Abstract
Hepatic glucose and lipid metabolism are altered in metabolic disease (e.g. obesity, metabolic syndrome, and Type 2 diabetes). Insulin-dependent regulation of glucose metabolism is impaired. In contrast, lipogenesis, hypertriglyceridemia, and hepatic steatosis are increased. Because insulin promotes lipogenesis and liver fat accumulation, to explain the elevation in plasma and tissue lipids, investigators have suggested the presence of pathway-selective insulin resistance. In this model, insulin signaling to glucose metabolism is impaired, but insulin signaling to lipid metabolism is intact. We discuss the evidence for the differential regulation of hepatic lipid and glucose metabolism. We suggest that the primary phenotypic driver is altered substrate delivery to the liver, as well as the repartitioning of hepatic nutrient handling. Specific alterations in insulin signaling serve to amplify the alterations in hepatic substrate metabolism. Thus, hyperinsulinemia and its resultant increased signaling may facilitate lipogenesis, but are not the major drivers of the phenotype of pathway-selective insulin resistance.
Keywords: Adipose Tissue, Glucose, Insulin, Lipid Synthesis, Liver, TOR Complex (TORC), Triacylglycerol
Introduction
Metabolic disease (i.e. obesity, metabolic syndrome, and Type 2 diabetes) is characterized by altered glucose homeostasis, hyperinsulinemia, and hypertriglyceridemia. In the fasting state, the hyperglycemia and hyperinsulinemia are driven by a failure of insulin to augment muscle glucose uptake and restrain hepatic glucose production. In the fed state, this insulin resistance results in a failure of the liver to switch from glucose production to glucose disposal, contributing to the exaggerated hyperglycemia. Hyperinsulinemia is thought to be driven by the accompanying insulin resistance in multiple tissues including liver, muscle, adipose tissue, vasculature, and the brain. Although overall brain glucose uptake is not regulated by insulin, specific regions in the brain can become resistant to insulin. This modifies neural circuits that regulate the insulin response of the liver and other peripheral tissues, thereby exacerbating hyperinsulinemia and impaired glucose metabolism (1).
The role of insulin resistance with regard to hepatic lipid metabolism is more complex. Insulin is required for hepatic lipid synthesis. Thus, one might hypothesize that hepatic insulin resistance would decrease hepatic triglyceride synthesis and therefore plasma triglycerides. In contrast, liver and plasma triglycerides are increased in metabolic disease. To resolve this paradox, investigators have proposed that there may be pathway-selective insulin resistance, which has been observed in vascular tissues (2–4). This would suggest that there are distinct insulin-sensitive signaling pathways that independently modulate glucose and lipid metabolism. Moreover, the model proposes that the pathways are differentially altered in metabolic disease. With selective insulin resistance, insulin fails to adequately suppress hepatic glucose production or augment hepatic glucose uptake, and yet still augments or at least sustains hepatic lipogenesis and TG2 accumulation, contributing to hypertriglyceridemia. We will examine the physiologic evidence that suggests selective hepatic insulin resistance exists and is the driver of the metabolic phenotype. We suggest that the alterations in hepatic substrate metabolism are not simply due to selective defects in insulin action, but to a large extent are driven by changes in substrate delivery to the liver that arise from impaired insulin signaling in liver, muscle, and adipose tissues.
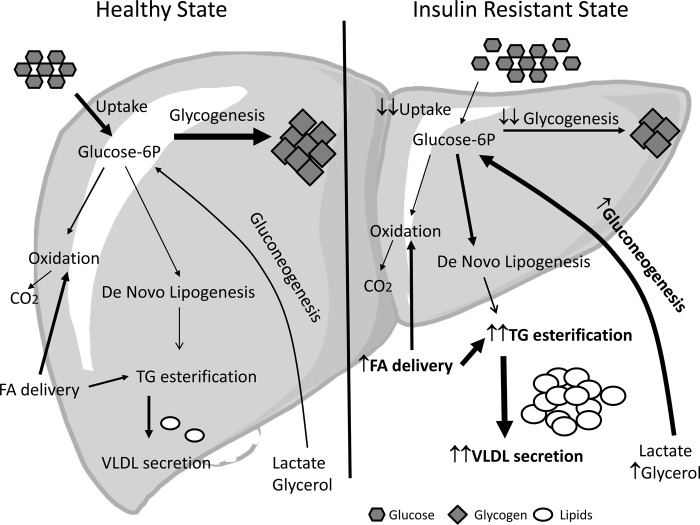
Throughout the 24-h feeding-fasting cycle, the liver handles a large fraction of the dietary nutrients. Metabolic disease markedly alters both the pattern and the absolute flux of those nutrients to the liver. There are six major metabolic pathways that are disrupted: two pathways are impaired (hepatic glucose uptake and glycogen deposition); two pathways are active, instead of inhibited due to the endocrine environment (gluconeogenesis and de novo lipogenesis); and two pathways are accelerated (fatty acid delivery and triglyceride esterification and secretion). As depicted in Fig. 1, it is the confluence of these dysregulated pathways in multiple tissues that precipitates the metabolic phenotype (i.e. impaired glucose tolerance, steatosis, and hypertriglyceridemia). In the following sections, we will discuss how each of these contributors to the phenotype of pathway-selective insulin resistance is altered.
FIGURE 1.
Hepatic substrate flux in the fed state in healthy and insulin-resistant subjects.
Hepatic Insulin Resistance and Glucose Homeostasis
With obesity and insulin resistance, hepatic glucose production after an overnight fast is normal or elevated despite the presence of hyperinsulinemia. This is because the ability of insulin to regulate hepatic glucose uptake and production is impaired (5). The defects observed in the fasted state are readily manifest during a hyperinsulinemic-euglycemic clamp, where in response to an increase in insulin infusion, hepatic glucose production fails to be suppressed. The inappropriately high rates of hepatic glucose production in the presence of hyperinsulinemia are associated with an increase in gluconeogenesis and glycogenolysis, with a greater contribution of gluconeogenesis to glucose production (6–9).
Hepatic glucose uptake and glycogen synthesis are also defective. Following a carbohydrate-containing meal, the liver rapidly switches from a net producer to a net consumer of glucose. A substantial portion of dietary carbohydrate is stored by the liver as glycogen (approximately one-third of the dietary glucose) and is then subsequently released during the post-absorptive state by activation of glycogenolysis (10). The magnitude of the hepatic glucose uptake is determined by the glucose and insulin concentration as well as by the route of glucose delivery (11). In addition, extrahepatic factors contribute to failed insulin suppression of hepatic glucose production. There is a failure of insulin to appropriately suppress glucagon secretion from the pancreas, as well as impaired suppression of adipose tissue lipolysis. Both of these exacerbate the impairment in liver glucose uptake in individuals with metabolic disease (12, 13). We will briefly discuss the molecular control points that contribute to the impaired hepatic glucose handling: 1) decreased glucose uptake, 2) decreased glycogen synthesis, and 3) persistent gluconeogenesis.
