Background: In adipogenesis, new adipocytes are generated from precursor cells and contribute to adipose tissue expansion.
Results: The A2b adenosine receptor (A2bAR) inhibits adipogenesis via expression of Krüppel-like factor 4 (KLF4).
Conclusion: A2bAR signaling regulates adipogenesis and is correlated tightly with KLF4.
Significance: A2bAR signaling via KLF4 may play an important role in adipose tissue biology.
Keywords: adenosine Receptor, Adipogenesis, Cell Differentiation, Kruppel-like Factor (KLF), Obesity
Abstract
Adipogenesis represents a key process in adipose tissue development and remodeling, including during obesity. Exploring the regulation of adipogenesis by extracellular ligands is fundamental to our understanding of this process. Adenosine, an extracellular nucleoside signaling molecule found in adipose tissue depots, acts on adenosine receptors. Here we report that, among these receptors, the A2b adenosine receptor (A2bAR) is highly expressed in adipocyte progenitors. Activation of the A2bAR potently inhibits differentiation of mouse stromal vascular cells into adipocytes, whereas A2bAR knockdown stimulates adipogenesis. The A2bAR inhibits differentiation through a novel signaling cascade involving sustained expression of Krüppel-like factor 4 (KLF4), a regulator of stem cell maintenance. Knockdown of KLF4 ablates the ability of the A2bAR to inhibit differentiation. A2bAR activation also inhibits adipogenesis in a human primary preadipocyte culture system. We analyzed the A2bAR-KLF4 axis in adipose tissue of obese subjects and, intriguingly, found a strong correlation between A2bAR and KLF4 expression in both subcutaneous and visceral human fat. Hence, our study implicates the A2bAR as a regulator of adipocyte differentiation and the A2bAR-KLF4 axis as a potentially significant modulator of adipose biology.
Introduction
The prevalence of obesity continues to rise among adults and adolescents in the United States and other developed nations (1). Obesity increases the risk of developing type 2 diabetes and cardiovascular disease (2, 3). A better understanding of the growth and expansion of adipose tissue is needed because it may lead to improved therapeutics and preventative measures for obesity and type 2 diabetes. As adipose tissue expands, it increases by hypertrophy and by hyperplasia (4). An increase in the number of adipocytes occurs from the differentiation of adipocyte precursors that reside along the vasculature in adipose tissue (5, 6). Adenosine, which is released continuously from fat cells (7, 8), can act on four G-protein coupled receptors, defined as adenylyl cyclase inhibitory A1 and A3 adenosine receptors (A1AR and A3AR) and adenylyl cyclase stimulatory A2a and A2b adenosine receptors (A2aAR5 and A2bAR). The A2aAR and A2bAR are expressed on preadipocytes from the mouse preadipose cell line Ob1771 and from rat adipose tissue-derived preadipocytes, whereas the A1AR is found on mature adipocytes (9, 10). This expression profile indicates that the A2aAR and A2bAR may play an important role in the early stages of adipogenesis.
Adipocyte differentiation has been studied extensively in culture using preadipocyte cell lines. These studies showed that early in adipocyte differentiation, the transcription factors CCAAT/enhancer-binding protein β (C/EBP-β) and C/EBP-δ are expressed transiently (11–15), which subsequently leads to the expression of C/EBP-α and peroxisome proliferator-activated receptor γ (PPARγ) (4, 16–19). Other early transcriptional events include elevation of Krüppel like factor 4 (KLF4) and a reduction in the expression of preadipocyte factor 1 (Pref1), as observed in 3T3-L1 cells (20, 21). Although the transcriptional cascade that leads to adipogenesis has been well studied, much is yet to be understood about potential regulators of early transcriptional events. In this investigation, we show that the A2bAR is an important early regulator of adipogenesis via a newly identified link between this receptor signaling and KLF4. An earlier study showed a transient increase in KLF4 during adipogenesis and that knockdown of KLF4 impairs adipogenesis in 3T3-L1 cells (20). Here we report that A2bAR-induced inhibition of adipogenesis is dependent on sustained expression of KLF4 in mouse primary preadipocytes. Intriguingly, we also identified a significant correlation between A2bAR and KLF4 expression in both subcutaneous and visceral adipose tissue derived from obese individuals. This suggests that the A2bAR-KLF4 axis may also play a key role in human adipose tissue biology.
EXPERIMENTAL PROCEDURES
Animals
All procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health. All animals in this study received humane care in agreement with the guidelines approved by the Institutional Animal Care and Use Committee of the Boston University School of Medicine. A2bAR KO mice were originally generated in our laboratory and bred into the C57BL/6J background, as confirmed by using gene marker analysis from MAX-BAX (Charles River Laboratories) (22). Age-, strain-, and sex-matched WT and A2bAR KO mice were used in all experiments (all bred in-house).
Stromal Vascular Cell (SVC) Isolation and Cell Culture
Six-week-old C57BL/6J male mice or A2bAR null mice (22) were euthanized, and the subcutaneous adipose tissue was extracted for isolation of SVCs, which included adipocyte precursors, as described earlier (23). Two-day post-confluent cells were induced to differentiate with proadipogenic factors, including 1.7 μm insulin, 1 μm dexamethasone, and 1 μm rosiglitazone. The media were changed every 3 days. Human preadipocytes were isolated and differentiated to adipocytes for 10 days as described previously (24, 25).
siRNA Knockdown
KLF4 or A2bAR OnTarget Plus siRNA (30 nm, Thermo Scientific) was transfected into SVCs using Lipofectamine RNAiMax (Invitrogen, catalog no. 13778-075) 48 h prior to induction of differentiation and/or treatment with agonists following the instructions of the manufacturer.
Oil Red O Staining of Lipid Droplets
To assess the formation of lipid droplets, cells were fixed in 4% formaldehyde and then stained with Oil Red O as described previously (26).
Cell Treatments and cAMP Measurement
To determine the effect of various signaling pathways on adipocyte differentiation, SVCs were pretreated with 1 unit/ml adenosine deaminase for 10 min and then treated with the proadipogenic mixture of 1.7 μm insulin, 1 μm dexamethasone, and 1 μm rosiglitazone and the indicated chemicals. RNA and protein were collected at various time points following treatment, as described below. Chemicals included 1 μm BAY 60-6583 (an A2bAR-specific agonist), 2 μm forskolin (FSK, an adenylyl cyclase activator), 10 μm CGS 21680 (an A2aAR-specific agonist), 10 μm 5′-(N-ethylcarboxamido) adenosine (an A2 adenosine receptor agonist), 500 μm 8-bromo-cAMP (a cAMP analog), 200 μm 8-(4-chlorophenylthio)-2′-O-Me-cAMP (an activator of exchange protein activated by cAMP, EPAC), 200 μm N6-monobutyryladenosine-cAMP (an activator of PKA), or vehicle (0.1% DMSO). SVCs were treated with chemicals on day 0 and day 3 of induction. For cAMP analysis, cells were pretreated with 1 unit/ml adenosine deaminase for 20 min and with 200 μm papavarine (a phosphodiesterase inhibitor) for 10 min prior to treatment with the chemicals. The cells were exposed to the chemicals and induction mixture for 10 min and then collected in 0.1 m HCl. cAMP determination was performed as described by the manufacturer (Enzo Life Sciences, catalog no. ADI-901066). Protein concentration was determined by Bradford assay and used for normalization.
Calcium Measurement
SVCs were grown to confluency on 35-mm glass bottom Petri dishes (MatTek, catalog no. P35 G-1.5-14-C) and incubated with 1 μm Fura-2/AM (Invitrogen, catalog no. F1221) and 0.01% pluoronic acid (Invitrogen, catalog no. P-6866) in Krebs buffer (5 mm glucose, 2 mm CaCl2, and 0.5% BSA) for 30 min in a 37 °C water bath. The buffer was replaced with fresh Krebs buffer, and the cells were incubated for 15 min in a 37 °C water bath. Fluorescence at 340 and 380 nm was assessed on an Olympus BX61 spinning disk confocal microscope. The analysis was performed using ImageJ (http://rsbweb.nih.gov/ij/). The 340:380 nm ratio was computed and averaged for all cells in the visual field (using a ×20 objective). Approximately 70 cells were analyzed.
