Abstract
Biotin-responsive basal ganglia disease (BBGD) is an autosomal recessive disorder, which is caused by mutations in the SLC19A3 gene. BBGD typically causes (sub)acute episodes with encephalopathy and subsequent neurological deterioration. If untreated, the clinical course may be fatal. Our report on a 6-year-old child with BBGD highlights that the disease is a crucial differential diagnosis of Leigh syndrome. Therefore, biotin and thiamine treatment is recommended for any patient with symmetrical basal ganglia lesions and neurological symptoms until BBGD is excluded. In addition, we exemplify that deformation-field-based morphometry of brain magnetic resonance images constitutes a novel quantitative tool, which might be very useful to monitor disease course and therapeutic effects in neurometabolic disorders.
Electronic supplementary material: The online version of this chapter (doi:10.1007/8904_2013_271) contains supplementary material, which is available to authorized users.
Introduction
Biotin-responsive basal ganglia disease (BBGD; OMIM 607483) is a rare autosomal recessive disorder, which is caused by mutations in the SLC19A3 gene, encoding a thiamine transporter (hTHTR2). The disease was first described in 1998 and later genetically characterized in 2005 (Ozand et al. 1998; Zeng et al. 2005). The first patients reported were of Saudi, Syrian, or Yemeni ancestry. However, in the following, also European and Japanese patients were identified, indicating that BBGD is a pan-ethnic disease (Debs et al. 2010; Tabarki et al. 2013). So far, the influence of SLC19A3 mutations on hTHTR2 function (i.e., transporter functionality, protein stability, targeting, or transport activity) and their impact on cell biology are unresolved (Subramanian et al. 2006).
BBGD frequently manifests in childhood with (sub)acute episodes of encephalopathy, dystonia, dysarthria, and seizures. If untreated, the clinical course may be fatal (Tabarki et al. 2013; Alfadhel et al. 2013). Brain magnetic resonance imaging (MRI) typically demonstrates bilateral hyperintensity of caudate nucleus and putamen on T2-weighted sequences. In addition, especially during acute neurometabolic crisis, diffuse cortical and subcortical changes are characteristic, which are thought to be caused by vasogenic edema (Tabarki et al. 2013).
Treatment of BBGD consists of high doses of biotin and additional thiamine. The efficacy of biotin treatment is still unclear because hTHTR2 is unable to transport biotin. However, it has been shown that biotin regulates gene expression of SLC19A3 (Debs et al. 2010). Therefore, a biotin-induced upregulation of hTHTR2 levels in combination with increased thiamine supplementation are most likely the therapeutic mechanisms.
Case Report
Here, we report on a 6-year-old girl, born to consanguineous Moroccan parents. She was the third child of the family. Two older sisters suffered from a progressive neurodegenerative disorder, which was classified as Leigh syndrome because of symmetrical brain lesions in the basal ganglia.
Leigh syndrome is a devastating disease caused by direct or indirect impairment of mitochondrial oxidative phosphorylation. The disorder typically causes symmetrical lesions in basal ganglia and/or brain stem and leads to a clinical course with rapid deterioration of cognitive and motor functions (Baertling et al. 2013).
However, in these two girls, biochemical diagnostics did not reveal any clear disturbance of mitochondrial metabolism. Both girls died during the course of the disease.
The girl reported here developed normal until the age of 3½ years. At that time, she suffered from a first encephalopathic episode with somnolence, dystonia, and dysarthria. Metabolic work-up was normal (including organic acids in urine, amino acids in blood and cerebrospinal fluid, acylcarnitines, etc.). Initial brain MRI showed symmetric basal ganglia lesions. In view of family history, Leigh syndrome was diagnosed.
At the age of 6 years, the child was seen at our department during an acute metabolic crisis (including somnolence and seizures). Brain MRI showed the previously described basal ganglia lesions (Fig. 1 a–b). In addition, single voxel spectroscopy demonstrated an intense lactate peak within the affected areas (see Supplementary Fig. 1). However, MRI also showed diffuse cortical and subcortical changes in addition to basal ganglia lesions, which appeared rather atypical for classical Leigh syndrome (Fig. 1 a–b). This finding brought up the differential diagnosis BBGD. Accordingly, the girl was treated with high-dose biotin (10 mg/kg/day) and additional thiamine (100 mg/day). This medication improved her clinical status dramatically. The child recovered from somnolence within several hours and seizures subsided completely.
Fig. 1.
