with rotation. Then, the wash was repeated twice followed by a 5-min wash in acetone. Filters were air-dried, and radioactivity was counted in 3 ml of liquid scintillation fluid. The counts without the SAMS peptide were used as the background.
Nucleotide Measurement
ATP concentrations were determined using an ATP determination kit (Molecular Probes, Invitrogen) with modifications. Reaction buffer (25 mm Tricine buffer, pH 7.8, 5 mm MgSO4, 0.1 mm EDTA, and 0.1 mm sodium azide) was also used to determine the ADP and AMP concentrations using the previously described methods (26, 27) with modifications. After the treatment, primary hepatocytes were harvested in 1 n cold perchloric acid followed by an 80-s sonication. Samples were centrifuged at 13,000 × g for 2 min. Supernatants were collected and neutralized with cold 1 n K2CO3 to keep the pH at about 6–8. First, AMP and ADP were converted to ATP in a 50-μl reaction containing 1 unit of myokinase, 0.25 unit of pyruvate kinase, 0.2 mm dCTP, 0.3 mm phosphoenolpyruvate, and 5 μl of samples and AMP standard. The reaction was incubated at room temperature for 30 min. Total ATP concentration was measured using a luciferase assay, and the reading was taken as A. Second, only ADP was converted to ATP in a 50-μl reaction containing 0.25 unit of pyruvate kinase, 0.3 mm P-enolpyruvate, and 5 μl of samples and ADP standard. The reaction was incubated at room temperature for 30 min. The ATP concentration was measured, and the reading was taken as B. Third, ATP concentrations in 5 μl of samples and ATP standard were determined, and the readings were taken as C. The AMP concentration equals A minus B, whereas ADP concentration equals B minus C, where C is the actual ATP concentration in the sample. To measure cAMP levels in primary hepatocytes, hepatocytes were washed with cold PBS once, and cells were harvested in 0.1 n HCL by scraping. Cellular lysates were passed 15 times through a syringe needle to break the cells on ice, and then cAMP levels were determined by using the Direct cAMP ELISA kit (Enzo Life Sciences) following the manufacturer's procedures.
AMPKα Phosphorylation at Ser-485
For AMPKα phosphorylation at Ser-485 by the PKA catalytic subunit, purified AMPKα1 was incubated with different amounts of PKA catalytic subunit (Millipore) at 30 °C for 30 min followed by heating at 95 °C to immediately inactivate the kinase activity.
Animal Experiments
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. C57 diet-induced obesity (DIO) mice at the age of 12 weeks were purchased from The Jackson Laboratory. Metformin was given to the mice through drinking water for 6 weeks (50 mg/kg of body weight). Mice were measured for weight gain every 7 days, and metformin concentrations in the drinking water were adjusted accordingly. Based on studies in The Jackson Laboratory, each DIO mouse drinks 7 ml of water per day. Mice were subjected to a 5-h fast before being sacrificed.
Real-Time PCR
The targeted mRNA levels were examined by quantitative real-time PCR using the CFX ConnectTM Detection System (Bio-Rad). Total RNA was prepared using TRIzol reagent (Invitrogen). The iScript cDNA synthesis kit (Bio-Rad) was used for cDNA synthesis. Relative cDNA levels were determined using the SYBR Green master mix Solution (Bio-Rad), and we used the ΔΔCt method to calculate relative levels of mRNA levels. A standard curve of 10-fold serial dilutions for each gene was generated to generate an efficiency value. Only data from experiments in which the efficiency was between 95–105% were used for data analysis.
Immunoblot
Immunoblots were conducted as described previously (23). Cellular lysates were passed 15 times through a syringe needle or were sonicated for 15 s three times sequentially, and immunoblotted to examine the target proteins with antibodies against AMPKα1, AMPKα2, phospho-AMPKα (Thr-172, Ser-485), CREB, phospho-CREB (Ser-133) (Cell Signaling), Pck1, AMPKα1, and LKB1 (Millipore) at the concentrations recommended by the manufacturers. Secondary antibodies were used at the concentrations recommended by the manufacturers at about 1:5000.
Statistical Analyses
Statistical significance was calculated with a Student's t test and analysis of variance test. Significance was accepted at the level of p < 0.05.
RESULTS
Metformin Suppresses Gluconeogenic Gene Expression
To assess whether metformin is able to inhibit the expression of genes related to gluconeogenesis, we conducted glucose production assays and measured gluconeogenic gene expression in primary hepatocytes treated with metformin and the cAMP analog Bt-cAMP simultaneously as was done in a previous study (20). In agreement with this study (20), simultaneous metformin treatment inhibited Bt-cAMP-stimulated glucose production and the mRNA levels of G6pc (glucose 6-phosphatase catalytic subunit) in a concentration-dependent manner; in contrast, Pck1 (phosphoenolpyruvate carboxykinase 1) mRNA levels were not affected by metformin (Fig. 1, a–c). Higher concentrations of metformin suppressed G6pc protein levels and mildly reduced Pck1 protein levels (Fig. 1e, left panel). The mRNA and protein levels of G6pc correlated well with glucose production in primary hepatocytes treated with Bt-cAMP and metformin, but the mRNA and protein levels of Pck1 did not follow the same pattern of change.
FIGURE 1.
