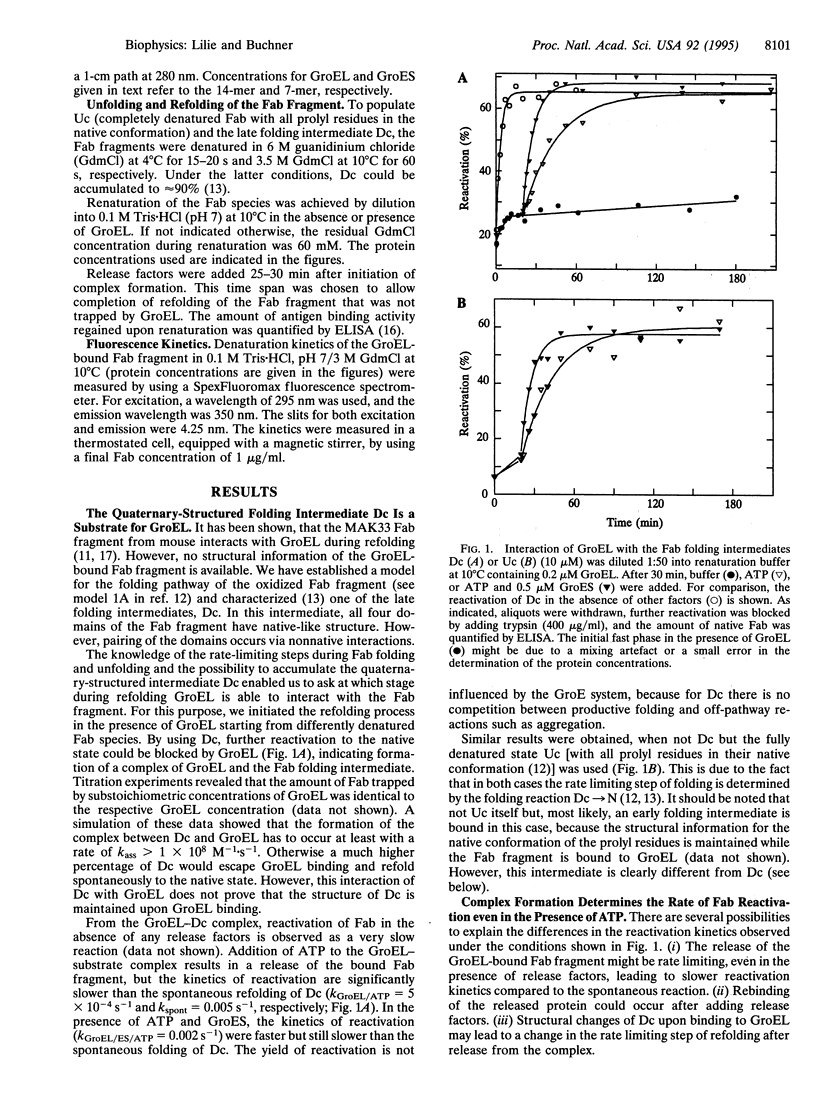
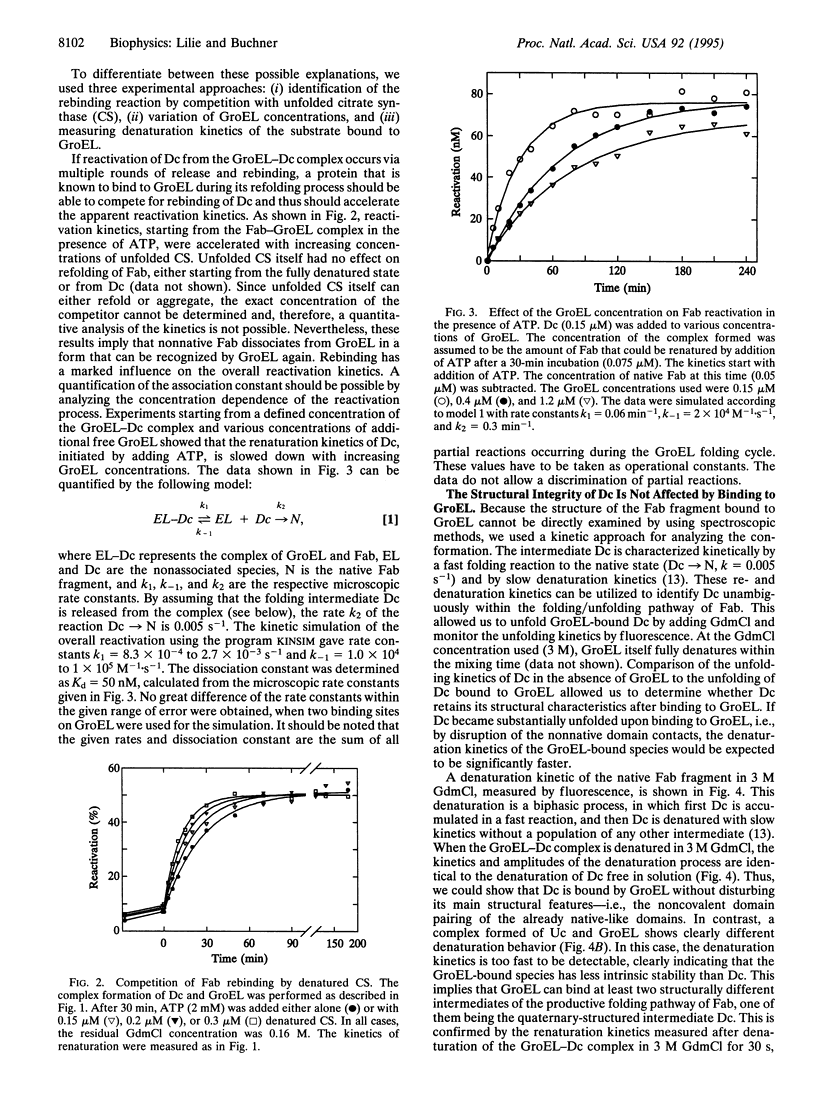
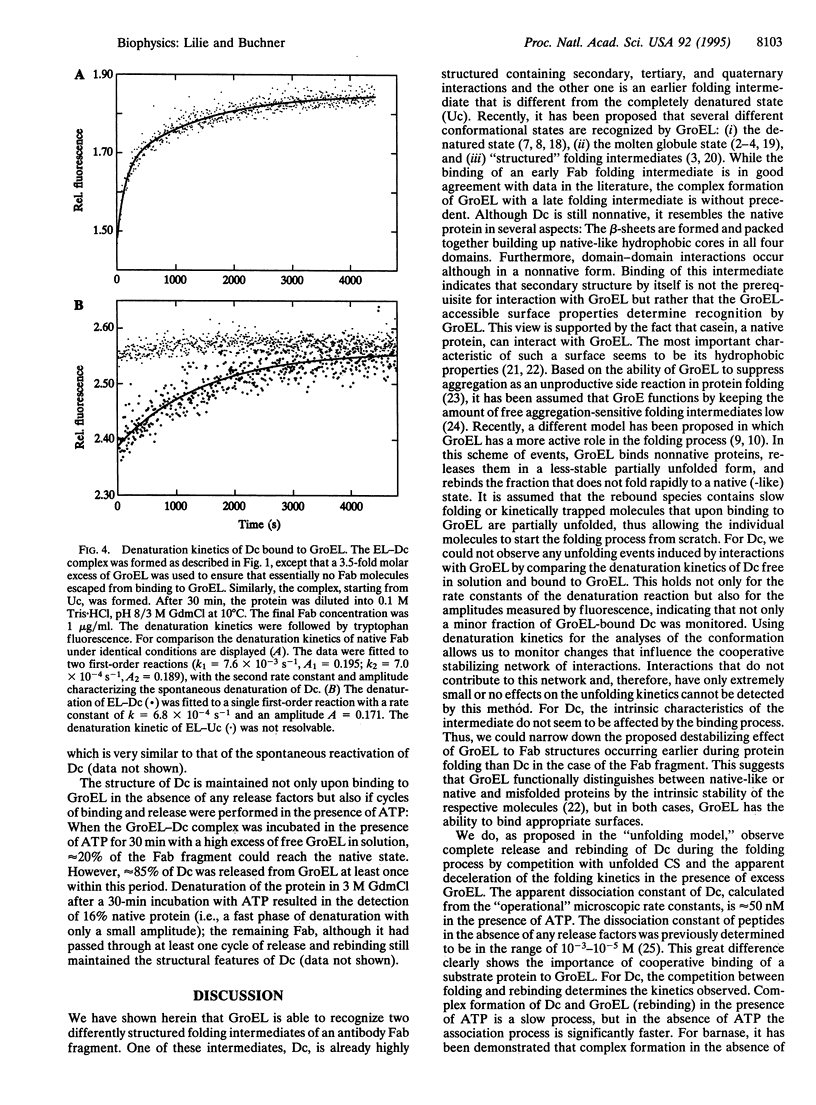
Abstract
The GroE proteins are molecular chaperones involved in protein folding. The general mechanism by which they facilitate folding is still enigmatic. One of the central open questions is the conformation of the GroEL-bound nonnative protein. Several suggestions have been made concerning the folding stage at which a protein can interact with GroEL. Furthermore, the possibility exists that binding of the nonnative protein to GroEL results in its unfolding. We have addressed these issues that are basic for understanding the GroE-mediated folding cycle by using folding intermediates of an Fab antibody fragment as molecular probes to define the binding properties of GroEL. We show that, in addition to binding to an early folding intermediate, GroEL is able to recognize and interact with a late quaternary-structured folding intermediate (Dc) without measurably unfolding it. Thus, the prerequisite for binding is not a certain folding stage of a nonnative protein. In contrast, general surface properties of nonnative proteins seem to be crucial for binding. Furthermore, unfolding of a highly structured intermediate does not necessarily occur upon binding to GroEL. Folding of Dc in the presence of GroEL and ATP involves cycles of binding and release. Because in this system no off-pathway reactions or kinetic traps are involved, a quantitative analysis of the reactivation kinetics observed is possible. Our results indicate that the association reaction of Dc and GroEL in the presence of ATP is rather slow, whereas in the absence of ATP association is several orders of magnitude more efficient. Therefore, it seems that ATP functions by inhibiting reassociation rather than promoting release of the bound substrate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badcoe I. G., Smith C. J., Wood S., Halsall D. J., Holbrook J. J., Lund P., Clarke A. R. Binding of a chaperonin to the folding intermediates of lactate dehydrogenase. Biochemistry. 1991 Sep 24;30(38):9195–9200. doi: 10.1021/bi00102a010. [DOI] [PubMed] [Google Scholar]
