Background: PECAM-1 participates in adhesion and signaling in blood and vascular cells.
Results: The adhesive properties of PECAM-1 in both cellular and artificial membranes can be regulated.
Conclusion: The binding affinity of PECAM-1 can be regulated by engagement of membrane-proximal Ig domain 6.
Significance: Modulating the adhesive properties of PECAM-1 offers possibility to control endothelial cell migration and barrier function in vascular permeability disorders.
Keywords: Cell Adhesion, Endothelium, Platelet, Platelet Endothelial Cell Adhesion Molecule (PECAM-1), Vascular Biology
Abstract
PECAM-1 is a 130-kDa member of the immunoglobulin (Ig) superfamily that is expressed on the surface of platelets and leukocytes, and at the intracellular junctions of confluent endothelial cell monolayers. Previous studies have shown that PECAM-1/PECAM-1 homophilic interactions play a key role in leukocyte transendothelial migration, in allowing PECAM-1 to serve as a mechanosensory complex in endothelial cells, in its ability to confer cytoprotection to proapoptotic stimuli, and in maintaining endothelial cell junctional integrity. To examine the adhesive properties of full-length PECAM-1 in a native lipid environment, we purified it from platelets and assembled it into phospholipid nanodiscs. PECAM-1-containing nanodiscs retained not only their ability to bind homophilically to PECAM-1-expressing cells, but exhibited regulatable adhesive interactions that could be modulated by ligands that bind membrane-proximal Ig Domain 6. This property was exploited to enhance the rate of barrier restoration in endothelial cell monolayers subjected to inflammatory challenge. The finding that the adhesive properties of PECAM-1 are regulatable suggests novel approaches for controlling endothelial cell migration and barrier function in a variety of vascular permeability disorders.
Introduction
Platelet endothelial cell adhesion molecule-1 (PECAM-1)3 is a 130-kDa transmembrane glycoprotein that is expressed on the surface of platelets and leukocytes, and at the intracellular junctions of confluent endothelial cell monolayers (1). PECAM-1 is comprised of six extracellular Ig-like domains, a 19-amino acid transmembrane domain and a 118-amino acid cytoplasmic domain that is subject to alternative splicing (2) and activation-dependent phosphorylation (3). In circulating platelets and leukocytes, PECAM-1 appears to function largely as an inhibitory receptor, relying on its cytoplasmic Immunoreceptor Tyrosine-based Inhibitory Motif (ITIM) to relay negative regulatory signals into the cell that limit cellular responsiveness to activating stimuli (4–6). In monolayer cells like the vascular endothelium, PECAM-1 functions in the terminal stages of leukocyte transendothelial migration (7, 8), as an anti-apoptotic protein that protects against Bax-mediated cell death (9–12), and as part of a shear-dependent mechanosensory complex (13–16). The ability of PECAM-1 to exert many of these functions is largely predicated on its ability to form homophilic interactions that allow PECAM-1 to concentrate at endothelial cell intercellular junctions in a process that has been termed “diffusion trapping” (17). Importantly, border-localized PECAM-1 is a major component of endothelial cell intercellular junctions, where it contributes importantly to barrier function and control of vascular permeability (18–22).
While it has been known for some time that PECAM-1-mediated homophilic interactions are primarily mediated by N-terminal Ig homology domain 1 (IgD1) (23–25), the molecular nature of such interactions, and whether they are subject to regulation, are unknown. Previous studies attempting to elucidate the biochemical properties that control PECAM-1-mediated adhesion have employed transfected cell lines (3, 26, 27), PECAM-1/IgG chimeric proteins (23, 28), and PECAM-1-containing proteoliposomes (23). While each of these approaches have distinct merits, they suffer from potential artifacts caused by supra-normal receptor density caused by overexpression, or altered avidity due to forced dimerization. Other approaches examining the binding of soluble recombinant forms of PECAM-1 containing only the six extracellular Ig-homology domains have observed only low-affinity interactions (29), perhaps due to loss of the transmembrane and cytoplasmic domain, or the absence of a lipid membrane environment. The adhesive properties of full-length PECAM-1 in its native environment, therefore, have been difficult to rigorously assess.
Phospholipid bilayer nanodiscs, pioneered by Sligar et al. (30–32), are a novel means of displaying membrane proteins having transmembrane domains embedded in a lipid bilayer. Nanodiscs are formed by incubating detergent-solubilized membrane proteins with phospholipids in the presence of an encircling amphipathic helical protein belt, termed a membrane scaffold protein (MSP). Following detergent removal, nanodiscs self-assemble into a discoid bilayer whose size is controlled by the length of the MSP. Membrane proteins presented in this way are soluble in aqueous solution and, importantly, exist in a near-native bilayer environment, where they remain monodisperse and functionally active. Nanodiscs have been successfully used to examine the physical and adhesive properties of a number of transmembrane receptors, including integrin αIIbβ3 (33) and the platelet GPIb complex (34). These proteins are simultaneously monomerized, solubilized, and incorporated into the well-defined membrane environment provided by nanodiscs.
The purpose of the present investigation was to adapt nanodisc technology to the study of the adhesive properties of full-length, monomeric PECAM-1 displayed in a natural membrane lipid environment. We provide evidence that individual PECAM-1 molecules, when embedded in phosphatidylcholine-containing nanodiscs retain not only their ability to bind homophilically to PECAM-1-expressing cells in an IgD1-dependent manner, but exhibit regulatable adhesive interactions that can be modulated by ligands that bind membrane-proximal IgD6. Insights provided by the knowledge that the adhesive properties of PECAM-1-containing nanodiscs are regulatable suggests novel approaches for controlling both endothelial cell migration during angiogenesis and barrier function in a variety of vascular permeability disorders.
EXPERIMENTAL PROCEDURES
Antibodies
Domain-specific mouse anti-human PECAM-1 monoclonal antibodies (mAbs) used in this study include: 235.1 (specific for the PECAM-1 C-terminal 15 amino acids), PECAM-1.2 (specific for PECAM-1 Ig Domain 6), PECAM-1.3 (specific for PECAM-1 Ig Domain 1), and have been previously described (23, 35, 36). Normal mouse IgG and secondary antibodies were purchased from Invitrogen (Grand Island, NY). Fab fragments were generated using immobilized papain (Pierce) according to the manufacturer's instructions and subjected to SDS-PAGE to confirm that no intact IgG remained in the preparations. Prior to their use, the reactivity of all anti-PECAM-1 Fab fragments was determined by enzyme-linked immunosorbent assay (ELISA) analysis using purified human platelet PECAM-1 as the target antigen. Alexa Fluor® 647-labeled mAb 235.1 was generated using a labeling kit purchased from Molecular Probes (Carlsbad, CA).