Quantitative RT-PCR
RNA was isolated using the RNeasy Plus mini kit (Qiagen, catalog no. 74136), reverse-transcribed (Invitrogen, catalog no. 4368814), and then the mRNA level was measured with TaqMan minor groove binder primers and TaqMan Master Mix (Invitrogen, catalog no. 4369016). 18 S rRNA (Invitrogen, catalog no. 4319413E) was used as an endogenous control, and relative mRNA expression was calculated by the ΔΔCT method.
Western Blotting
Cells were lysed with radioimmunoprecipitation assay buffer with protease and phosphatase inhibitor mixtures (Roche Diagnostics). Equal amounts of protein per sample were heated in 2× sample buffer, electrophoresed on 10–12% SDS polyacrylamide gels, and transferred to Immobilon-P PVDF membranes. Western blotting was performed as described previously (27). The following antibodies were used at the following dilutions: PPARγ (Cell Signaling Technology, catalog no. 81B8, 1:1000); aP2/FABP4 (Cell Signaling Technology, catalog no. D25B3, 1:5000); C/EBP-α (Cell Signaling Technology, catalog no. 2295, 1:1000); KLF4 (MBL International, catalog no. PM057, 1:5000); α-tubulin (Sigma, catalog no. T6199, 1:10,000); β-actin (Sigma, catalog no. A5441, 1:10,000); anti-rabbit IgG, HRP-linked (Cell Signaling Technology, catalog no. 7074, 1:5000); and anti-mouse IgG, HRP-linked (Cell Signaling Technology, catalog no. 7076; 1:5000).
Determination of Adipocyte Number and Adipose Tissue Mass
Visceral adipose tissue was extracted from 8-week-old WT and A2bAR KO male mice, weighed, fixed in 4% paraformaldehyde, and embedded in paraffin. 5-μm sections were stained with hematoxylin and eosin. Adipocyte diameters were measured using Adiposoft (28). Adipocyte cell number was calculated as described previously (29). Briefly, the weighted mean adipocyte diameter was used to calculate mean adipocyte volume. Mean adipocyte weight was determined using the density of lipid as 0.915 g/ml. Adipose tissue mass was determined by weighing the fat pads immediately following isolation. The mass of the adipose tissue depot that is due to lipid was determined by multiplying the wet weight of the adipose tissue by the percent of lipid in the adipose tissue (measured by chloroform:methanol extraction as described in Ref. 30). The number of adipocytes was calculated by dividing the adipose tissue lipid mass by the mean adipocyte weight.
Human Study Population and Adipose Tissue Collection
Subcutaneous (n = 73) and visceral (omental) (n = 19) adipose tissue was collected via percutaneous needle biopsy or intraoperatively during planned bariatric surgery, as we described previously (31), from obese men and women who were receiving care at the Boston Medical Center Nutrition and Weight Management Center. The study was approved by the Boston Medical Center Institutional Review Board, and all subjects gave written informed consent. Adipose tissue was placed immediately into RNALater (Qiagen, catalog no. 76104) and stored at −80 °C until further processing. Total RNA was extracted using the Qiagen RNeasy lipid kit. cDNA synthesis was performed with the Quantitect cDNA reverse transcription kit and amplified with TaqMan PreAmp Master Mix (Invitrogen, catalog no. 4391128). TaqMan gene expression assays were used for RT-PCR reactions as described above.
Statistical Analysis
Data are presented as mean ± S.E. Each n represents an individual batch of cells or an individual mouse or person. Statistical comparison of two means was performed by two-tailed Student's t test. Determination of statistical significance of more than two means was calculated by one- or two-way ANOVA with Bonferroni multiple comparison correction. The association between two variables was performed by Spearman correlation. Analyses were performed with GraphPad Prism 4.
RESULTS
A2bAR Agonism Inhibits Adipogenesis of SVCs
We first explored the expression profile and functionality of the A2bAR in adipocyte precursors found in mouse SVCs. The A2bAR-selective ligand BAY 60-6583 (32) elevates cAMP levels in SVCs (Fig. 1A), indicative of a functional receptor. Furthermore, as these cells are induced to differentiate into adipocytes, the expression of the A2bAR decreases (Fig. 1B). The expression of the A2aAR is 80% less than that of the A2bAR in SVCs at baseline (Fig. 1B), and an agonist of the A2 type (A2a and A2b) adenosine receptors, 5′-N-ethycarboxamidoadenosine, does not increase cAMP levels above that of BAY 60-6583 (Fig. 1A), suggesting that there is little functional A2aAR on these cells. The concentration of BAY used here and in subsequent experiments (1 μm) is on the basis of preliminary studies showing a significant effect on cAMP and differentiation.
FIGURE 1.
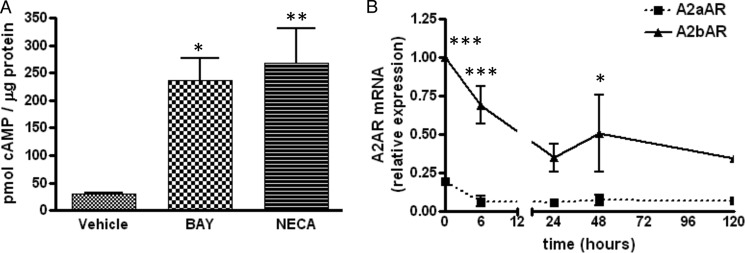
A2bAR expression and function in SVCs. SVCs were isolated from mouse inguinal adipose tissue and, upon confluence, induced to differentiate to adipocytes as described under “Experimental Procedures.” A, cAMP levels were determined following 10-min treatment of SVCs with induction mixture and vehicle (DMSO), 1 μm BAY 60-6583 (BAY), or 10 μm 5′-(N-ethylcarboxamido) adenosine (NECA). *, p < 0.05; **, p < 0.01 compared with vehicle (n = 4). B, the expression of A2aAR and A2bAR was determined by quantitative PCR at various time points during differentiation. Relative mRNA expression was determined using the ΔΔCT method and normalized to 18 S rRNA. *, p < 0.05; ***, p < 0.001 compared with the 0 h time point (n = 4). Data are mean ± S.E. Analyses were performed by one- or two-way ANOVA with Bonferroni multiple comparison post hoc test.