- Badcoe I. G., Smith C. J., Wood S., Halsall D. J., Holbrook J. J., Lund P., Clarke A. R. Binding of a chaperonin to the folding intermediates of lactate dehydrogenase. Biochemistry. 1991 Sep 24;30(38):9195–9200. doi: 10.1021/bi00102a010. [DOI] [PubMed] [Google Scholar]
- Braig K., Otwinowski Z., Hegde R., Boisvert D. C., Joachimiak A., Horwich A. L., Sigler P. B. The crystal structure of the bacterial chaperonin GroEL at 2.8 A. Nature. 1994 Oct 13;371(6498):578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- Buchner J., Schmidt M., Fuchs M., Jaenicke R., Rudolph R., Schmid F. X., Kiefhaber T. GroE facilitates refolding of citrate synthase by suppressing aggregation. Biochemistry. 1991 Feb 12;30(6):1586–1591. doi: 10.1021/bi00220a020. [DOI] [PubMed] [Google Scholar]
- Buckel P., Hübner-Parajsz C., Mattes R., Lenz H., Haug H., Beaucamp K. Cloning and nucleotide sequence of heavy- and light-chain cDNAs from a creatine-kinase-specific monoclonal antibody. Gene. 1987;51(1):13–19. doi: 10.1016/0378-1119(87)90469-0. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Christeller J. T., Gatenby A. A., Lorimer G. H. Reconstitution of active dimeric ribulose bisphosphate carboxylase from an unfoleded state depends on two chaperonin proteins and Mg-ATP. Nature. 1989 Dec 21;342(6252):884–889. doi: 10.1038/342884a0. [DOI] [PubMed] [Google Scholar]
- Gray T. E., Eder J., Bycroft M., Day A. G., Fersht A. R. Refolding of barnase mutants and pro-barnase in the presence and absence of GroEL. EMBO J. 1993 Nov;12(11):4145–4150. doi: 10.1002/j.1460-2075.1993.tb06098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayer-Hartl M. K., Ewbank J. J., Creighton T. E., Hartl F. U. Conformational specificity of the chaperonin GroEL for the compact folding intermediates of alpha-lactalbumin. EMBO J. 1994 Jul 1;13(13):3192–3202. doi: 10.1002/j.1460-2075.1994.tb06618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima K. The molten globule state as a clue for understanding the folding and cooperativity of globular-protein structure. Proteins. 