Metformin inhibited gluconeogenic gene expression in mouse primary hepatocytes. a–d, pretreatment (Pre-T) with the indicated amounts of metformin (Met) for 3 or 24 h prior to the addition of 0.2 mm Bt-cAMP had a much stronger effect on glucose production (a), G6pc (b), Pck1 (c), and Fbp1 (d) mRNA levels than the treatment in which metformin and Bt-cAMP were added simultaneously. Primary hepatocytes were treated as described under “Experimental Procedures” (n = 4). Same-T, same-time treatment. e, pretreatment of metformin for 3 h greatly activated AMPK (p-AMPKα) and had a stronger inhibition of 0.2 mm Bt-cAMP (4-h incubation)-stimulated G6pc and Pck1 protein levels in primary hepatocytes. * signifies that groups with the same treatment are significantly different (p < 0.05). Error bars indicate mean ± S.D.
Simultaneous treatment of primary hepatocytes with metformin and cAMP analogs, however, does not recapitulate usual therapy for patients with diabetes. Metformin is generally administered with food at night for the purpose of controlling the morning fasting blood glucose level, indicating that metformin achieves peak concentrations prior to an elevation in blood glucagon levels. We, therefore, treated primary hepatocytes with metformin 3 h before the addition of Bt-cAMP. As shown in Fig. 1, a–c, a 3-h period of metformin pretreatment led to greater decreases in glucose production and mRNA levels for G6pc and Pck1 gene. Pretreatment with metformin for 3 h also led to a greater inhibition of the protein levels of G6pc and Pck1 (Fig. 1e, right panel). Moreover, 24 h of pretreatment with metformin suppressed Bt-cAMP-stimulated glucose production and gluconeogenic gene expression to a greater degree (Fig. 1, a–c). In contrast, metformin did not significantly suppress Fbp1 mRNA levels (Fig. 1d).
To examine whether metformin is able to suppress gluconeogenic gene expression in vivo, high fat diet-fed (DIO) mice were administered orally with low dose of metformin (50 mg/kg) for 6 weeks. We found that administration of low dose of metformin significantly suppressed the mRNA levels of G6pc and Pck1 genes (Fig. 2, a and b). In comparison, metformin administration did not significantly affect GAPDH mRNA levels, which served as an endogenous control (Fig. 2c).
FIGURE 2.

Metformin suppressed gluconeogenic gene expression in the liver of DIO mice. a–c, administration of metformin (50 mg/kg of body weight) for 6 weeks significantly decreased mRNA levels of G6pc (a) and Pck1 (b) in the liver of DIO mice (n = 4). c, the mRNA levels of GAPDH in the liver of same DIO mice as in a and c. Error bars indicate mean ± S.D. RD, regular diet; HFD, high fat diet.
Depletion of AMPK Catalytic α Subunits Decreased the Effect of Metformin to Inhibit Glucose Production in Primary Hepatocytes
Numerous studies have shown that metformin increases phosphorylation of the AMPK catalytic α subunit at Thr-172, which then elevates AMPK enzymatic activity (12, 28). LKB1 is a major kinase for Thr-172 phosphorylation, and accordingly, loss of LKB1 in the liver prevents AMPK activation by metformin (13). Indeed, metformin failed to phosphorylate the AMPKα at Thr-172 after depletion of LKB1 by adenoviral shRNAs (Fig. 3a). To assess whether AMPK is necessary for suppression of HGP by metformin, we employed adenoviral shRNAs to deplete the AMPKα1 and AMPKα2 subunits in primary hepatocytes (Fig. 3b). Depletion of AMPKα subunits significantly decreased Thr-172 phosphorylation (Fig. 3b) and inhibition of Bt-cAMP-stimulated glucose production by metformin (Fig. 3c). These data suggest that at least part of the suppression of HGP by metformin is mediated by the AMPK pathway.
FIGURE 3.
Depletion of AMPK catalytic α subunits decreased the effect of metformin to inhibit glucose production in primary hepatocytes. a, two sets of LKB1 adenoviral (ad-shLKB1#1 and ad-shLKB1#2) shRNAs were added in Hepa1–6 cells for 48 h followed by the addition of 0.25 mm metformin (Met) for 6 h. ad-shSCR, adenoviral scramble shRNA; p-AMPK, phospho-AMPK. b, 48 h after the addition of adenoviral shRNAs to deplete AMPKα1 and AMPKα2, primary hepatocytes were treated with the indicated amount of metformin for 3 h during serum starvation and then incubated with 0.2 mm Bt-cAMP and metformin for 4 h. ACC, acetyl-CoA carboxylase; pACC, phospho-ACC; pCREB, phospho-CREB. c, 48 h after adenoviral shRNAs mediated depletion of AMPKα1 and AMPKα2, metformin was added to medium during serum starvation. After washing with PBS, both metformin and 0.2 mm Bt-cAMP were added in glucose production medium (n = 3). Pre-T, pretreatment. * signifies that groups with the same treatment are significantly different (p < 0.05). Error bars indicate mean ± S.D.