Hepatic Glucose Transport and Phosphorylation
In the liver, the primary regulated step that determines glucose uptake is glucose phosphorylation and not glucose transport. In contrast to muscle and adipose tissue, liver glucose transport (primarily through GLUT2) is not regulated by insulin and is not a significant barrier for glucose uptake (or release) by the liver. The liver expresses hexokinase IV (i.e. glucokinase (GK)), which is inactive when associated with glucokinase regulatory protein (GKRP) in the nucleus. The interaction of GK with GKRP is regulated by metabolic intermediates (glucose-6-phosphate and fructose-1-phosphate) that accumulate in response to hyperglycemia and dietary carbohydrates (e.g. fructose). These glycolytic intermediates compete with the binding of GK to GKRP; thus, this step is not directly controlled by glucose (14). Allosteric regulation of GK allows the liver to be very responsive to changes in glucose concentration (15, 16). GK transcription is positively regulated by insulin signaling, but this does not play a role in the rapid regulation of the glucose phosphorylation capacity of the liver (17, 18). Therefore, the GK activity is controlled by the partnership between insulin signaling and substrate-dependent regulation. Metabolic disease impairs GK activity (19, 20). This can be partially reversed if chronic hyperglycemia is corrected, suggesting that possible post-transcriptional modification may occur (21).
Alterations in the dephosphorylation of glucose 6-phosphate (G6P) by glucose-6-phosphatase (G6Pase) can also contribute to the observed defects in liver glucose uptake in metabolic disease. G6Pase is part of a multimeric complex located in the endoplasmic reticulum membrane, which includes G6Pase and transporters for glucose, inorganic phosphate, and glucose 6-phosphate. The regulation of this complex is poorly understood (22). Deficiency of G6Pase or the G6P transporter leads to glycogen storage disease and hypoglycemia (23). G6Pase activity is increased by glucagon and hyperglycemia and inhibited by insulin (24, 25). In mild metabolic disease, hepatic expression of G6Pase is not increased (26). However, the normal activity is inappropriate for the prevailing hyperinsulinemia, indicating predominant hepatic insulin resistance. Thus, sustained G6Pase activity opposes GK and limits the capacity of the liver to take up glucose.
Liver Glycogen Synthesis
Hepatic glycogen deposition is impaired in metabolic disease. The normal activation of net glycogen deposition requires a coordinated suppression of glycogen phosphorylase and activation of glycogen synthase. The rapid entry of glucose, facilitated by hyperglycemia and glucose activation of GK, increases G6P, suppresses glycogen phosphorylase, and activates glycogen synthase. In addition, both enzymes are regulated by phosphorylation and dephosphorylation reactions that are reciprocally regulated by insulin and glucagon (27). However, plasma glucose entry via GK is not the only source of G6P for glycogen synthesis. Gluconeogenesis is active in the fed state and accounts for about 50% of the glycogen synthesized in the liver after a carbohydrate-containing meal (10, 28–30). In metabolic disease, hepatic glycogen synthesis is decreased, and it is accompanied by a failure to appropriately suppress glycogen phosphorylase and activate glycogen synthase (31). As overexpression of a constitutively active glycogen synthase can overcome this impairment, defects upstream of glycogen synthase (e.g. GK) are not likely the sole determinant of the impairment in glycogen synthesis (31, 32). As glycogen synthesis is the major metabolic fate of glucose in the liver, glucose carbon that cannot be deposited as glycogen must be released, oxidized, or diverted to lipid synthetic pathways.
Hepatic Carbohydrate Oxidation
Impairments in hepatic glycogen synthesis in metabolic disease divert carbohydrate carbon flux to de novo lipogenesis (DNL) and oxidation. Although glycogen is a main metabolic fate of glucose after a meal, a small portion (<25%) of the glucose taken up by the liver is oxidized, and an even smaller amount is diverted to lipid synthesis. Pyruvate dehydrogenase (PDH) activity in the liver, which is negatively regulated by phosphorylation (PDK2 and PDK4), plays a central role in regulating pyruvate oxidation (33). Targeted removal of PDK4 improves pyruvate dehydrogenase activity and glucose homeostasis and the ability of insulin to suppress gluconeogenic gene expression in insulin-resistant mice (34). Although the liver has the capacity to convert glucose carbon to lipid (i.e. DNL) in whole body physiology, this is a relatively minor metabolic pathway, except with carbohydrate overfeeding (35, 36). In obese diabetic animal models, glycogen synthesis is impaired. Thus, even in the fed state, the failure to augment glycogen synthesis combined with normal or elevated G6Pase activity allows more gluconeogenic-derived carbon to be diverted to glucose release, rather than to storage as glycogen (37, 38), with potential spillover of these carbons to DNL. Animals and humans lacking normal glycogen synthase are prone to hepatic lipid accumulation even on a normal diet (39, 40).
Gluconeogenesis
Acutely, gluconeogenesis is determined by availability of the gluconeogenic precursors, the first pass extraction of the precursors by the liver, and the gluconeogenic capacity of liver. In contrast to glycogenolysis, physiologic control of gluconeogenesis is primarily determined by hepatic gluconeogenic substrate uptake and not by gene expression (41–44). Although insulin inhibits phosphoenolpyruvate carboxykinase (PEPCK) expression, acute increases in insulin do not inhibit gluconeogenic flux (e.g. pyruvate to G6P); gluconeogenic carbon is directed to glycogen rather than glucose. Insulin does potently suppress lipolysis, which would decrease glycerol (a gluconeogenic precursor) flux to the liver. In metabolic disease, lipolysis can persist despite hyperinsulinemia, which helps to sustain gluconeogenesis (38, 45). Thus, the abnormal carbohydrate handling by the liver is driven by interplay between altered nutrient disposal in many tissues and insulin signaling.
Hepatic Insulin Resistance and Triglyceride Metabolism
Elevated fasting plasma triglyceride concentration (hypertriglyceridemia) is commonly seen in metabolic disease. Circulating triglycerides are primarily associated with very low density lipoprotein (VLDL) in the fasting state and with chylomicrons in the fed state (46). An increase in VLDL secretion is the main cause of the fasting hypertriglyceridemia (47, 48). Each VLDL particle is loaded with one ApoB molecule. The lipid associated with VLDL is derived from multiple sources (fatty acids from adipose tissue, intrahepatic lipid stores, hepatic clearance of chylomicron remnants, and DNL (49, 50)). Their relative contribution to VLDL flux is dependent on fasting status, the composition of the diet in the fed state, and the capacity of peripheral tissue to remove the dietary nutrients (51, 52). Re-esterification of lipolysis-derived fatty acids compromises the majority of the VLDL-associated TG in the fasted setting (49). In a net sense, hepatic lipid stores are not appreciably changing as humans transition into an overnight fast (53). Thus, mobilization of hepatic lipid stores is not contributing to fasting VLDL production. An exception to this would be rodents where overnight fasting can induce a hepatic steatosis. Rodents are nocturnal feeders; overnight fasting is a major metabolic stress, especially in mice. This results in a mobilization of adipose-derived lipids that can accumulate in the liver.