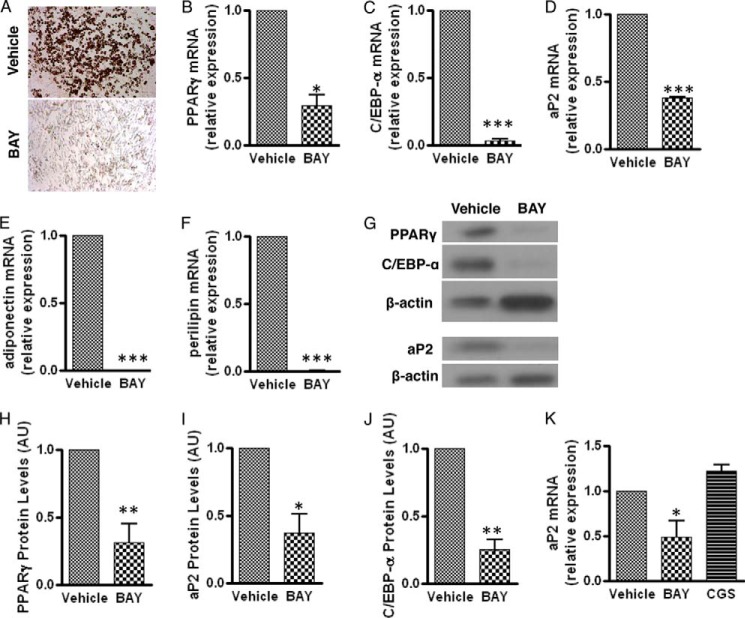
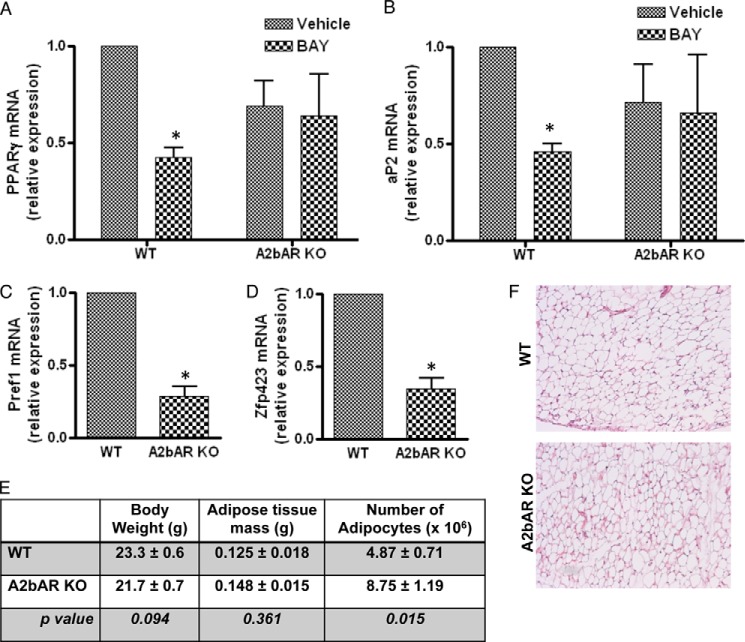
Intriguingly, A2bAR activation in SVCs prevents lipid accumulation compared with vehicle-treated cells, as measured by Oil Red O staining (Fig. 2A). Moreover, following induction of differentiation and agonist treatment on day 0 and day 3 of differentiation, BAY 60-6583 reduces the mRNA expression of the proadipogenic transcription factors PPARγ and C/EBP-α (Fig. 2, B and C) and the fat-specific genes fatty acid-binding protein 4 (FABP4/aP2), adiponectin, and perilipin (Fig. 2, D–F) compared with cultures treated with vehicle. Activation of the A2bAR with BAY 60-6583 also reduces the protein levels of PPARγ, aP2, and C/EBP-α (Fig. 2, G–J). The late transcriptional changes that occur following A2bAR activation with BAY 60-6583 do not occur with an A2aAR agonist (CGS 21680) (Fig. 2K). This highlights the importance of the A2bAR in regulating these factors and differentiation of primary adipocytes. Moreover, the effect of BAY 60-6583 on the differentiation of SVCs is specific to the A2bAR because BAY 60-6583 does not reduce the expression of PPARγ and aP2 in A2bAR KO SVCs (Fig. 3, A and B). Of note, the SVCs isolated from A2bAR KO mice had a lower expression of Pref1 and Zfp423, markers of preadipocytes (21, 33) (Fig. 3, C and D). This suggests that there are fewer preadipocytes in the extracted SVCs from A2bAR KO mice and makes a direct comparison between the adipogenic potential of WT and A2bAR KO mice difficult. Earlier reports showed that increased adipogenesis in vivo leads to the depletion of adipocyte progenitors (34–36). Considering the above data related to progenitor markers and the inhibitory effect of A2bAR activation on preadipocyte differentiation, we postulated that A2bAR KO mice have a greater number of fat tissue adipocytes. To test this contention, the number of adipocytes was determined within the visceral adipose tissue of 8-week-old WT and A2bAR KO male mice. WT and A2bAR KO mice had similar body weight and adipose tissue mass (Fig. 3E). However, A2bAR KO mice had an increased number of adipocytes in the adipose tissue compared with WT mice (Fig. 3, E and F). This suggests a greater extent of adipogenesis in the visceral adipose tissue of mice that lack the A2bAR.
FIGURE 2.
A2bAR agonism inhibits adipogenesis of SVCs. SVCs were isolated from the subcutaneous adipose tissue of WT mice, treated with vehicle (DMSO), 1 μm BAY 60-6583 (BAY), or 10 μm CGS 21680 (CGS) on day 0 and day 3 of induction, and induced to differentiate as described under “Experimental Procedures.” A, representative image of Oil Red O staining performed 6 days after induction of differentiation. B–F, mRNA expression of PPARγ (B), C/EBP-α, (C), aP2 (D), adiponectin (E), and perilipin (F) after 6 days of induction. Agonist treatment was determined using the ΔΔCT method and normalized to 18 S rRNA values (n = 3). G, representative Western blot analysis for PPARγ (57 kDa), aP2 (15 kDa), and C/EBP-α (45 kDa) performed with β-actin (45 kDa) as a loading control. (The blot was stripped and reprobed twice, including with anti β-actin.) H–J, quantification of Western blot analysis results was performed with ImageJ software and normalized to β-actin (n = 4). AU, arbitrary units. K, mRNA expression of aP2 after 6 days of induction and agonist treatment was determined using the ΔΔCT method and normalized to 18 S rRNA values (n = 4). Data are mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with vehicle. Analyses were performed by Student's t test (B—F and H–J) or by one-way ANOVA with Bonferroni multiple comparisons post hoc test (K).
FIGURE 3.
A2bAR Regulates Adipogenesis in vitro and in vivo. A and B, SVCs were isolated from the subcutaneous adipose tissue of WT and A2bAR KO mice, treated with DMSO (vehicle) or 1 μm BAY 60-6583 (BAY) on day 0 and day 3 of induction, and induced to differentiate as described under “Experimental Procedures.” mRNA expression of PPARγ (A) and aP2 (B) after 6 days of induction and agonist treatment was determined using the ΔΔCT method and was normalized to 18 S rRNA values (n = 4). C and D, the stromal vascular fraction was isolated from the adipose tissue of 8-week-old WT and A2bAR KO mice. mRNA expression of Pref1 (C) and Zfp423 (D) was determined using the ΔΔCT method and normalized to 18 S rRNA values (n = 3 WT, n = 3 A2bAR KO). E, the body weight, adipose tissue mass, and number of adipocytes in the visceral adipose tissue of WT and A2bAR KO 8-week-old male mice were analyzed and calculated as described under “Experimental Procedures” (n = 7 WT, n = 6 A2bAR KO). F, representative H&E-stained section from the visceral adipose tissue of WT and A2bAR KO mice. Data are mean ± S.E. *, p < 0.05 compared with vehicle treatment within the same genotype. Analyses were performed by two-way ANOVA with Bonferroni multiple comparisons post hoc test (A and B) or by Student's t test (C–E).
A2bAR Agonism Increases the Expression of Early Adipogenic Transcription Factors
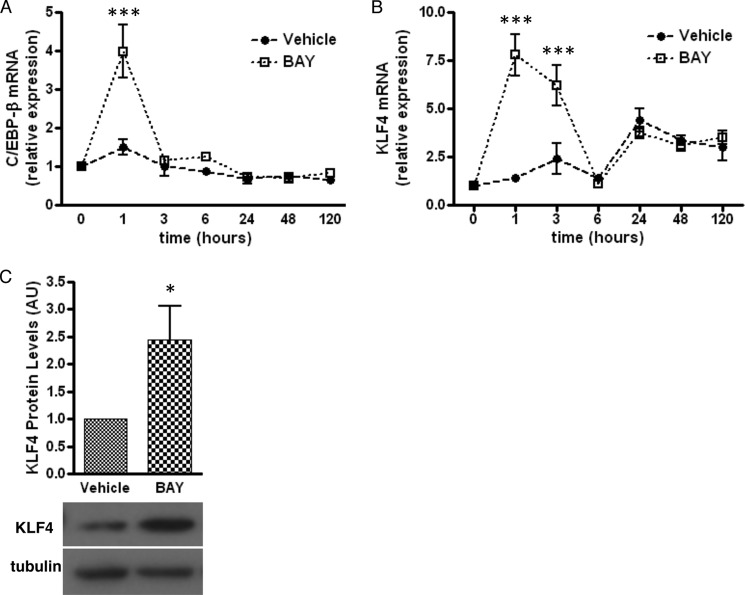
Next, we explored the mechanism by which A2bAR controls the differentiation of mouse SVC preadipocytes into adipocytes. Activation of the A2bAR rapidly and transiently increases the expression of the early differentiation marker C/EBP-β (Fig. 4A). KLF4 is a transcription factor upstream of C/EBP-β (20). As early as 1 h after treatment with BAY 60-6583, KLF4 mRNA levels are 8-fold higher than those of vehicle-treated controls (Fig. 4B). KLF4 protein levels remain 2-fold greater than those of vehicle-treated controls following 2 days of treatment with BAY 60-6583 (Fig. 4C).