Cells
Cell culture reagents were obtained from Mediatech (Manassas, VA), unless otherwise specified. Immortalized human umbilical vein endothelial cells (iHUVEC, generated by transducing HUVECs with the recombinant retrovirus LXSN16 E6/E7) and PEC02 cells (generated by transducing iHUVECs with a lentivirus expressing a PECAM1-specific siRNA PEC02) were maintained as previously described (22). PECAM-1-transfected HEK293 cells expressing full-length human PECAM-1 were maintained in RPMI medium containing 10% FBS and 0.5 mg/ml G418 (Invitrogen).
Preparation of PECAM-1-containing Nanodiscs
PECAM-1 was purified from detergent-solubilized single-donor platelet apheresis products using a monoclonal antibody (mAb) PECAM-1.3 affinity chromatography, as previously described (23). The MSP1D1 plasmid encoding a histidine-tagged form of membrane scaffold protein was obtained from Addgene (Cambridge, MA). MSP1D1 was expressed in Escherichia coli BL21 cells and purified by Ni affinity chromatography. The yield of PECAM-1 from 500 ml PRP was ∼100 μg, while the yield of MSP1D1 from one liter of transformed BL21 E. coli cells was ∼20 mg. 1, 2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC, Avanti Polar Lipids, Alabster, AL) dissolved in chloroform was dried to a thin film in a glass vial using a gentle nitrogen stream, and then placed under vacuum to remove residual solvent. The dried lipid was redissolved with hydration buffer (20 mm Tris-HCl, 100 mm NaCl, and 100 mm sodium cholate (pH 7.4)), and then sonicated until clear at a final concentration of 5 mm. Empty nanodiscs (Enano) and PECAM-1-containing nanodiscs (Pnano) were assembled by incubating a 1.4 mm DMPC, 16 μm MSP1D1, and 12.8 mm sodium cholate without or with 0.9 μm PECAM-1 at room temperature for 60 min in a final volume of 1 ml. Assembly of nanodiscs was initiated by the addition of 0.2 g of damp Biobeads SM-2 (Bio-Rad), and the reaction complex was gently rocked for an additional 16 h. at 20 °C. After assembly, the Biobeads were removed by centrifugation at 10,000 × g for 1 min, and the supernatant applied to a Superdex 200 10/300 GL size exclusion column (GE Healthcare) calibrated by molecular mass standards (Bio-Rad) to analyze the mixture and to purify assembled nanodiscs.
Transmission Electron Microscopy (TEM)
Soluble recombinant PECAM-1 was purified from the culture medium of stably-transfected Chinese hamster ovary (CHO) cells according to previously established methods (37). Preparations for TEM were diluted in 30% glycerol, sprayed onto carbon-coated mica discs, rotary shadowed with carbon-platinum and examined in a Philips 400-T electron microscope at 60 kV.
Scanning Transmission Electron Microscopy (STEM)
PECAM-1 extracellular domain was imaged at the Brookhaven National Laboratory Facility (Upton, NY) using previously described methods (38). Briefly, specimens were diluted to a concentration of 10 μg/ml in 0.1 m NaCl, 20 mm Hepes, pH 7.0 buffer. Three μl aliquots were injected into a 3 μl buffer droplet on a carbon-film coated microscope grid and the specimen allowed to attach for 1 min. Fluid on the grid surface was exchanged 8–10 times with 150 mm ammonium acetate solution, the specimen frozen in liquid nitrogen, freeze dried, and transferred under vacuum to the microscope stage. These uncontrasted specimens were imaged at the Brookhaven STEM facility using a 40-kv probe focused at 0.25 nm. Monomolecular or bimolecular forms were verified by mass analysis of individual objects.
TEM Imaging of Negatively Contrasted Specimens
Purified nanodiscs were diluted to a concentration of 60 μg/ml in 20 mm Tris-HCl (pH 7.4) and applied to a copper EM grid containing a carbon film support (TED PELLA). Specimens were stained with 1.5% uranyl acetate, and images acquired on a Tecnai T12 transmission electron microscope (FEI, Hillsboro, OR) at a magnification of ×200,000 and recorded on a 4k × 4k Gatan Ultrascan that was binned by 2 for 2048 × 2048 resolution.
ELISA
Purified nanodiscs at a concentration of 10 μg/ml were immobilized in a 96-well Immulon 2-HB flat bottom 96-well microtiter plate (Thermo, Franklin) at 4 °C overnight, after which the wells were blocked with phosphate-buffered saline containing 1.5% bovine serum albumin (BSA). mAbs PECAM-1.3, PECAM-1.2, and 235.1 (2 μg/ml) were then added to the wells, followed by alkaline phosphatase (AP)-conjugated goat anti-mouse IgG. Normal mouse IgG (2 μg/ml) was used as a negative control. After incubation at room temperature for 1 h., wells were washed three times, 150 μl of pNPP-Substrate (Zymed Laboratories Inc., San Francisco, CA) added to each well, and the plates incubated for an additional 30 min. The reaction was stopped by addition of 25 μl of 1 m NaOH, and the plate read at 405 nm in a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA).
Flow Cytometry
PECAM-1-transfected HEK293 cells were lifted with 0.25% trypsin-EDTA and resuspended in 100 μl culture media at a density of 5 × 105/ml. Nanodiscs were then added to the cell suspension at a final concentration of 40 μg/ml, incubated for 30 min at 37 °C, and binding detected by the addition of 10 μg/ml Alexa Fluor 647-labeled mAb 235.1 for 20 min. Flow cytometry was performed using a Becton-Dickenson LSRII (BD Biosciences, San Jose, CA).
Fluorescence Microscopy
Nanodiscs were added to PECAM-1-transfected HEK293 monolayers that had been plated at a density of 1 × 105/ml on top of fibronectin-coated slide chambers (BD Biosciences). After a 40-min incubation, wells were rinsed with PBS, and the cells were fixed with 2% paraformaldehyde, and blocked with PBS containing 3% BSA. Monolayers were then incubated with mAb 235.1 (2 μg/ml) at 4 °C overnight. Binding was detected using Alexa Fluor 488 anti-mouse IgG (Invitrogen) and images obtained using a FluoView FV1000 multi-photon emission microscope (Olympus, Center Valley, PA).
Nanodisc Binding to Cells in the Presence of anti-PECAM-1 Fab Fragments
Confluent monolayers of iHUVEC and PEC02 cells were lifted with 0.25% trypsin-EDTA, resuspended in 100 μl of culture media containing varying concentrations of monoclonal antibody Fab fragments, and then washed once before addition of nanodiscs at a final concentration of 40 μg/ml. Following a 30-min incubation at 37 °C, the cells were stained with Alexa-Fluor-647-labeled mAb 235.1 (final concentration 10 μg/ml) for 20 min, and the association of nanodiscs and cells determined by flow cytometry.