A Decrease in cAMP Levels Is Not Necessary for Suppression of Glucose Production by Metformin
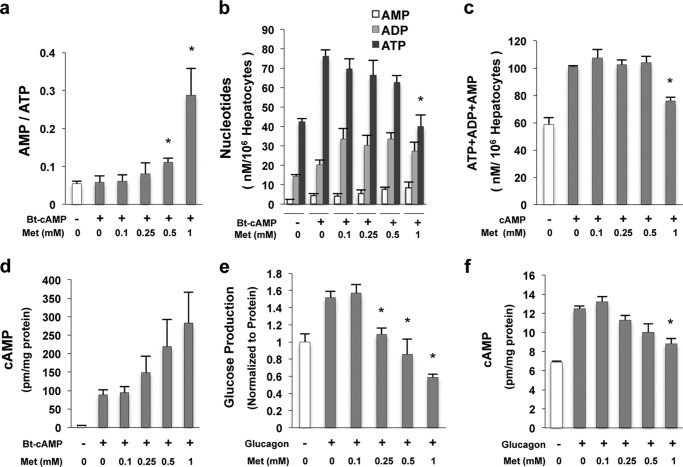
A recent study proposed that metformin suppresses hepatic glucose production through inhibition of adenylyl cyclase by elevated AMP levels (29). We and others demonstrate that metformin is still able to suppress glucose production stimulated by Bt-cAMP, a hydrolysis-resistant cAMP analog (Fig. 1a) (20), arguing that inhibition of adenylyl cyclase is not the mechanism for metformin suppression of glucose production. We next determined adenine nucleotide levels in primary hepatocytes treated simultaneously with Bt-cAMP and metformin. Only at metformin concentrations that are unlikely to be achieved in the portal vein (0.5, 1.0 mm) are the AMP/ATP ratios increased significantly (Fig. 4a), and only 1 mm metformin significantly decreased ATP levels (Fig. 4, b and c). In addition, metformin did not decrease cAMP levels in primary hepatocytes pretreated with metformin 3 h before the addition of Bt-cAMP (Fig. 4d). Furthermore, we only observed significant decrease in cAMP levels after 1 mm metformin treatment, yet glucagon-stimulated glucose production was suppressed by much lower concentrations of metformin (Fig. 4, e and f). These data do not support the notion that metformin inhibits HGP by decreasing cAMP levels (29).
FIGURE 4.
A decrease in cAMP levels is not necessary for suppression of glucose production by metformin. a, the AMP/ATP ratio in primary hepatocytes treated with Bt-cAMP and the indicated amount of metformin (Met) simultaneously for 3 h (n = 4). b and c, primary hepatocytes were treated with Bt-cAMP and metformin simultaneously for 3 h. A high concentration of metformin (1 mm) decreased ATP (b) and total nucleotide levels (c) (n = 4). d, metformin did not decrease the cAMP levels in primary hepatocytes pretreated with metformin for 3 h and then treated with metformin and 0.2 mm Bt-cAMP together for another 3 h (n = 4). e and f, metformin significantly suppressed glucagon-stimulated glucose production at 0.25, 0.5, and 1 mm (e) after 4 h of 5 nm glucagon together with the indicated metformin treatment; however, only a high concentration of metformin (1 mm) significantly decreased cAMP levels (f) in primary hepatocytes (n = 4). Error bars indicate mean ± S.D.
High Metformin Concentrations Are Not Required to Suppress Gluconeogenic Gene Expression and Glucose Production in Primary Hepatocytes
High concentrations of metformin are routinely used in in vitro studies, which are 10–100-fold higher than found in the hepatic portal vein (20, 29). In contrast, it was reported that low metformin concentrations (<50 μm) are able to activate AMPK activity (12), and cAMP and AMP/ATP ratio are not affected at these concentrations (20, 29). We confirmed that treatment with low concentrations of metformin resulted in the elevation of AMPK phosphorylation at Thr-172 and AMPK activity in primary hepatocytes (Fig. 5, a and b). Moreover, a 24-h pretreatment with low metformin concentrations significantly suppressed Bt-cAMP-stimulated glucose production (Fig. 5c) together with an increase in phosphorylation of AMPKα at Thr-172 (Fig. 5d). We employed a metformin concentration of 80 μm, which corresponds to the maximal therapeutic metformin concentration in hepatic portal vein (8, 30). Pretreatment with 80 μm metformin for 24 h increased AMPK activity in primary hepatocytes (Fig. 5b) and significantly decreased the mRNA levels of G6pc and Pck1 after Bt-cAMP stimulation (Fig. 5, e and f), without affecting the GAPDH mRNA levels (Fig. 5g).
FIGURE 5.
Activation of AMPK by low metformin concentrations inhibited gluconeogenic gene expression. a, pretreatment with low concentration of metformin (Met, 80 μm) increased the phosphorylation of AMPKα (p-AMPK) at Thr-172. Hepa1–6 cells were grown in DMEM medium (5.5 mm glucose) plus 10% FBS and metformin for the indicated times. b, AMPK activity in primary hepatocytes after 24 h of metformin treatment (n = 3). c and d, primary hepatocytes were grown in DMEM medium plus 10% FBS and metformin for 21 h and then FBS-free DMEM and metformin for 3 h followed by glucose production medium supplemented with 0.2 mm Bt-cAMP and metformin for another 3 h. Glucose production was measured in the medium (c) (n = 4), and cellular lysates were subjected to immunoblot (d). pCREB, phospho-CREB. e–g, suppression of the Bt-cAMP-stimulated mRNA levels of G6pc (e), Pck1 (f), and GAPDH (g) in the primary hepatocytes pretreated with 80 μm metformin and then with Bt-cAMP (n = 4). h, in primary hepatocytes, 36 h after the addition of adenoviral shRNAs of AMPKα1 and AMPKα2 (ad-shAMPK1/2), metformin was added into DMEM medium plus 10% FBS for 21 h and then added to FBS-free DMEM for 3 h and glucose production medium together with 0.2 mm Bt-cAMP for another 3 h (n = 3). * signifies that groups with the same treatment are significantly different (p < 0.05). NS, not significant. Error bars indicate mean ± S.D. ad-shSCR, adenoviral scramble shRNA.