1989;6(2):87–103. doi: 10.1002/prot.340060202. [DOI] [PubMed] [Google Scholar]
- Landry S. J., Jordan R., McMacken R., Gierasch L. M. Different conformations for the same polypeptide bound to chaperones DnaK and GroEL. Nature. 1992 Jan 30;355(6359):455–457. doi: 10.1038/355455a0. [DOI] [PubMed] [Google Scholar]
- Lilie H., Lang K., Rudolph R., Buchner J. Prolyl isomerases catalyze antibody folding in vitro. Protein Sci. 1993 Sep;2(9):1490–1496. doi: 10.1002/pro.5560020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilie H., Rudolph R., Buchner J. Association of antibody chains at different stages of folding: prolyl isomerization occurs after formation of quaternary structure. J Mol Biol. 1995 Apr 21;248(1):190–201. doi: 10.1006/jmbi.1995.0211. [DOI] [PubMed] [Google Scholar]
- Martin J., Langer T., Boteva R., Schramel A., Horwich A. L., Hartl F. U. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature. 1991 Jul 4;352(6330):36–42. doi: 10.1038/352036a0. [DOI] [PubMed] [Google Scholar]
- Mendoza J. A., Butler M. C., Horowitz P. M. Characterization of a stable, reactivatable complex between chaperonin 60 and mitochondrial rhodanese. J Biol Chem. 1992 Dec 5;267(34):24648–24654. [PubMed] [Google Scholar]
- Okazaki A., Ikura T., Nikaido K., Kuwajima K. The chaperonin GroEL does not recognize apo-alpha-lactalbumin in the molten globule state. Nat Struct Biol. 1994 Jul;1(7):439–446. doi: 10.1038/nsb0794-439. [DOI] [PubMed] [Google Scholar]
- Peralta D., Hartman D. J., Hoogenraad N. J., Høj P. B. Generation of a stable folding intermediate which can be rescued by the chaperonins GroEL and GroES. FEBS Lett. 1994 Feb 14;339(1-2):45–49. doi: 10.1016/0014-5793(94)80381-1. [DOI] [PubMed] [Google Scholar]