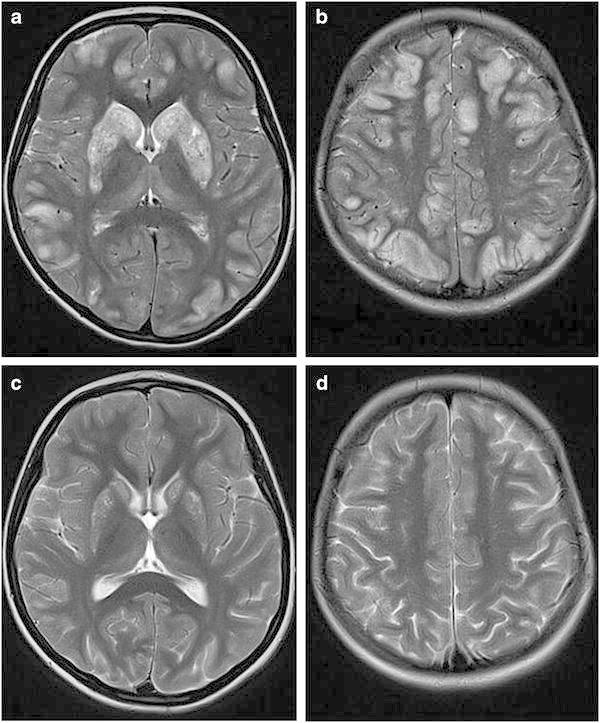
MRI findings in biotin-responsive basal ganglia disease. (a–b), T2-weighted sequences during acute metabolic crisis demonstrating bilateral hyperintensity and swelling of caudate nucleus and putamen as well as abnormal signals in cortical-subcortical areas. (c–d) T2-weighted sequences 3 months after start of treatment with biotin and thiamine showing clear improvement of basal ganglia alterations and disappearance of cortical abnormalities
Molecular genetic studies confirmed BBGD, demonstrating a mutation in the SLC19A3 gene (c.1264G>A, p.T422A, in exon 5; mutation previously described by Zeng et al. 2005). In addition, molecular genetic studies were also carried out in cultured skin fibroblasts of the oldest sister, which allowed a postmortem diagnosis also in this child.
The further clinical course of the girl was favorable. Follow-up brain MRI, 3 months after the acute metabolic crisis revealed a clear regression of lesions in basal ganglia and other cortical and subcortical regions (Fig. 1 c–d).
In order to enable a quantitative evaluation of the disease course with respect to different brain areas, we applied deformation-field-based morphometry of the MR scans and cytoarchitectonic probabilistic maps to localize volume changes (Fig. 2; Pieperhoff et al. 2008). The superimposition of the MRI scans obtained prior and after 3 months of therapy showed a widespread involvement of brain regions, extending those of the previously identified changes in the caudate nucleus and putamen. The caudate nucleus and putamen were among those regions with most pronounced volume decrease under therapy. Other regions with volume decrease were the fundus striati, the ventral globus pallidus, the pulvinar of the thalamus, the periaqueductal gray matter, but also cortical areas PG and PGa of the angular gyrus. Volume increases were found in the ventricles, but also in the thalamus (paratenial nucleus, mammillary body, ventral anterior nuclei, and habenula).
Fig. 2.
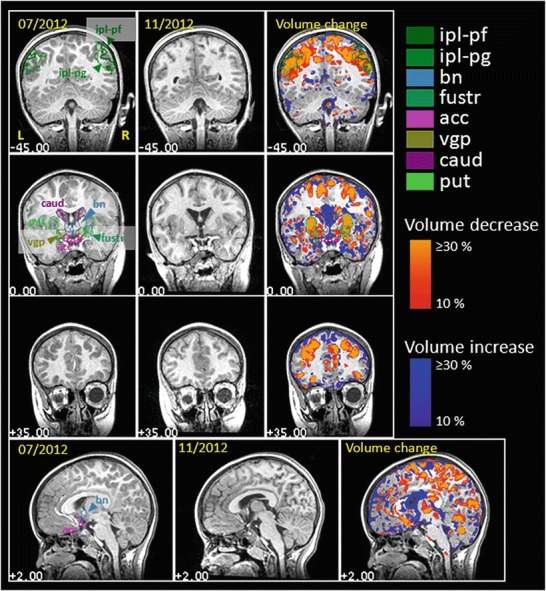
Deformation-field-based morphometry of the MR scans. Volume changes in time as assed by means of deformation-field-based morphometry (DFM, Pieperhoff et al. 2008). The first and second pictures in each row show MR sections, which were acquired within an interval of ~3 months. The third picture in each row shows the difference in volume as compared to the first MR image, where the brain was imaged for the first time. Volume decreases and increases of more than 10 % are colored in red to yellow, and in blue, respectively. Contours of the following anatomical regions are labeled: ipl-pf, ipl-pg = areas PF and PG of the inferior parietal lobule (Caspers et al. 2008); bn bed nucleus, fustr fundus striati, acc ncl. accumbens, vgp ventral globus pallidus, caud ncl. caudatus, put putamen
These findings are in accordance with current MRI studies on BBGD (Tabarki et al. 2013) and indicate that brain pathology of the disease is much more complex than previously thought. The volume changes observed most probably constitute a combination of recovery processes (e.g., disappearance of vasogenic edema and normalization of cell metabolism) and residual tissue damage/atrophy.