Consistent with the concept that activation of AMPK restores cellular energy balance, pretreatment with low concentration of metformin (80 μm) increased the levels of nucleotides and ATP in primary hepatocytes without increasing the AMP/ATP ratio and the change of adenylate energy charge (Fig. 6, a–d). Moreover, pretreatment with 80 μm metformin decreased glucose production stimulated by glucagon and did not affect the cAMP levels (Fig. 7, a and b). Furthermore, metformin elevated adenine nucleotide levels in primary hepatocytes treated with glucagon without increasing the AMP/ATP ratio and the change of adenylate energy charge (Fig. 7, c–f). Finally, depletion of AMPKα1 and AMPKα2 abolished metformin inhibition of either Bt-cAMP-stimulated or glucagon-stimulated glucose production, indicating that the metformin effect is AMPK-dependent at this concentration (Figs. 5h and 7g).
FIGURE 6.

Low metformin concentration increased ATP levels in primary hepatocytes. a–c, metformin (Met) was added into DMEM medium plus 10% FBS for 21 h and then added to FBS-free DMEM for 3 h and glucose production medium together with 0.2 mm Bt-cAMP for another 3 h. Metformin increased the total nucleotides (a) and ATP levels (b) without affecting AMP/ATP ratio (c) and adenylate energy charge (d) (n = 6). * signifies that groups with the same treatment are significantly different (p < 0.05). Error bars indicate mean ± S.D.
FIGURE 7.
Suppression of glucagon-stimulated glucose production by low metformin concentration in primary hepatocytes. a and b, pretreatment with 80 μm metformin (Met) for 24 h significantly inhibited 5 nm glucagon-stimulated glucose production in primary hepatocytes (a) without affecting the cAMP levels (b). c–e, total nucleotides (c), ATP levels (d), AMP/ATP ratio (e), and adenylate energy charge (f) in primary hepatocytes treated as in a and b (n = 3). g, in primary hepatocytes, 36 h after the addition of adenoviral shRNAs of AMPKα1 and AMPKα2, metformin was added into DMEM medium plus 10% FBS for 21 h and then added to FBS-free DMEM for 3 h and glucose production medium together with 5 nm glucagon for another 3 h (n = 3). * signifies that groups with the same treatment are significantly different (p < 0.05). NS, not significant. Error bars indicate mean ± S.D.
The Phosphorylation of AMPK Catalytic α Subunit at Ser-485/497 by the cAMP-PKA Pathway Decreased Metformin-mediated AMPK Activation
Pretreatment with metformin resulted in greater inhibition of Bt-cAMP-stimulated glucose production and gluconeogenic gene expression than simultaneous treatment (Fig. 1, a–c). Furthermore, metformin pretreatment led to greater AMPK phosphorylation at Thr-172, AMPK activation, and inhibition of Pck1 protein levels than simultaneous treatment (Fig. 8, a and b). In addition, previous in vivo and in vitro studies have shown that activation of the β-adrenergic receptor decreased AMPKα phosphorylation at Thr-172 and AMPK activity in adipose tissue and cardiomyocytes (31–33). In light of these data, we reasoned that cAMP might antagonize AMPK activation by metformin, and this might be important for clinical metformin usage because diabetic patients often have hyperglucagonemia. Furthermore, this might offer an explanation for why high concentrations of metformin in previous studies were needed to suppress the glucose production in hepatocytes (20, 29). Therefore, we employed higher metformin concentrations that were used in previous studies (20, 29). As anticipated, Bt-cAMP reduced Thr-172 phosphorylation of AMPKα by metformin during a time-course experiment (Fig. 8c), and glucagon also reduced AMPKα phosphorylation at Thr-172 in a concentration-dependent manner (Fig. 8d). To further prove that cAMP negatively affects AMPK activity, we overexpressed a FLAG-tagged AMPKα1 subunit in Hepa1–6 cells. The FLAG-tagged AMPKα1 subunit was immunoprecipitated from the cell lysates using an anti-FLAG antibody after the treatment with cAMP and/or metformin. We observed significant decreases in AMPK enzymatic activities by cAMP in both control-treated and metformin-treated groups (Fig. 8e), which correlated with Thr-172 phosphorylation. Blockade of the cAMP-PKA pathway with PKA inhibitors, H89, or a competitive cAMP inhibitor (Rp)-cAMP, increased AMPKα phosphorylation at Thr-172 (Fig. 8, f–h).
FIGURE 8.