FIGURE 4.
A2bAR activation increases the expression of early proadipogenic factors. SVCs were isolated from the subcutaneous adipose tissue of WT mice, treated with DMSO (vehicle) or 1 μm BAY 60-6583 (BAY) on day 0 and day 3 of induction and induced to differentiate as described under “Experimental Procedures.” A and B, mRNA expression of C/EBP-β (A) and KLF4 (B) following induction and agonist treatment was determined using the ΔΔCT method and was normalized to 18 S rRNA values (n = 4). C, Western blot analysis for KLF4 (65 kDa) after 2 days of induction with tubulin (52 kDa) as a loading control (n = 4). Quantification of Western blot analysis results was performed with ImageJ software and normalized to tubulin. AU, arbitrary units. Data are mean ± S.E. *, p < 0.05; ***, p < 0.001 compared with vehicle at the same time point. Analyses were performed by two-way ANOVA with Bonferroni multiple comparison post hoc test (A and B) or by Student's t test (C).
Knockdown of A2bAR in Preadipocytes Enhances Adipocyte Differentiation
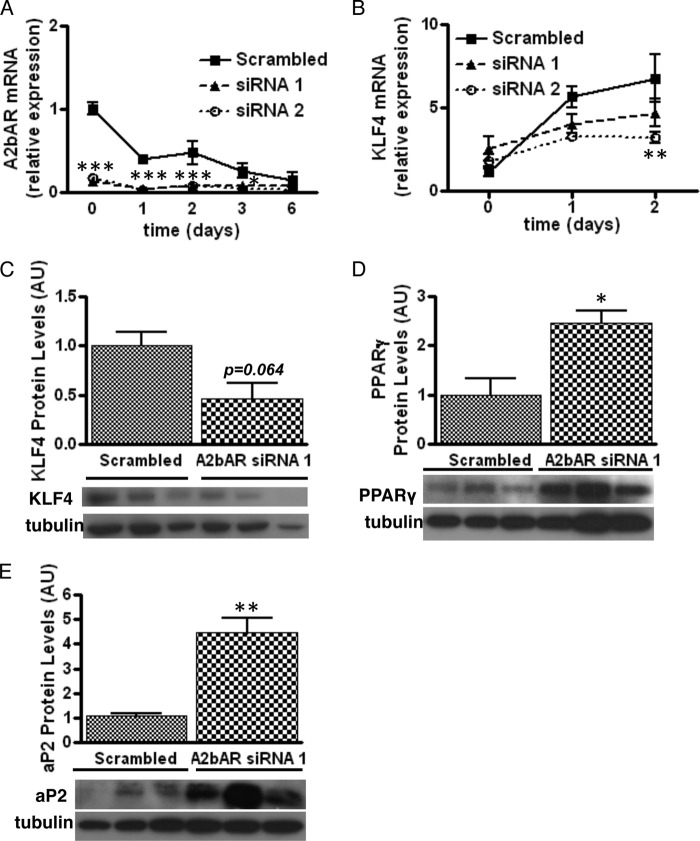
Considering the possibility that SVCs from A2bAR KO mice contain fewer preadipocytes compared with WT samples (Fig. 3, C and D), we were unable to compare the in vitro adipogenic potential of WT and A2bAR KO cells. Therefore, we elected to knock down A2bAR expression in WT SVCs. A2bAR knockdown was more than 80% effective for at least up to 2 days following induction of adipocyte differentiation (Fig. 5A). As expected, knockdown of A2bAR reduced KLF4 mRNA expression and protein levels (Fig. 5, B and C) and increased PPARγ and aP2 protein levels (Fig. 5, D and E).
FIGURE 5.
Lack of A2bAR improves adipocyte differentiation. SVCs were isolated from the subcutaneous adipose tissue of WT mice. siRNA targeting A2bAR was used to knock down A2bAR expression, and then cells were induced to differentiate as described under “Experimental Procedures.” A and B, mRNA expression of A2bAR (A) and KLF4 (B) was determined using the ΔΔCT method and normalized to 18 S rRNA values (n = 4). C–E, Western blot analyses for KLF4 (C) after 1 day of induction and PPARγ (D) and aP2 (E) after 6 days of differentiation with tubulin as loading control (n = 3). Quantification of Western blot results was performed with ImageJ software and normalized to tubulin. AU, arbitrary units. Data are mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with scrambled siRNA at the same time point. Analyses were performed by two-way ANOVA with Bonferroni multiple comparisons post-hoc test (A and B) and by Student's t test (C–E).
A2bAR Activation Inhibits Adipocyte Differentiation via KLF4
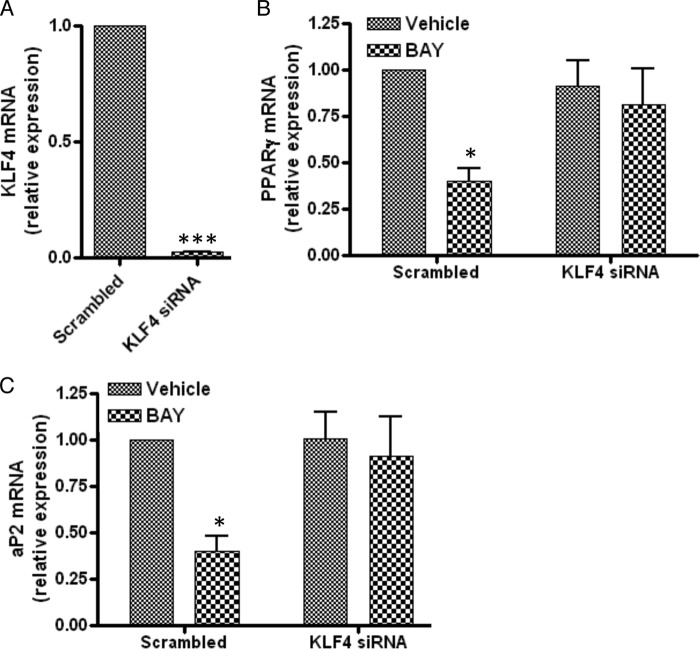
To determine whether A2bAR regulation of KLF4 plays a central role in the ability of A2bAR activation to inhibit adipocyte differentiation, the expression of KLF4 in SVCs was knocked down by siRNA, followed by induction of adipocyte differentiation in the presence and absence of BAY 60-6583. KLF4 expression was reduced significantly following siRNA knockdown (Fig. 6A). Lack of KLF4 prevented BAY 60-6583 from inhibiting the expression of PPARγ (Fig. 6B) and aP2 (Fig. 6C) (note that the incubation time was shorter than in Fig. 2 to examine the cells while the KLF4 was still ablated).
FIGURE 6.
KLF4 is required for the action of BAY 60-6583. SVCs were isolated from the subcutaneous adipose tissue of WT mice. Cells received scrambled siRNA (Scrambled) or siRNA targeting KLF4 (KLF4 siRNA) and were treated with DMSO (Vehicle) or 1 μm BAY 60-6583 (BAY) on day 0 and day 3 of induction and induced to differentiate as described under “Experimental Procedures.” A–C, mRNA expression of KLF4 (A) at 0 h and PPARγ (B) and aP2 (C) after 3 days of differentiation was determined using the ΔΔCT method and normalized to 18 S rRNA values (n = 4). Data are mean ± S.E. *, p < 0.05; ***, p < 0.001 compared with scrambled (A) or vehicle treatment given the same siRNA (B and C). Analyses were performed by Student's t test (A) and by two-way ANOVA with Bonferroni multiple comparisons post hoc test (B and C).