ECIS Measurements of Endothelial Barrier Function
Endothelial cells were grown to confluence on gold electrodes that had been coated overnight with 50 μg/ml bovine fibrinogen (Invitrogen) and subjected to two different Electric Cell-substrate Impedance Sensing (ECIS) functional assays using an ECIS ZTheta Instrument (Applied Biophysics, Troy, NY). To measure PECAM-1 mediated endothelial cell barrier function, cells were grown in 450 μl of human endothelial serum free medium on 8W10E+ electrode arrays, stimulated with 1 unit of human thrombin (Sigma), and endothelial barrier disruption and restoration measured in real time. PECAM-1-mediated cell migration was determined by growing cells in 8W1E electrode array chambers, subjecting them to a defined elevated field pulse (1400 mA, 40000 HZ, 14 s), and measuring their rate of migration into the electrically wounded area. In both assays, measurements were continuingly recorded at multiple frequencies and modeled with ECIS software (Applied Biophysics) to obtain the barrier function parameter, Rb, which is expressed as the average basal electrical resistances (in Ω/cm2). The effects of anti-PECAM-1 antibody binding on both measures of PECAM-1-mediated endothelial cell function was determined by adding 40 μg/ml of mAbs PECAM-1.2, PECAM-1.3 or normal mouse IgG Fab fragments to wells at the nadir of Rb following thrombin- or electrical-induced injury.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA). Data were analyzed by one-way ANOVA followed by Bonferroni's multiple-comparisons test. Multiple comparisons tests were only applied when a significant difference was determined in the ANOVA (p < 0.05). Results are expressed as mean ± S.D.
RESULTS
Generation and Adhesive Properties of PECAM-1-containing Phospholipid Nanodiscs
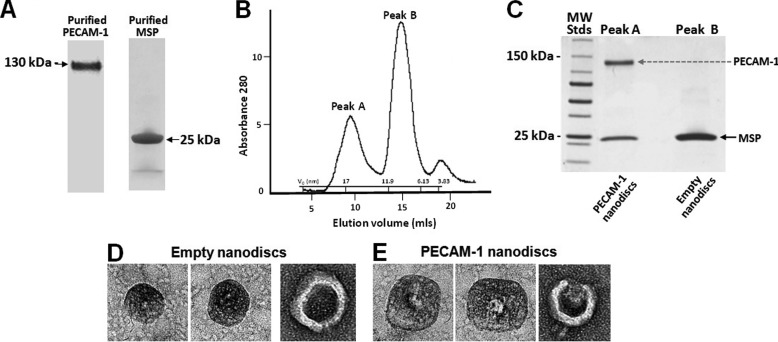
Full-length PECAM-1 purified from human platelet concentrates and the MSP1D1 form of membrane scaffold protein (Fig. 1A) were assembled at a ratio of 1:87.5:0.05625 MSP1D1:DMPC:PECAM-1 into phosphatidylcholine-containing nanodiscs as described in “Experimental Procedures,” separated from empty nanodiscs by gel filtration chromatography (Fig. 1B), and the resulting products analyzed by SDS-PAGE to confirm purity (Fig. 1C). Empty nanodiscs were eluted with a Stoke diameter of ∼9 nm, while well-separated Peak A, containing PECAM-1 nanodiscs, eluted with an apparent stokes diameter of ∼16 nm. The molar ratio of PECAM-1 to MSP1D1 was calculated as ∼0.47:1 by calibrating the difference between the Coomassie Blue staining efficiency of PECAM-1 and MSP1D1 in SDS-PAGE gels (not shown). Because nanodiscs contain two copies of MSP1D1 per nanodisc, these data are consistent with most nanodiscs containing only one copy of PECAM-1. To confirm this biochemical prediction, Enanos and Pnanos were negatively-stained and visualized by transmission electron microscopy. As shown Fig. 1, D and E, Enanos appear smaller than Pnanos, and Pnanos contain a single high-density refraction shadow in the center of each disc, consistent with the presence of an embedded PECAM-1 protein. The right-most image of Fig. 1E is tilted, exposing what appears to be both the extracellular and cytoplasmic domains of PECAM-1.
FIGURE 1.
Preparation and characterization of PECAM-1-containing nanodiscs. A, PECAM-1 was affinity-purified from human platelets, while recombinant MSP was purified from bacterial lysates. B, after self-assembly in the presence of phosphatidylcholine, PECAM-1-containing nanodiscs were separated from empty nanodiscs by gel-filtration chromatography and their Stokes diameters calculated by their elution relative to a series of known molecular mass standards using y = −0.7043× + 21.351. C, SDS-PAGE analysis of faster eluting Peak A shows the presence of ∼16 nm particles containing both PECAM-1 and MSP, as well slower-eluting Peak B comprised of ∼9 nm particles containing only the MSP protein. D and E, representative negative stained electron micrographs of empty (D) and PECAM-1-containing (E) nanodiscs. Note the single, centrally-placed electron dense entity present in the PECAM-1, but not the empty, nanodiscs.
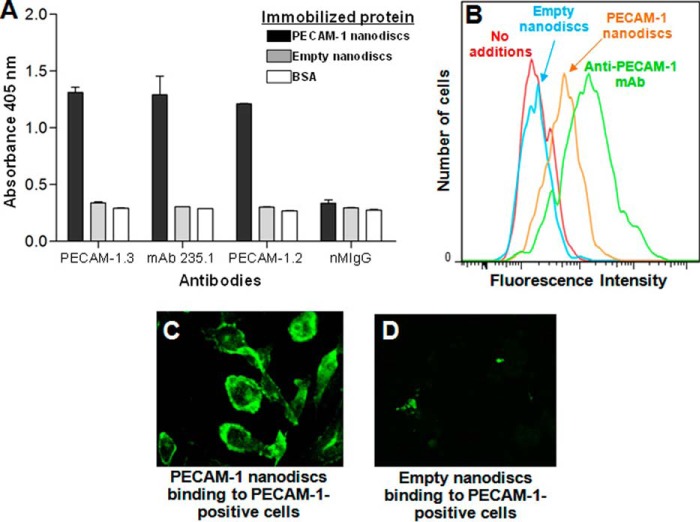
To ensure that the PECAM-1-containing nanodiscs retained both the extracellular and cytoplasmic domains of the receptor, we immobilized them in microtiter wells and evaluated their ability to be recognized by a series antibodies specific for different domains of PECAM-1. As shown in Fig. 2A, mAbs that recognize N-terminal IgD1 (PECAM-1.3), membrane-proximal IgD6 (PECAM-1.2), and the last 15 amino acids of the cytoplasmic domain (235.1) were all strongly positive by ELISA. Pnanos, but not Enanos, also bound to PECAM-1-expressing cells, as shown by FACS analysis (Fig. 2B) and immunofluorescence microscopy (Fig. 2, C and D).