The cAMP-PKA pathway negatively regulates AMPK activity. a, primary hepatocytes were treated with 0.25 mm metformin 3 h prior to the addition of 0.2 mm Bt-cAMP or metformin and Bt-cAMP at the same time (n = 3). Same-T, same treatment; Pre-T, pretreatment. b, in primary hepatocytes, 0.25 mm metformin (Met) was added to FBS-free medium during serum starvation in pretreatment group (Pre-Met). After washing with PBS, both 0.25 mm metformin and 0.2 mm Bt-cAMP were added in glucose production medium for another 3 h. p-AMPKα, phospho-AMPKα; ACC, acetyl-CoA carboxylase; pACC, phospho-ACC; pCREB, phospho-CREB. c, primary hepatocytes were treated with 0.5 mm metformin or together with 0.2 mm Bt-cAMP and harvested at the indicated time points. d, Hepa1–6 cells were grown in DMEM medium (5.5 mm glucose) plus 10% FBS for 16 h and then subjected to serum starvation for 2 h, and 0.5 mm metformin and the indicated amounts of glucagon were added for 4 h. e, 48 h after the addition of adenoviral FLAG-tagged AMPKα1, Hepa1–6 cells were incubated with 0.5 mm metformin and 0.2 mm Bt-cAMP for 4 h. ip, immunoprecipitation. f, blockade of PKA by H89 increased phosphorylation of AMPKα at Thr-172. Primary hepatocytes were treated with 20 μm H89 or vehicle (Veh) for 30 min prior to the addition of 100 nm glucagon. Cells were incubated for 3 h before harvest. g, the PKA competitive inhibitor-(Rp)-cAMP (100 μm) also blocked the negative effect of Bt-cAMP on AMPK phosphorylation at Thr-172. (Rp)-cAMP was administrated 1 h before the addition of 0.2 mm Bt-cAMP in Hepa1–6 cells. h, H89 accentuated calyculin (protein phosphatase inhibitor)-mediated phosphorylation of AMPKα1 at Thr-172. Primary hepatocytes were incubated with H89 or H89 plus 50 nm calyculin (Cell Signaling) for 75 min before harvest. Error bars indicate mean ± S.D.
Previous studies have shown that PKA phosphorylates the AMPKα subunit at Ser-173, Ser-485, and Ser-497 (34), and these phosphorylation events reduce Thr-172 phosphorylation and decrease AMPK enzymatic activity (34–36). Using LCMS/MS analysis, we mapped phosphorylation of these sites in FLAG-tagged AMPKα1 subunit protein isolated from primary hepatocytes treated with Bt-cAMP (Fig. 9a). We detected phosphorylation of AMPKα1 subunit at Ser-485 and Ser-497 (Fig. 9, b and c), but not at Ser-173 (data not shown), in primary hepatocytes. In an in vitro assay, PKA catalytic subunit phosphorylated purified α1 subunit at Ser-485 (Fig. 9d). Next, we investigated further whether phosphorylation of AMPKα1 subunit at Ser-485 or Ser-497 by cAMP impedes phosphorylation of AMPKα subunit at Thr-172 using AMPKα1 mutants (S485A and S497A). Mutation of either site abolished the negative effect of cAMP on AMPKα phosphorylation at Thr-172, and the S497A mutant also blocked phosphorylation of AMPKα1 at Ser-485 (Fig. 9, e and f). These data are in good agreement with a previous study showing that mutation of either site prevented phosphorylation of the other site (37). Moreover, Bt-cAMP had no effect on basal and metformin-stimulated AMPK activity and phosphorylation at Thr-172 of AMPKα1S485A (Fig. 10a). These data demonstrate that the cAMP-PKA pathway negatively regulates AMPK activity through phosphorylation of AMPKα at Ser-485. We went a step further to examine the effect of the α1S485A mutation on glucose production; overexpression of adenoviral AMPKα1S485A led to an increase in metformin-mediated AMPKα1 phosphorylation at Thr-172, and enhanced suppression of Bt-cAMP-stimulated glucose production in primary hepatocytes (Fig. 10b).
FIGURE 9.
Phosphorylation of AMPKα at Ser-485/497 by cAMP decreased metformin-mediated AMPK activation. a, FLAG-tagged AMPKα1 was immunoprecipitated (FLAG ip) from primary hepatocytes treated with 0.2 mm Bt-cAMP and was used to map the phosphorylation site(s). p-AMPKα, phospho-AMPKα; pCREB, phospho-CREB. b and c, identification of AMPKα1 phosphorylation at Ser-485/497 by cAMP in LCMS/MS analysis. d, purified AMPKα1 was incubated with different amounts of PKA catalytic subunit (PKAc; Millipore) at 30 °C for 30 min. e and f, 48 h after the addition of adenoviral FLAG-tagged AMPKα1 and its mutants, Hepa1–6 cells were subjected to 90 min of serum starvation, and then 0.5 mm metformin (Met) and 0.2 mm Bt-cAMP were added for 4 h. FLAG-tagged AMPKα1 and its mutants were expressed at levels 5-fold higher than endogenous α1 levels.
FIGURE 10.