Following a meal, hepatic handling of fatty acids is amplified despite a fall in lipolysis as both chylomicron remnant uptake and DNL increase (50). Although dietary or obesity-induced hepatic steatosis does occur in humans, in most cases it develops slowly over an extended period of time (months to years). The liver is very efficient at resisting changes in hepatic lipids, despite the fact that intrahepatic stores are rapidly turning over throughout the feeding-fasting cycle (80%/h in mouse and 10%/h in human). For example, to triple hepatic lipid stores in 2 weeks in mice would require only a 1.5% difference between the net esterification rate of hepatic lipid (DNL + re-esterification − oxidation) and rate of release of VLDL.
The molecular events that are important in the acute and chronic regulation of hepatic lipid metabolism have been defined in multiple species (54). We will divide the regulation of hepatic lipid metabolism into three sections: 1) de novo lipogenesis, 2) fatty acid esterification (TG synthesis), and 3) VLDL secretion. Using gene targeting and pharmacological approaches, investigators have identified the signaling events and control points that seem to be major targets of insulin and that have the potential to be dysregulated in metabolic disease (54). A number of excellent review articles have discussed these pathways, so we will only highlight the key control points and the evidence suggesting that these molecules are important in hepatic lipid metabolism in vivo.
De Novo Lipogenesis
As already mentioned, the synthesis of newly synthesized (de novo) lipid in the liver is normally relatively low (<5% of palmitate is newly synthesized) unless animals are severely obese (55). In individuals with diabetes, it is also low in most but not all studies (56, 57). Because palmitate is the primary product of DNL and represents ∼20% of VLDL-associated fatty acids, the percentage of VLDL-associated fatty acids that are derived from DNL will be even lower. The coordinated regulation of DNL requires complex fatty acid synthetic machinery, which is dependent on liver X receptor (LXR), SREBP-1c, ChREBP, and others. They combine to increase the DNL capacity of the liver (58). Insulin increases hepatic SREBP-1c, promoting expression of lipogenic genes and fatty acid accumulation in liver (59).
A key regulated step in hepatic DNL is acetyl CoA carboxylase (ACC). Initial studies indicated that ACC2 global KO mice are protected from high fat diet-induced obesity (improved overnight fasting glucose, insulin concentrations, and glucose tolerance) (60, 61). However, in a separately generated animal line, there was no glucose phenotype (62). Liver-specific loss of ChREBP using a ChREBP-specific antisense oligonucleotide in fructose fed rats did not alter hepatic insulin action on glucose metabolism. These rats had a fall in plasma FFA, suggesting that lipolysis was decreased, which likely explains the accompanying decrease in VLDL flux. Although lipogenic gene expression was decreased, hepatic lipid content was unaltered (63). In genetically obese (ob/ob) mice, hepatic lipids were improved, as well as tracer-determined DNL, when ChREBP was suppressed using adenovirus expressing shChREBP (64). Moreover, as in the prior study, plasma triglycerides and circulating fatty acids were also decreased, thus leaving in question the contribution of DNL to a decrease in plasma triglycerides and the specific regulatory role of ChREBP.
Investigators using genetic manipulations have identified a number of control points for DNL. However, these control points are not the sole determinants of the rate of DNL in metabolic disease. Interestingly, although hepatic DNL gene (Fasn, Elovl6) expression correlated with markers of steatosis and insulin resistance, it was not correlated with plasma TG (65). This suggests that plasma TG levels are driven by the combined effects of hepatic and extrahepatic events. In contrast to adipose tissue, changes in the absolute rate of DNL in the liver are less responsive to rapid changes of glucose and/or insulin that might occur throughout the feeding-fasting cycle and are more tightly linked to the rate of substrate delivery to and uptake by the liver (66, 67). An additional confounder is that many of the transcription factors that are important for DNL also affect hepatic carbohydrate metabolism and vice versa. Thus, it is often difficult to ascertain whether insulin regulation of DNL is modulated independent of the effects on other metabolic pathways. For example, patients with genetic defects in gluconeogenic enzymes, such as PCK1, PCK2, G6Pase, G6PC, FBP1, or pyruvate carboxylase, manifest hypoglycemia, hepatomegaly, and fatty liver (OMIM (Online Mendelian Inheritance in Man) database).
TG Esterification and Secretion
The contribution of dysfunctional regulation of fatty acid esterification into hepatic TG toward fatty liver and dyslipidemia is unclear (68). DGAT is the final step in the conversion of diacylglycerol to TG. The liver expresses both DGAT1 and DGAT2. Overexpression of either one will increase TG content in the liver, but not plasma TG or VLDL secretion (69). Although genes involved in DNL were increased with DGAT overexpression, the primary driver of the elevation in hepatic TG is likely due to diminished hepatic clearance (export) of stored TG onto VLDL. Whether DGAT has a role in modulating insulin action is controversial; DGAT overexpression has been reported to have no phenotype and an insulin-resistant phenotype (69, 70). Suppression of DGAT2 (but not DGAT1) in liver and adipose tissue improved hepatic and peripheral tissue insulin action (71). DGAT has been evaluated as a therapeutic target. However, the primary effect may not be on the liver because these therapeutics limited weight gain (72).
After assembly of fatty acids onto TG, the lipid has to be packaged in VLDL and exported; this process is inhibited by insulin. As opposed to DNL and TG esterification, which are increased by insulin, VLDL assembly and secretion are inhibited by insulin in part though limiting apoB availability (73, 74). ApoB is synthesized in excess of what is needed for VLDL secretion, and the remainder is degraded. The rate of apoB degradation is rapidly stimulated by insulin. Increases in insulin thus limit VLDL secretion and favor TG accumulation in the liver. Insulin also suppresses microsomal triglyceride transfer protein (MTP) by translocating FoxO1 out of the nucleus (75, 76). Overexpression of MTP augments VLDL flux, but this increase is dependent on the presence of apoB as well as excess hepatic lipid stores (77). Not surprisingly, MTP inhibitors limit export of TG from newly esterified fatty acids and lower plasma TG, but also cause hepatic steatosis (78). Interestingly, sustained (15 h) hyperglycemia (and concomitant hyperinsulinemia) can increase VLDL flux if FFA delivery is not suppressed. In contrast, if lipolysis is allowed to decrease, acute increases in insulin and glucose (2–3 h) suppress VLDL flux. In insulin-resistant settings, there is a failure to suppress lipolysis and apoB degradation persists (75, 79–82). The sustained availability of fatty acids may combine with an impaired insulin signaling to sustain VLDL flux (83). Thus, the alterations in lipid handling by peripheral tissues (failed suppression of lipolysis) are a major contributor to the altered hepatic lipid metabolism in insulin-resistant states.