FIGURE 2.
PECAM-1 nanodiscs contain intact extracellular and cytoplasmic domains and bind to PECAM-1-expressing cells. A, PECAM-1 ELISA. PECAM-1 nanodiscs, empty nanodiscs, or BSA was immobilized on microtiter wells and incubated with monoclonal antibodies specific for extracellular IgD1 (PECAM-1.3), extracellular IgD6 (PECAM-1.2), or the last 15 amino acids of the cytoplasmic domain (mAb 235.1). B, FACS analysis of nanodisc binding to PECAM-1-transfected HEK293 cells. Cells were incubated with PECAM-1-containing or empty nanodiscs, washed, and detected with Alexa Fluor 647-labeled mAb 235.1. Only nanodiscs containing PECAM-1 bound to the cells. mAb PECAM-1.3 (green line) was used as a positive control. C and D, visualization of nanodisc binding to PECAM-1-transfected cells. PECAM-1-transfected HEK293 cells were incubated with PECAM-1-containing or empty nanodiscs, washed, fixed, and incubated with mAb 235.1 and Alexa Fluor 488 secondary antibody to visualize binding.
The Adhesive Properties of PECAM-1 Are Subject to Affinity Modulation
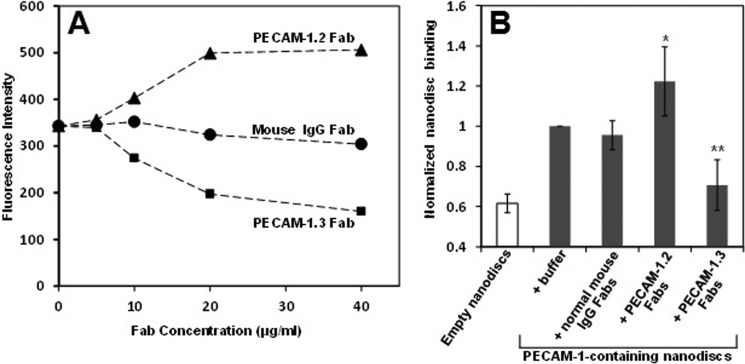
To examine whether the adhesive properties of PECAM-1 might be subject to regulation, we added nanodiscs to HUVECs in the presence or absence of domain-specific anti-PECAM-1 Fabs. As shown in Fig. 3, A and B, PECAM-1.3 Fabs, which bind IgD1 and inhibit PECAM-1-mediated homophilic binding (1), block binding of PECAM-1-containing nanodiscs to human endothelial cells, as expected. In contrast, the IgD6-specific mAb, PECAM-1.2, enhanced Pnano binding by more than 50%. Similar enhancement occurred in the presence of Fab fragments of the PECAM-1 IgD6-specific mAb, 4G6 (not shown). Pnanos did not bind to iHUVECs in which PECAM-1 had been knocked down with an siRNA (not shown). Because the PECAM-1 molecules contained in the nanodiscs are monomeric, and the Fabs are monovalent, these data are most consistent with a model in which the Fab fragments engage PECAM-1 IgD6 and increase the homophilic binding affinity of PECAM-1.
FIGURE 3.
Affinity modulation of PECAM-1. A, PECAM-1 nanodiscs were added to HUVECs in the presence of Fab fragments of the indicated antibodies. Fabs from the IgD6-specific mAb, PECAM-1.2 (▴) consistently increased nanodisc binding, while Fabs from the IgD1-specific mAb, PECAM-1.3 (■), inhibited nanodisc binding. Fabs from normal mouse IgG (●) was used as a control. A representative experiment is shown. B, level of nanodisc binding across five independent experiments, performed as in panel A, was normalized to the level of Pnano binding to iHUVECs in the absence of any added mAb. Note that PECAM-1.2 Fabs increased Pnano binding (*, p = 0.03), while PECAM-1.3 Fabs inhibited Pnano binding (**, p = 0.006). Both mAbs were added at a final concentration of 40 μg/ml. Similar augmentation was seen using the IgD6-specific mAb 4G6 (not shown).
Regulation of Endothelial Cell Barrier Function by Antibody-driven Affinity Modulation of PECAM-1
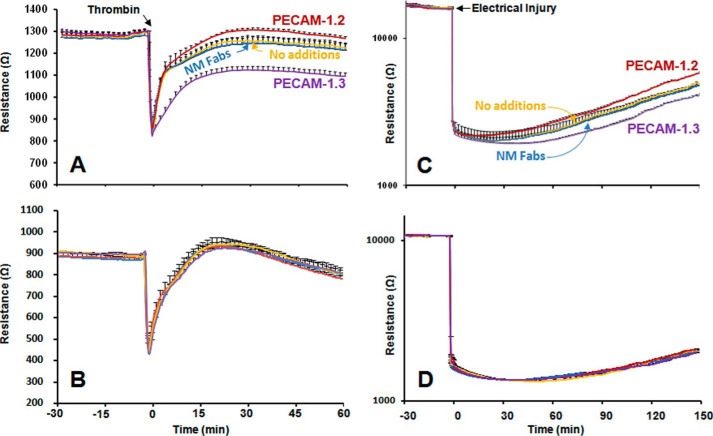
Numerous studies over the past 20 years have established that PECAM-1, which is highly-enriched at endothelial cell-cell junctions (39), contributes importantly to vascular integrity in a manner that is dependent on its homophilic binding properties (18–22). PECAM-1 has also been shown to influence the rate of endothelial cell migration (40–42). In light of the observation (Fig. 3) that the homophilic binding affinity of single PECAM-1 molecules residing in nanodiscs is subject to modulation, we employed Electric Cell-substrate Impedance Sensing (ECIS) technology to examine the ability of these same two domain-specific anti-PECAM-1 Fab fragments to influence both of these PECAM-1-dependent functions in the context of an intact endothelial cell. Endothelial cell monolayers were grown on gold-plated electrodes and stimulated with thrombin to disrupt junctional integrity, and the effects anti-PECAM-1 domain-specific Fab fragments on the rate and extent of junctional restoration recorded in real time. As shown in Fig. 4A, whereas Fab fragments from the IgD1-specific mAb, PECAM-1.3, interfered with the rate and extent of junctional restoration, IgD6-specific PECAM-1.2 Fabs enhanced both. mAb 4G6, which also binds IgD6, also enhanced the kinetics of barrier reformation (not shown). Neither PECAM-1.3 nor PECAM-1.2 had an effect on PECAM-1-negative HUVECs (Fig. 4B) in which PECAM-1 expression had been knocked down with an siRNA (described in Ref. 22). These antibodies exerted similar inhibitory and enhancing effects, respectively, on the rate of endothelial cell migration into an electrically wounded area (Fig. 4C). Similar to panel B, Fabs had no effect in PECAM-1-negative cells (panel D). Taken together, these data demonstrate that agents that block homophilic binding disrupt reformation of endothelial cell-cell junctions and suppress endothelial cell migration, while engaging IgD6 results in marked enhancement of homophilic binding and improved rates of endothelial cell migration and barrier restoration.