AMPKα1 mutant (S485A) abolished the negative effect of cAMP on AMPKα1 activation. a, 48 h after the addition of adenoviral FLAG-tagged AMPKS485A mutant in Hepa1–6 cells, 0.5 mm metformin (Met) and 0.2 mm Bt-cAMP were added, and cells were harvested 5 h after the incubation. FLAG-tagged AMPKS485A was purified by using FLAG immunoprecipitation (FLAG ip) kit (Sigma). pCREB, phospho-CREB. b, 36 h after adenoviral FLAG-tagged AMPKα1 and S485A mutant were added to primary hepatocytes, cells underwent 3 h of serum starvation, and then medium was changed to glucose production medium supplemented with 0.25 mm metformin and 0.2 mm Bt-cAMP for another 4 h (n = 3). p-AMPKα1, phospho-AMPKα1. * signifies that groups with the same treatment are significantly different (p < 0.05). NS, not significant. Error bars indicate mean ± S.D.
DISCUSSION
AMPK plays a key role as a master regulator in cellular and whole organism energy homeostasis (12, 38). To date, both AMPK-dependent and AMPK-independent mechanisms for suppression of hepatic glucose production by metformin have been proposed. We and others have proposed that metformin suppresses hepatic glucose production through activation of AMPK pathway to inhibit gluconeogenic gene expression (11, 13, 15). In contrast, a study showing that metformin suppresses hepatic glucose production through AMPK-independent pathway is based on results from AMPKα1/α2 knockout mice (20). It is worth noting that metformin treatment completely suppressed Pck1 protein levels in primary hepatocytes from AMPKα1/α2 knock-out mice (20), and this did not occur in primary hepatocytes from wild type control mice and also did not occur in primary hepatocytes with acute depletion of AMPKα1/2 by adenoviral shRNAs (Fig. 3b). Because Pck1 protein is a rate-limited enzyme in the gluconeogenic pathway, these results in the AMPKα1/α2 knock-out mice make other data in this study difficult to interpret. In addition, high concentrations of metformin were employed in studies in which metformin suppression of glucose production via the inhibition of mitochondrial respiratory chain complex 1 was proposed. However, it remains unknown how such a high concentration could be achieved in the portal vein given current dosing constrains. We found, in contrast, that low metformin concentration (≤80 μm) is able to suppress Bt-cAMP- or glucagon-stimulated glucose production in primary hepatocytes (Figs. 5c and 7a). Foretz et al. (20) also suggested that metformin suppresses hepatic glucose production by decreasing ATP levels through inhibition of complex 1 in the electron transport chain. However, other in vivo experiments in animals and in humans have shown that complex 1 activity was not inhibited and ATP levels were not affected by metformin, although AMPKα phosphorylation at Thr-172 was significantly increased (21, 22). Moreover and in contrast to these results, low metformin concentrations actually increased ATP levels (Figs. 6b and 7c) when compared with the ATP levels in hepatocytes treated with high concentrations of metformin (Fig. 4b). These data are in good agreement with the concept that activation of AMPK restores energy levels and as a result also inhibits gluconeogenesis.
Acute depletion of both AMPKα1 and AMPKα2 significantly decreased the efficacy of metformin to suppress Bt-cAMP-stimulated glucose production (Fig. 3c), which indicates that AMPK is important in mediating metformin suppression of glucose production. This notion is further supported by data showing that depletion of both AMPKα1 and AMPKα2 eliminated suppression of glucose production by low concentration of metformin (80 μm) (Figs. 5h and 7g). Low metformin concentrations (<50 μm) activated AMPK activity (12), and cAMP levels and AMP/ATP ratio were not affected at these concentrations (20, 29), highlighting the essential role of AMPK in mediating the suppression of HGP by metformin at low concentrations observed in the portal vein.
Foretz et al. (20) also proposed that metformin administration did not affect Pck1 mRNA levels in primary hepatocytes. However, metformin significantly inhibited Pck1 and G6pc mRNA levels in vivo (Fig. 2, a and b) (11, 22). The reason why high concentrations of metformin did not inhibit Pck1 mRNA in primary hepatocytes (20) is due to the fact that metformin and Bt-cAMP were added simultaneously. However, pretreatment with metformin significantly suppressed Pck1 and G6pc mRNA levels, and Bt-cAMP stimulated glucose production in a concentration-dependent manner (Fig. 1, a–c). Importantly, pretreatment with low metformin concentrations also significantly suppressed Pck1 mRNA levels (Figs. 1c and 5f). These data suggest that activation of cAMP-PKA results in a negative effect on metformin action. Indeed, phosphorylation of AMPKα at Ser-485/497 by activated PKA negatively regulates the phosphorylation of AMPKα at Thr-172 and the activation of AMPK (Fig. 8, c–e). Of note, activation of PKA by Bt-cAMP and phosphorylation of AMPKα at Ser-485/497 precedes the phosphorylation of AMPKα at Thr-172 (30 min versus 2 h) (Fig. 8c), and the AMPKα S485A mutant negated the negative regulation of Bt-cAMP on AMPK activity, resulting in the increased suppression of Bt-cAMP-stimulated glucose production by metformin (Fig. 10, a and b).