Is Insulin the Conductor or Part of the Orchestra?
We suggest that the abnormal regulation of hepatic glucose and lipid metabolism is not dependent on alterations in the actions of insulin with regard to metabolic process in the liver, but rather an interaction of altered insulin signaling and nutrient fluxes within the liver, from the diet, and from extrahepatic tissues. Moreover, the differing “sensitivities” of the liver, muscle, and adipose to insulin have to be considered (84–86). Compensatory hyperinsulinemia, in an attempt to control one variable, can mask specific abnormalities in that specific substrate (e.g. obese nondiabetic individuals have near normal glucose concentrations), whereas abnormalities in other substrates can still be evident (e.g. increased plasma fatty acids). As already mentioned, substrates can be diverted to alternative pathways (e.g. DNL). Thus, if a metabolic blockade is present in a preferred pathway (e.g. glycogen synthesis or glucose dephosphorylation) (87, 88), hepatic lipid storage and DNL flux can increase without a change in VLDL flux in vivo. A targeted inhibition (or activation) of a single pathway (or insulin-mediated signal) can generate secondary metabolic signals (e.g. carbohydrate sensing via ChREBP) that drive another process that may or may not be regulated directly by insulin (75). Moreover, the response of the liver is also dependent on the delivery and subsequent uptake of substrates derived from the diet and/or released (or not cleared) by extrahepatic tissues. If insulin-responsive peripheral tissues fail to efficiently clear dietary nutrients, the liver will have to remove an even greater fraction of the dietary nutrients. Therefore, altered nutrient handling by peripheral tissues might contribute to the lipid phenotype that is mistakenly attributed primarily to selective hepatic resistance to insulin.
Hepatic DNL does require insulin signaling. Humans with rare mutations and mice lacking hepatic insulin receptor do not develop steatosis or hypertriglyceridemia (89). However, it may be that except in these extreme cases, hepatic insulin signaling serves more of a permissive role in allowing excess carbon to be diverted to DNL. Any alterations in the absolute rate of DNL may be determined more by the hepatic availability of substrates for DNL. Hence, it is possible that limitation of gluconeogenesis in a setting of impaired glycogen synthesis could protect the liver from substrate-driven DNL. An additional confounder is dietary fructose. It bypasses any existing defect in glucokinase, is preferentially taken up by the liver, and provides additional carbohydrate carbon for DNL. This is especially true if glycogen disposition (the preferred fate) is impaired (15, 90, 91). There have been some molecular explanations for pathway-selective insulin resistance. Akt induces the phosphorylation of FoxO1, which removes this transcription factor from the nucleus, shutting off gluconeogenic genes such as Pck (92). A normal function of insulin is triggering lipogenesis in the liver. Pathways downstream of the insulin receptor and Akt that stimulate the lipid synthesis include the phosphorylation of ATP-citrate lyase (ACLY) through glycogen synthase kinase 3β (GSK3β) and the transcription of genes regulated by SREBP-1c, whose transcription is under the control of mTORC1 (93, 94). Inhibition of mTORC1 blocks insulin-induced up-regulation of lipogenic gene expression, but does not affect insulin-mediated suppression of gluconeogenic gene expression (95). This is one explanation for selective hepatic insulin resistance. It is also possible that a significant portion of hepatic lipogenesis is independent of insulin signaling. A recent study suggests that hepatic lipogenic gene expression may be driven by an Akt-independent mechanism possibly through mTORC2 (96). However, as with many studies, only hepatic gene expression was assessed; the rates of the lipid esterification and DNL pathway in vivo were not assessed.
Nutrient sensing can limit or modify insulin resistance-induced defects. In the liver knock-out of the insulin receptor (complete insulin resistance), hepatic DNL and plasma VLDL were markedly decreased, but so was total glucokinase activity. Furthermore, the liver is still responsive to nutrient sensing; the SREBP response to refeeding was intact (97). Another sensor is ChREBP, which is activated by increased glucose influx in liver and, together with SREBP-1, drives expression of genes involved in fatty acid synthesis and esterification (98). ChREBP overexpression decreases the expression of gluconeogenic genes and reduces hepatic glycogen storage in mice. In many ways, ChREBP mirrors the insulin-like effects. In humans, ChREBP expression is positively correlated with hepatosteatosis, but negatively related with insulin resistance (99). On the contrary, ChREBP knock-out mice develop insulin resistance, hyperglycemia, hyperinsulinemia, and increased hepatic glycogen storage, without changes in body weight (100). Lipid metabolites derived from DNL may serve as ligands to drive metabolic changes. Mice lacking fatty acid synthase (FAS) specifically in liver showed hypoglycemia, hepatosteatosis, and reduced glycogen storage in fasted states, without affecting their body weight. Those changes are reversed when mice are treated with a ligand of peroxisome proliferator-activated receptor α (PPARα), suggesting that fatty acid synthase is involved in the generations of PPARα ligands (101). An additional explanation, however, for the reversal of steatosis is that the lipid and carbohydrate load presented to the liver was decreased by the PPARα ligands so that steatosis did not occur. Thus, events occurring in multiple tissues alter substrate availability that contributes to the metabolic phenotype.
A number of physiologic modulators that are known to improve hepatic insulin action and lower plasma TG require combined improvements in lipid metabolism in both hepatic and peripheral tissues. Weight loss decreases VLDL flux; this is due primarily to a decrease in lipolysis (102). In obese patients with nonalcoholic fatty liver disease, exercise in the absence of weight loss lowers hepatic lipid content, but does not correct the increase in VLDL flux (103). The persistent VLDL flux is likely mediated by the failure of exercise in the absence of weight loss to decrease lipolysis and FFA availability. Recent work suggests that estrogen signaling in the liver protects against fatty liver and improves insulin action; also, modulation of FoxO1 can regulate glucose-dependent VLDL flux (75, 104). This could explain why menopausal women are at greater risk of metabolic disease than premenopausal women.