Forskolin and PKA Activation Partially Mimic the Effect of A2bAR Activation on Adipogenesis
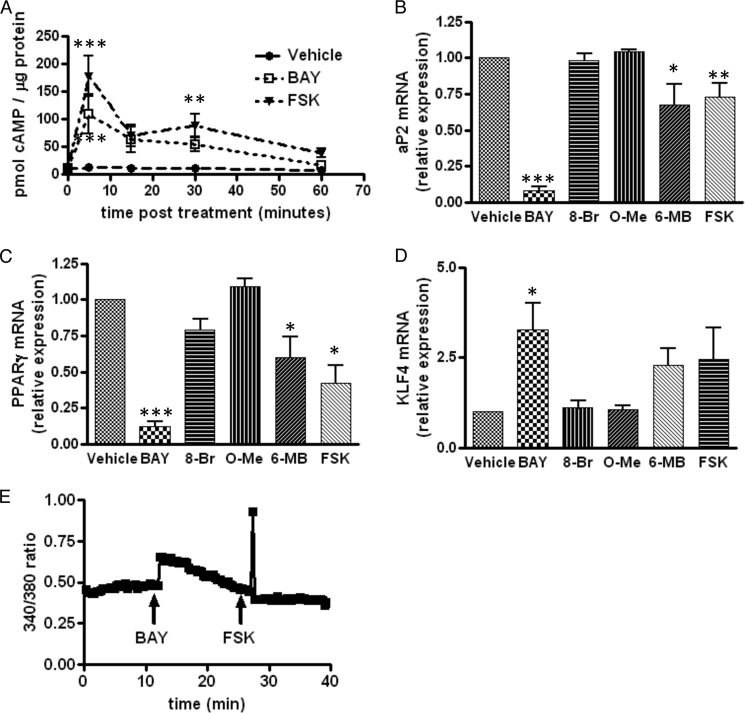
Agonism of the A2bAR activates Gs and adenylyl cyclase to increase cAMP levels (Fig. 7A). Therefore, we sought to determine whether other effectors known to elevate cAMP mimic the inhibitory effect of the A2bAR on adipogenesis. To this end, we employed agonists that directly activate adenylyl cyclase (FSK) or selectively activate PKA (6-MB-cAMP) (37) or EPACs (8-CPT-2′-O-Me-cAMP) (38), two downstream targets of cAMP. Only FSK or activation of PKA by 6-MB-cAMP mimicked, to some extent, the effect of BAY 60-6583 (Fig. 7, B–D). More specifically, 6-MB-cAMP and FSK, but not 8-CPT-2′-O-Me-cAMP, reduced PPARγ and aP2 expression (Fig. 7, B and C). The agonists FSK and 6-MB-cAMP also tended to increase KLF4 expression. The discrepancies in the extent of the effect of BAY 60-6583 and FSK on adipocyte differentiation may be due to differences in the level of cAMP elevation (Fig. 7A). It is also possible that A2bAR activation-mediated changes in intracellular calcium over time, via Gq signaling (39) (Fig. 7E), contribute to its significant inhibitory effect on adipogenesis. Indeed, increased intracellular calcium has been shown to inhibit adipogenesis (40, 41).
FIGURE 7.
PKA and not EPAC activation mimics A2bAR agonism by BAY 60-6583. SVCs were isolated from the subcutaneous adipose tissue of WT mice, treated with DMSO (Vehicle), 1 μm BAY 60-6583 (BAY), 2 μm FSK, 500 μm 8-bromo-cAMP (activates EPAC and PKA, 8-Br), 200 μm 8-CPT-2′-O-Me-cAMP (activates EPAC, O-Me), or 200 μm 6-MB cAMP (activates PKA, 6-MB) on day 0 and day 3 of induction and induced to differentiate as described under “Experimental Procedures.” A, cAMP levels were assessed as described under “Experimental Procedures” (n = 4). B–D, mRNA expression of aP2 (B) and PPARγ (C) after 6 days of differentiation and of KLF4 (D) after 6 h of differentiation was determined using the ΔΔCT method and normalized to 18 S rRNA values (n = 3). E, SVCs were treated with 1 μm BAY 60-6583 and 2 μm FSK. Fluorescence at 340 and 380 nm was assessed throughout the treatments, and the 340:380 nm ratio was calculated using ImageJ software. Data are mean ± S.E. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with vehicle treatment at the same time point. Analyses were performed by two-way ANOVA with Bonferroni multiple comparisons post hoc test (A), by one-way ANOVA with Bonferroni multiple comparisons post hoc test (B and C), or by Student's t test (D).
A2bAR and KLF4 Expression Are Highly Correlated in Human Adipose Tissue
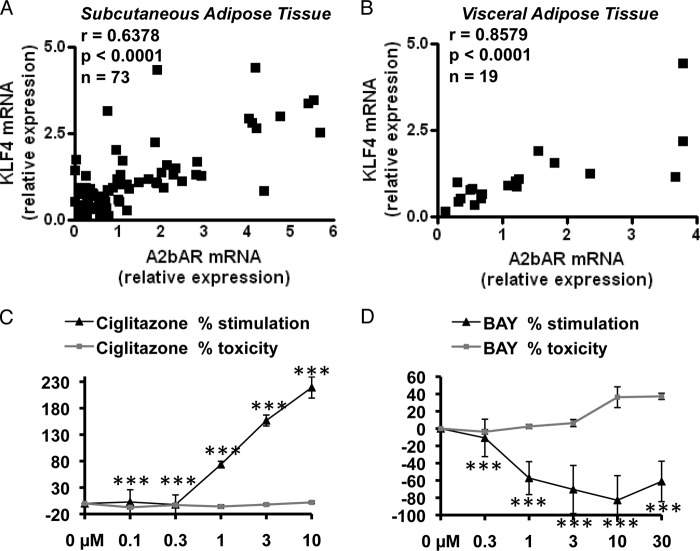
Because we observed a strong relationship between A2bAR and KLF4 in differentiating cultured adipocytes, we next sought to investigate the translational relevance in human disease and examined gene expression in adipose tissue biopsy samples collected from obese humans. Subcutaneous adipose tissue samples from 73 obese individuals (body mass index, 46 ± 1 kg/m2; age, 41 ± 1 years; 80% female) and visceral (omental) fat samples from 19 obese individuals (body mass index, 46 ± 2 kg/m2; age, 43 ± 3 years, 70% female) were available for analysis. We have shown previously that the A2bAR is elevated in the adipose tissue of obese individuals (42). Intriguingly, we now found a strong positive correlation between mRNA expression of A2bAR and KLF4 in both subcutaneous (Fig. 8A) and visceral (Fig. 8B) adipose tissue of obese individuals, as assessed by the Spearman correlation coefficient (r) (42). Of note, using a human primary preadipocyte culture system (isolated as described in Ref. 24), activation of the A2bAR with BAY 60-6583 effectively inhibited adipogenesis (Fig. 8, C and D) in accordance with the mouse culture data.
FIGURE 8.
KLF4 and A2bAR are correlated significantly in human adipose tissue. Subcutaneous (A, n = 73) and visceral (B, n = 19) adipose tissues were obtained from obese individuals. As described under “Experimental Procedures,” mRNA was extracted from the samples, and mRNA expression was determined by using the ΔΔCT method and normalized to 18 S rRNA values. Spearman correlation coefficients (r) were calculated comparing mRNA expression of A2bAR to KLF4. C and D, human preadipocytes were isolated and differentiated to adipocytes for 10 days as described previously (24, 25). During differentiation, cells were treated with increasing concentrations (0, 0.1, 0.3, 1, 3, and 10 μm) of ciglitazone, a thiazolidinedione and potent and selective PPARγ ligand (C), BAY 60-6583 (BAY) (D), or vehicle control (DMSO) every other day during induction. On day 10, adipocytes were stained with BODIPY fluorescent fatty acid (which becomes incorporated into lipid filled cells), and relative fluorescence compared with vehicle treatment was measured using a Tecan microplate reader. Cell viability was determined by CellTiter-Blue assay. The percent stimulation of adipogenesis (measured by adipocyte staining) and toxicity over control treatment was determined for each concentration (n = 3). Data are mean ± S.E. ***, p < 0.001 compared with vehicle treatment at the same concentration. Analyses were performed by Spearman correlation coefficient (A and B) or by Student's t test (C and D).