Moreover, a recent study demonstrated that the ATP and cAMP levels and the AMP/ATP ratio did not change in the liver of rats treated with metformin (39). Of note, metformin did not affect the protein levels of phosphoenolpyruvate carboxykinase C (PEPCK-C) in the liver of healthy rats; however, the protein levels of G6pc, which is more sensitive to metformin (Fig. 1e, left), were not provided. These investigators proposed that metformin inhibits the enzymatic activity of glycerol-3-phosphate dehydrogenase to decrease ATP synthesis in mitochondria by blocking the NADH shuttle. This mechanism of metformin action may be important for the suppression of hepatic production in diabetic patients with high levels of serum lactate (39).
In summary, our results confirm our previous report that metformin inhibits expression of gene related to gluconeogenesis (Figs. 1, b and c, and 2, a and b) (15, 40), and other investigators have also shown that gluconeogenic gene expression is suppressed by metformin in euglycemic clamp experiments (11) and in mice with long term metformin administration (22). Importantly, low metformin concentrations are able to suppress glucose production and gluconeogenic gene expression if hepatocytes are pretreated with metformin (Figs. 5, c, e, and f, and 7a) without increasing the AMP/ATP ratio (Figs. 6c and 7e).
This work was supported in part National Institutes of Health Grant R00DK085142 through the NIDDK (to L. H.), The Johns Hopkins University-University of Maryland Diabetes Research Center, National Institutes of Health Grant P60DK079637 from NIDDK, and an award from the Johns Hopkins Institute for Clinical and Translational Research funded by the National Center for Advancing Translational Sciences Grant UL1TR000424-06 (to L. H.).
This article was selected as a Paper of the Week.
Cancer research report (2013) Metformin: Can a diabetes drug help prevent cancer? http://www.cancer.gov/cancertopics/research-updates/2013/metformin.
- HGP
- hepatic glucose production
- AMPK
- AMP-activated protein kinase
- CREB
- cAMP-response element-binding protein
- Bt-cAMP
- dibutyryl-cAMP
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- DIO
- diet-induced obesity
- (Rp)-cAMP
- a PKA competitive inhibitor.
REFERENCES
- 1. Witters L. A. (2001) The blooming of the French lilac. J. Clin. Invest. 108, 1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Evans J. M., Donnelly L. A., Emslie-Smith A. M., Alessi D. R., Morris A. D. (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li D., Yeung S. C., Hassan M. M., Konopleva M., Abbruzzese J. L. (2009) Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137, 482–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Libby G., Donnelly L. A., Donnan P. T., Alessi D. R., Morris A. D., Evans J. M. (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32, 1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landman G. W., Kleefstra N., van Hateren K. J., Groenier K. H., Gans R. O., Bilo H. J. (2010) Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 33, 322–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Higgins J. W., Bedwell D. W., Zamek-Gliszczynski M. J. (2012) Ablation of both organic cation transporter (OCT)1 and OCT2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab. Dispos. 40, 1170–1177 [DOI] [PubMed] [Google Scholar]
- 7. Stocker S. L., Morrissey K. M., Yee S. W., Castro R. A., Xu L., Dahlin A., Ramirez A. H., Roden D. M., Wilke R. A., McCarty C. A., Davis R. L., Brett C. M., Giacomini K. M. (2013) The effect of novel promoter variants in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin. Pharmacol. Ther. 93, 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilcock C., Bailey C. J. (1994) Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 24, 49–57 [DOI] [PubMed] [Google Scholar]
- 9. Tucker G. T., Casey C., Phillips P. J., Connor H., Ward J. D., Woods H. F. (1981) Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br. J. Clin. Pharmacol. 12, 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hundal R. S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S. E., Schumann W. C., Petersen K. F., Landau B. R., Shulman G. I. (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takashima M., Ogawa W., Hayashi K., Inoue H., Kinoshita S., Okamoto Y., Sakaue H., Wataoka Y., Emi A., Senga Y., Matsuki Y., Watanabe E., Hiramatsu R., Kasuga M. (2010) Role of KLF15 in regulation of hepatic gluconeogenesis and metformin action. Diabetes 59, 1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 15. He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M. A., Radovick S., Wondisford F. E. (2009) Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell 137, 635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Owen M. R., Doran E., Halestrap A. P. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 [PMC free article] [PubMed] [Google Scholar]
- 17. El-Mir M. Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 [DOI] [PubMed] [Google Scholar]
- 18. Oakhill J. S., Steel R., Chen Z. P., Scott J. W., Ling N., Tam S., Kemp B. E. (2011) AMPK is a direct adenylate charge-regulated protein kinase. Science 332, 1433–1435 [DOI] [PubMed] [Google Scholar]