Summary
The association of hepatic insulin resistance with persistent glucose production, lipogenic capacity, steatosis, and elevated VLDL flux seems at first an anomaly. However, this is only an anomaly if one assumes that insulin signaling or impaired insulin signaling are the major drivers of these processes. Insulin works in partnership with a number of physiologic regulators of carbohydrate and lipid metabolism both in liver and in peripheral tissues. Although insulin signaling plays a central role in the overall control of glucose homeostasis, its impact on lipid metabolism is more complex and requires coordinated intrahepatic regulatory mechanisms that, in some ways, are independent of insulin and work in concert with actions in peripheral tissues. Thus, although selectively altered insulin signaling does exist, the phenotype of metabolic disease is substrate-driven, and insulin signaling alterations may play more of a facilitating role.
This work was supported, in whole or in part, by National Institutes of Health Grants DK043748 and DK078188 (to O. P. M.). This work was also supported by Department of Veterans Affairs Career Development Award and Merit Award BX002223 (to J. M. S.), the Vanderbilt Diabetes and Research and Training Center (Grant DK020593), and the Vanderbilt Mouse Metabolic Phenotyping Center (Grant DK059637).
- TG
- triglyceride
- GK
- glucokinase
- GKRP
- glucokinase-associated regulatory protein
- G6P
- glucose 6-phosphate
- G6Pase
- glucose-6-phosphatase
- PDK
- pyruvate dehydrogenase kinase
- DNL
- de novo lipogenesis
- SREBP
- sterol regulatory element-binding protein
- ChREBP
- carbohydrate response element-binding protein
- DGAT
- diacylglycerol acyltransferase
- MTP
- microsomal triglyceride transfer protein
- mTORC
- mammalian target of rapamycin complex
- PPARα
- peroxisome proliferator-activated receptor α.
REFERENCES
- 1. Carey M., Kehlenbrink S., Hawkins M. (2013) Evidence for central regulation of glucose metabolism. J. Biol. Chem. 288, 34981–34988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown M. S., Goldstein J. L. (2008) Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 [DOI] [PubMed] [Google Scholar]
- 3. King G. L., Brownlee M. (1996) The cellular and molecular mechanisms of diabetic complications. Endocrinol. Metab. Clin. North Am. 25, 255–270 [DOI] [PubMed] [Google Scholar]
- 4. Jiang Z. Y., Lin Y. W., Clemont A., Feener E. P., Hein K. D., Igarashi M., Yamauchi T., White M. F., King G. L. (1999) Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Invest. 104, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cryer P. E. (2012) Minireview: Glucagon in the pathogenesis of hypoglycemia and hyperglycemia in diabetes. Endocrinology 153, 1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basu A., Shah P., Nielsen M., Basu R., Rizza R. A. (2004) Effects of type 2 diabetes on the regulation of hepatic glucose metabolism. J. Investig. Med. 52, 366–374 [DOI] [PubMed] [Google Scholar]
- 7. Rizza R. A. (2010) Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: implications for therapy. Diabetes 59, 2697–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Basu R., Chandramouli V., Dicke B., Landau B., Rizza R. (2005) Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes 54, 1942–1948 [DOI] [PubMed] [Google Scholar]
- 9. Bock G., Chittilapilly E., Basu R., Toffolo G., Cobelli C., Chandramouli V., Landau B. R., Rizza R. A. (2007) Contribution of hepatic and extrahepatic insulin resistance to the pathogenesis of impaired fasting glucose: role of increased rates of gluconeogenesis. Diabetes 56, 1703–1711 [DOI] [PubMed] [Google Scholar]
- 10. Pagliassotti M. J., Cherrington A. D. (1992) Regulation of net hepatic glucose uptake in vivo. Annu. Rev. Physiol. 54, 847–860 [DOI] [PubMed] [Google Scholar]
- 11. Moore M. C., Cherrington A. D., Wasserman D. H. (2003) Regulation of hepatic and peripheral glucose disposal. Best Pract. Res. Clin. Endocrinol. Metab. 17, 343–364 [DOI] [PubMed] [Google Scholar]
- 12. Shah P., Vella A., Basu A., Basu R., Adkins A., Schwenk W. F., Johnson C. M., Nair K. S., Jensen M. D., Rizza R. A. (2002) Effects of free fatty acids and glycerol on splanchnic glucose metabolism and insulin extraction in nondiabetic humans. Diabetes 51, 301–310 [DOI] [PubMed] [Google Scholar]
- 13. Shah P., Vella A., Basu A., Basu R., Adkins A., Schwenk W. F., Johnson C. M., Nair K. S., Jensen M. D., Rizza R. A. (2003) Elevated free fatty acids impair glucose metabolism in women: decreased stimulation of muscle glucose uptake and suppression of splanchnic glucose production during combined hyperinsulinemia and hyperglycemia. Diabetes 52, 38–42 [DOI] [PubMed] [Google Scholar]
- 14. Anderka O., Boyken J., Aschenbach U., Batzer A., Boscheinen O., Schmoll D. (2008) Biophysical characterization of the interaction between hepatic glucokinase and its regulatory protein: impact of physiological and pharmacological effectors. J. Biol. Chem. 283, 31333–31340 [DOI] [PubMed] [Google Scholar]
- 15. McGuinness O. P., Cherrington A. D. (2003) Effects of fructose on hepatic glucose metabolism. Curr. Opin. Clin. Nutr. Metab. Care 6, 441–448 [DOI] [PubMed] [Google Scholar]
- 16. Van Schaftingen E., Detheux M., Veiga da Cunha M. (1994) Short-term control of glucokinase activity: a role of a regulatory protein. FASEB J. 8, 414–419 [DOI] [PubMed] [Google Scholar]
- 17. Jetton T. L., Liang Y., Pettepher C. C., Zimmerman E. C., Cox F. G., Horvath K., Matschinsky F. M., Magnuson M. A. (1994) Analysis of upstream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J. Biol. Chem. 269, 3641–3654 [PubMed] [Google Scholar]
- 18. Postic C., Shiota M., Niswender K. D., Jetton T. L., Chen Y., Moates J. M., Shelton K. D., Lindner J., Cherrington A. D., Magnuson M. A. (1999) Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic β cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 274, 305–315 [DOI] [PubMed] [Google Scholar]
- 19. Torres T. P., Fujimoto Y., Donahue E. P., Printz R. L., Houseknecht K. L., Treadway J. L., Shiota M. (2011) Defective glycogenesis contributes toward the inability to suppress hepatic glucose production in response to hyperglycemia and hyperinsulinemia in Zucker diabetic fatty rats. Diabetes 60, 2225–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torres T. P., Catlin R. L., Chan R., Fujimoto Y., Sasaki N., Printz R. L., Newgard C. B., Shiota M. (2009) Restoration of hepatic glucokinase expression corrects hepatic glucose flux and normalizes plasma glucose in Zucker diabetic fatty rats. Diabetes 58, 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujimoto Y., Torres T. P., Donahue E. P., Shiota M. (2006) Glucose toxicity is responsible for the development of impaired regulation of endogenous glucose production and hepatic glucokinase in Zucker diabetic fatty rats. Diabetes 55, 2479–2490 [DOI] [PubMed] [Google Scholar]