FIGURE 4.
IgD6-specific PECAM-1. 2 Fabs enhance endothelial cell function. A and B, restoration of endothelial cell barrier integrity. iHUVECs were grown to confluence in ECIS wells and treated with thrombin to disrupt endothelial cell-cell junctions in the presence of 40 mg/ml of the indicated Fab fragments. Addition of PECAM-1.2 Fabs to PECAM-1-positive iHUVECs (panel A) improved both the rate and extent of barrier recovery, while PECAM-1.3 Fabs, which interfere with homophilic binding, interfered with junctional restoration. No such effect was seen in PECAM-1-negative HUVECs (panel B) in which PECAM-1 expression had been knocked down with siRNA. Note that the baseline resistance is lower in PECAM-1-negative cells (compare panels A and B), as previously reported by Privratsky et al. (22), in keeping with its role in maintaining vascular integrity. The tracings shown represent the normalized mean ± S.D. of five independent experiments. The slope of recovery between 0 and 30 min, obtained by linear regression analysis, was significantly faster (p = 0.002) in the presence of PECAM-1.2 Fab fragments versus no additions, and significantly slower (p < 0.001) in the presence of PECAM-1.3 Fabs. C and D, recovery into an electrically injured area. iHUVEC monolayers were subjected to elevated field intensity in a discrete region of the ECIS chamber and the rate of barrier restoration followed over a 3-h period in the presence of 40 mg/ml of the indicated Fab fragments. The tracings shown represent the normalized mean ± S.D. of five independent experiments. The slope of recovery between 0 and 90 min obtained by linear regression analysis was significantly faster (p = 0.032) in the presence of PECAM-1.2 Fabs versus no additions, and significantly slower (p < 0.005) in the presence of PECAM-1.3 Fab fragments. Fabs had no effect in PECAM-1-negative cells (panel D).
DISCUSSION
Examining the structure and function of isolated integral membrane proteins is complicated by the insolubility of their transmembrane domains in an aqueous environment, a property that has traditionally been overcome by maintaining continued presence of non-ionic detergents, or by inducing self-assembly of membrane proteins into detergent-lipid micelles (43) or proteoliposomes (23, 44). While we and others have used such technologies in the past to investigate the adhesive properties of the cell adhesion and signaling receptor, PECAM-1 phospholipid nanodiscs offer several previously unavailable advantages. First, it is a simple matter to control their size, and therefore the number of membrane proteins inserted per nanodisc, by using membrane scaffold proteins of different lengths (30), and by adjusting the ratio of lipid:MSP:transmembrane receptor. In this study, we used the 1D1 version of MSP, which is a 25 kDa N-terminal truncation of apolipoprotein A-1, that when circularized can accommodate 80 mole DMPC lipid/mole of MSP and a single PECAM-1 molecule (Fig. 1). By examining the adhesive properties of nanodiscs containing only one PECAM-1 molecule each, we were able to interpret the effects of modulators of PECAM-1 homophilic binding affinity without the added complication of having to consider the potential role of avidity. Second, the small size and geometry of nanodiscs permits ready simultaneous access of both the extracellular and cytoplasmic domains to biochemical manipulation. Issues like phosphorylation of the cytoplasmic domain (45) and the potential role of carbohydrate chains in homophilic binding (46) can now be explored at a molecular level using isolated, soluble forms of full-length PECAM-1. Finally, previous studies have revealed lineage-specific differences in the apparent molecular mass of PECAM-1 in differentiated leukemic cell lines, in endothelial cells, and in leukocyte populations (47). Whether such variations translate into differing adhesive properties can now readily be evaluated using nanodisc technology.
The observation that the binding of nanodiscs containing monomeric, monovalent molecules of PECAM-1 can be inhibited with agents that bind N-terminal IgD1, but enhanced with agents that bind IgD6 (Fig. 3), establishes principles of PECAM-1-mediated cellular adhesion that we exploited to regulate the adhesive and migratory properties of PECAM-1-expressing endothelial cells. Similar to a previous report in which an IgD1-specific reagent inhibited angiogenesis associated with solid tumor growth (40, 48), we found that the IgD1-specific mAb, PECAM-1.3 delayed not only endothelial cell migration into an electrically-wounded area, but also suppressed junctional restoration (Fig. 4). Might such reagents be of similar value in controlling the growth of hemangiomas? Conversely, we found that the PECAM-1/PECAM-1 homophilic interactions that contribute to the junctional integrity of endothelial cell-cell junctions could be enhanced by molecules that bind membrane-proximal IgD6. Might such reagents be used to speed the restoration of junctional integrity in inflammation-induced vascular leak in conditions such as septic shock (19–21). Additional studies are required to assess the ability of PECAM-1-specific reagents to regulate vascular permeability using in vivo models of vascular challenge.
In addition to IgD6-specific Fabs, modulation of PECAM-1 homophilic binding affinity might have an in vivo correlate that is operable during leukocyte diapedesis. In 2007, the first physiologically-relevant heterophilic binding partner for PECAM-1 was described as a “contaminant” of a preparation of the neutrophil surface protein NB1 (= CD177), a glycosylated-phosphatidylinositol (GPI)-linked membrane protein that is expressed on the surface of human neutrophils (49, 50). The contaminant was later identified as Proteinase 3 (PR3), a neutrophil-specific protease that is stored in and released from azurophilic granules and presented on the surface of activated neutrophils (50). Interestingly, Sachs and co-workers found that the PR3/NB1 complex binds tightly to PECAM-1 IgD6 (29), raising the intriguing possibility that NB1 directs at least a subpopulation of PR3 molecules to endothelial cell-cell junctions where PECAM-1 is localized. Since neutrophils release PR3 during the process of transendothelial migration (51), it is tempting to speculate that NB1-bound PR3 binds to PECAM-1 IgD6 and increases PECAM-1 homophilic binding capacity, thereby helping to reseal endothelial cell junctions as neutrophils pass through. Studies are in progress to determine whether PR3 and NB1/PR3 complexes might have such effects on PECAM-1-mediated adhesion.