DISCUSSION
Identifying novel factors that regulate adipocyte differentiation may advance our knowledge of the molecular mechanisms involved in the pathogenesis of obesity. Activation of the A2bAR inhibits adipogenesis. Using siRNA knockdown of KLF4, we show that this effect is dependent on an A2bAR-induced increase in KLF4 levels. A2bAR couples to Gs and increases intracellular cAMP levels. Activating adenylyl cyclase or PKA partially mimics the inhibitory effect of A2bAR on adipogenesis and increases KLF4 levels. Lack of A2bARs promotes adipogenesis both in vitro and in vivo, as demonstrated by A2bAR knockdown of SVCs and determination of the number of adipocytes in the adipose tissue of WT and A2bAR KO mice. Importantly, the association between the A2bAR and KLF4 is relevant in human adipose tissue because there is a strong correlation between A2bAR and KLF4 expression in the adipose tissue of obese individuals.
We propose that A2bAR signaling, via a sustained elevation in KLF4, is an upstream determinant of preadipocyte fate. Activation of the A2bAR inhibits adipocyte differentiation and terminal expression of proadipogenic transcription factors (PPARγ and C/EBP-α) and fat-specific genes (e.g. aP2, perilipin, and adiponectin) and reduces lipid accumulation. A2bAR activation greatly increases the expression of the transcription factor KLF4, which is part of a family of zinc finger transcription factors implicated in the regulation of proliferation and differentiation (43). KLF proteins have been shown to play a role in adipogenesis (44–46). A previous study in 3T3-L1 cells reported that cAMP induced an increase in KLF4 expression soon after induction and that KLF4 enhanced adipocyte differentiation as a coactivator of C/EBP-β expression (20). However, in a supplemental figure, the authors showed that high levels of KLF4 also inhibited adipogenesis and concluded that the level and timing of KLF4 expression need to be regulated tightly during differentiation (20). In this sense, our results are in accordance with this finding, although our studies are performed in primary progenitors isolated from the adipose tissue of mice, which could have a somewhat different response to KLF4 elevation than 3T3-L1 cells. The novelty in our report also resides in showing that the inhibitory action of A2bAR on adipogenesis is dependent on KLF4.
The inhibitory effect of A2bAR on adipocyte differentiation appears to be partially through an increase in cAMP and activation of PKA. The role of cAMP in adipogenesis has been highly debated (47). Because methylisobutylxanthine, a phosphodiesterase inhibitor, is commonly used to induce adipogenesis in culture, cAMP has been considered to be proadipogenic. Furthermore, cAMP response element-binding protein has been shown to be necessary and sufficient for adipogenesis in 3T3-L1 cells by activating adipogenic inducers (like C/EBP-β) and repressing adipogenic inhibitors (48–52). However, several reports have shown that expression of the Gα subunit inhibits adipogenesis in 3T3-L1 and 3T3-F442A cells (53–58). In our studies with primary SVCs, treatment with forskolin, 6-MB-cAMP, or BAY 60-6583 inhibits adipocyte differentiation, albeit to varying degrees. The differential effect of cAMP inducers on adipocyte differentiation may be due to different timing and levels of cAMP induction or due to compartmentalization of the A2bAR, adenylate cyclase, and effector molecules in microdomains (59). Increased intracellular calcium has been shown to inhibit adipogenesis (40, 41, 60). Therefore, the ability of A2bAR to increase cAMP as well as intracellular calcium via coupling to both Gs and Gq (39) may allow a more effective inhibition of adipogenesis.
Importantly, we show that A2bAR activation inhibits adipocyte differentiation from human preadipocyte precursors. Furthermore, A2bAR and KLF4 expression are strongly correlated in the subcutaneous and visceral adipose tissue of obese individuals. This suggests that the A2bAR-KLF4 axis is relevant in human pathology, and modifying the activity of the receptor may be a potential therapeutic avenue in controlling KLF4 levels and adipogenesis. In light of evolving research regarding the use of A2bAR agonists and antagonists in treating human disease (61, 62), it is important to consider the other consequences of modulating A2bAR signaling in vivo.
Adenosine is released from adipocytes within the adipose tissue (7, 63). ATP, which is released from cells following inflammation and ischemia, is converted to adenosine extracellularly by ectonucleotidases, which are present on the extracellular membrane of adipocytes (64, 65). Adenosine, in this sense, is a cell stress sensor and may act to prevent adipocyte differentiation during times when there is local cellular stress.
It has been shown that changes in bone and fat masses are correlated inversely (66–69). Considering our previous report of enhanced osteoblast differentiation by A2bAR signaling (70) and our current finding that activation of the A2bAR inhibits adipogenesis and increases the expression of KLF4, a stem cell factor, it follows that the A2bAR may be an important mediator of lineage determination. A comparison of the gene expression profiles of differentiated fibroblasts and human mesenchymal stem cells (bone marrow- and adipose tissue-derived) identified KLF4 as a key gene regulating stem cell fate (71). Therefore, our study suggests that signaling through the A2bAR is important in modulating KLF4 expression and, consequently, stem cell determination.
Taken together, the strong relationship between the A2bAR and KLF4 in human adipose tissue may have significant implications for adipose tissue biology and pathology. Furthermore, the newly identified link between A2bAR and KLF4 expression suggests a larger role for the A2bAR in coordinating and regulating stem cell determination and differentiation. Hence, our study implicates the A2bAR as a regulator of adipocyte differentiation and, on a broader scale, stem cell fate.
Acknowledgments
We thank Dr. Susan Fried for discussions and insights regarding methods of measurement of fat tissue cell number, Dr. Sarah Haigh Molina and Ada Kane at the Boston University School of Medicine High Throughput Core (fee-for-service core), and the Boston Nutrition Obesity Research Center at the Boston University School of Medicine for assistance with the analysis of human adipocyte cultures.
This work was supported, in whole or in part, by NHLBI, National Institutes of Health Grant HL93149 (to K. R.) and National Institutes of Health Grant R01HL114675-02) (to N. G.). This work was also supported by Boston Nutrition Obesity Research Center Pilot Grant DK046200 (to K. R.).
- A2aAR
- A2b adenosine receptor
- A2bAR
- A2b adenosine receptor
- C/EBP
- CCAAT/enhancer binding protein
- PPAR
- peroxisome proliferator-activated receptor
- SVC
- stromal vascular cell
- FSK
- forskolin
- EPAC
- exchange protein activated by cAMP
- ANOVA
- analysis of variance
- DMSO
- dimethyl sulfoxide.