- 22. Nordlie R. C., Foster J. D. (2010) A retrospective review of the roles of multifunctional glucose-6-phosphatase in blood glucose homeostasis: genesis of the tuning/retuning hypothesis. Life Sci. 87, 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Froissart R., Piraud M., Boudjemline A. M., Vianey-Saban C., Petit F., Hubert-Buron A., Eberschweiler P. T., Gajdos V., Labrune P. (2011) Glucose-6-phosphatase deficiency. Orphanet. J. Rare Dis. 6, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clore J. N., Stillman J., Sugerman H. (2000) Glucose-6-phosphatase flux in vitro is increased in type 2 diabetes. Diabetes 49, 969–974 [DOI] [PubMed] [Google Scholar]
- 25. Hornbuckle L. A., Everett C. A., Martin C. C., Gustavson S. S., Svitek C. A., Oeser J. K., Neal D. W., Cherrington A. D., O'Brien R. M. (2004) Selective stimulation of G-6-Pase catalytic subunit but not G-6-P transporter gene expression by glucagon in vivo and cAMP in situ. Am. J Physiol. Endocrinol. Metab. 286, E795–E808 [DOI] [PubMed] [Google Scholar]
- 26. Samuel V. T., Beddow S. A., Iwasaki T., Zhang X. M., Chu X., Still C. D., Gerhard G. S., Shulman G. I. (2009) Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc. Natl. Acad. Sci. U.S.A. 106, 12121–12126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roach P. J., Depaoli-Roach A. A., Hurley T. D., Tagliabracci V. S. (2012) Glycogen and its metabolism: some new developments and old themes. Biochem. J. 441, 763–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moore M. C., Cherrington A. D., Cline G., Pagliassotti M. J., Jones E. M., Neal D. W., Badet C., Shulman G. I. (1991) Sources of carbon for hepatic glycogen synthesis in the conscious dog. J. Clin. Invest. 88, 578–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giaccari A., Rossetti L. (1992) Predominant role of gluconeogenesis in the hepatic glycogen repletion of diabetic rats. J. Clin. Invest. 89, 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chueh F. Y., Malabanan C., McGuinness O. P. (2006) Impact of portal glucose delivery on glucose metabolism in conscious, unrestrained mice. Am. J. Physiol. Endocrinol. Metab. 291, E1206–E1211 [DOI] [PubMed] [Google Scholar]
- 31. Ros S., Zafra D., Valles-Ortega J., García-Rocha M., Forrow S., Domínguez J., Calbó J., Guinovart J. J. (2010) Hepatic overexpression of a constitutively active form of liver glycogen synthase improves glucose homeostasis. J. Biol. Chem. 285, 37170–37177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ros S., García-Rocha M., Calbó J., Guinovart J. (2011) Restoration of hepatic glycogen deposition reduces hyperglycaemia, hyperphagia and gluconeogenic enzymes in a streptozotocin-induced model of diabetes in rats. Diabetologia 54, 2639–2648 [DOI] [PubMed] [Google Scholar]
- 33. Jeong J. Y., Jeoung N. H., Park K. G., Lee I. K. (2012) Transcriptional regulation of pyruvate dehydrogenase kinase. Diabetes Metab. J. 36, 328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tao R., Xiong X., Harris R. A., White M. F., Dong X. C. (2013) Genetic inactivation of pyruvate dehydrogenase kinases improves hepatic insulin resistance induced diabetes. PLoS ONE 8, e71997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lewis G. F. (1997) Fatty acid regulation of very low density lipoprotein production. Curr. Opin. Lipidol. 8, 146–153 [DOI] [PubMed] [Google Scholar]
- 36. Chong M. F. F., Fielding B. A., Frayn K. N. (2007) Metabolic interaction of dietary sugars and plasma lipids with a focus on mechanisms and de novo lipogenesis. Proc. Nutr. Soc. 66, 52–59 [DOI] [PubMed] [Google Scholar]
- 37. Prahl A., Guptill L., Glickman N. W., Tetrick M., Glickman L. T. (2007) Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J. Feline Med. Surg. 9, 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Brien T. D. (2002) Pathogenesis of feline diabetes mellitus. Mol. Cell. Endocrinol. 197, 213–219 [DOI] [PubMed] [Google Scholar]
- 39. Irimia J. M., Meyer C. M., Peper C. L., Zhai L., Bock C. B., Previs S. F., McGuinness O. P., DePaoli-Roach A., Roach P. J. (2010) Impaired glucose tolerance and predisposition to the fasted state in liver glycogen synthase knock-out mice. J. Biol. Chem. 285, 12851–12861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bandsma R. H., Prinsen B. H., van Der Velden Mde S., Rake J. P., Boer T., Smit G. P., Reijngoud D. J., Kuipers F. (2008) Increased de novo lipogenesis and delayed conversion of large VLDL into intermediate density lipoprotein particles contribute to hyperlipidemia in glycogen storage disease type 1a. Pediatr. Res. 63, 702–707 [DOI] [PubMed] [Google Scholar]
- 41. Edgerton D. S., Ramnanan C. J., Grueter C. A., Johnson K. M. S., Lautz M., Neal D. W., Williams P. E., Cherrington A. D. (2009) Effects of insulin on the metabolic control of hepatic gluconeogenesis in vivo. Diabetes 58, 2766–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ramnanan C. J., Edgerton D. S., Rivera N., Irimia-Dominguez J., Farmer B., Neal D. W., Lautz M., Donahue E. P., Meyer C. M., Roach P. J., Cherrington A. D. (2010) Molecular characterization of insulin-mediated suppression of hepatic glucose production in vivo. Diabetes 59, 1302–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burgess S. C., He T., Yan Z., Lindner J., Sherry A. D., Malloy C. R., Browning J. D., Magnuson M. A. (2007) Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab. 5, 313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Catchpole B., Kennedy L. J., Davison L. J., Ollier W. E. R. (2008) Canine diabetes mellitus: from phenotype to genotype. J. Small Anim. Pract. 49, 4–10 [DOI] [PubMed] [Google Scholar]
- 45. Jungas R. L., Halperin M. L., Brosnan J. T. (1992) Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol. Rev. 72, 419–448 [DOI] [PubMed] [Google Scholar]
- 46. Grundy S. M. (2000) Metabolic complications of obesity. Endocrine 13, 155–165 [DOI] [PubMed] [Google Scholar]
- 47. Grundy S. M., Mok H. Y., Zech L., Steinberg D., Berman M. (1979) Transport of very low density lipoprotein triglycerides in varying degrees of obesity and hypertriglyceridemia. J. Clin. Invest. 63, 1274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fabbrini E., Mohammed B. S., Magkos F., Korenblat K. M., Patterson B. W., Klein S. (2008) Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology 134, 424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barrows B. R., Parks E. J. (2006) Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J. Clin. Endocrinol. Metab. 91, 1446–1452 [DOI] [PubMed] [Google Scholar]