IgD6 has been the subject of a number of other intriguing, but largely unexplained, phenomena over the past 20 years. Three different laboratories found that IgD6-derived peptides have potent immunomodulatory properties, and proposed their use as therapeutic immunosuppressive agents for treating conditions ranging from graft-versus-host disease (52, 53) to atherosclerosis (54). Where such peptides bind, and how they exert their effects is not known. Monoclonal antibodies (mAbs) 4G6 and PECAM-1.2, whose epitopes lie within IgD6 (35), have been reported to have the unusual property of trapping transmigrating leukocytes beneath the endothelial cell monolayer following transmigration (55, 56). The mechanism by which this occurs is also not understood, but the findings are consistent with the possibility that binding of these mAbs increased the strength of PECAM-1 homophilic interactions, thereby delaying the final stages of extravasation. Finally, mAb PECAM-1.2 has the effect of enhancing ADP-induced platelet aggregation via a mechanism that may involve, in addition to its ability to augment integrin function (57), its newly revealed role in increasing PECAM-1 adhesiveness.
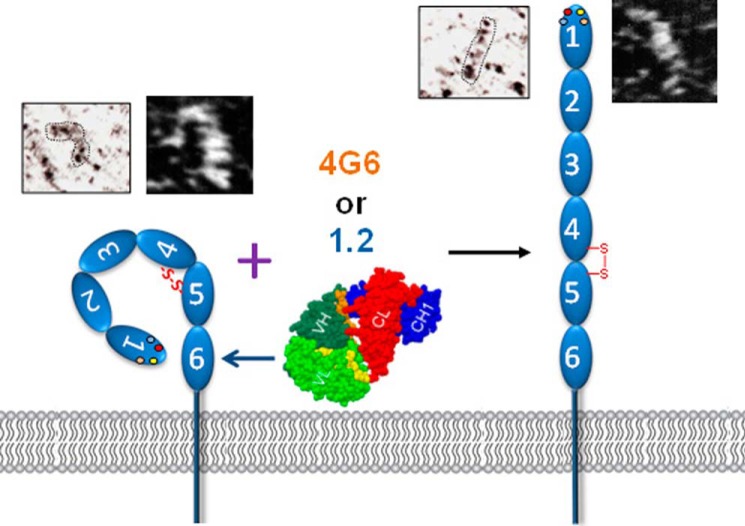
The mechanism by which IgD6-specific reagents effect affinity modulation of this Ig superfamily member is not known. Because the PECAM-1 molecules contained in the nanodiscs are monomeric, and the Fabs are monovalent, affinity, rather than avidity, is likely to have been increased. Whether affinity modulation is due to subtle versus more obvious conformational modulation is not known. Interestingly, preliminary rotary shadowed and STEM images of soluble recombinant PECAM-1 (Fig. 5) suggest the presence of both straight and bent forms of this molecule. While long-range conformational change as the mechanism of affinity modulation for members of the integrin family is widely accepted, there are but a few examples of this within Ig superfamily CAMs, most notably Axonin 1 (58) and the neural cell adhesion molecule L1 (59). Future structural studies are planned to examine the detailed conformational states of this novel vascular adhesion and signaling receptor.
FIGURE 5.
Hypothetical model of PECAM-1 affinity modulation. The model, which borrows conceptually from integrin biology, takes into account the observation that IgD6-specific Fabs are able to increase the homophilic binding capacity of PECAM-1-containing nanodiscs. In support of this model, also shown are preliminary rotary shadowed and STEM images of PECAM-1 (described under “Experimental Procedures”) showing the presence of both straight and bent forms.
In conclusion, we successfully assembled full-length PECAM-1 molecules into phospholipid nanodiscs, characterized their morphology, and showed that PECAM-1 is capable of affinity modulation. We expect that the principles established by examining the adhesive properties of PECAM-1 embedded in phospholipid nanodiscs will find broad applicability in the biology of this novel member of the Ig-CAM family in the blood and vascular cells in which it is expressed.
Acknowledgment
We thank Dr. James Morrissey for helpful protocols for nanodisc preparation.
This work was supported, in whole or in part, by National Institutes of Health Grant HL40926 (to P. J. N.) and AHA Grant 13POST16840095 (to H. M.). This work is the result of an ongoing jointly-sponsored Research Partnership between the Blood Research Institute, Blood Center of Wisconsin and the Institute of Hematology, Union Hospital, Huazhong University of Science and Technology, Professor Yu Hu, M.D., Ph.D., Director.
- PECAM
- platelet endothelial cell adhesion molecule
- Pnano
- PECAM-1 containing nanodisc
- Enano
- empty nanodisc
- MSP
- membrane scaffold protein.
REFERENCES
- 1. Newman P. J., Berndt M. C., Gorski J., White G. C., 2nd, Lyman S., Paddock C., Muller W. A. (1990) PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 247, 1219–1222 [DOI] [PubMed] [Google Scholar]
- 2. Baldwin H. S., Shen H. M., Yan H.-C., DeLisser H. M., Chung A., Mickanin C., Trask T., Kirschbaum N., Newman P. J., Albelda S. M., Buck C. A. (1994) Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): Alternatively spliced, functionally distinct isoforms expressed during early mammalian cardiovascular development. Development 120, 2539–2553 [DOI] [PubMed] [Google Scholar]
- 3. Albelda S. M., Muller W. A., Buck C. A., Newman P. J. (1991) Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J. Cell Biol. 114, 1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Newton-Nash D. K., Newman P. J. (1999) A new role for Platelet-Endothelial Cell Adhesion Molecule-1 (CD31): Inhibition of TCR-mediated signal transduction. J. Immunol. 163, 682–688 [PubMed] [Google Scholar]
- 5. Newman P. J. (1999) Switched at birth: a new family for PECAM-1. J. Clin. Invest. 103, 5–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patil S., Newman D. K., Newman P. J. (2001) Platelet endothelial cell adhesion molecule-1 serves as an inhibitory receptor that modulates platelet responses to collagen. Blood 97, 1727–1732 [DOI] [PubMed] [Google Scholar]
- 7. Muller W. A., Weigl S. A., Deng X., Phillips D. M. (1993) PECAM-1 is required for transendothelial migration of leukocytes. J. Exp. Med. 178, 449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaporciyan A. A., DeLisser H. M., Yan H.-C., Mendiguren I. I., Thom S. R., Jones M. L., Ward P. A., Albelda S. M. (1993) Involvement of Platelet Endothelial Cell Adhesion Molecule-1 in neutrophil recruitment in vivo. Science 262, 1580–1582 [DOI] [PubMed] [Google Scholar]
- 9. Gao C., Sun W., Matsuyama S., Newman P. J. (2001) PECAM-1 is a specific and potent inhibitor of Bax-induced apoptosis. Blood 98, 796a. [DOI] [PubMed] [Google Scholar]
- 10. Evans P. C., Taylor E. R., Kilshaw P. J. (2001) Signaling through CD31 protects endothelial cells from apoptosis. Transplantation 71, 457–460 [DOI] [PubMed] [Google Scholar]
- 11. Bird I. N., Taylor V., Newton J. P., Spragg J. H., Simmons D. L., Salmon M., Buckley C. D. (1999) Homophilic PECAM-1(CD31) interactions prevent endothelial cell apoptosis but do not support cell spreading or migration. J. Cell Sci. 112, 1989–1997 [DOI] [PubMed] [Google Scholar]