REFERENCES
- 1. Flegal K. M., Carroll M. D., Ogden C. L., Curtin L. R. (2010) Prevalence and trends in obesity among US adults, 1999–2008. JAMA 303, 235–241 [DOI] [PubMed] [Google Scholar]
- 2. Malnick S. D., Knobler H. (2006) The medical complications of obesity. QJM 99, 565–579 [DOI] [PubMed] [Google Scholar]
- 3. Orpana H. M., Berthelot J. M., Kaplan M. S., Feeny D. H., McFarland B., Ross N. A. (2010) BMI and mortality: results from a national longitudinal study of Canadian adults. Obesity 18, 214–218 [DOI] [PubMed] [Google Scholar]
- 4. Rosen E. D., MacDougald O. A. (2006) Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 [DOI] [PubMed] [Google Scholar]
- 5. Tang W., Zeve D., Suh J. M., Bosnakovski D., Kyba M., Hammer R. E., Tallquist M. D., Graff J. M. (2008) White fat progenitor cells reside in the adipose vasculature. Science 322, 583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodeheffer M. S., Birsoy K., Friedman J. M. (2008) Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 [DOI] [PubMed] [Google Scholar]
- 7. Fredholm B. B. (1976) Release of adenosine-like material from isolated perfused dog adipose tissue following sympathetic nerve stimulation and its inhibition by adrenergic α-receptor blockade. Acta Physiol. Scand. 96, 122–130 [DOI] [PubMed] [Google Scholar]
- 8. Schwabe U., Ebert R., Erbler H. C. (1973) Adenosine release from isolated fat cells and its significance for the effects of hormones on cyclic 3′,5′-AMP levels and lipolysis. Naunyn Schmiedebergs Arch. Pharmacol. 276, 133–148 [DOI] [PubMed] [Google Scholar]
- 9. Vassaux G., Gaillard D., Mari B., Ailhaud G., Negrel R. (1993) Differential expression of adenosine A1 and A2 receptors in preadipocytes and adipocytes. Biochem. Biophys. Res. Commun. 193, 1123–1130 [DOI] [PubMed] [Google Scholar]
- 10. Børglum J. D., Vassaux G., Richelsen B., Gaillard D., Darimont C., Ailhaud G., Négrel R. (1996) Changes in adenosine A1- and A2-receptor expression during adipose cell differentiation. Mol. Cell Endocrinol. 117, 17–25 [DOI] [PubMed] [Google Scholar]
- 11. Cao Z., Umek R. M., McKnight S. L. (1991) Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5, 1538–1552 [DOI] [PubMed] [Google Scholar]
- 12. Darlington G. J., Ross S. E., MacDougald O. A. (1998) The role of C/EBP genes in adipocyte differentiation. J. Biol. Chem. 273, 30057–30060 [DOI] [PubMed] [Google Scholar]
- 13. Yeh W. C., Cao Z., Classon M., McKnight S. L. (1995) Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 9, 168–181 [DOI] [PubMed] [Google Scholar]
- 14. Hamm J. K., Park B. H., Farmer S. R. (2001) A role for C/EBPβ in regulating peroxisome proliferator-activated receptor γ activity during adipogenesis in 3T3-L1 preadipocytes. J. Biol. Chem. 276, 18464–18471 [DOI] [PubMed] [Google Scholar]
- 15. Wu Z., Bucher N. L., Farmer S. R. (1996) Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Mol. Cell Biol. 16, 4128–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kubota N., Terauchi Y., Miki H., Tamemoto H., Yamauchi T., Komeda K., Satoh S., Nakano R., Ishii C., Sugiyama T., Eto K., Tsubamoto Y., Okuno A., Murakami K., Sekihara H., Hasegawa G., Naito M., Toyoshima Y., Tanaka S., Shiota K., Kitamura T., Fujita T., Ezaki O., Aizawa S., Kadowaki T. (1999) PPAR γ mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol. Cell 4, 597–609 [DOI] [PubMed] [Google Scholar]
- 17. Tontonoz P., Hu E., Devine J., Beale E. G., Spiegelman B. M. (1995) PPAR γ 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol. Cell Biol. 15, 351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tontonoz P., Hu E., Spiegelman B. M. (1994) Stimulation of adipogenesis in fibroblasts by PPAR γ 2, a lipid-activated transcription factor. Cell 79, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 19. Tontonoz P., Hu E., Spiegelman B. M. (1995) Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor γ. Curr. Opin. Genet. Dev. 5, 571–576 [DOI] [PubMed] [Google Scholar]
- 20. Birsoy K., Chen Z., Friedman J. (2008) Transcriptional regulation of adipogenesis by KLF4. Cell Metab. 7, 339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smas C. M., Sul H. S. (1993) Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell 73, 725–734 [DOI] [PubMed] [Google Scholar]
- 22. Yang D., Zhang Y., Nguyen H. G., Koupenova M., Chauhan A. K., Makitalo M., Jones M. R., St Hilaire C., Seldin D. C., Toselli P., Lamperti E., Schreiber B. M., Gavras H., Wagner D. D., Ravid K. (2006) The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 116, 1913–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hausman D. B., Park H. J., Hausman G. J. (2008) Isolation and culture of preadipocytes from rodent white adipose tissue. Methods Mol. Biol. 456, 201–219 [DOI] [PubMed] [Google Scholar]
- 24. Lee M. J., Wu Y., Fried S. K. (2012) A modified protocol to maximize differentiation of human preadipocytes and improve metabolic phenotypes. Obesity 20, 2334–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu G., Floyd Z. E., Wu X., Hebert T., Halvorsen Y. D., Buehrer B. M., Gimble J. M. (2011) Adipogenic differentiation of adipose-derived stem cells. Methods Mol. Biol. 702, 193–200 [DOI] [PubMed] [Google Scholar]
- 26. Fink T., Zachar V. (2011) Adipogenic differentiation of human mesenchymal stem cells. Methods Mol. Biol. 698, 243–251 [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y., Sun S., Wang Z., Thompson A., Kaluzhny Y., Zimmet J., Ravid K. (2002) Signaling by the Mpl receptor involves IKK and NF-κB. J. Cell Biochem. 85, 523–535 [DOI] [PubMed] [Google Scholar]
- 28. Galarraga M., Campión J., Muñoz-Barrutia A., Boqué N., Moreno H., Martínez J. A., Milagro F., Ortiz-de-Solórzano C. (2012) Adiposoft: automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 53, 2791–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Girolamo M., Mendlinger S., Fertig J. W. (1971) A simple method to determine fat cell size and number in four mammalian species. Am. J. Physiol. 221, 850–858 [DOI] [PubMed] [Google Scholar]
- 30. Folch J., Lebaron F. N. (1956) The isolation from brain tissue of a trypsin-resistant protein fraction containing combined inositol, and its relation to neurokeratin. J. Neurochem. 1, 101–108 [DOI] [PubMed] [Google Scholar]
- 31. Farb M. G., Bigornia S., Mott M., Tanriverdi K., Morin K. M., Freedman J. E., Joseph L., Hess D. T., Apovian C. M., Vita J. A., Gokce N. (2011) Reduced adipose tissue inflammation represents an intermediate cardiometabolic phenotype in obesity. J. Am. Coll. Cardiol. 58, 232–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koupenova M., Johnston-Cox H., Vezeridis A., Gavras H., Yang D., Zannis V., Ravid K. (2012) A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 125, 354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta R. K., Arany Z., Seale P., Mepani R. J., Ye L., Conroe H. M., Roby Y. A., Kulaga H., Reed R. R., Spiegelman B. M. (2010) Transcriptional control of preadipocyte determination by Zfp423. Nature 464, 619–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Joe A. W., Yi L., Even Y., Vogl A. W., Rossi F. M. (2009) Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 27, 2563–2570 [DOI] [PubMed] [Google Scholar]
- 35. Vroegrijk I. O., van Klinken J. B., van Diepen J. A., van den Berg S. A., Febbraio M., Steinbusch L. K., Glatz J. F., Havekes L. M., Voshol P. J., Rensen P. C., van Dijk K. W., van Harmelen V. (2013) CD36 is important for adipocyte recruitment and affects lipolysis. Obesity 21, 2037–2045 [DOI] [PubMed] [Google Scholar]
- 36. Tang W., Zeve D., Seo J., Jo A. Y., Graff J. M. (2011) Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 14, 116–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kopperud R., Krakstad C., Selheim F., Døskeland S. O. (2003) cAMP effector mechanisms. Novel twists for an “old” signaling system. FEBS Lett. 546, 121–126 [DOI] [PubMed] [Google Scholar]