- 50. Lambert J. E., Parks E. J. (2012) Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta 1821, 721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Timlin M. T., Barrows B. R., Parks E. J. (2005) Increased dietary substrate delivery alters hepatic fatty acid recycling in healthy men. Diabetes 54, 2694–2701 [DOI] [PubMed] [Google Scholar]
- 52. Stanhope K. L., Bremer A. A., Medici V., Nakajima K., Ito Y., Nakano T., Chen G., Fong T. H., Lee V., Menorca R. I., Keim N. L., Havel P. J. (2011) Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J. Clin. Endocrinol. Metab. 96, E1596–E1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vedala A., Wang W., Neese R. A., Christiansen M. P., Hellerstein M. K. (2006) Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J. Lipid Res. 47, 2562–2574 [DOI] [PubMed] [Google Scholar]
- 54. Strable M. S., Ntambi J. M. (2010) Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 45, 199–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jensen K. K., Previs S. F., Zhu L., Herath K., Wang S.-P., Bhat G., Hu G., Miller P. L., McLaren D. G., Shin M. K., Vogt T. F., Wang L., Wong K. K., Roddy T. P., Johns D. G., Hubbard B. K. (2012) Demonstration of diet-induced decoupling of fatty acid and cholesterol synthesis by combining gene expression array and 2H2O quantification. Am. J. Physiol. Endocrinol. Metab. 302, E209–E217 [DOI] [PubMed] [Google Scholar]
- 56. Hellerstein M. K. (1999) De novo lipogenesis in humans: metabolic and regulatory aspects. Eur. J Clin. Nutr. 53, Suppl. 1, S53–S65 [DOI] [PubMed] [Google Scholar]
- 57. Beysen C., Murphy E. J., Nagaraja H., Decaris M., Riiff T., Fong A., Hellerstein M. K., Boyle P. J. (2008) A pilot study of the effects of pioglitazone and rosiglitazone on de novo lipogenesis in type 2 diabetes. J. Lipid Res. 49, 2657–2663 [DOI] [PubMed] [Google Scholar]
- 58. Beaven S. W., Matveyenko A., Wroblewski K., Chao L., Wilpitz D., Hsu T. W., Lentz J., Drew B., Hevener A. L., Tontonoz P. (2013) Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance. Cell Metab. 18, 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shimomura I., Bashmakov Y., Ikemoto S., Horton J. D., Brown M. S., Goldstein J. L. (1999) Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. U.S.A. 96, 13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Abu-Elheiga L., Wu H., Gu Z., Bressler R., Wakil S. J. (2012) Acetyl-CoA carboxylase 2−/− mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions. J. Biol. Chem. 287, 12578–12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abu-Elheiga L., Oh W., Kordari P., Wakil S. J. (2003) Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc. Natl. Acad. Sci. U.S.A. 100, 10207–10212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Olson D. P., Pulinilkunnil T., Cline G. W., Shulman G. I., Lowell B. B. (2010) Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc. Natl. Acad. Sci. U.S.A. 107, 7598–7603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Erion D. M., Popov V., Hsiao J. J., Vatner D., Mitchell K., Yonemitsu S., Nagai Y., Kahn M., Gillum M. P., Dong J., Murray S. F., Manchem V. P., Bhanot S., Cline G. W., Shulman G. I., Samuel V. T. (2013) The role of the carbohydrate response element-binding protein in male fructose-fed rats. Endocrinology 154, 36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J. R., Girard J., Postic C. (2006) Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes 55, 2159–2170 [DOI] [PubMed] [Google Scholar]
- 65. Eissing L., Scherer T., Tödter K., Knippschild U., Greve J. W., Buurman W. A., Pinnschmidt H. O., Rensen S. S., Wolf A. M., Bartelt A., Heeren J., Buettner C., Scheja L. (2013) De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 4, 1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. (1976) Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem. J. 160, 413–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Otero Y. F., Lundblad T. M., Ford E. A., House L. M., McGuinness O. P. (2014) Liver but not adipose tissue is responsive to the pattern of enteral feeding. Physiol. Rep. 2, e00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cryer P. E. (2010) Hypoglycemia in type 1 diabetes mellitus. Endocrinol. Metab. Clin. North Am. 39, 641–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jornayvaz F. R., Birkenfeld A. L., Jurczak M. J., Kanda S., Guigni B. A., Jiang D. C., Zhang D., Lee H.-Y., Samuel V. T., Shulman G. I. (2011) Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc. Natl. Acad. Sci. U.S.A. 108, 5748–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Sr., Hevener A. L., Farese R. V., Jr. (2007) Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6, 69–78 [DOI] [PubMed] [Google Scholar]
- 71. Choi C. S., Savage D. B., Kulkarni A., Yu X. X., Liu Z. X., Morino K., Kim S., Distefano A., Samuel V. T., Neschen S., Zhang D., Wang A., Zhang X. M., Kahn M., Cline G. W., Pandey S. K., Geisler J. G., Bhanot S., Monia B. P., Shulman G. I. (2007) Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282, 22678–22688 [DOI] [PubMed] [Google Scholar]
- 72. Cao J., Zhou Y., Peng H., Huang X., Stahler S., Suri V., Qadri A., Gareski T., Jones J., Hahm S., Perreault M., McKew J., Shi M., Xu X., Tobin J. F., Gimeno R. E. (2011) Targeting acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. J. Biol. Chem. 286, 41838–41851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Banarer S., Cryer P. E. (2004) Hypoglycemia in type 2 diabetes. Med. Clin. North Am. 88, 1107–1116 [DOI] [PubMed] [Google Scholar]
- 74. Sparks J. D., Sparks C. E., Adeli K. (2012) Selective hepatic insulin resistance, VLDL overproduction, and hypertriglyceridemia. Arterioscler. Thromb. Vasc. Biol. 32, 2104–2112 [DOI] [PubMed] [Google Scholar]
- 75. Wu K., Cappel D., Martinez M., Stafford J. M. (2010) Impaired-inactivation of FoxO1 contributes to glucose-mediated increases in serum very low-density lipoprotein. Endocrinology 151, 3566–3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kamagate A., Qu S., Perdomo G., Su D., Kim D. H., Slusher S., Meseck M., Dong H. H. (2008) FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J. Clin. Invest. 118, 2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen Z., Newberry E. P., Norris J. Y., Xie Y., Luo J., Kennedy S. M., Davidson N. O. (2008) ApoB100 is required for increased VLDL-triglyceride secretion by microsomal triglyceride transfer protein in ob/ob mice. J. Lipid Res. 49, 2013–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rizzo M., Wierzbicki A. S. (2011) New lipid modulating drugs: the role of microsomal transport protein inhibitors. Curr. Pharm. Des. 17, 943–949 [DOI] [PubMed] [Google Scholar]