- 12. Bergom C., Goel R., Paddock C., Gao C., Newman D. K., Matsuyama S., Newman P. J. (2006) The cell-adhesion and signaling molecule PECAM-1 is a molecular mediator of resistance to genotoxic chemotherapy. Cancer Biol. Ther. 5, 1699–1707 [DOI] [PubMed] [Google Scholar]
- 13. Osawa M., Masuda M., Kusano K., Fujiwara K. (2002) Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J. Cell Biol. 158, 773–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tzima E., Irani-Tehrani M., Kiosses W. B., Dejana E., Schultz D. A., Engelhardt B., Cao G., DeLisser H., Schwartz M. A. (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 [DOI] [PubMed] [Google Scholar]
- 15. Collins C., Guilluy C., Welch C., O'Brien E. T., Hahn K., Superfine R., Burridge K., Tzima E. (2012) Localized tensional forces on PECAM-1 elicit a global mechanotransduction response via the integrin-RhoA pathway. Curr. Biol. 22, 2087–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Conway D. E., Breckenridge M. T., Hinde E., Gratton E., Chen C. S., Schwartz M. A. (2013) Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23, 1024–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sun J., Paddock C., Shubert J., Zhang H., Amin K., Newman P. J., Albelda S. M. (2000) Contributions of the extracellular and cytoplasmic domains of platelet- endothelial cell adhesion molecule-1 (PECAM-1/CD31) in regulating cell- cell localization. J. Cell Sci. 113, 1459–1469 [DOI] [PubMed] [Google Scholar]
- 18. Ferrero E., Ferrero M. E., Pardi R., Zocchi M. R. (1995) The platelet endothelial cell adhesion molecule-1 (PECAM1) contributes to endothelial barrier function. FEBS Lett. 374, 323–326 [DOI] [PubMed] [Google Scholar]
- 19. Graesser D., Solowiej A., Bruckner M., Osterweil E., Juedes A., Davis S., Ruddle N. H., Engelhardt B., Madri J. A. (2002) Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM-1-deficient mice. J. Clin. Invest. 109, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maas M., Stapleton M., Bergom C., Mattson D. L., Newman D. K., Newman P. J. (2005) Endothelial cell PECAM-1 confers protection against endotoxic shock. Am. J. Physiol. Heart Circ. Physiol. 288, H159–H164 [DOI] [PubMed] [Google Scholar]
- 21. Carrithers M., Tandon S., Canosa S., Michaud M., Graesser D., Madri J. A. (2005) Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling in CD31-deficient mice. Am. J. Pathol. 166, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Privratsky J. R., Paddock C. M., Florey O., Newman D. K., Muller W. A., Newman P. J. (2011) Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J. Cell Sci. 124, 1477–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun Q.-H., DeLisser H. M., Zukowski M. M., Paddock C., Albelda S. M., Newman P. J. (1996) Individually distinct Ig homology domains in PECAM-1 regulate homophilic binding and modulate receptor affinity. J. Biol. Chem. 271, 11090–11098 [DOI] [PubMed] [Google Scholar]
- 24. Sun J., Williams J., Yan H.-C., Amin K. M., Albelda S. M., DeLisser H. M. (1996) Platelet Endothelial Cell Adhesion Molecule-1 (PECAM-1) homophilic adhesion is mediated by immunoglobulin-like domains 1 and 2 and depends on the cytoplasmic domain and the level of surface expression. J. Biol. Chem. 271, 18561–18570 [DOI] [PubMed] [Google Scholar]
- 25. Newton J. P., Buckley C. D., Jones E. Y., Simmons D. L. (1997) Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites on PECAM-1/CD31. J. Biol. Chem. 272, 20555–20563 [DOI] [PubMed] [Google Scholar]
- 26. Muller W. A., Berman M. E., Newman P. J., DeLisser H. M., Albelda S. M. (1992) A heterophilic adhesion mechanism for platelet/endothelial cell adhesion molecule 1 (CD31). J. Exp. Med. 175, 1401–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao T., Newman P. J. (2001) Integrin activation by regulated dimerization and oligomerization of platelet endothelial cell adhesion molecule (PECAM)-1 from within the cell. J. Cell Biol. 152, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fawcett J., Buckley C., Holness C. L., Bird I. N., Spragg J. H., Saunders J., Harris A., Simmons D. L. (1995) Mapping the homotypic binding sites in CD31 and the role of CD31 adhesion in the formation of interendothelial cell contacts. J. Cell Biol. 128, 1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sachs U. J., Andrei-Selmer C. L., Maniar A., Weiss T., Paddock C., Orlova V. V., Choi E. Y., Newman P. J., Preissner K. T., Chavakis T., Santoso S. (2007) The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). J. Biol. Chem. 282, 23603–23612 [DOI] [PubMed] [Google Scholar]
- 30. Denisov I. G., Grinkova Y. V., Lazarides A. A., Sligar S. G. (2004) Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477–3487 [DOI] [PubMed] [Google Scholar]
- 31. Ritchie T. K., Grinkova Y. V., Bayburt T. H., Denisov I. G., Zolnerciks J. K., Atkins W. M., Sligar S. G. (2009) Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 464, 211–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nath A., Atkins W. M., Sligar S. G. (2007) Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry 46, 2059–2069 [DOI] [PubMed] [Google Scholar]
- 33. Ye F., Hu G., Taylor D., Ratnikov B., Bobkov A. A., McLean M. A., Sligar S. G., Taylor K. A., Ginsberg M. H. (2010) Recreation of the terminal events in physiological integrin activation. J. Cell Biol. 188, 157–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yan R., Mo X., Paredes A. M., Dai K., Lanza F., Cruz M. A., Li R. (2011) Reconstitution of the platelet glycoprotein Ib-IX complex in phospholipid bilayer nanodiscs. Biochemistry 50, 10598–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yan H.-C., Pilewski J. M., Zhang Q., DeLisser H. M., Romer L., Albelda S. M. (1995) Localization of multiple functional domains on human PECAM-1 (CD31) by monoclonal antibody epitope mapping. Cell Adhesion Commun. 3, 45–66 [DOI] [PubMed] [Google Scholar]
- 36. Newman D. K., Hoffman S., Kotamraju S., Zhao T., Wakim B., Kalyanaraman B., Newman P. J. (2002) Nitration of PECAM-1 ITIM tyrosines abrogates phosphorylation and SHP-2 binding. Biochem. Biophys. Res. Commun. 296, 1171–1179 [DOI] [PubMed] [Google Scholar]