- 19. Xiao B., Sanders M. J., Underwood E., Heath R., Mayer F. V., Carmena D., Jing C., Walker P. A., Eccleston J. F., Haire L. F., Saiu P., Howell S. A., Aasland R., Martin S. R., Carling D., Gamblin S. J. (2011) Structure of mammalian AMPK and its regulation by ADP. Nature 472, 230–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen S., Rabøl R., Hansen C. N., Madsbad S., Helge J. W., Dela F. (2012) Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia 55, 443–449 [DOI] [PubMed] [Google Scholar]
- 22. Martin-Montalvo A., Mercken E. M., Mitchell S. J., Palacios H. H., Mote P. L., Scheibye-Knudsen M., Gomes A. P., Ward T. M., Minor R. K., Blouin M. J., Schwab M., Pollak M., Zhang Y., Yu Y., Becker K. G., Bohr V. A., Ingram D. K., Sinclair D. A., Wolf N. S., Spindler S. R., Bernier M., de Cabo R. (2013) Metformin improves healthspan and lifespan in mice. Nat. Commun. 4, 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He L., Naik K., Meng S., Cao J., Sidhaye A. R., Ma A., Radovick S., Wondisford F. E. (2012) Transcriptional co-activator p300 maintains basal hepatic gluconeogenesis. J. Biol. Chem. 287, 32069–32077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He L., Cao J., Meng S., Ma A., Radovick S., Wondisford F. E. (2013) Activation of basal gluconeogenesis by co-activator p300 maintains hepatic glycogen storage. Mol. Endocrinol. 27, 1322–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Witters L. A., Kemp B. E. (1992) Insulin activation of acetyl-CoA carboxylase accompanied by inhibition of the 5′-AMP-activated protein kinase. J. Biol. Chem. 267, 2864–2967 [PubMed] [Google Scholar]
- 26. Brovko L. Yu., Romanova N. A., Ugarova N. N. (1994) Bioluminescent assay of bacterial intracellular AMP, ADP, and ATP with the use of a coimmobilized three-enzyme reagent (adenylate kinase, pyruvate kinase, and firefly luciferase). Anal. Biochem. 220, 410–414 [DOI] [PubMed] [Google Scholar]
- 27. Gorman M. W., Marble D. R., Ogimoto K., Feigl E. O. (2003) Measurement of adenine nucleotides in plasma. Luminescence 18, 173–181 [DOI] [PubMed] [Google Scholar]
- 28. Fryer L. G., Parbu-Patel A., Carling D. (2002) The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277, 25226–25232 [DOI] [PubMed] [Google Scholar]
- 29. Miller R. A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M. J. (2013) Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 494, 256–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jang E. H., Kim H. K., Park C. S., Kang J. H. (2010) Increased expression of hepatic organic cation transporter 1 and hepatic distribution of metformin in high-fat diet-induced obese mice. Drug Metab. Pharmacokinet. 25, 392–397 [DOI] [PubMed] [Google Scholar]
- 31. Pulinilkunnil T., He H., Kong D., Asakura K., Peroni O. D., Lee A., Kahn B. B. (2011) Adrenergic regulation of AMP-activated protein kinase in brown adipose tissue in vivo. J. Biol. Chem. 286, 8798–8809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soraya H., Farajnia S., Khani S., Rameshrad M., Khorrami A., Banani A., Maleki-Dizaji N., Garjani A. (2012) Short-term treatment with metformin suppresses Toll like receptors (TLRs) activity in isoproterenol-induced myocardial infarction in rat: are AMPK and TLRs connected? Int. Immunopharmacol. 14, 785–791 [DOI] [PubMed] [Google Scholar]
- 33. Tsuchiya Y., Denison F. C., Heath R. B., Carling D., Saggerson D. (2012) 5′-AMP-activated protein kinase is inactivated by adrenergic signalling in adult cardiac myocytes. Biosci. Rep. 32, 197–213 [DOI] [PubMed] [Google Scholar]
- 34. Djouder N., Tuerk R. D., Suter M., Salvioni P., Thali R. F., Scholz R., Vaahtomeri K., Auchli Y., Rechsteiner H., Brunisholz R. A., Viollet B., Mäkelä T. P., Wallimann T., Neumann D., Krek W. (2010) PKA phosphorylates and inactivates AMPKα to promote efficient lipolysis. EMBO J. 29, 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hurley R. L., Barré L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., Witters L. A. (2006) Regulation of AMP-activated protein kinase by multisite phosphorylation in response to agents that elevate cellular cAMP. J. Biol. Chem. 281, 36662–36672 [DOI] [PubMed] [Google Scholar]
- 36. Ning J., Xi G., Clemmons D. R. (2011) Suppression of AMPK activation via S485 phosphorylation by IGF-I during hyperglycemia is mediated by AKT activation in vascular smooth muscle cells. Endocrinology 152, 3143–3154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Garcia-Haro L., Garcia-Gimeno M. A., Neumann D., Beullens M., Bollen M., Sanz P. (2012) Glucose-dependent regulation of AMP-activated protein kinase in MIN6 beta cells is not affected by the protein kinase A pathway. FEBS Lett. 586, 4241–4247 [DOI] [PubMed] [Google Scholar]
- 38. Dagon Y., Hur E., Zheng B., Wellenstein K., Cantley L. C., Kahn B. B. (2012) p70S6 kinase phosphorylates AMPK on serine 491 to mediate leptin's effect on food intake. Cell Metab. 16, 104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Madiraju A. K., Erion D. M., Rahimi Y., Zhang X. M., Braddock D. T., Albright R. A., Prigaro B. J., Wood J. L., Bhanot S., MacDonald M. J., Jurczak M. J., Camporez J. P., Lee H. Y., Cline G. W., Samuel V. T., Kibbey R. G., Shulman G. I. (2014) Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 10.1038/nature13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. He L., Meng S., Germain-Lee E. L., Radovick S., Wondisford F. E. (2014) Potential biomarker of metformin action. J. Endocrinol. 221, 363–369 [DOI] [PMC free article] [PubMed] [Google Scholar]