- 79. Sidossis L. S., Mittendorfer B., Walser E., Chinkes D., Wolfe R. R. (1998) Hyperglycemia-induced inhibition of splanchnic fatty acid oxidation increases hepatic triacylglycerol secretion. Am. J. Physiol. 275, E798–E805 [DOI] [PubMed] [Google Scholar]
- 80. Mittendorfer B., Patterson B. W., Klein S., Sidossis L. S. (2003) VLDL-triglyceride kinetics during hyperglycemia-hyperinsulinemia: effects of sex and obesity. Am. J. Physiol. Endocrinol. Metab. 284, E708–E715 [DOI] [PubMed] [Google Scholar]
- 81. Sørensen L. P., Søndergaard E., Nellemann B., Christiansen J. S., Gormsen L. C., Nielsen S. (2011) Increased VLDL-triglyceride secretion precedes impaired control of endogenous glucose production in obese, normoglycemic men. Diabetes 60, 2257–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Smith G. I., Magkos F., Reeds D. N., Okunade A. L., Patterson B. W., Mittendorfer B. (2013) One day of mixed meal overfeeding reduces hepatic insulin sensitivity and increases VLDL particle but not VLDL-triglyceride secretion in overweight and obese men. J. Clin. Endocrinol. Metab. 98, 3454–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lewis G. F., Uffelman K. D., Szeto L. W., Weller B., Steiner G. (1995) Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J. Clin. Invest. 95, 158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Conte C., Fabbrini E., Kars M., Mittendorfer B., Patterson B. W., Klein S. (2012) Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care 35, 1316–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Basu A., Dalla Man C., Basu R., Toffolo G., Cobelli C., Rizza R. A. (2009) Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 32, 866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Miles J. M., Wooldridge D., Grellner W. J., Windsor S., Isley W. L., Klein S., Harris W. S. (2003) Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 52, 675–681 [DOI] [PubMed] [Google Scholar]
- 87. Bandsma R. H. J., Wiegman C. H., Herling A. W., Burger H.-J., ter Harmsel A., Meijer A. J., Romijn J. A., Reijngoud D.-J., Kuipers F. (2001) Acute inhibition of glucose-6-phosphate translocator activity leads to increased de novo lipogenesis and development of hepatic steatosis without affecting VLDL production in rats. Diabetes 50, 2591–2597 [DOI] [PubMed] [Google Scholar]
- 88. Mutel E., Abdul-Wahed A., Ramamonjisoa N., Stefanutti A., Houberdon I., Cavassila S., Pilleul F., Beuf O., Gautier-Stein A., Penhoat A., Mithieux G., Rajas F. (2011) Targeted deletion of liver glucose-6 phosphatase mimics glycogen storage disease type 1a including development of multiple adenomas. J. Hepatol. 54, 529–537 [DOI] [PubMed] [Google Scholar]
- 89. Semple R. K., Sleigh A., Murgatroyd P. R., Adams C. A., Bluck L., Jackson S., Vottero A., Kanabar D., Charlton-Menys V., Durrington P., Soos M. A., Carpenter T. A., Lomas D. J., Cochran E. K., Gorden P., O'Rahilly S., Savage D. B. (2009) Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Invest. 119, 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hudgins L. C., Parker T. S., Levine D. M., Hellerstein M. K. (2011) A dual sugar challenge test for lipogenic sensitivity to dietary fructose. J. Clin. Endocrinol. Metab. 96, 861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stanhope K. L., Havel P. J. (2009) Fructose consumption: considerations for future research on its effects on adipose distribution, lipid metabolism, and insulin sensitivity in humans. J. Nutr. 139, 1236S–1241S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. O'Brien R. M., Streeper R. S., Ayala J. E., Stadelmaier B. T., Hornbuckle L. A. (2001) Insulin-regulated gene expression. Biochem. Soc. Trans. 29, 552–558 [DOI] [PubMed] [Google Scholar]
- 93. Leavens K. F., Easton R. M., Shulman G. I., Previs S. F., Birnbaum M. J. (2009) Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 10, 405–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Laplante M., Sabatini D. M. (2010) mTORC1 activates SREBP-1c and uncouples lipogenesis from gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 3281–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li S., Brown M. S., Goldstein J. L. (2010) Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 3441–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yuan M., Pino E., Wu L., Kacergis M., Soukas A. A. (2012) Identification of Akt-independent regulation of hepatic lipogenesis by mammalian target of rapamycin (mTOR) complex 2. J. Biol. Chem. 287, 29579–29588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Haas J. T., Miao J., Chanda D., Wang Y., Zhao E., Haas M. E., Hirschey M., Vaitheesvaran B., Farese R. V., Jr., Kurland I. J., Graham M., Crooke R., Foufelle F., Biddinger S. B. (2012) Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 15, 873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Poupeau A., Postic C. (2011) Cross-regulation of hepatic glucose metabolism via ChREBP and nuclear receptors. Biochim. Biophys. Acta 1812, 995–1006 [DOI] [PubMed] [Google Scholar]
- 99. Benhamed F., Denechaud P. D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J., Girard J., Guillou H., Postic C. (2012) The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 122, 2176–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Iizuka K., Bruick R. K., Liang G., Horton J. D., Uyeda K. (2004) Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. U.S.A. 101, 7281–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chakravarthy M. V., Pan Z., Zhu Y., Tordjman K., Schneider J. G., Coleman T., Turk J., Semenkovich C. F. (2005) “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1, 309–322 [DOI] [PubMed] [Google Scholar]
- 102. Mittendorfer B., Patterson B. W., Klein S. (2003) Effect of weight loss on VLDL-triglyceride and apoB-100 kinetics in women with abdominal obesity. Am. J. Physiol. Endocrinol. Metab. 284, E549–E556 [DOI] [PubMed] [Google Scholar]
- 103. Sullivan S., Kirk E. P., Mittendorfer B., Patterson B. W., Klein S. (2012) Randomized trial of exercise effect on intrahepatic triglyceride content and lipid kinetics in nonalcoholic fatty liver disease. Hepatology 55, 1738–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhu L., Brown W. C., Cai Q., Krust A., Chambon P., McGuinness O. P., Stafford J. M. (2013) Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62, 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]