- 37. Goldberger A., Middleton K. A., Oliver J. A., Paddock C., Yan H.-C., DeLisser H. M., Albelda S. M., Newman P. J. (1994) Biosynthesis and processing of the cell adhesion molecule PECAM-1 includes production of a soluble form. J. Biol. Chem. 269, 17183–17191 [PubMed] [Google Scholar]
- 38. Mosesson M. W., Siebenlist K. R., Hainfeld J. F., Wall J. S. (1995) The covalent structure of factor XIIIa crosslinked fibrinogen fibrils. J. Struct. Biol. 115, 88–101 [DOI] [PubMed] [Google Scholar]
- 39. Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. (1989) A human endothelial cell-restricted externally disposed plasmalemmal protein enriched in intercellular junctions. J. Exp. Med. 170, 399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cao G., O'Brien C. D., Zhou Z., Sanders S. M., Greenbaum J. N., Makrigiannakis A., DeLisser H. M. (2002) Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am. J. Physiol. Cell Physiol. 282, C1181–C1190 [DOI] [PubMed] [Google Scholar]
- 41. DeLisser H. M., Christofidou-Solomidou M., Strieter R. M., Burdick M. D., Robinson C. S., Wexler R. S., Kerr J. S., Garlanda C., Merwin J. R., Madri J. A., Albelda S. M. (1997) Involvement of endothelial PECAM-1/CD31 in angiogenesis. Am. J. Pathol. 151, 671–677 [PMC free article] [PubMed] [Google Scholar]
- 42. Sheibani N., Newman P. J., Frazier W. A. (1997) Thrombospondin-1, a natural inhibitor of angiogenesis, regulates Platelet-Endothelial Cell Adhesion Molecule-1 expression and endothelial cell morphogenesis. Mol. Biol. Cell 8, 1329–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hornemann S., von Schroetter C., Damberger F. F., Wüthrich K. (2009) Prion protein-detergent micelle interactions studied by NMR in solution. J. Biol. Chem. 284, 22713–22721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Baldassare J. J., Kahn R. A., Knipp M. A., Newman P. J. (1985) Reconstitution of platelet proteins into phospholipid vesicles. Functional proteoliposomes. J. Clin. Invest. 75, 35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Paddock C., Lytle B. L., Peterson F. C., Holyst T., Newman P. J., Volkman B. F., Newman D. K. (2011) Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood 117, 6012–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kitazume S., Imamaki R., Ogawa K., Komi Y., Futakawa S., Kojima S., Hashimoto Y., Marth J. D., Paulson J. C., Taniguchi N. (2010) α2,6-sialic acid on platelet endothelial cell adhesion molecule (PECAM) regulates its homophilic interactions and downstream antiapoptotic signaling. J. Biol. Chem. 285, 6515–6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goldberger A., Middleton K. A., Newman P. J. (1994) Changes in expression of the cell adhesion molecule PECAM-1 (CD31) during differentiation of human leukemic cell lines. Tissue Antigens 44, 285–293 [DOI] [PubMed] [Google Scholar]
- 48. Zhou Z., Christofidou-Solomidou M., Garlanda C., DeLisser H. M. (1999) Antibody against murine PECAM-1 inhibits tumor angiogenesis in mice. Angiogenesis 3, 181–188 [DOI] [PubMed] [Google Scholar]
- 49. Stroncek D. (2002) Neutrophil-specific antigen HNA-2a (NB1, CD177): serology, biochemistry, and molecular biology. Vox Sang 83, 359–361 [DOI] [PubMed] [Google Scholar]
- 50. von Vietinghoff S., Tunnemann G., Eulenberg C., Wellner M., Cristina Cardoso M., Luft F. C., Kettritz R. (2007) NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood 109, 4487–4493 [DOI] [PubMed] [Google Scholar]
- 51. Kuckleburg C. J., Tilkens S. B., Santoso S., Newman P. J. (2012) Proteinase 3 contributes to transendothelial migration of NB1-positive neutrophils. J. Immunol. 188, 2419–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zehnder J. L., Hirai K., Shatsky M., McGregor J. L., Levitt L. J., Leung L. L. K. (1992) The cell adhesion molecule CD31 is phosphorylated after cell activation. Down-regulation of CD31 in activated T lymphocytes. J. Biol. Chem. 267, 5243–5249 [PubMed] [Google Scholar]
- 53. Zehnder J. L., Shatsky M., Leung L. L. K., Butcher E. C., McGregor J. L., Levitt L. J. (1995) Involvement of CD31 in lymphocyte-mediated immune responses: Importance of the membrane-proximal immunoglobulin domain and identification of an inhibiting CD31 peptide. Blood 85, 1282–1288 [PubMed] [Google Scholar]
- 54. Fornasa G., Clement M., Groyer E., Gaston A. T., Khallou-Laschet J., Morvan M., Guedj K., Kaveri S. V., Tedgui A., Michel J. B., Nicoletti A., Caligiuri G. (2012) A CD31-derived peptide prevents angiotensin II-induced atherosclerosis progression and aneurysm formation. Cardiovasc. Res. 94, 30–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liao F., Huynh H. K., Eiroa A., Greene T., Polizzi E., Muller W. A. (1995) Migration of monocytes across endothelium and passage through extracellular matrix involve separate molecular domains of PECAM-1. J. Exp. Med. 182, 1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wakelin M. W., Sanz M.-J., Dewar A., Albelda S. M., Larkin S. W., Boughton-Smith N., Williams T. J., Nourshargh S. (1996) An anti-Platelet-Endothelial Cell Adhesion Molecule-1 antibody inhibits leukocyte extravasation from mesenteric microvessels in vivo by blocking the passage through the basement membrane. J. Exp. Med. 184, 229–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Varon D., Jackson D. E., Shenkman B., Dardik R., Tamarin I., Savion N., Newman P. J. (1998) Platelet/Endothelial Cell Adhesion Molecule-1 serves as a co-stimulatory agonist receptor that modulates integrin-dependent adhesion and aggregation of human platelets. Blood 91, 500–507 [PubMed] [Google Scholar]
- 58. Freigang J., Proba K., Leder L., Diederichs K., Sonderegger P., Welte W. (2000) The crystal structure of the ligand binding module of axonin-1/TAG-1 suggests a zipper mechanism for neural cell adhesion. Cell 101, 425–433 [DOI] [PubMed] [Google Scholar]
- 59. Arevalo E., Shanmugasundararaj S., Wilkemeyer M. F., Dou X., Chen S., Charness M. E., Miller K. W. (2008) An alcohol binding site on the neural cell adhesion molecule L1. Proc. Natl. Acad. Sci. U.S.A. 105, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]