- Robinson C. V., Gross M., Eyles S. J., Ewbank J. J., Mayhew M., Hartl F. U., Dobson C. M., Radford S. E. Conformation of GroEL-bound alpha-lactalbumin probed by mass spectrometry. Nature. 1994 Dec 15;372(6507):646–651. doi: 10.1038/372646a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Buchner J. Interaction of GroE with an all-beta-protein. J Biol Chem. 1992 Aug 25;267(24):16829–16833. [PubMed] [Google Scholar]
- Schmidt M., Bücheler U., Kaluza B., Buchner J. Correlation between the stability of the GroEL-protein ligand complex and the release mechanism. J Biol Chem. 1994 Nov 11;269(45):27964–27972. [PubMed] [Google Scholar]
- Schmidt M., Rutkat K., Rachel R., Pfeifer G., Jaenicke R., Viitanen P., Lorimer G., Buchner J. Symmetric complexes of GroE chaperonins as part of the functional cycle. Science. 1994 Jul 29;265(5172):656–659. doi: 10.1126/science.7913554. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Viitanen P. V., Lorimer G. H. Dynamics of the chaperonin ATPase cycle: implications for facilitated protein folding. Science. 1994 Jul 29;265(5172):659–666. doi: 10.1126/science.7913555. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kashi Y., Fenton W. A., Horwich A. L. GroEL-mediated protein folding proceeds by multiple rounds of binding and release of nonnative forms. Cell. 1994 Aug 26;78(4):693–702. doi: 10.1016/0092-8674(94)90533-9. [DOI] [PubMed] [Google Scholar]
- Zahn R., Axmann S. E., Rücknagel K. P., Jaeger E., Laminet A. A., Plückthun A. Thermodynamic partitioning model for hydrophobic binding of polypeptides by GroEL. I. GroEL recognizes the signal sequences of beta-lactamase precursor. J Mol Biol. 1994 Sep 16;242(2):150–164. doi: 10.1006/jmbi.1994.1566. [DOI] [PubMed] [Google Scholar]
- Zahn R., Spitzfaden C., Ottiger M., Wüthrich K., Plückthun A. Destabilization of the complete protein secondary structure on binding to the chaperone GroEL. Nature. 1994 Mar 17;368(6468):261–265. doi: 10.1038/368261a0. [DOI] [PubMed] [Google Scholar]