- 38. Enserink J. M., Christensen A. E., de Rooij J., van Triest M., Schwede F., Genieser H. G., Døskeland S. O., Blank J. L., Bos J. L. (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat. Cell Biol. 4, 901–906 [DOI] [PubMed] [Google Scholar]
- 39. Linden J., Thai T., Figler H., Jin X., Robeva A. S. (1999) Characterization of human A(2B) adenosine receptors: radioligand binding, Western blotting, and coupling to G(q) in human embryonic kidney 293 cells and HMC-1 mast cells. Mol. Pharmacol. 56, 705–713 [PubMed] [Google Scholar]
- 40. Graham S. J., Black M. J., Soboloff J., Gill D. L., Dziadek M. A., Johnstone L. S. (2009) Stim1, an endoplasmic reticulum Ca2+ sensor, negatively regulates 3T3-L1 pre-adipocyte differentiation. Differentiation 77, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ntambi J. M., Takova T. (1996) Role of Ca2+ in the early stages of murine adipocyte differentiation as evidenced by calcium mobilizing agents. Differentiation 60, 151–158 [DOI] [PubMed] [Google Scholar]
- 42. Johnston-Cox H., Koupenova M., Yang D., Corkey B., Gokce N., Farb M. G., LeBrasseur N., Ravid K. (2012) The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS ONE 7, e40584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghaleb A. M., Nandan M. O., Chanchevalap S., Dalton W. B., Hisamuddin I. M., Yang V. W. (2005) Kruppel-like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 15, 92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li D., Yea S., Li S., Chen Z., Narla G., Banck M., Laborda J., Tan S., Friedman J. M., Friedman S. L., Walsh M. J. (2005) Kruppel-like factor-6 promotes preadipocyte differentiation through histone deacetylase 3-dependent repression of DLK1. J. Biol. Chem. 280, 26941–26952 [DOI] [PubMed] [Google Scholar]
- 45. Mori T., Sakaue H., Iguchi H., Gomi H., Okada Y., Takashima Y., Nakamura K., Nakamura T., Yamauchi T., Kubota N., Kadowaki T., Matsuki Y., Ogawa W., Hiramatsu R., Kasuga M. (2005) Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J. Biol. Chem. 280, 12867–12875 [DOI] [PubMed] [Google Scholar]
- 46. Oishi Y., Manabe I., Tobe K., Tsushima K., Shindo T., Fujiu K., Nishimura G., Maemura K., Yamauchi T., Kubota N., Suzuki R., Kitamura T., Akira S., Kadowaki T., Nagai R. (2005) Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 1, 27–39 [DOI] [PubMed] [Google Scholar]
- 47. Eisenstein A., Ravid K. (2013) G protein-coupled receptors and adipogenesis: a focus on adenosine receptors. J. Cell. Physiol. 229, 414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reusch J. E., Colton L. A., Klemm D. J. (2000) CREB activation induces adipogenesis in 3T3-L1 cells. Mol. Cell Biol. 20, 1008–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fox K. E., Fankell D. M., Erickson P. F., Majka S. M., Crossno J. T., Jr., Klemm D. J. (2006) Depletion of cAMP-response element-binding protein/ATF1 inhibits adipogenic conversion of 3T3-L1 cells ectopically expressing CCAAT/enhancer-binding protein (C/EBP) α, C/EBP β, or PPAR γ 2. J. Biol. Chem. 281, 40341–40353 [DOI] [PubMed] [Google Scholar]
- 50. Zhang J. W., Klemm D. J., Vinson C., Lane M. D. (2004) Role of CREB in transcriptional regulation of CCAAT/enhancer-binding protein β gene during adipogenesis. J. Biol. Chem. 279, 4471–4478 [DOI] [PubMed] [Google Scholar]
- 51. Tang Y., Osawa H., Onuma H., Nishimiya T., Ochi M., Makino H. (1999) Improvement in insulin resistance and the restoration of reduced phosphodiesterase 3B gene expression by pioglitazone in adipose tissue of obese diabetic KKAy mice. Diabetes 48, 1830–1835 [DOI] [PubMed] [Google Scholar]
- 52. Fox K. E., Colton L. A., Erickson P. F., Friedman J. E., Cha H. C., Keller P., MacDougald O. A., Klemm D. J. (2008) Regulation of cyclin D1 and Wnt10b gene expression by cAMP-responsive element-binding protein during early adipogenesis involves differential promoter methylation. J. Biol. Chem. 283, 35096–35105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang H., Johnson G. L., Liu X., Malbon C. C. (1996) Repression of adipogenesis by adenylyl cyclase stimulatory G-protein α subunit is expressed within region 146–220. J. Biol. Chem. 271, 22022–22029 [DOI] [PubMed] [Google Scholar]
- 54. Liu X., Malbon C. C., Wang H. Y. (1998) Identification of amino acid residues of Gsα critical to repression of adipogenesis. J. Biol. Chem. 273, 11685–11694 [DOI] [PubMed] [Google Scholar]
- 55. Wang H., Malbon C. C. (1999) G(s)α repression of adipogenesis via Syk. J. Biol. Chem. 274, 32159–32166 [DOI] [PubMed] [Google Scholar]
- 56. Denis-Henriot D., de Mazancourt P., Morot M., Giudicelli Y. (1998) Mutant α-subunit of the G protein G12 activates proliferation and inhibits differentiation of 3T3-F442A preadipocytes. Endocrinology 139, 2892–2899 [DOI] [PubMed] [Google Scholar]
- 57. Zhang L., Paddon C., Lewis M. D., Grennan-Jones F., Ludgate M. (2009) Gsα signalling suppresses PPARγ2 generation and inhibits 3T3L1 adipogenesis. J. Endocrinol. 202, 207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang H. Y., Watkins D. C., Malbon C. C. (1992) Antisense oligodeoxynucleotides to GS protein α-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature 358, 334–337 [DOI] [PubMed] [Google Scholar]
- 59. Sitaraman S. V., Wang L., Wong M., Bruewer M., Hobert M., Yun C. H., Merlin D., Madara J. L. (2002) The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation. J. Biol. Chem. 277, 33188–33195 [DOI] [PubMed] [Google Scholar]
- 60. Kim Y., Kelly O. J., Ilich J. Z. (2013) Synergism of α-linolenic acid, conjugated linoleic acid and calcium in decreasing adipocyte and increasing osteoblast cell growth. Lipids 48, 787–802 [DOI] [PubMed] [Google Scholar]
- 61. Gao Z. G., Jacobson K. A. (2011) Emerging adenosine receptor agonists: an update. Expert Opin. Emerg. Drugs 16, 597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Haskó G., Linden J., Cronstein B., Pacher P. (2008) Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7, 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fredholm B. B., Sollevi A. (1981) The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J. Physiol. 313, 351–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eltzschig H. K., Carmeliet P. (2011) Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fredholm B. B. (2007) Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 14, 1315–1323 [DOI] [PubMed] [Google Scholar]
- 66. Kawai M., Devlin M. J., Rosen C. J. (2009) Fat targets for skeletal health. Nat. Rev. Rheumatol. 5, 365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tuominen J. T., Impivaara O., Puukka P., Rönnemaa T. (1999) Bone mineral density in patients with type 1 and type 2 diabetes. Diabetes Care 22, 1196–1200 [DOI] [PubMed] [Google Scholar]
- 68. Meunier P., Aaron J., Edouard C., Vignon G. (1971) Osteoporosis and the replacement of cell populations of the marrow by adipose tissue: a quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 80, 147–154 [DOI] [PubMed] [Google Scholar]
- 69. Rozman C., Feliu E., Berga L., Reverter J. C., Climent C., Ferrán M. J. (1989) Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp. Hematol. 17, 34–37 [PubMed] [Google Scholar]
- 70. Carroll S. H., Wigner N. A., Kulkarni N., Johnston-Cox H., Gerstenfeld L. C., Ravid K. (2012) A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J. Biol. Chem. 287, 15718–15727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Saulnier N., Puglisi M. A., Lattanzi W., Castellini L., Pani G., Leone G., Alfieri S., Michetti F., Piscaglia A. C., Gasbarrini A. (2011) Gene profiling of bone marrow- and adipose tissue-derived stromal cells: a key role of Kruppel-like factor 4 in cell fate regulation. Cytotherapy 13, 329–340 [DOI] [PubMed] [Google Scholar]