Discussion
In conclusion, our report highlights that BBGD is an important treatable differential diagnosis of Leigh syndrome. The clinical picture may completely mimic a mitochondrial disorder, including lactic acidemia (which was present in one of the girls during acute metabolic crisis) and detection of a lactate peak on magnetic resonance spectroscopy (as demonstrated in the index patient). Importantly, atypical brain imaging findings (i.e., cortical signal alterations; indications of vasogenic edema) should alert clinicians to the differential diagnosis BBGD. Generally, biotin and thiamine treatment is suggested in any patient with symmetrical basal ganglia lesions and neurological symptoms until BBGD is excluded or a mitochondrial dysfunction has been clearly identified. Moreover, our morphometry analysis exemplifies that this novel quantitative tool might be very useful to monitor disease course and therapeutic effects in neurometabolic disorders.
Electronic Supplementary Material
Supplementary Fig. 1 Single voxel spectroscopy during acute metabolic crisis, demonstrating an intense lactate peak at 1.35 ppm and a reduced N-acetyl-aspartate peak
Synopsis
Biotin and thiamine treatment is lifesaving in patients with biotin-responsive basal ganglia disease.
Compliance with Ethics Guidelines
Conflict of Interest
Felix Distelmaier, Peter Huppke, Peter Pieperhoff, Katrin Amunts, Jörg Schaper, Eva Morava, Ertan Mayatepek, Jürgen Kohlhase, and Michael Karenfort declare that they have no conflict of interest.
Informed Consent
Additional informed consent was obtained from all patients, for which identifying information is included in this article.
Animal Rights
This article does not contain any studies with animal subjects performed by any of the authors.
Footnotes
Competing interests: None declared
Contributor Information
Felix Distelmaier, Email: felix.distelmaier@med.uni-duesseldorf.de1.
Collaborators: Johannes Zschocke and K Michael Gibson
References
- Alfadhel M, Almuntashri M, Jadah RH, et al. Biotin-responsive basal ganglia disease should be renamed biotin-thiamine-responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J Rare Dis. 2013;8:83. doi: 10.1186/1750-1172-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baertling F, Rodenburg RJ, Schaper J, et al (2013) A guide to diagnosis and treatment of Leigh syndrome. J Neurol Neurosurg Psychiatry Jun 14. [Epub ahead of print]. doi:10.1136/jnnp-2012-304426 [DOI] [PubMed]
- Caspers S, Eickhoff SB, Geyer S, et al. The human inferior parietal lobule in stereotaxic space. Brain Struct Funct. 2008;212:481–495. doi: 10.1007/s00429-008-0195-z. [DOI] [PubMed] [Google Scholar]
- Debs R, Depienne C, Rastetter A, et al. Biotin-responsive basal ganglia disease in ethnic Europeans with novel SLC19A3 mutations. Arch Neurol. 2010;67:126–130. doi: 10.1001/archneurol.2009.293. [DOI] [PubMed] [Google Scholar]
- Ozand PT, Gascon GG, Al Essa M, et al. Biotin-responsive basal ganglia disease: a novel entity. Brain. 1998;121:1267–1279. doi: 10.1093/brain/121.7.1267. [DOI] [PubMed] [Google Scholar]
- Pieperhoff P, Südmeyer M, Hömke L, Zilles K, Schnitzler A, Amunts K. Detection of structural changes of the human brain in longitudinally acquired MR images by deformation field morphometry: methodological analysis, validation and application. NeuroImage. 2008;43:269–287. doi: 10.1016/j.neuroimage.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Subramanian VS, Marchant JS, Said HM. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR2: biotin is not a substrate for hTHTR2. Am J Physiol Cell Physiol. 2006;291:C851–C859. doi: 10.1152/ajpcell.00105.2006. [DOI] [PubMed] [Google Scholar]
- Tabarki B, Al-Shafi S, Al-Shahwan S, et al. Biotin-responsive basal ganglia disease revisited: clinical, radiologic, and genetic findings. Neurology. 2013;80:261–267. doi: 10.1212/WNL.0b013e31827deb4c. [DOI] [PubMed] [Google Scholar]
- Zeng WQ, Al-Yamani E, Acierno JS, Jr, et al. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am J Hum Genet. 2005;77:16–26. doi: 10.1086/431216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 Single voxel spectroscopy during acute metabolic crisis, demonstrating an intense lactate peak at 1.35 ppm and a reduced N-acetyl-aspartate peak


